Abstract
Neurogenic inflammation is induced by inflammatory mediators released in peripheral tissue from primary afferent nociceptors. Our previous studies suggest that neurogenic inflammation induced by intradermal injection of capsaicin results from the enhancement of dorsal root reflexes (DRRs), which involve antidromic activation of dorsal root ganglion (DRG) neurons. Numerous studies have reported the important role of glial modulation in pain. However, it remains unclear whether glial cells participate in the process of neurogenic inflammation-induced pain. Here we tested the role of DRG satellite glial cells (SGCs) in this process in anesthetized rats by administration of a glial inhibitor, minocycline. Electrical stimuli (ES, frequency 10 Hz; duration 1 ms; strength 3 mA) were applied to the cut distal ends of the L4-5 dorsal roots. The stimuli evoked antidromic action potentials designed to mimic DRRs. Local cutaneous blood flow in the hindpaw was measured using a Doppler flow meter. Antidromic ES for 10 min evoked a significant vasodilation that could be inhibited dose-dependently by local administration of the calcitonin gene-related peptide receptor antagonist, CGRP8-37. Pretreatment with capsaicin intradermally injected into the hindpaw 2 h before the ES enhanced greatly the vasodilation evoked by antidromic ES, and this enhancement could be reversed by minocycline pretreatment. Our findings support the view that neurogenic inflammation following capsaicin injection involves antidromic activation of DRG neurons via the generation of DRRs. Inhibition of neurogenic inflammation by minocycline is suggested to be associated with its inhibitory effect on SGCs that are possibly activated following capsaicin injection.
Keywords: neurogenic inflammation, capsaicin, minocycline, satellite glial cells
Neurogenic inflammation is produced by overstimulation of primary afferent nociceptive terminals due to injury or inflammation of peripheral tissues [35]. Upon stimulation, a variety of inflammatory mediators, such as neuropeptides, are released from terminals of these nociceptors [21,28]. Neuropeptides, including calcitonin gene-related peptide (CGRP) and substance P (SP), are synthesized and stored in medium- and small-sized primary afferent nociceptive neurons with lightly myelinated Aδ and unmyelinated C axonal fibers in dorsal root ganglia (DRG). It has been accepted by our and other groups that the release of neuropeptides is driven by antidromic stimulation of primary afferent nociceptive fibers, which is mainly mediated by dorsal root reflexes (DRRs) [46]. In an animal model of neurogenic inflammation induced by intradermal injection of capsaicin (CAP), our group has demonstrated that neurogenic inflammation and the resulting sensitization of primary afferent nociceptors evoked by CAP injection result from antidromic activation of DRG neurons [26,28]. In this process, the expression of CGRP in DRG neurons is increased [48].
Recent findings have highlighted the active role of glia in the pathogenesis of pain. Peripheral painful stimulation induces activation of spinal microglial cells [3,7,34,41], in which a cross-talk between glial cells and sensory neurons linked by pro-inflammatory agents, such as cytokines, released from activated microglial cells contribute critically to the development and maintenance of pain [32,42]. In sensory ganglia, activated satellite glial cells (SGCs) are found to play a major role in neuropathic pain [43]. However, whether SGCs are involved in neurogenic inflammation remains unclear. Experiments in this study were designed to test if neurogenic inflammation (vasodilation) induced by antidromic activation of DRG neurons involves the activation of SGCs. Some preliminary data have been published in abstract form [10,27].
Experiments were conducted on male Sprague-Dawley rats, weighing 250–350 g. They were housed two per cage, with free access to food and water, in the animal facility center with a 12-h alternating light-dark cycle. All experimental protocols were approved by the Animal Care and Use Committee of the University of Texas at Arlington and were in accordance with the guidelines of the National Institutes of Health and the International Association for the Study of Pain.
Rats were initially anesthetized with sodium pentobarbital (50 mg/kg, i.p.). The external jugular vein was cannulated, and anesthesia was maintained by continuous infusion of sodium pentobarbital (5–8 mg/kg/h). Anesthesia was kept at a sufficient level as judged by the absence of corneal reflexes and withdrawal reflexes. Rectal temperature was monitored using a rectal probe and maintained at 37°C by a servo-controlled heating blanket.
