Abstract
Anthrax lethal toxin (LT) is an important virulence factor for Bacillus anthracis. In mice, LT lyses macrophages from certain inbred strains in less than two hours by activating the Nlrp1b inflammasome and caspase-1, while macrophages from other strains remain resistant to the toxin’s effects. We analyzed LT effects in toxin-sensitive and resistant rat macrophages to test if a similar pathway was involved in rat macrophage death. LT activates caspase-1 in rat macrophages from strains harboring LT-sensitive macrophages in a manner similar to that in toxin-sensitive murine macrophages. This activation of caspase-1 is dependent on proteasome activity, and sensitive macrophages are protected from LT’s lytic effects by lactacystin. Proteasome inhibition also delayed the death of rats in response to LT, confirming our previous data implicating the rat Nlrp1 inflammasome in animal death. Quinidine, caspase-1 inhibitors, the cathepsin B inhibitor CA-074Me, and heat shock also protected rat macrophages from LT toxicity. These data support the existence of an active functioning LT-responsive Nlrp1 inflammasome in rat macrophages. The activation of the rat Nlrp1 inflammasome is required for LT-mediated rat macrophage lysis and contributes to animal death.
Keywords: anthrax, lethal toxin, inflammasome, caspase-1, Nlrp1
Introduction
Bacillus anthracis lethal toxin (LT) is composed of lethal factor (LF) and protective antigen (PA). PA binds to cell receptors and delivers LF into the cytosol (for review see [1]). In the cytosol LF cleaves and inactivates members of the mitogen-activated protein kinase kinase (MAPKK or MEK) family. The role of this cleavage event in the pathogenesis induced by toxin is unknown. Injection of LT results in the death of most inbred mice over a period of days [2; 3]and that of select inbred rats in one hour [4; 5]. LT is best known for inducing rapid lysis of murine macrophages through activation of caspase-1 (for review see [6]), although this lysis alone does not control mouse susceptibility to toxin challenge [2; 3; 7]. Furthermore, macrophage sensitivity in mice is correlated with relative resistance to spore infection [7] although the same is not true in rats (Moayeri, unpublished data).
Sensitivity of murine macrophages to LT-mediated lysis is controlled by a single gene, Nlrp1b (mNlrp1b) [8]. NOD-like receptor proteins, including Nlrp1, are intracellular sensors of danger signals. Their activation is required for assembly of the inflammasome, a multiprotein complex which provides the scaffold for the recruitment and cleavage of pro-caspase-1 [9]. mNlrp1b has five highly polymorphic alleles [8], making it difficult to distinguish the regions of the protein responsible for determining sensitivity to LT. Further complicating matters is the expression of two paralogs, mNlrp1a and mNlrp1c which are expressed and may be active in certain mouse strains. It is unclear how polymorphisms in the mNlrp1b protein result in such striking variation in the ability of LT to activate caspase-1 (and subsequently induce cell death) in murine macrophages. However, the mNlrp1b inflammasome-mediated activation of caspase-1 is necessary for LT-mediated murine macrophage cell death [8; 10–12]. It has recently been reported that mNlrp1b and caspase-1 are alone sufficient to render fibroblasts sensitive to LT [13].
We recently mapped macrophage and animal susceptibility to LT in rats using a recombinant inbred rat panel. We found that LT-dependent rat macrophage cell death and, surprisingly, the rapid death of animals (which can occur in as little as 37 min, [4]) are both controlled by a single locus on chromosome 10 which harbors the mNlrp1b homolog (rNlrp1) [5]. We found a perfect correlation between each rat strain’s sensitivity to LT and the susceptibility of its macrophages. Polymorphisms in rNlrp1 that correlated with sensitivity to LT were restricted to a small region in the first 100 amino acids of the protein that has no known function [5].
Although it appears that rNlrp1 controls rat macrophage (and animal) susceptibility to LT, the functionality of the rat Nlrp1 protein/inflammasome in rat macrophages has not been investigated. In the current report, we show that LT-mediated rat macrophage death requires activation of the rNlrp1 inflammasome and caspase-1 activation. This cell death also requires proteasome activity and is inhibited by caspase-1 inhibitors, potassium channel inhibition, cathepsin B inhibition, and heat shock. Our data indicate that the rNlrp1 inflammasome responds to LT in rat macrophages, and its activation by this toxin is required for rat macrophage death through a pathway similar to that initiated by LT in murine macrophages. Furthermore, activation of this inflammasome in response to the toxin is also a contributor to animal death.
