Abstract
We analyzed the contribution of individual ocular components to vision-induced ametropias in 210 rhesus monkeys. The primary contribution to refractive-error development came from vitreous chamber depth; a minor contribution from corneal power was also detected. However, there was no systematic relationship between refractive error and anterior chamber depth or between refractive error and any crystalline lens parameter. Our results are in good agreement with previous studies in humans, suggesting that the refractive errors commonly observed in humans are created by vision-dependent mechanisms that are similar to those operating in monkeys. This concordance emphasizes the applicability of rhesus monkeys in refractive-error studies.
Keywords: hyperopia, myopia, anisometropia, emmetropization
Introduction
In a simplistic sense, the primary biometric variables that potentially contribute to the eye’s refractive status are the refracting powers of the cornea and crystalline lens and the axial dimensions of the anterior chamber, lens and vitreous chamber. To understand the contributions of these variables to ocular refraction, a substantial number of primarily cross-sectional studies have examined the dimensions and distributions of these ocular components in populations of emmetropic and ametropic eyes, the interrelations between these ocular components in emmetropic and ametropic eyes, and the correlations between individual ocular components and refractive error (Bullimore et al., 1992; Fledelius, 1988; Fledelius, 1995; Goss et al., 1990; Grosvenor & Scott, 1991; Grosvenor & Scott, 1993; Jensen, 1991; Larsen, 1971a; Larsen, 1971b; Larsen, 1971c; Larsen, 1971d; Mayer et al., 2001; McBrien & Millodot, 1987; Mutti et al., 2005; Sorsby et al., 1957; Sorsby et al., 1961; Sorsby et al., 1966; Stenstrom, 1948; Zadnik et al., 2003). For example, correlation analyses have shown that the primary ocular components that influence refractive error are interdependent and that during early development these components grow in a coordinated manner to move the eye toward emmetropia (Carroll, 1981; Carroll, 1982; Hirsch, 1947; Hirsch & Weymouth, 1947; Stenstrom, 1948; van Alphen, 1961). In other words, compensatory alterations in related parameters occur to promote emmetropia. In particular, the concept of the inflatable globe evolved from these studies and the notion that aspects of emmetropization are passive consequences of eye growth (Hofstetter, 1969; Koretz et al., 1995; Mutti et al., 1998; Wallman & Adams, 1987), specifically that increases in axial length during early development are counterbalanced by concomitant decreases in corneal power, lens thickness and lens power. These studies have also provided insights into the nature of refractive errors, in essence, how ametropic eyes, in particular myopic eyes, differ from emmetropic eyes. The results from these investigations have demonstrated the relative importance of individual ocular components in determining the eye’s refraction and provided insights into the mechanisms that are associated with the development of common refractive errors.
It has been consistently shown that elongation of the vitreous chamber contributes to myopia (Wildsoet, 1998). However, each of the major ocular components has been shown to potentially contribute to myopic refractions. The degree of influence for a given component is somewhat dependent on the analysis methods and possibly the age of the sample studied (Wildsoet, 1998). For instance, in his classic study, Stenstrom (1948), using correlation analyses, showed that refraction was significantly correlated with corneal radius (r = +0.18), anterior chamber depth (r = −0.34), and especially axial length (r = −0.76); therefore, he concluded that axial length had the greatest influence on ocular refraction and that most myopia was axial in nature. Using Stenstrom’s data and partial correlation analyses, Hirsch and Weymouth (1947) reported that axial length accounted for 47% of the variance in refractive error, while corneal power and anterior chamber depth were responsible for 24% and 7% of the variability of refraction, respectively. Van Alphen (1961) reanalyzed Stenstrom’s data using partial correlation coefficients and factor analysis and described the myopic eye as one with a longer than normal axial length and/or a more powerful cornea with a flatter crystalline lens. More recent investigations employing analyses based on structural models (Scott & Grosvenor, 1993) or quantitative analyses of growth curves (Jones et al., 2005) for individual ocular components have shown that in comparison to emmetropic eyes, myopic eyes have higher corneal powers, higher lens powers, and greater anterior and vitreous chamber depths.