Cutaneous blood flow in the hindpaw was detected as blood cell flux by a laser Doppler flowmeter (Moor Instruments, UK) and vasodilation (a major characteristic of inflammation) in response to antidromic activation of primary afferents was evaluated by measuring changes in blood flow using a laser Doppler flow meter probe attached to the skin of the foot, as described previously [28,29]. The output showing blood flow level was then recorded by a computer data acquisition system (CED 1401+, with Spike-2 software) in mV units [28] (also see Figs. 1 and 2).
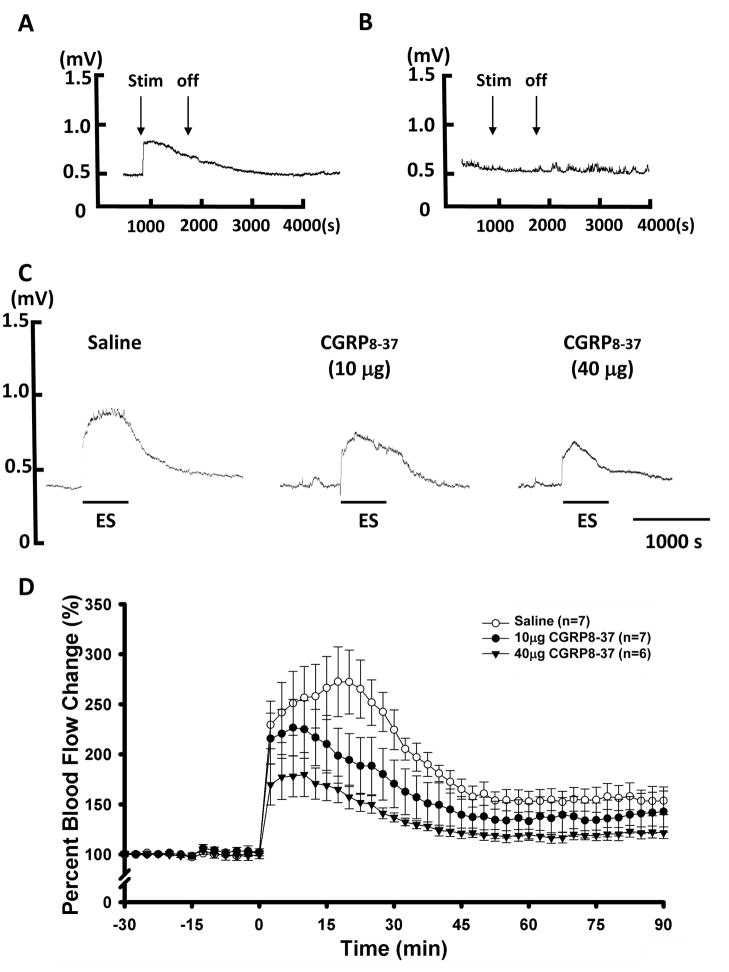
Figure 1.
Increase in cutaneous blood flow in the hindpaw evoked by antidromic electrical stimulus (ES) of L4 and L5 dorsal roots and the effects of blockade of peripheral CGRP receptors by intra-arterial injection of CGRP8-37. A: Sample of the laser Doppler flowmeter trace that shows the increase in blood flow in the hindpaw evoked by antidromic ES. B: Sample of the trace that shows no change in blood flow in the forepaw when L4 and L5 dorsal roots were antidromically stimulated. C: Samples of the traces showing the effects of blockade of CGRP receptors on the antidromic ES evoked vasodilation by pretreatment of the periphery with two different doses of CGRP8-37. Saline pretreatment using the vehicle for dissolving CGRP8-37 served as a control (left panel). Horizontal lines below traces show times of application of the ES. D: Grouped data summarizing that CGRP8-37 inhibited dose-dependently the increase in blood flow evoked by antidromic ES.
