Abstract
YB-1 is a member of the cold shock domain family, with complex roles in DNA structure, gene transcription and translation. YB-1 promotes chromosomal instability, and mammary gland transgenic expression induces tumors with 100% penetrance. YB-1 is linked to poor prognosis in breast carcinoma and is a strong predictor of relapse and disease-specific survival. Survival is directly tied to the extent of local invasion and distal metastasis, processes dependent upon the activity of the membrane type I-matrix metalloproteinase, MT1-MMP. Non-invasive MCF-7 breast adenocarcinoma cells were transfected with YB-1/EGFP. YB-1 protein was detected in the invadopodia of cells with a migratory phenotype. There was increased expression of MT1-MMP protein concentrated at the leading edges of motile cells, which were highly invasive in collagen three-dimensional culture. The rates of MT1-MMP protein endocytosis and recycling to the cell surface were elevated in clones expressing higher levels of YB-1 protein. Control MCF-7 cells formed nonfatal, noninvasive, differentiated adenocarcinomas in vivo. MCF-7 cells expressing a two-fold increase in YB-1 formed highly anaplastic tumors with local invasion, pulmonary metastases and high lethality. We conclude that YB-1 contributes to the development of an invasive, metastatic breast carcinoma phenotype by enhanced presentation of MT1-MMP at the sites of cellular invasion.
Words: Breast cancer, invasion, metastasis, MT1-MMP, cold-shock protein, YB-1
Introduction
The Y-box binding protein is a member of a highly conserved protein family characterized by a nucleic acid binding cold shock domain. Activity of YB-1 as a transcription factor was first demonstrated by regulatory binding to the MHC class II promoter [1]. YB-1 regulates the transcription of many genes, including the multidrug resistance gene-1 (MDR-1), the epidermal growth factor receptor, DNA polymerase-α and matrix metalloproteinase-2 (MMP-2) [2–5]. YB-1 has additional levels of activity, including regulation of pre-mRNA splicing, mRNA-translocation, and regulation of mRNA translation [6–7]. YB-1 also interacts with structural elements, including tubulin and actin, and can act as an actin-bundling protein [8,9]. YB-1 has a complex cellular distribution, including nuclear, cytoplasmic and invadopodial localization [2,10]. In addition, YB-1 is a bona fide oncogene: transgenic expression of YB-1 in murine mammary tissue results in breast tumor development with 100% penetrance [11]. Furthermore, measurement of YB-1 expression in breast cancer tissues is superior to estrogen receptor or HER-2 status as a predictor of relapse and disease-specific survival [12].
Membrane type 1 matrix metalloproteinase (MT1-MMP), has a pivotal role in tumor cell invasion and metastasis, including breast adenocarcinomas [13,14]. We previously demonstrated that YB-1 induces MMP-2 transcription through an interaction with a non-canonical binding site located in the MMP-2 proximal promoter [15]. We initiated similar studies of the MT1-MMP promoter to determine whether YB-1 affected MT1-MMP transcription by MCF-7 breast adenocarcinoma cells. In contrast to our findings with MMP-2, YB-1 had no effect on MT1-MMP transcription rates or transcript stability. As detailed herein, YB-1 converted well differentiated MCF-7 cells into an anaplastic and invasive phenotype through subtle alterations in MT1-MMP cellular localization and turnover. These observations further broaden the spectrum of the tumor-promoting activities of this remarkably pleiotropic molecule.
MATERIALS AND METHODS
Cell line and culture conditions
MCF-7 cells were obtained from ATCC and maintained in MEM Eagle’s supplemented with 20% fetal calf serum, 1% non-essential amino acids, 1% sodium pyruvate and 0.1 μg/ml insulin.
Construction of YB-1-EGFP and MT1-MMP-DsRed2 expression plasmids
A human YB-1 cDNA was subcloned into the EcoR1 site of pEGFP-N1 (Clontech). The YB-1 cDNA was expressed as a fusion with the N-terminus of EGFP. Human MT1-MMP cDNA was cloned into the vector pDsRed2-N1 (Clontech). The MT1-MMP cDNA was expressed as a fusion with the N-terminus of the DsRed2 red fluorescence protein.
Generation of stable YB-1-EGFP MCF-7 transfectants
Cultures were transfected with the YB-1-EGFP plasmid using Fugene 6 (Roche Diagnostics). Transfectants were selected using antibiotic G418 at 1 mg/ml. MCF-7 cells expressing a control pEGFP-N1 plasmid were selected using G418 at 500 μg/ml.