Longitudinal studies of the changes in ocular components that occur during the onset and/or progression of myopia have emphasized the contribution of vitreous chamber elongation to myopic refractions (Fledelius, 1988; Grosvenor & Scott, 1993; Gwiazda et al., 2003; Jensen, 1991; Mutti et al., 2005). In both juveniles (Fledelius, 1988; Grosvenor & Scott, 1993; Gwiazda et al., 2003; Jensen, 1991) and adults (Grosvenor & Scott, 1993; McBrien & Adams, 1997), the onset and progression of myopia are strongly correlated with increases in axial length and, specifically, vitreous chamber depth. There is little or no evidence that increases in either corneal power (Fledelius, 1988; Goss & Erickson, 1987; Grosvenor & Scott, 1993; Jensen, 1991; McBrien & Adams, 1997; Parssinen, 1993) or lens power (Bullimore et al., 1992; Grosvenor & Scott, 1991; Jensen, 1991; Larsen, 1971c; McBrien & Adams, 1997; McBrien & Millodot, 1987) contribute to myopic progression. However, the growth curves for the anterior chamber and corneal power for myopic children are different in shape than those for emmetropic children (Jones et al., 2005).
There are a number of parallels between the structural characteristics of refractive errors in humans and those in animals with experimentally induced ametropias. In particular, in a wide variety of animal species, experimentally induced refractive errors are associated with alterations in vitreous chamber depth and axial length. For example, myopia produced by form deprivation or optical defocus is associated with vitreous chamber elongation in chicks (Schaeffel et al., 1988; Wallman & Adams, 1987; Wildsoet & Wallman, 1995), tree shrews (Marsh-Tootle & Norton, 1989; McBrien & Norton, 1992; Norton et al., 2006), guinea pigs (Howlett & McFadden, 2006; Jiang et al., 2009), marmosets (Graham & Judge, 1999; Troilo & Judge, 1993), and macaques (Greene, 1990; Hung et al., 1995; Qiao-Grider et al., 2004; Smith et al., 1999a; Smith et al., 1987; Smith & Hung, 2000; Smith et al., 2002a; Tigges et al., 1990; Wiesel & Raviola, 1977). However, the associations between experimental refractive errors and other ocular component changes are less consistent between species and, in some cases, between studies. For example, experimental myopia has been associated with increases in corneal power in guinea pigs (Howlett & McFadden, 2006). When form deprivation myopia is produced by lid closure, decreases in corneal power have been reported for chicks (Troilo et al., 1995), marmosets (Troilo & Judge, 1993), and tree shrews (Marsh-Tootle & Norton, 1989; McBrien & Norton, 1992; Norton et al., 2006). However, when spectacle lenses are employed to produce myopia, corneal power is not affected in macaques (Hung et al., 1995; Smith & Hung, 1999; Smith et al., 1994), chicks (Irving et al., 1995), marmosets (Graham & Judge, 1999) or tree shrews (Norton et al., 2006), which suggests that lid closure can have confounding mechanical effects on the cornea. Experimental myopia is associated with increases in anterior chamber depth in guinea pigs (Howlett & McFadden, 2006) and decreases in anterior chamber depth in tree shrews (Marsh-Tootle & Norton, 1989; McBrien & Norton, 1992; Norton et al., 2006). However, anterior chamber alterations have not been consistently observed in chicks (Schaeffel et al., 1988; Wallman & Adams, 1987; Wildsoet & Wallman, 1995), marmosets (Troilo & Judge, 1993) or macaques (Smith & Hung, 1999). Increases and decreases in crystalline lens thickness have been observed, respectively, in guinea pigs (Howlett & McFadden, 2006) and tree shrews with experimental myopia (Marsh-Tootle & Norton, 1989; McBrien & Norton, 1992; Norton et al., 2006; Siegwart & Norton, 1998), but no consistent changes in lens thickness have been found in chicks (Irving et al., 1995; Troilo et al., 1995), marmosets (Graham & Judge, 1999; Troilo & Judge, 1993) or macaques (Greene, 1990; Hung et al., 1995; Tigges et al., 1990; Wiesel & Raviola, 1977). However, experimental myopia does increase the variability of lens power in chickens with experimental myopia (Priolo et al., 2000).
Examining the nature of refractive errors in animals with experimentally induced refractive errors, particularly vision-induced ametropias, is important because it is a critical step in determining the applicability of animal data to the human condition. Moreover, if ocular development in these animals is similar to that of humans, the results from these animal investigations can identify which ocular components are affected by alterations in visual experience and, thus, provide the foundation for understanding the effects of visual experience on refractive development in humans. Refractive development and the optical organization of macaque eyes are very similar to those of humans (Bradley et al., 1999; Greene, 1990; Qiao-Grider et al., 2007b). However, previous studies of the nature of experimental refractive errors in macaques have employed limited numbers of subjects and have not measured all of the key ocular components. The purpose of this study was to determine the structural features of experimentally induced refractive errors in a large number of infant rhesus monkeys.