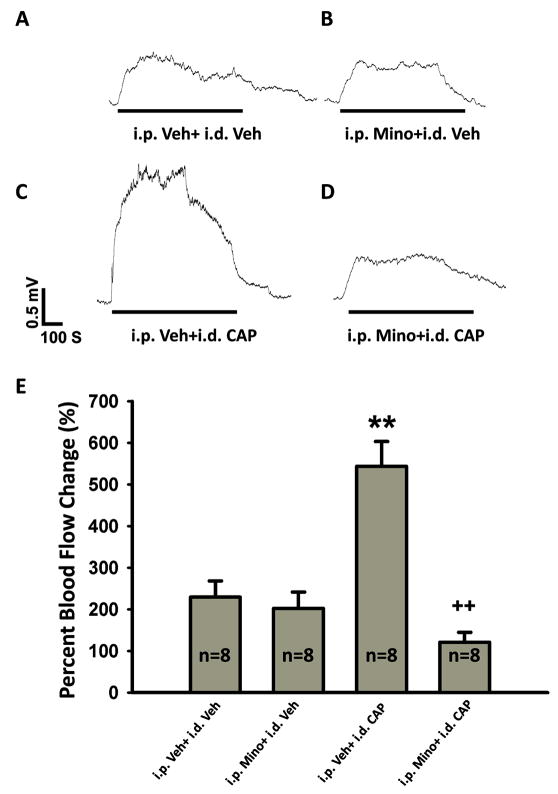
Figure 2.
Enhancement of the antidromic ES-evoked vasodilation following CAP injection and the effects of glial cell inhibition by pretreatment with minocycline. A: Antidromic ES-evoked vasodilation without minocycline and CAP treatments [treatments with vehicle (Veh) for minocycline (Mino) and CAP treatments]. B: Antidromic ES-evoked vasodilation with minocycline and without CAP treatment. C: Antidromic ES-evoked vasodilation without minocycline and with CAP treatment. D: Antidromic ES-evoked vasodilation with minocycline and CAP treatments. Horizontal lines below traces show times of application of the ES. E: Grouped data summarizing the changes in antidromic ES-evoked vasodilation following CAP injection and the effects of minocycline treatment. ** p<0.01, compared with the group without minocycline and CAP treatments (i.p. Veh+i.d. Veh). ++ P<0.01, compared with the group without minocycline treatment (i.p. Veh+i.d. CAP).
In order to perform antidromic electrical stimulation (ES) of primary afferents, the L4 and L5 dorsal roots were exposed by laminectomy and their identity was confirmed by their relation to the corresponding vertebra. Dorsal rhizotomy was performed on the ipsilateral side where the blood flow in the hindpaw was measured, as described before [28]. A bipolar silver electrode was placed on the cut distal ends of dorsal rootlets. Warm mineral oil pools contained by skin flaps kept the nerves from drying and cooling. The parameters for antidromic ES were 10 Hz, 1 ms, and 3 mA, which have been shown to activate C-fibers [4,15].
Close-by intra-arterial injection was used to deliver a CGRP receptor antagonist, CGRP8-37, to the periphery [26]. To do this, one branch of the femoral artery on the side ipsilateral to the blood flow measurement was carefully isolated from connective tissue and ligated proximally. The artery was then cannulated distally by a small-sized polyethylene tube that was connected with a Hamilton syringe. CGRP8-37 (from TOCRIS), dissolved in saline at doses of 10 or 40 μg [26], was injected intra-arterially in a volume of 25 μl at 5 min before antidromic ES. For control purposes, saline (25 μl) was given using the same procedure.
One percent CAP (from Sigma/Aldrich, 20 μl, prepared in a solution of 7% Tween 80 and 93% saline) was injected intradermally (i.d.) into the plantar surface of the foot after dorsal rhizotomy [29]. The vehicle (7% Tween 80 and 93% saline) was injected i.d. as a control, which has been shown in our previous study to produce no obvious changes in blood flow [29].
Experiments on the effects of glial cell inhibition were conducted on rats that had undergone a pretreatment with minocycline, a glial inhibitor (from Sigma/Aldrich), that was injected intraperitoneally (i.p.) at a dose of 50 mg/kg daily for 7 days. The administration schedule we used in the study was based on our preliminary studies in which glial inhibitor pretreatment could inhibit neurogenic inflammation and the resulting sensitization of primary afferent nociceptors [10,27]. For control purposes, saline used for dissolving minocycline was administered using the same procedure.