Selection of YB-1-EGFP expressing MCF-7 populations
To avoid clonal selection bias, control MCF-7 cells transfected with the pEGFP-N1 plasmid and MCF-7 cells transfected with the YB-1-EGFP plasmid were suspended in PBS (1x106 cells/ml) and sorted (FACS Calibur, BD Biosciences). A population of YB-1-EGFP expressing cells was collected with the same fluorescence intensity as a population of control EGFP-expressing cells and denoted population “A”. A second population of YB-1-EGFP-expressing MCF-7 cells was collected with twice the fluorescence intensity of population “A” and was denoted population “B”. Each set of MCF-7 cells was plated, grown to near confluence and resorted. Thereafter, each cell population was expanded, harvested into aliquots and stored in liquid nitrogen. For experiments, cells were thawed, expanded through no more than two passages and used as detailed.
Electophoretic mobility shift analyses (EMSA)
EMSA was performed as reported [5], using nuclear and cytoplasmic protein fractions from controls and from populations “A” and “B”. The probe consisted of the single stranded YB-1 binding sequence,TGAGGCTGATTGGCTGGGCA, from the MDR1 gene promoter [16].
MT1-MMP inhibition studies
Control cells and population “B” cells were cultured in OptiMEM (Invitrogen) and were incubated for 48 hours with a murine monoclonal IgG1κ directed against the MT1-MMP catalytic domain (10 μg/ml, clone 114-6G6, Chemicon) or with control murine IgG1 at the same concentration. Cells were stained for expression of E-cadherin and vimentin as detailed below.
MCF-7 membrane fraction preparation
Triton X114 detergent extraction of MCF-7 cellular membranes was performed as reported (17). Membrane protein concentrations were determined with BCA (Pierce).
Collagen gel culture
The respective MCF-7 cell populations were plated (1 × 105 cells/dish) on Type I collagen gel (Upstate) in culture medium and examined by phase contrast microscopy for invasive activity at 72 hours. To determine the effect of MT1-MMP inhibition on invasion, monoclonal anti-MT1-MMP was included in the collagen matrix (10 μg/ml).
Western blots
MCF-7 membrane fractions (20 μg/sample) were electrophoresed, transferred to PVDF membranes (GE Amersham), and blocked (StartBlock, Pierce). The blots were incubated sequentially with rabbit anti-human MT1-MMP antibody (1 μg/ml, Chemicon) and HRP-coupled goat anti-rabbit F(ab’)2 (20 ng/ml, Zymed) in StartBlock and developed with ECL Plus (GE Amersham).
Immunohistochemistry
Cells were fixed for 20 minutes at 4°C with 4% paraformaldehyde. For vimentin staining cells were permeabilized in 0.5% Triton X-100 for 10 minutes. Cells were blocked with 5% goat serum for 30 minutes (Vector), and an avidin/biotin kit (Vector). For vimentin staining cells were incubated with anti-vimentin IgG1 (10 μg/ml, clone RV202, Abcam), biotinylated goat anti-mouse IgG F(ab’)2 (5 μg/ml, Zymed) in 1% goat serum/PBS at for 2 hours, and streptavidin-rhodamine (0.5 μg/ml, Jackson ImmunoResearch) in 0.1% BSA/PBS for thirty minutes. For E-cadherin detection, cells were incubated with anti-E-cadherin IgG2a (10 μg/ml, clone 36, Transduction Laboratories) followed by rhodamine-conjugated rat anti-IgG2a (5 μg/ml, Caltag) in 1% goat serum/PBS for 2 hours.
For co-localization of YB-1 and actin, MCF-7 cells expressing EGFP alone, or expressing YB-1-EGFP were fixed in paraformaldehyde, rinsed and permeabilized in 0.1% Triton X-100 in PBS for 3 minutes, followed by staining with rhodamine-phalloidin (Molecular Probes).
To localize the MT1-MMP protein, control MCF-7 and cells expressing YB-1 were transfected with the plasmid MT1-MMP-DsRed2 and examined at 48 hours following transfection.
Quantitative RT-PCR of MT1-MMP
RNA was isolated from the respective cell populations, and analyzed with the Agilent 2100 Bioanalyzer. The cDNA templates were generated by oligo-dT priming (Transcriptor, Roche) and RT-PCR performed using SYBR Green incorporation (Applied Biosystems). The primer pairs for MT1-MMP were 5′-GGGGCTTTCACAGTTAGAAG-3′/5′-TTCCAATGCTGCTGCACGTC-3′, for GAPDH 5′-TGACATCAAGAAGGTGGTGAAGCAGGCAT-3′/ 5′-CACCCTGTTGCTGTAGCCGTATTCATTGTCAT-3′. Reactions were performed in triplicate to quadruplicate and quantified by the ΔΔCt method. Results are normalized to fold-change compared to control MCF-7 cells.