Materials and Methods
Materials
PA and LF were purified from B. anthracis as previously described [14; 15]. The LF used here is a recombinant protein having an N-terminal sequence beginning HMAGG. Doses and concentrations of LT given for each experiment correspond to that of each toxin component (i.e., 10 μg LT is 10 μg PA + 10 μg LF). Anti-caspase-1 p10 (sc-514) and anti-MEK3 NT (sc-959) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-IL-1β (AF-401-NA) and Z-Val-Ala-Asp (OMe)-fluoromethylketone (Z-VAD-FMK) were from R&D Systems (Minneapolis, MN). Anti-MEK1 NT antibody, ultra-pure LPS, nigericin, muramyl dipeptide (MDP), and lactacystin were purchased from Calbiochem (San Diego, CA). The anti-rabbit infrared dye secondary antibody (800CW) was from Licor Biosciences (Lincoln, NE). Anti-goat infrared dye (IRDye 800CW) secondary antibody was from Rockland (Gilbertsville, PA). L-3-trans-(propylcarbamoyl)oxirane-2-carbonyl-L-isoleucyl-L-proline methyl ester (CA-074Me), ATP, polyinosinic-polycytidylic acid (poly(I:C)), and quinidine were from Sigma (St Louis, MO). Boc-Asp (OBzl)-chloromethylketone (Boc-D-CMK) was purchased from AnaSpec (San Jose, CA). Bortezomib (Velcade®) was from Millenium Pharmaceuticals (Cambridge, MA).
Cell culture
L929 mouse fibroblast cells were a gift from Dr. Antonio Rothfuchs [12] and were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 10 mM HEPES, and 50 μg/ml gentamicin (all obtained from Invitrogen, Carlsbad, CA) at 37°C in 5% CO2. Bone marrow-derived macrophages (BMDMs) were cultured in complete DMEM (as described above) with 30% L929 cell culture supernatant. BMDMs were grown for 7–9 days to allow time for differentiation before use in assays.
Animal studies
Adult female rats (150–180 g) were purchased from Charles River Laboratories (Wilmington, MA). Rats were acclimated for four-five days prior to experiments. Bortezomib or PBS (vehicle) was injected with 0.2 mg/kg (n=8) or 0.1 mg/kg (n=4), 25–35 min prior to IV challenge with LT (10 ug; 1× LD100). Rats were monitored continuously for 5 h followed by a 24-h check of surviving animals. All animal experiments were performed in strict accordance with guidelines from the NIH and the Animal Welfare Act, under protocols approved by the Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Cytotoxicity assays
BMDMs were plated in 96-well plates 24 h prior to assays at 90% confluence. For basic macrophage LT sensitivity testing, cells were exposed to LT at the indicated concentrations and times. For protection assays, cells were pretreated with lactacystin, quinidine, Z-VAD-FMK, Boc-D-CMK, or CA-074Me at the indicated concentrations for 45 min and then treated with 1 μg/ml LT for 3.5 h. Viability was assessed by addition of MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide] (USB Corporation, Cleveland, OH) to a final concentration of 0.6 mg/ml in DMEM. Following 30–45 min of incubation with MTT dye, cell culture medium was removed and cells were dissolved with 0.5% SDS, 25 mM HCl in 90% isopropanol and A570 was measured. Percent viabilities were calculated relative to medium-treated controls. Rat IL-1β ELISAs were also performed on LPS-primed (1 μg/ml, 2 h) BMDMs in parallel to LT cytotoxicity assays. Supernatants were analyzed with a Quantikine rat IL-1β ELISA (R&D Systems) according to the manufacturer’s instructions.