All values are presented as means±SEM. Baseline blood flow level (pre-antidromic ES) was expressed as 100% and percentage changes after antidromic ES were compared for groups of animals that received different treatments. One-way ANOVA was used to compare the difference among the groups with different treatments, and values were considered to be significantly different when P < 0.05. All statistical calculations were performed with a statistical software package (SPSS, Version 13.0).
When ES were applied antidromically to the cut distal ends of L4 and L5 dorsal roots, an immediate increase in cutaneous blood flow was evoked in the hindpaw skin ipsilateral to the ES side (Fig. 1A). The increased blood flow lasted for about 10 min and then recovered gradually even though the stimuli were continuously given (Fig. 1A). Thus, 10-min of ES was chosen to test the responses of blood flow to antidromic activation of primary afferents. Changes in the blood flow in the forepaw skin during antidromic ES of the cut distal ends of dorsal roots were monitored simultaneously, and no changes were found (Fig. 1B), which means that the evoked blood flow changes in the hindpaw were site and stimulation specific, not the result of a stimulation-induced systemic effect.
To see if vasodilation evoked by antidromic activation of primary afferents was mediated by the release of neuropeptides, such as CGRP, intra-arterial administration of the CGRP receptor antagonist, CGRP8-37, was applied to the hindpaw ipsilateral to the ES at 5 min prior to antidromic ES. We found that CGRP8-37 pretreatment could inhibit the increase in blood flow evoked by antidromic ES in a dose-dependent manner. The peak increase was significantly reduced from 272.6±34.8% (saline pre-treated, n=7) to 228.7±27.4% after 10 μg CGRP8-37 was administered as a pretreatment (p<0.05, compared with saline pre-treated, n=7), and to 181.2±13.3% (p<0.01, compared with saline pretreatment, n=6) after 40 μg CGRP8-37 was given (Fig. 1C and D).
In the following experiments, we wanted to test further if antidromic ES-evoked vasodilation is affected by intradermal injection of CAP and effects of pretreatment with minocycline. Blood flow responses to antidromic ES without minocycline pretreatment and CAP injection [vehicle (Veh) treatments] were tested to serve as controls for minocycline (Mino) and/or CAP treatments. Consistent with the results shown in Fig. 1A, antidromic ES evoked a significant vasodilation in the rats without minocycline and CAP treatments (i.p. Veh+i.d. Veh, in Fig. 2A and the first bar in 2E), and the peak increase was 229.4±38.9% (n=8, the first bar in Fig. 2E). In the group with minocycline pretreatment (i.p. Mino+i.d. Veh, n=8), antidromic ES induced an increase in blood flow for 202.6±38.9% (Fig. 2B and the second bar in 2E). However, there was no statistically significant difference between these two groups (p=0.966, Fig. 2E). The above experiments were further performed under the condition where the hindpaw was injected intradermally with CAP at 2 h before the experiments. Vasodilation was produced following CAP injection, and returned nearly completely to the control level 2 h after injection (data not shown), which was consistent with our previous observations [29]. Interestingly, 2 h after CAP injection, the vasodilation evoked by antidromic ES was greatly enhanced (Fig. 2C). The test was made after saline pretreatment, and the peak increase was 543.8±59.5% (the third bar in Fig. 2E, p<0.01, compared with saline injection). Importantly, the enhancement of evoked vasodilation was profoundly inhibited after pretreatment with minocycline (Fig. 2D and the fourth bar in Fig. 2E). The peak increase dropped to 121.0±23.8% (n=8, p<0.01, compared with the saline pretreatment group having CAP injection).
The main findings of the present study are that vasodilation in the hindpaw evoked by antidromic ES can be enhanced by intradermal injection of CAP. This enhancement is significantly prevented by minocycline pretreatment that inhibits glial cells. Thus, activation of SGCs in DRG is suggested to make an important contribution to the CAP-induced neurogenic inflammation.