MT1-MMP endocytosis and recycling assay
Cell surface biotinylation and recycling assays were performed as detailed by Remacle et al. [18]. In brief, MCF-7 cell surfaces were biotinylated with NHS-SS-biotin (Pierce). To assess endocytosis, cells were quenched with glycine, washed and incubated for 15 minutes at 37 º C to permit endocytosis. Non-endocytosed surface biotin was cleaved with 2-mercaptoethane sulfonic acid (MESNA) and the cells lysed and immunoprecipitated using mouse monoclonal anti-biotin IgG (Zymed) coupled to protein G agarose (Sigma-Aldrich). Bound complexes were eluted into sample buffer followed by Western blot analysis for MT1-MMP. To assess recycling of internalized MT1-MMP to the cell surface MESNA-treated cells were incubated at 37 º C for 30 minutes, followed by lysis, biotinnylated complex retrieval and Western blot analysis for MT1-MMP as detailed above.
In vivo subcutaneous tumor assay
Control MCF-7 cells and the two populations of YB-1-EGFP expressing cells (1 × 106) were suspended in a 1:1 mixture of medium/Matrigel (BD Biosciences) and injected subcutaneously into groups of six female 8 week old athymic nu/nu mice (Jackson Laboratory) preimplanted with 1.7 mg of 17-β-estradiol pellets (Innovative Research of America). At the time of death or sacrifice (8 weeks) the primary tumors, liver, kidneys and lungs were excised, fixed in 4% paraformaldehyde, sectioned and stained with hematoxylin/eosin.
RESULTS
Characterization of YB-1-expressing MCF-7 cells
MCF-7 cells retain many features of differentiated mammary epithelium, including estrogen receptor positivity and ability to form domes in culture. We generated MCF-7 cells in which the levels of YB-1 expression were modulated within a narrow range (up to 2-fold). To avoid selection bias we used FACS to select MCF-7 cell populations, such that one population denoted “A” expressed YB-1-EGFP at the levels of controls expressing EGFP alone. A second population denoted “B”, in which YB-1-EGFP expression was approximately two-times the level of the controls, was selected. We quantified biologically active YB-1 in the cells as determined by specific binding to the single-stranded oligonucleotide sequence 5′-TGAGGCTGATTG-GCTGGGCA-3′ of the MDR1 promoter [16]. YB-1 binding activity was detected in both cytoplasmic and nuclear fractions of control MCF-7 cells (Figure 1, Panel I.). The ratio of cytoplasmic to nuclear YB-1 binding activity was approximately 2:1 when focusing on complex 1. EMSA of population “A” cells revealed small increases in both cytoplasmic and nuclear fractions, with a faster migrating second complex in the nuclear fraction (complex denoted <2). Nuclear YB-1 binding activities were increased approximately two-fold in population “B” cells as compared to control MCF-7 cells. Selection of transfected MCF-7 cells required twice as much G418 antibiotic as the controls (1 mg G418/ml vs. 500 μg G418/ml) consistent with YB-1-mediated drug resistance, confirming the biologic activity of the transfected YB-1.
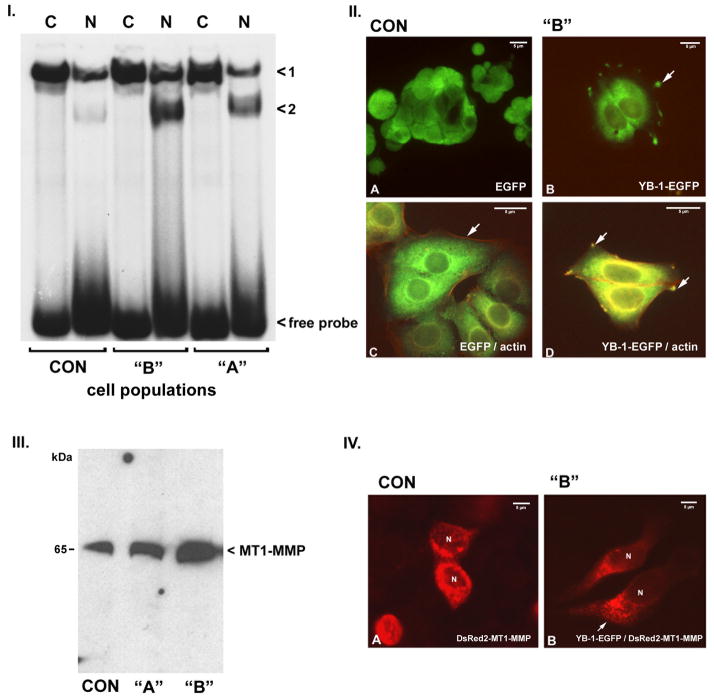
Figure 1. Characterization of YB-1 transfected MCF-7 cells.