Westerns blots
Cells were pretreated with 1 μg/ml LPS for 2 h and then treated with 1 μg/ml LT for various lengths of time, or with the following: 10 μg/ml poly (I:C) for 6 h, or 3 μg/ml MDP for 6 h prior to treatment with nigericin (at indicated concentrations and times) or 5 mM ATP for 30 min. Lysates were prepared with RIPA lysis buffer (1% Nonidet-P40, 0.5% sodium deoxycholate, and 0.1% SDS in PBS) containing EDTA-free Complete™ protease inhibitor cocktail (Roche Diagnostics). Protein concentrations were quantified using a BCA protein assay (Pierce, Rockford, IL) prior to electrophoresis and Western blotting using anti-IL-1β (1:700) or anti-caspase-1 p10 (1:250). Primary antibodies were detected using an appropriate IR-dye-conjugated secondary antibody (anti-rabbit 800CW, 1:30,000; anti-goat 800CW, 1:5000) and the Odyssey Infrared Imaging System (Licor Biosciences, Lincoln, NE).
Results and Discussion
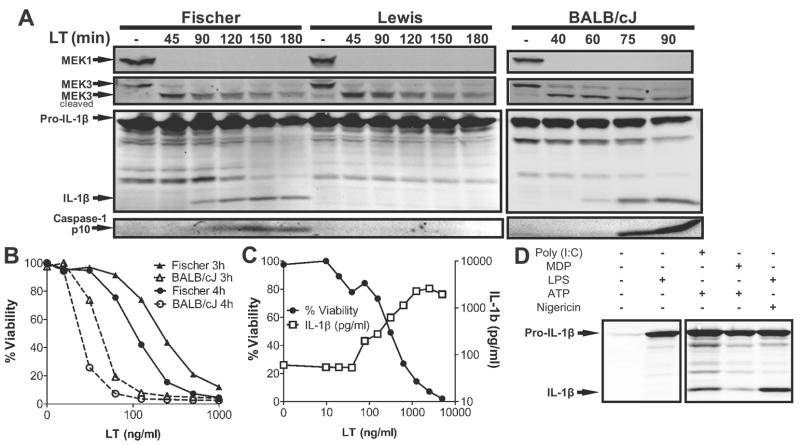
We previously showed that LT sensitivity of rat strains and their bone marrow-derived macrophages (BMDMs) was a qualitative dichotomous phenotype. Macrophages and animals either succumbed rapidly or were completely resistant to this toxin [5]. Consistent with rNlrp1 being the LT susceptibility locus [5], we now show that toxin treatment of LT-sensitive Fischer rat BMDMs, but not LT-resistant Lewis rat BMDMs led to the activation of caspase-1 and maturation of IL-1β (Figure 1A). This activation consistently occurred 30–45 min later in toxin sensitive Fischer BMDMs than in LT-sensitive mouse (BALB/cJ) BMDMs (Figure 1A). In a parallel observation, rat macrophages also succumbed at later times than mouse macrophages treated with the same LT concentrations (Figure 1B). Surprisingly, despite the delay in killing of rat macrophages, toxin entry and cleavage of MEK proteins occurred equivalently and with the same timing in Fischer (sensitive), Lewis (resistant) and BALB/cJ (sensitive) BMDMs (Figure 1A). This finding indicates that the events downstream of MEK cleavage control inflammasome assembly in macrophages. It is unclear if the cleavage of MEK proteins is required for inflammasome assembly, but sensed differentially by sensitive and resistant rNlrp1 proteins, or if rat macrophage death occurs completely independent of MEK cleavage. However, the delay in activation of the rNlrp1 inflammasome may be a direct consequence of the extensive differences between LT-sensitive rNlrp1 and mNlrp1b sequences [5].
FIGURE 1. LT activates the inflammasome in sensitive rat macrophages.
(A) Fischer, Lewis, and BALB/cJ BMDMs were primed with 1 μg/ml LPS for 2 h and then treated with 1 μg/ml LT for varying amounts of time. Western blots were performed with MEK1, MEK3, IL-1β and caspase-1 p10 antibodies. (B) BALB/cJ and Fischer BMDMs were treated with the same range of toxin concentrations and viability was assessed 3 h or 4 h following LT addition relative to untreated controls. (C) LPS-primed (1 μg/ml, 2 h) Fischer BMDMs were treated with LT at the indicated concentrations for 2 h prior to measuring IL-1β in the supernatant via ELISA and cell viability via MTT assay. (D) Fischer BMDMs were pretreated with LPS (1 μg/ml, 2 h), poly(I:C) (10 μg/ml, 6 h), or MDP (3 μg/ml, 6 h) prior to treatment with nigericin (20 μM, 10 min or 10 μM, 40 min) or ATP (5 mM, 30 min). Western blots were performed with an IL-1β antibody.