Antidromic primary afferent stimulation-evoked inflammatory responses are characterized by vasodilation and/or edema. These responses are stimulus strength dependent and most significant when the strength is sufficient to activate C-fibers [19,20,39]. In the current study, a significant vasodilation was evoked by antidromic ES of dorsal roots using the parameters of ES similar to those used by other groups [4,19,36]. It is well accepted that inflammatory responses evoked by antidromic activation of primary nociceptive afferents are due to the release of pro-inflammatory agents, such as CGRP and/or SP, in the target tissue [21,22,24,25,37]. Antidromic activation of primary afferents was presumed to mimic the vasodilation induced by triggering of DRRs [8,28,30]. Data obtained from our and other groups and the current study demonstrate that neurogenic inflammation evoked by antidromic activation of primary afferent nociceptive fibers is partially explained by CGRP release because a blockade of peripheral CGRP receptors inhibited dose-dependently the evoked vasodilation [8,28].
The current study has also shown that the antidromic ES-evoked vasodilation could be significantly enhanced by CAP injection. Since the enhancement was seen around 2 h after CAP injection, it does not seem to be due to a direct effect on the peripheral endings of primary afferents. Many studies have shown that plastic changes in DRG neurons occur after peripheral injury, inflammation or persistent nociceptive stimulation, which is due to up-regulation of nociceptive molecules, such as the transient receptor potential vanilloid-1 (TRPV1) receptors, and/or CGRP and SP, in the neurons [16,18,33,48]. Studies by our group using a rat model of intradermal CAP injection suggest strongly that up-regulation of TRPV1 receptors and hyperactive synthesis of CGRP in DRG neurons plays a critical role in DRR-mediated neurogenic inflammation and the resulting sensitization of primary afferent nociceptors [27,28,47,48]. Therefore, plastic changes in DRG nociceptive neurons should contribute to the mechanism of DRR-mediated neurogenic inflammation.
Studies on pain models, particularly the neuropathic pain models, have indicated that sensitization of sensory neurons in responses to peripheral injury, inflammation or persistent nociceptive stimulation is closely associated with activation of microglial cells in the spinal cord and SGCs in DRG [6,14,44]. It has been well documented that neuron-glia signaling promotes hyperexcitability of sensory neurons by the release of a variety of mediators, such as pro-inflammatory cytokines, between neurons and glia. For example, adenosine 5′-triphosphate released from the cell bodies of DRG neurons can activate the surrounding SGCs [50]. CAP injection leads to an enhancement of neuronal-satellite glial signaling in trigeminal ganglia [5,9,40]. Upon activation, glial cells synthesize a plethora of compounds, such as tumor necrosis factor-α (TNFα), interleukin-1β (IL-1β), and cyclooxygenase [32,34,45,49]; these have been widely reported to cause hyperexcitablity of sensory neurons [1,11,12].
In the present study, we found that the CAP-induced enhancement of neurogenic inflammation could be dramatically inhibited by pretreatment with minocycline, a glial inhibitor. However, minocycline treatment did not produce any effect on vasodilation evoked by antidromic stimulation if CAP was not injected. Minocycline is a broad-spectrum antibiotic that inhibit microglial activation and proliferation [13,31,32,38]. The effects of minocycline have been suggested to involve multiple mechanisms of action specifically on glial cells, which include suppression of microglial phosphorylated-p38 mitogen-activated protein kinase (MAPK) [2,17] and down-regulation of microglial production of pro-inflammatory cytokines, such as IL-1β and TNF-α [23], and prostaglandin E2 [51]. It has been demonstrated that minocycline can alleviate pain through the above mechanisms without evidence of direct effects on neurons either at spinal or DRG levels [17,31]. It has been reported that preemptive and repeated injection of minocycline can produce a cumulative drug effect that is more effective than acute administration in inhibition of activated glial cells [2,31]. Thus, it is strongly indicated in the present study that there is activation of glial cells following CAP injection, which participates in the process of neurogenic inflammation. Based on the experimental manipulation of dorsal rhizotomy in which dorsal roots at lumbosacral level have been sectioned, we favor the opinion that the glia activated and involved in neurogenic inflammation are mainly the SGCs in the DRG. Direct evidence remains to be provided in future plan by anatomically investigating satellite glial activation following CAP injection and its contribution to neurogenic inflammation.