I. Electrophoretic mobility shift analyses of cytosolic (C) and nuclear (N) fractions from control (CON) and populations “A” and “B” YB-1-EGFP transfected cells. Labeled oligonucleotide comprised of the single-stranded DNA YB-1 binding sequence within the MDR1 gene promoter. Two distinct complexes were detected, denoted “<1” and “<2”. Compared to controls, extracts from population “A” cells have moderate increases in both cytosolic and nuclear YB-1, with C > N for complex 1 and N > C for complex 2. Population “B” cells, which express twice as much YB-1-EGFP protein by FACS analysis, have approximate two-fold increases in nuclear YB-1, forming complex 2. Free probe is indicated. Relative amounts of expressed YB-1-GFP were assessed by Western Blots in all cell batches used for experiments.
II. Fluorescence microscopy of control MCF-7 cells (A) expressing EGFP alone. EGFP signal is diffusely present throughout the cytosol. (B) In cells expressing YB-1-EGFP the protein is present in both, cytosolic as well as nuclear fractions, with C > N. In addition YB-1-EGFP protein is concentrated at the tips of invadopodial structures (arrow). Dual imaging of EGFP and actin (red signal) in control MCF-7 cells (C) shows no co-localization of fluorescent signals. (D) In contrast EGFP-tagged YB-1 protein co-localizes with pericellular and invadopodial actin in the transfected MCF-7 cells (yellow signal). Magnification 300 to 450-fold in A-D, magnification bars are indicated.
III. Western blot of membrane extracts for MT1-MMP protein in control (CON) and population “A” and “B” YB-1-EGFP transfected cells. As compared to controls, population “A” cells have an approximate 2-fold increase in membrane-associated MT1-MMP protein, while population “B” cells have an approximate 6-fold increase. Relative increase of MT1-MMP protein was found in all tested cell batches.
IV. YB-1-transfected MCF-7 cells have an altered distribution of DsRed2-tagged YB-1 protein. Panel A: Tagged MT1-MMP protein is symmetrically distributed in a perinuclear pattern in control MCF-7 cells. Panel B: DsRed2-tagged MT1-MMP protein is asymmetrically concentrated at the leading edge of migratory YB-1-EGFP transfected MCF-7 cells. Magnification 450-fold.
Control MCF-7 cells transfected with EGFP (Figure 1, Panel II., A) revealed diffuse, cytoplasmic signal. In contrast, fluorescent signal in MCF-7 cells transfected with YB-1-EGFP was concentrated in a filamentous perinuclear pattern (B). In addition, YB-1-EGFP signal was localized within the cellular invadopodia. We co-stained the MCF-7 cells with rodamine-phalloidin to visualize actin localization in relation to YB-1 (C and D). In the control MCF-7 cells actin was located in a subcortical distribution (C), while in the YB-1 transfected cells there was colocalization of the YB-1-EGFP protein with perinuclear and cytoplasmic actin, and within the invadopodial structures (D).
Western blots of membrane extracts revealed an approximate two-fold increase in MT1-MMP protein in population “A” MCF-7 cells, while membrane extracts from population “B” MCF-7 cells indicated a 5-6-fold increase in MT1-MMP protein as compared to controls (Figure 1, panel III.). Quantitative RT-PCR determined MT1-MMP transcript abundance, where control MCF-7 cell MT1-MMP transcript abundance was assigned a value of 1. Population “A” cells had a MT1-MMP transcript abundance of 1.12 ± 0.03; population “B” cells had a MT1-MMP transcript abundance of 1.08 ± 0.15, P > 0.05. We examined MT1-MMP transcription rates using a murine MT1-MMP promoter-luciferase construct and did not detect upregulation of MT1-MMP transcription in the YB-1-transfected cells (data not shown). Thus, the increased quantities of MT1-MMP protein present within the membrane extracts of the YB-1 transfected MCF-7 cells are not the consequence of YB-1-mediated MT1-MMP transcriptional activation or enhanced mRNA stability.
YB-1-transfected MCF-7 cells demonstrated an altered cellular distribution of DsRed2-tagged MT1-MMP protein (Figure 1, Panel IV.). The DsRed2-tagged MT1-MMP protein was symmetrically distributed in a granular perinuclear pattern in the control MCF-7 cells (A). In contrast, the MT1-MMP protein in the YB-1-EGFP transfected cells was asymmetrically concentrated in the leading edges of cells with an elongated, migratory phenotype (B). Pull-down experiments did not demonstrate a physical association of the YB-1 and MT1-MMP proteins (data not shown).