LT-dependent IL-1β release following activation of caspase-1 by the toxin was dose-dependent and paralleled BMDM cell death (Figure 1C). We found that the more commonly studied Nlrp3 inflammasome, also not yet studied in rat macrophages, is also functional in rat BMDMs, as shown by the response of these cells to the classic stimuli previously demonstrated to result in its activation in mouse cells (Figure 1D). Thus, rat BMDMs appear to be similar to murine BMDMs in activation of multiple inflammasome pathways. Macrophages isolated from ten independent human donors, however, exhibited complete resistance to LT-mediated lysis (data not shown). It is tempting to speculate that this resistance may be related to the presence of an N-terminal pyrin domain in hNlrp1 which is absent in rodents, since this N-terminus has been shown to play a role in protein-protein interactions in the human inflammasome. However, hNlrp1 polymorphisms are associated with a number of human diseases [16–18], so that examination of more donors may reveal LT-sensitive hNlrp1 alleles.
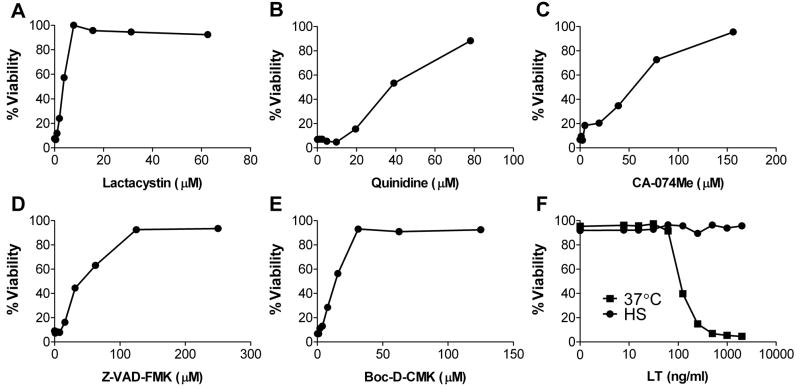
The mNlrp1b inflammasome in mice has both similarities and differences from the other, better-studied inflammasomes. We and others have previously demonstrated that activation of the mNlrp1b inflammasome requires proteasome activity (unlike the Nlrp3 inflammasome) [11; 12; 19]. This activation also involves K+ effluxes [12] and is accompanied by lysosomal membrane permeabilization and resulting increases in cytoplasmic cathepsin B activity [20; 21], in a manner similar to that of some Nlrp3 inflammasome stimuli. As previously shown for LT-sensitive mouse BMDMs [11; 12; 19], rat BMDMs were protected against LT-induced cytotoxicity by dose-dependent inhibition of the proteasome (lactacystin, Figure 2A), K+ fluxes (quinidine, Figure 2B), cathepsin B (CA-074Me, Figure 2C), or caspase-1 (Z-VAD-FMK or Boc-D-CMK, Figures 2D and 2E, respectively). Our previous findings also showed that heat shock results in the mobilization of murine procaspase-1 to a high mobility complex which prevents its activation by both Nlrp1 and Nlrp3 stimuli without any effect on LT translocation or activity [22]. Heat shock also protected LT-sensitive rat BMDMs against toxin-induced cytotoxicity (Figure 2F). These data show that despite extensive sequence differences between mNlrp1 and rNlrp1 LT-sensitivity-conferring alleles [5], this toxin kills rat macrophages through a rNlrp1 inflammasome-dependent process similar to that which causes mNlrp1b-mediated murine macrophage death.
FIGURE 2.
(A–E) Fischer BMDMs were pretreated with the indicated drugs for 45 min prior to LT treatment at 1 μg/ml for 3.5 h. (F) Fischer BMDMs were heat shocked at 45°C or incubated at 37°C for 1 h prior to treatment with a range of toxin doses for 2.5 h. Cell viability was assessed by MTT assay as described in Materials and Methods.