In summary, this study supports the view that neurogenic inflammation following CAP injection involves antidromic activation of DRG neurons via the generation of DRRs. Importantly, minocycline inhibition of neurogenic inflammation is likely associated with its inhibitory effect on SGCs that are possibly activated following CAP injection. Thus, we propose that this should be an important component of the pathological mechanisms of neurogenic inflammation.
Research Highlights.
Cutaneous vasodilation evoked by antidromic electrical stimulation of the primary afferents can be enhanced by intradermal injection of capsaicin.
Capsaicin induced enhancement is significantly prevented by chronic minocycline pretreatment that inhibits glial cells.
Inhibition of neurogenic inflammation by minocycline is suggested to be associated with its inhibitory effect on satellite glial cells that are possibly activated following capsaicin injection.
Acknowledgments
We would like to thank Drs. Richard Coggeshall and William Willis for their valuable suggestions and criticism on the preparation of this manuscript. This work was supported by the National Institutes of Health Grant NS 040723 to Q. Lin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1β sensors. J Neurosci. 2008;28:14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang YW, Waxman SG. Minocycline attenuates mechanical allodynia and central sensitization following peripheral second-degree burn injury. J Pain. 2010 doi: 10.1016/j.jpain.2010.02.010. in press. [DOI] [PubMed] [Google Scholar]
- 3.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De KY. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 4.Dai Y, Iwata K, Fukuoka T, Kondo E, Tokunaga A, Yamanaka H, Tachibana T, Liu Y, Noguchi K. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J Neurosci. 2002;22:7737–7745. doi: 10.1523/JNEUROSCI.22-17-07737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damodaram S, Thalakoti S, Freeman SE, Garrett FG, Durham PL. Tonabersat inhibits trigeminal ganglion neuronal-satellite glial cell signaling. Headache. 2009;49:5–20. doi: 10.1111/j.1526-4610.2008.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dublin P, Hanani M. Satellite glial cells in sensory ganglia: their possible contribution to inflammatory pain. Brain Behav Immun. 2007;21:592–598. doi: 10.1016/j.bbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Fu KY, Light AR, Maixner W. Relationship between nociceptor activity, peripheral edema, spinal microglial activation and long-term hyperalgesia induced by formalin. Neuroscience. 2000;101:1127–1135. doi: 10.1016/s0306-4522(00)00376-6. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Nicas E, Laird JM, Cervero F. Vasodilatation in hyperalgesic rat skin evoked by stimulation of afferent Aβ-fibers: further evidence for a role of dorsal root reflexes in allodynia. Pain. 2001;94:283–291. doi: 10.1016/S0304-3959(01)00365-7. [DOI] [PubMed] [Google Scholar]
- 9.Garrett FG, Durham PL. Differential expression of connexins in trigeminal ganglion neurons and satellite glial cells in response to chronic or acute joint inflammation. Neuron Glia Biol. 2008;4:295–306. doi: 10.1017/S1740925X09990093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong K, Yue Y, Zou X, Lin Q. Minocycline attenuates sensitization of primary afferent nociceptors evoked by antidromic stimulation of primary afferents: a possible contribution of satellite glial cells to the neurogenic inflammation induced pain. Soc. Neurosci. Program No. 586. 2010 (Abstract) [Google Scholar]