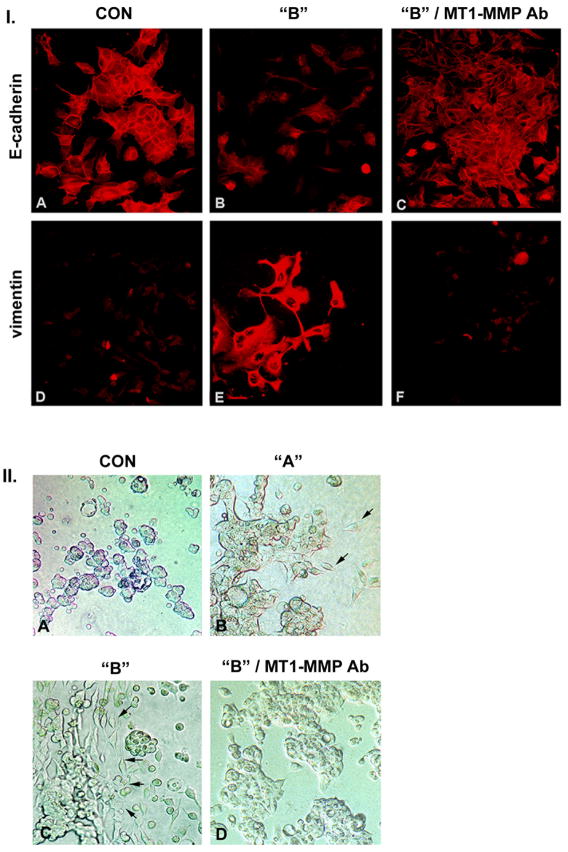
YB-1-transfected MCF-7 cells underwent epithelial-mesenchymal transition and developed a migratory phenotype (Figure 2, panel I.). As compared to controls (A), the epithelial marker E-cadherin was reduced in the YB-1 transfected cells (B). The mesenchymal marker vimentin was barely detectable in the control MCF-7 cells (D), but was prominently expressed within the YB-1 transfected cells (E). Inclusion of a monoclonal antibody directed to the catalytic site of MT1-MMP reverted the YB-1 transfected MCF-7 cells to an epithelial phenotype, with reconstitution of E-cadherin expression (C) and suppression of vimentin expression (F). There was no effect on YB-1-dependent epithelial-mesenchymal transition following inclusion of a control antibody not directed against the MT1-MMP catalytic site (data not shown).
Figure 2. YB-1 transfected MCF-7 cells undergo epithelial to mesenchymal transition.
I. The epithelial marker E-cadherin is reduced in YB-1-EGFP transfected cells (B) as compared to control cells (A) and has a primarily cytosolic distribution. Incubation with a monoclonal antibody directed against the catalytic site of MT1-MMP reverts the YB-1 transfected cells to a normal epithelial phenotype with restitution of E-cadherin expression (C). Staining for vimentin reveals the contrary pattern with detection of vimentin in cells over expressing YB-1-EGFP (E) compared to control cells (D). With inclusion of the antibody targeting the catalytic MT1-MMP site the vimentin expression is down regulated (F). Magnification 450-fold.
II. YB-1 promotes invasive activity of MCF-7 cells in three dimensional collagen gels. Control MCF-7 cells grow in multicellular spherules (A). Population “A” cells grow primarily in spherules with occasional single cells invading the collagen gel (B), while population “B” cells are present as elongated individual cells with cohort or invasion front characteristics (arrows) (C). Inclusion of monoclonal anti-MT1-MMP antibody in the collagen gel reverts population “B” MCF-7 cells to an epithelial, non-invasive phenotype (D). Magnification 200-fold.
We evaluated the ability of YB-1-transfected MCF-7 cells to grow in three-dimensional collagen gel. As shown in Figure 2, panel II. (A), control MCF-7 cells grew as multicellular spherules, with no invasive activity into the gel. YB-1-transfected MCF-7 populations demonstrated a graded increase in invasive activity. Population “A” MCF-7 cells grew primarily in spherules, but individual cells invading the collagen gel were present (B). Population “B” cells grew as individual cells with evidence for cohort, or invasion front, activity (C). Inclusion of antibody to the MT1-MMP catalytic site in the collagen gel blocked the invasive activity of the YB-1-transfected cells and reverted the cells to growth in spherules (D).