Interestingly, there is a complete correlation between rat death and inflammasome-dependent rat macrophage sensitivity to LT [5]. To test if rNlrp1-dependent rat death also requires the proteasome-dependent activation of this inflammasome, we investigated the effects of proteasome inhibition on animal death. Treatment of LT-sensitive Fischer rats with bortezomib, a potent proteasome inhibitor (0.2 mg/kg or 0.1 mg/kg, IV, 25–35 min prior to challenge with 10 ug LT, IV, n=12) resulted in a statistically significant (P < 0.001) delay in TTD. Drug treated animals succumbed at later times (128–346 min; average 209 min, n=12) relative to vehicle-treated controls (68–79 min, n=4) (Figure 3). We suggest it is unlikely, however, that macrophage lysis in the rat is responsible for the rapid animal death, as rat macrophages do not lyse in under 2 h, while the Fischer rat can be killed in 37 min when a saturating dose of toxin is administered [4]. Furthermore, unlike Balb/cJ mice, where LT-mediated lysis of toxin-sensitive macrophages in vivo leads to a systemic IL-1β release in animals [2; 3], we have been unable to detect any IL-1β in Fischer rat serum following toxin treatment (data not shown). Therefore, we hypothesize that rNlrp1 may have a role in the response of other cell types to toxin. LT-induced animal death of both rats and mice has been linked to changes in cardiac function [23; 24] and it is possible that rNlrp1 plays a role in the heart. A better understanding of the role of different Nlrp1 isoforms in various cell types is needed to fully understand the mechanisms by which the inflammasome activation described here contributes to rat death.
FIGURE 3.
Bortezomib or PBS (vehicle) was injected IV in Fischer rats at 0.2 mg/kg (n=12) or 0.1 mg/kg (n=4), 25–35 min prior to IV administration of LT (10 μg; 1× LD100). Rats were monitored continuously following toxin injection.
Conclusions
Anthrax LT-mediated rat macrophage death requires activation of caspase-1. This activation occurs differentially in rats harbouring sensitive or resistant rNlrp1alleles, resulting in susceptibility or resistance of macrophages. This inflammasome-mediated cell death requires proteasome activity and is inhibited by caspase-1 inhibitors, potassium channel inhibition, cathepsin B inhibition, and heat shock. Thus we conclude the rNlrp1 inflammasome responds to LT in rat macrophages, and its activation by this toxin is required for rat macrophage death through a pathway similar to that initiated by LT in murine macrophages. A potent proteasome inhibitor can delay the time to death of LT-treated rats, suggesting the toxin-mediated caspase-1 activation is also involved in rat death.
Acknowledgments
We thank Rasem Fattah for toxin preparation. This research was supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases.
Abbreviations
- LT
lethal toxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Young JA, Collier RJ. Anthrax toxin: receptor-binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
- 2.Moayeri M, Martinez NW, Wiggins J, Young HA, Leppla SH. Mouse susceptibility to anthrax lethal toxin is influenced by genetic factors in addition to those controlling macrophage sensitivity. Infect Immun. 2004;72:4439–4447. doi: 10.1128/IAI.72.8.4439-4447.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moayeri M, Haines D, Young HA, Leppla SH. Bacillus anthracis lethal toxin induces TNF-á-independent hypoxia-mediated toxicity in mice. J Clin Invest. 2003;112:670–682. doi: 10.1172/JCI17991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta PK, Moayeri M, Crown D, Fattah RJ, Leppla SH. Role of N-terminal amino acids in the potency of anthrax lethal factor. PLoS ONE. 2008;3:e3130. doi: 10.1371/journal.pone.0003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman ZL, Printz MP, Liu S, Crown D, Breen L, Miller-Randolph S, Flodman P, Leppla SH, Moayeri M. Susceptibility to anthrax lethal toxin-induced rat death is controlled by a single chromosome 10 locus that includes rNlrp1. PLoS Pathog. 2010;6:e1000906. doi: 10.1371/journal.ppat.1000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moayeri M, Leppla SH. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol Aspects Med. 2009;30:439–455. doi: 10.1016/j.mam.