- 11.Gustafson-Vickers SL, Lu VB, Lai AY, Todd KG, Ballanyi K, Smith PA. Long-term actions of interleukin-1β on delay and tonic firing neurons in rat superficial dorsal horn and their relevance to central sensitization. Mol Pain. 2008;4:63. doi: 10.1186/1744-8069-4-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagenacker T, Czeschik JC, Schafers M, Busselberg D. Sensitization of voltage activated calcium channel currents for capsaicin in nociceptive neurons by tumor-necrosis-factor-α. Brain Res Bull. 2010;81:157–163. doi: 10.1016/j.brainresbull.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Hains LE, Loram LC, Weiseler JL, Frank MG, Bloss EB, Sholar P, Taylor FR, Harrison JA, Martin TJ, Eisenach JC, Maier SF, Watkins LR. Pain intensity and duration can be enhanced by prior challenge: initial evidence suggestive of a role of microglial priming. J Pain. 2010 doi: 10.1016/j.jpain.2010.01.271. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Hathway GJ, Vega-Avelaira D, Moss A, Ingram R, Fitzgerald M. Brief, low frequency stimulation of rat peripheral C-fibres evokes prolonged microglial-induced central sensitization in adults but not in neonates. Pain. 2009;144:110–118. doi: 10.1016/j.pain.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong Y, Liu Y, Chabot JG, Fournier A, Quirion R. Upregulation of adrenomedullin in the spinal cord and dorsal root ganglia in the early phase of CFA-induced inflammation in rats. Pain. 2009;146:105–113. doi: 10.1016/j.pain.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci. 2005;22:2431–2440. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- 18.Ikeuchi M, Kolker SJ, Sluka KA. Acid-sensing ion channel 3 expression in mouse knee joint afferents and effects of carrageenan-induced arthritis. J Pain. 2009;10:336–342. doi: 10.1016/j.jpain.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jänig W, Lisney SJ. Small diameter myelinated afferents produce vasodilatation but not plasma extravasation in rat skin. J Physiol. 1989;415:477–486. doi: 10.1113/jphysiol.1989.sp017732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenins P. Identification of the unmyelinated sensory nerves which evoke plasma extravasation in response to antidromic stimulation. Neurosci Lett. 1981;25:137–141. doi: 10.1016/0304-3940(81)90321-9. [DOI] [PubMed] [Google Scholar]
- 21.Kress M, Guthmann C, Averbeck B, Reeh PW. Calcitonin gene-related peptide and prostaglandin E2 but not substance P release induced by antidromic nerve stimulation from rat skin in vitro. Neuroscience. 1999;89:303–310. doi: 10.1016/s0306-4522(98)00280-2. [DOI] [PubMed] [Google Scholar]
- 22.Lam FY, Ferrell WR. Acute inflammation in the rat knee joint attenuates sympathetic vasoconstriction but enhances neuropeptide-mediated vasodilatation assessed by laser Doppler perfusion imaging. Neuroscience. 1993;52:443–449. doi: 10.1016/0306-4522(93)90170-k. [DOI] [PubMed] [Google Scholar]
- 23.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Lewin GR, Lisney SJ, Mendell LM. Neonatal anti-NGF treatment reduces the Aδ- and C-fibre evoked vasodilator responses in rat skin: evidence that nociceptor afferents mediate antidromic vasodilatation. Eur J Neurosci. 1992;4:1213–1218. doi: 10.1111/j.1460-9568.1992.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 25.Lewin GR, Ritter AM, Mendell LM. On the role of nerve growth factor in the development of myelinated nociceptors. J Neurosci. 1992;12:1896–1905. doi: 10.1523/JNEUROSCI.12-05-01896.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li D, Ren Y, Xu X, Zou X, Fang L, Lin Q. Sensitization of primary afferent nociceptors induced by intradermal capsaicin involves the peripheral release of calcitonin gene-related peptide driven by dorsal root reflexes. J Pain. 2008;9:1155–1168. doi: 10.1016/j.jpain.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Q, Gong K, Zou X, Yue Y. Satellite glial cell in dorsal root ganglion modulate the neurogenic inflammation induced by capsaicin injection. 13th World Congress on Pain; Montreal: IASP; Program No. PT140 (2010) (Abstract) [Google Scholar]
- 28.Lin Q, Li D, Xu X, Zou X, Fang L. Roles of TRPV1 and neuropeptidergic receptors in dorsal root reflex-mediated neurogenic inflammation induced by intradermal injection of capsaicin. Mol Pain. 2007;3:30. doi: 10.1186/1744-8069-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin Q, Wu J, Willis WD. Dorsal root reflexes and cutaneous neurogenic inflammation after intradermal injection of capsaicin in rats. J Neurophysiol. 1999;82:2602–2611. doi: 10.1152/jn.1999.82.5.2602. [DOI] [PubMed] [Google Scholar]
- 30.Lin Q, Zou X, Willis WD. Aδ and C primary afferents convey dorsal root reflexes after intradermal injection of capsaicin in rats. J Neurophysiol. 2000;84:2695–2698. doi: 10.1152/jn.2000.84.5.2695. [DOI] [PubMed] [Google Scholar]