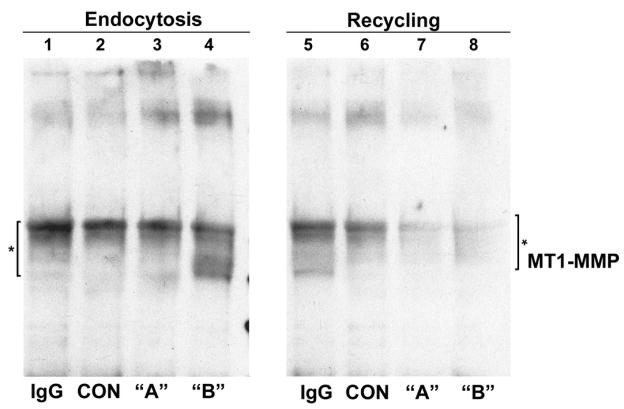
Our findings to this point indicated that increased MT1-MMP protein in the YB-1-transfected MCF-7 cells was not due to enhanced transcriptional rates or transcript abundance. MT1-MMP is subjected to complex post-translational regulation, including endocytosis by caveolae-dependent and clathrin-dependent pathways [18]. Internalized MT1-MMP is degraded within lysosomes or recycled back to the cell surface to facilitate migration and invasion. Internalization and recycling controls the amount of MT1-MMP enzyme at the plasma membrane. Using a cell surface biotinylation technique, [18], we examined the relative rates of MT1-MMP endocytosis and recycling. Representative results are depicted in Figure 3. To assess rates of MT1-MMP endocytosis, MCF7 cell surfaces were biotinylated on ice, and then warmed for 15 minutes at 37º C to permit internalization. Cells were then cooled and the remaining biotinylated MT1-MMP on the cell surface was removed with nonpermeant MESNA, followed by MT1-MMP Western blot analysis of biotinylated complexes recovered with immobilized monoclonal anti-biotin. Decreased MT1-MMP signal reflects increased endocytosis. The rate of endocytosis of MT1-MMP protein was increased in population “B” MCF-7 cells, while the endocytosis rates in the relatively non-invasive population “A” cells were equivalent to controls. To assess MT1-MMP recycling of internalized protein back to the cell surface, cells were treated as described for endocytosis through the first MESNA cleavage step, but in this case followed by incubation at 37 ºC for 30 minutes to permit recycling. Thereafter, the cells were treated with a second course of MESNA to cleave the biotin present on proteins recycled to the cell surface, followed by MT1-MMP Western blot analysis of recovered biotinylated proteins. In this case, decreased MT1-MMP signal reflects increased recycling. Both populations, “A” and “B” cells, had increased rates of cell surface recycling as compared to the controls, indicated by decreased band intensities (Figure 3, compare lanes 7 and 8 with 5 and 6). Thus, YB-1 affects both MT1-MMP endocytosis and recycling, particularly in the invasive population “B” cells.
Figure 3. Assessment of MT1-MMP protein endocytosis and recycling in control and YB-1 transfected MCF-7 cells.
Assessment of MT1-MMP protein turnover rates was performed as detailed in Materials and Methods. Endocytosis rates of population “A” cells expressing YB-1-EGFP at low levels does not differ from control MCF-7 cells (lane 3). Endocytosis rates in population “B” cells are increased approximately 2-fold as compared to controls and population “A” cells. Recycling rates were equally increased in both populations, “A” and “B” cells, as compared to MCF-7 control cells. * indicates endocytosis complex. IgG, control antibody.
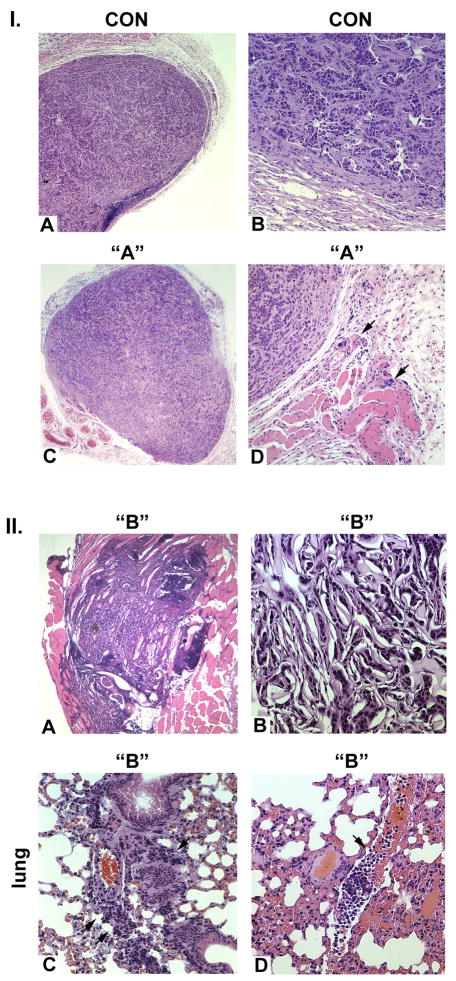
We examined the ability of MCF-7 and the YB-1-expressing MCF-7 cells to form tumors in athymic female nu/nu mice. At eight weeks all six mice in the control group were alive; four had tumors. Control MCF-7 cell tumors generated well-differentiated adenocarcinomas, (Figure 4 Panel I., A and B) with dense capsules, negligible neoangiogenesis and absent invasion. Population “A” derived tumors were also well-differentiated adenocarcinomas, but did manifest modest degrees of neoangiogenesis and limited local invasion (Panel I., C and D). All six mice in this group survived to eight weeks following injection. In contrast, five of six mice injected with the population “B” YB-1-expressing MCF-7 cells were dead by eight weeks. The tumors were highly anaplastic, lacked capsules, and manifested local invasion and neoangiogenesis. (Figure 4, panel II., A and B). Metastases were observed in the liver, peritoneum, kidney and lung. Examples of pulmonary metastases are shown in C and D, wherein there is peribronchial infiltration of anaplastic tumor cells, along with masses of tumor cells within the pulmonary vasculature, consistent with hematogenous dissemination.