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terra JK, Cote CK, France B, Jenkins AL, Bozue JA, Welkos SL, Levine SM, Bradley KA. Cutting edge: Resistance to Bacillus anthracis infection mediated by a lethal toxin sensitive allele of Nalp1b/Nlrp1b. J Immunol. 2010;184:17–20. doi: 10.4049/jimmunol.0903114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 9.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 10.Muehlbauer SM, Evering TH, Bonuccelli G, Squires RC, Ashton AW, Porcelli SA, Lisanti MP, Brojatsch J. Anthrax lethal toxin kills macrophages in a strain-specific manner by apoptosis or caspase-1-mediated necrosis. Cell Cycle. 2007;6:758–766. doi: 10.4161/cc.6.6.3991. [DOI] [PubMed] [Google Scholar]
- 11.Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc Natl Acad Sci SA. 2008;105:4312–4317. doi: 10.1073/pnas.0707370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wickliffe KE, Leppla SH, Moayeri M. Anthrax lethal toxin-induced inflammasome formation and caspase-1 activation are late events dependent on ion fluxes and the proteasome. Cell Microbiol. 2008;10:332–343. doi: 10.1111/j.1462-5822.2007.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao KC, Mogridge J. Expression of Nlrp1b inflammasome components in human fibroblasts confers susceptibility to anthrax lethal toxin. Infect Immun. 2009;77:4455–4462. doi: 10.1128/IAI.00276-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park S, Leppla SH. Optimized production and purification of Bacillus anthracis lethal factor. Protein Expr Purif. 2000;18:293–302. doi: 10.1006/prep.2000.1208. [DOI] [PubMed] [Google Scholar]
- 15.Varughese M, Chi A, Teixeira AV, Nicholls PJ, Keith JM, Leppla SH. Internalization of a Bacillus anthracis protective antigen-c-Myc fusion protein mediated by cell surface anti-c-Myc antibodies. Mol Med. 1998;4:87–95. [PMC free article] [PubMed] [Google Scholar]
- 16.Jin Y, Mailloux CM, Gowan K, Riccardi SL, Laberge G, Bennett DC, Fain PR, Spritz RA. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356:1216–1225. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- 17.Taieb A. NALP1 and the inflammasomes: challenging our perception of vitiligo and vitiligo-related autoimmune disorders. Pigment Cell Res. 2007;20:260–262. doi: 10.1111/j.1600-0749.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- 18.Magitta NF, Boe Wolff AS, Johansson S, Skinningsrud B, Lie BA, Myhr KM, Undlien DE, Joner G, Njolstad PR, Kvien TK, Forre O, Knappskog PM, Husebye ES. A coding polymorphism in NALP1 confers risk for autoimmune Addison’s disease and type 1 diabetes. Genes Immun. 2009;10:120–124. doi: 10.1038/gene.2008.85. [DOI] [PubMed] [Google Scholar]
- 19.Squires RC, Muehlbauer SM, Brojatsch J. Proteasomes control caspase-1 activation in anthrax lethal toxin-mediated cell killing. J Biol Chem. 2007;282:34260–34267. doi: 10.1074/jbc.M705687200. [DOI] [PubMed] [Google Scholar]
- 20.Newman ZL, Leppla SH, Moayeri M. CA-074Me protection against anthrax lethal toxin. Infect Immun. 2009;77:4327–4336. doi: 10.1128/IAI.00730-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Averette KM, Pratt MR, Yang Y, Bassilian S, Whitelegge JP, Loo JA, Muir TW, Bradley KA. Anthrax lethal toxin induced lysosomal membrane permeabilization and cytosolic cathepsin release is Nlrp1b/Nalp1b-dependent. PLoS ONE. 2009;4:e7913. doi: 10.1371/journal.pone.0007913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin TC, Wickliffe KE, Leppla SH, Moayeri M. Heat shock inhibits caspase-1 activity while also preventing its inflammasome-mediated activation by anthrax lethal toxin. Cell Microbiol. 2008;10:2434–2446. doi: 10.1111/j.1462-5822.2008.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson LE, Kuo SR, Katki K, Dang T, Park SK, Dostal DE, Tang WJ, Leppla SH, Frankel AE. Anthrax toxins induce shock in rats by depressed cardiac ventricular function. PLoS ONE. 2007;2:e466. doi: 10.1371/journal.pone.0000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moayeri M, Crown D, Dorward DW, Gardner D, Ward JM, Li Y, Cui X, Eichacker P, Leppla SH. The heart is an early target of anthrax lethal toxin in mice: a protective role for neuronal nitric oxide synthase (nNOS) PLoS Pathog. 2009;4:e1000456. doi: 10.1371/journal.ppat.1000456. [DOI] [PMC free article] [PubMed] [Google Scholar]