- 31.Mika J, Rojewska E, Makuch W, Przewlocka B. Minocycline reduces the injury-induced expression of prodynorphin and pronociceptin in the dorsal root ganglion in a rat model of neuropathic pain. Neuroscience. 2010;165:1420–1428. doi: 10.1016/j.neuroscience.2009.11.064. [DOI] [PubMed] [Google Scholar]
- 32.Ohtori S, Takahashi K, Moriya H, Myers RR. TNF-α and TNF-α receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine (Phila Pa 1976 ) 2004;29:1082–1088. doi: 10.1097/00007632-200405150-00006. [DOI] [PubMed] [Google Scholar]
- 33.Qiao LY, Grider JR. Up-regulation of calcitonin gene-related peptide and receptor tyrosine kinase TrkB in rat bladder afferent neurons following TNBS colitis. Exp Neurol. 2007;204:667–679. doi: 10.1016/j.expneurol.2006.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raghavendra V, Tanga FY, Deleo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 35.Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302:839–845. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- 36.Sato A, Sato Y, Shimura M, Uchida S. Calcitonin gene-related peptide produces skeletal muscle vasodilation following antidromic stimulation of unmyelinated afferents in the dorsal root in rats. Neurosci Lett. 2000;283:137–140. doi: 10.1016/s0304-3940(00)00932-0. [DOI] [PubMed] [Google Scholar]
- 37.Scott DT, Lam FY, Ferrell WR. Acute inflammation enhances substance P-induced plasma protein extravasation in the rat knee joint. Regul Pept. 1992;39:227–235. doi: 10.1016/0167-0115(92)90543-4. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu K, Guo W, Wang H, Zou S, LaGraize SC, Iwata K, Wei F, Dubner R, Ren K. Differential involvement of trigeminal transition zone and laminated subnucleus caudalis in orofacial deep and cutaneous hyperalgesia: the effects of interleukin-10 and glial inhibitors. Mol Pain. 2009;5:75. doi: 10.1186/1744-8069-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szolcsanyi J. Antidromic vasodilatation and neurogenic inflammation. Agents Actions. 1988;23:4–11. doi: 10.1007/BF01967170. [DOI] [PubMed] [Google Scholar]
- 40.Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, Durham PL. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vit JP, Jasmin L, Bhargava A, Ohara PT. Satellite glial cells in the trigeminal ganglion as a determinant of orofacial neuropathic pain. Neuron Glia Biol. 2006;2:247–257. doi: 10.1017/s1740925x07000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 45.Whitehead KJ, Smith CG, Delaney SA, Curnow SJ, Salmon M, Hughes JP, Chessell IP. Dynamic regulation of spinal pro-inflammatory cytokine release in the rat in vivo following peripheral nerve injury. Brain Behav Immun. 2010;24:569–576. doi: 10.1016/j.bbi.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Willis WD., Jr Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res. 1999;124:395–421. doi: 10.1007/s002210050637. [DOI] [PubMed] [Google Scholar]
- 47.Xu X, Wang P, Zou X, Li D, Fang L, Gong K, Lin Q. The effects of sympathetic outflow on upregulation of vanilloid receptors TRPV1 in primary afferent neurons evoked by intradermal capsaicin. Exp Neurol. 2010;222:93–107. doi: 10.1016/j.expneurol.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu X, Wang P, Zou X, Li D, Fang L, Lin Q. Increases in transient receptor potential vanilloid-1 mRNA and protein in primary afferent neurons stimulated by protein kinase C and their possible role in neurogenic inflammation. J Neurosci Res. 2009;87:482–494. doi: 10.1002/jnr.21844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang FY, Wan Y, Zhang ZK, Light AR, Fu KY. Peripheral formalin injection induces long-lasting increases in cyclooxygenase 1 expression by microglia in the spinal cord. J Pain. 2007;8:110–117. doi: 10.1016/j.jpain.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci U S A. 2007;104:9864–9869. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao P, Waxman SG, Hains BC. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J Neurosci. 2007;27:2357–2368. doi: 10.1523/JNEUROSCI.0138-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]