Figure 4. Assessment of tumor formation of control and YB-1 transfected MCF-7 cells.
I. Control MCF-7 tumors are thickly encapsulated and histologically characterized as highly differentiated adenocarcinomas (A, B) with no neoangiogenesis or local invasion. Population “A” tumors (C, D) are encapsulated, histologically characterized as well differentiated adenocarcinomas with limited local invasion (arrows). A and C: 50-fold; B and D: 200-fold magnification.
II. Population “B” MCF-7 cells are unencapsulated with prominent local invasion and neovascularization (A) and are highly anaplastic in nature (B). Panel C illustrates distal pulmonary metastases with peribronchial infiltration of anaplastic MCF-7 cells. Panel D demonstrates prominent masses of tumor cells within the pulmonary vasculature, consistent with hematogenous dissemination. A: 50-fold; B: 200-fold; C and D: 150-fold magnification.
DISCUSSION
Transfection of MCF-7 adenocarcinoma cells with YB-1 has profound effects on cellular phenotypes. MCF-7 cells expressing modest, two-fold increases in YB-1 are anaplastic and form tumors characterized by aggressive local invasion and metastases.
The cellular distribution of EGFP-tagged YB-1 protein is complex, with YB-1-EGFP present within nuclear and cytosolic fractions. YB-1-EGFP protein was also concentrated within invadopodia in association with actin. YB-1’s activity as a actin bundling protein suggests a structural function in membrane endocytosis and recycling [9].
YB-1-mediated development of an invasive phenotype was dependent upon the activity of MT1-MMP. MT1-MMP protein content within membrane-enriched fractions of YB-1 transfected cells was increased, but we were not able to demonstrate significant differences from control cells in terms of MT1-MMP transcription rates or steady-state levels of the MT1-MMP transcripts. Isolated invadopodia contain the full array of cellular components required to support translation, [10], and YB-1 plays a key role in the regulation of translation through binding to the 5’-UTR [6,7]. Within the context of MCF-7 cells, Dong et al., [19], recently demonstrated that YB-1 has RNA-binding sequence specificity, but that binding, per se, does not clearly regulate translation of the mRNA targets. In this regard, we were unable to demonstrate specific binding of YB-1 to the 5’UTR of the MT1-MMP mRNA (data not shown), suggesting that the increased MT1-MMP observed in YB-1-transfected cells was not dependent on enhanced translation within the invadopodial compartment.
Bravo-Cordero et al. [20] have presented a detailed analysis of MT1-MMP endo- and exocytosis and proposed a mechanism whereby the relative targeting of MT1-MMP to lysosomal degradation vs. recycling to the cell surface determines the net amount of MT1-MMP present within invadopodia. Although speculative at this point, our findings suggest that YB-1 redirects, perhaps in association with subcortical actin, the endocytosis of MT1-MMP protein away from the lysosomal compartment and back into the cellular membrane, resulting in increased effective concentrations of MT1-MMP protein at sites of interaction with the extracellular matrix.
In summary, we have shown that YB-1 induces an anaplastic, invasive and metastatic phenotype in model MCF-7 breast adenocarcinoma cells through a relatively subtle alteration in the cellular localization and internalization dynamics of the MT1-MMP protein. Future studies to examine the precise cellular mechanisms involved in MT1-MMP protein turnover modification by YB-1 could provide the basis for the therapeutic targeting of YB-1 effects, as opposed to the molecule, per se.
Acknowledgments
This study was supported by NIDDK grants RO1-DK39776 (DHL), KO8-DK59381 (SC) and SFB854 project 1/SFB542 (PRM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Didier DK, Schiffenbauer J, Woulfe SLM, et al. Characterization of the cDNA encoding a protein binding to the major histocompatibility complex class II Y box. Proc Natl Acad Sci U S A. 1988;85:7322–6. doi: 10.1073/pnas.85.19.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohno K, Izumi H, Uchiumi T, et al. The pleiotropic functions of the Y-box-binding protein, YB-1. Bioessays. 2003;25:691–8. doi: 10.1002/bies.10300. [DOI] [PubMed] [Google Scholar]
- 3.Wu J, Lee C, Yokom D, et al. Disruption of the Y-box binding protein-1 results in suppression of the epidermal growth factor receptor and HER-2. Cancer Res. 2006;66:4872–9. doi: 10.1158/0008-5472.CAN-05-3561. [DOI] [PubMed] [Google Scholar]
- 4.En-Nia A, Yilmaz E, Klinge U, Lovett DH, Stefanidis I, Mertens PR. Transcription factor YB-1 mediates DNA polymerase alpha gene expression. J Biol Chem. 2005;280:7702–11. doi: 10.1074/jbc.M413353200. [DOI] [PubMed] [Google Scholar]
- 5.Mertens PR, Harendza S, Pollock AS, Lovett DH. Glomerular mesangial cell-specific transactivation of matrix metalloproteinase 2 transcription is mediated by YB-1. J Biol Chem. 1997;272:22905–12. doi: 10.1074/jbc.272.36.22905. [DOI] [PubMed] [Google Scholar]
- 6.Evdokimova V, Ruzanov P, Imataka H, et al. The major mRNA-associated protein YB-1 is a potent 5' cap-dependent mRNA stabilizer. Embo J. 2001;20:5491–502. doi: 10.1093/emboj/20.19.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raffetseder U, Frye B, Rauen T, et al. Splicing factor SRp30c interaction with Y-box protein-1 confers nuclear YB-1 shuttling and alternative splice site selection. J Biol Chem. 2003;278:18241–8. doi: 10.1074/jbc.M212518200. [DOI] [PubMed] [Google Scholar]
- 8.Chernov KG, Mechulam A, Popova NVD, et al. YB-1 promotes microtubule assembly in vitro through interaction with tubulin and microtubules. BMC Biochem. 2008;9:23. doi: 10.1186/1471-2091-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruzanov PV, Evdokimova VM, Korneeva NL, et al. Interaction of the universal mRNA-binding protein, p50, with actin: a possible link between mRNA and microfilaments. J Cell Sci. 1999;112( Pt 20):3487–96. doi: 10.1242/jcs.112.20.3487. [DOI] [PubMed] [Google Scholar]
- 10.Jia Z, Barbier L, Stuart H, et al. Tumor cell pseudopodial protrusions. Localized signaling domains coordinating cytoskeleton remodeling, cell adhesion, glycolysis, RNA translocation, and protein translation. J Biol Chem. 2005;280:30564–73. doi: 10.1074/jbc.M501754200. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann S, Royer-Pokora B, Fietze E, et al. YB-1 provokes breast cancer through the induction of chromosomal instability that emerges from mitotic failure and centrosome amplification. Cancer Res. 2005;65:4078–87. doi: 10.1158/0008-5472.CAN-04-4056. [DOI] [PubMed] [Google Scholar]
- 12.Habibi G, Leung S, Law JH, et al. Redefining prognostic factors for breast cancer: YB-1 is a stronger predictor of relapse and disease-specific survival than estrogen receptor or HER-2 across all tumor subtypes. Breast Cancer Res. 2008;10:R86. doi: 10.1186/bcr2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotary KB, Allen ED, Brooks PC, et al. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 14.Sounni NE, Devy L, Hajitou A, et al. MT1-MMP expression promotes tumor growth and angiogenesis through an up-regulation of vascular endothelial growth factor expression. Faseb J. 2002;16:555–64. doi: 10.1096/fj.01-0790com. [DOI] [PubMed] [Google Scholar]
- 15.Mertens PR, Alfonso-Jaume MA, Steinmann K, Lovett DH. A synergistic interaction of transcription factors AP2 and YB-1 regulates gelatinase A enhancer-dependent transcription. J Biol Chem. 1998;273:32957–65. doi: 10.1074/jbc.273.49.32957. [DOI] [PubMed] [Google Scholar]
- 16.Bargou RC, Jurchott K, Wagener C, et al. Nuclear localization and increased levels of transcription factor YB-1 in primary human breast cancers are associated with intrinsic MDR1 gene expression. Nat Med. 1997;3:447–50. doi: 10.1038/nm0497-447. [DOI] [PubMed] [Google Scholar]
- 17.Turck J, Pollock AS, Lee LK, Marti HP, Lovett DH. Matrix metalloproteinase 2 (gelatinase A) regulates glomerular mesangial cell proliferation and differentiation. J Biol Chem. 1996;271:15074–15083. doi: 10.1074/jbc.271.25.15074. [DOI] [PubMed] [Google Scholar]
- 18.Remacle A, Murphy G, Roghi C. Membrane type I-matrix metalloproteinase (MT1-MMP) is internalised by two different pathways and is recycled to the cell surface. J Cell Sci. 2003;116:3905–16. doi: 10.1242/jcs.00710. [DOI] [PubMed] [Google Scholar]
- 19.Dong J, Akcakanat A, Stivers DN, et al. RNA-binding specificity of Y-box protein 1. RNA Biol. 2009;6:59–64. doi: 10.4161/rna.6.1.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bravo-Cordero JJ, Marrero-Diaz R, Megias D, et al. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. Embo J. 2007;26:1499–510. doi: 10.1038/sj.emboj.7601606. [DOI] [PMC free article] [PubMed] [Google Scholar]