Materials and Methods
Subjects
Data are presented for 210 infant rhesus monkeys (Macaca mulatta). The subject population included most of the animals that we used in previous studies of the effects of visual experience on refractive development and for which we had complete biometric data (Hung et al., 1995; Hung & Smith, 1996; Hung et al., 2000; Kee et al., 2005; Kee et al., 2007; Kee et al., 2004; Kee et al., 2002; Kee et al., 2003; Qiao-Grider et al., 2004; Qiao-Grider et al., 2007a; Qiao-Grider et al., 2007b; Ramamirtham et al., 2007; Ramamirtham et al., 2006; Smith et al., 2001; Smith et al., 2003; Smith et al., 2005; Smith et al., 2007; Smith, 1998a; Smith, 1998b; Smith & Hung, 1999; Smith & Hung, 2000; Smith et al., 1994; Smith et al., 1999b; Smith et al., 2000; Smith et al., 2002a). The animals were obtained at 2 to 3 weeks of age and housed in our primate nursery that was maintained on a 12-hour light/12-hour dark lighting cycle. Animals reared under continuous lighting conditions were excluded from our analyses because evidence from chickens indicate that continuous light can produce alterations in the ocular components of the eye that are very different from those produced by form deprivation or optical defocus and that may be secondary to alterations in intraocular pressure (Lauber, 1987; Lauber & McGinnis, 1966; Li et al., 1995). Our rearing and experimental procedures were reviewed and approved by the University of Houston’s Institutional Animal Care and Use Committee and were in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
With the exception of 18 animals that were reared with unrestricted vision, all of the animals were subjected to one of a variety of different experimental rearing strategies that employed either form deprivation or optically imposed defocus to examine the effects of visual experience on refractive development. Although evidence in chickens indicates that the effects of form deprivation and optically imposed defocus, in particular hyperopic defocus, are not mediated by identical mechanisms (Kee et al., 2001), it is likely that the vision-dependent mechanisms that mediate the effects of form deprivation and optical defocus have many components in common. In this respect, in monkeys the effects of form deprivation and hyperopic defocus are both mediated by local retinal mechanisms, dominated by visual signals from the peripheral retina, and produce similar changes in the shapes of the posterior globe (Huang et al., 2009; Hung et al., 2009; Smith et al., 2009a; Smith et al., 2009b) and the anterior cornea (Kee et al., 2005). Additionally, multi-linear regression analyses using treatment regimen as a dummy variable indicated that there were no significant differences between the effects of form deprivation and optical defocus (p = 0.415 to 0.972). Consequently, for the purposes of this study, we have assumed that form deprivation and optical defocus affect the ocular components of refractions in a qualitatively similar fashion.
The experimental rearing procedures were started at 2 to 4 weeks of age (see Table 1 for details). The duration of the experimental rearing procedures varied between 14 and 21 weeks and encompassed the rapid early phase of ocular growth and emmetropization, which in normal infant monkeys is largely complete by 150 days of age (Bradley et al., 1999; Hung et al., 1995; Qiao-Grider et al., 2007a). Although the treated subjects represent a heterogeneous group, all of the experimental manipulations were optically induced. In part, because the nature and degree of altered visual experience differed between monkeys, our rearing strategies resulted in a wide range of spherical refractive errors. Thus, it was possible to examine the ocular changes in monkeys that developed moderate to high levels of myopia and hyperopia.
Table 1.
Experimental Rearing Regimens
| Group | Comments | Number of Monkeys | References |
|---|---|---|---|
| Normal Control | Unrestricted Vision | 18 (I = 12, C = 6) | (Qiao-Grider et al., 2007a, Qiao-Grider et al., 2007b, Ramamirtham et al., 2006) |
| Plano Lens Control | Plano lenses binocularly | 6 (I = 5, C = 1) | (Smith, 1998b, Smith & Hung, 1999, Smith et al., 1999b) |
| Binocular, Full-field, Spherical Spectacle Lenses | Lens power ranged from −6 D to +12 D | 62 (I = 58, C = 4) | (Hung et al., 1995, Hung et al., 2000, Kee et al., 2007, Smith, 1998b, Smith & Hung, 1999, Smith et al., 1999b) |
| Binocular Peripheral Defocus | Lens power ranged from −3 D to +3 D (6 mm central aperture) | 15 (I = 0, C = 15) | (Smith, et al., 2007a) |
| Monocular Spherical Spectacle Lenses | Lens power ranged from −6 D to +6 D | 6 (I = 6, C = 0) | (Hung et al., 1995, Hung et al., 2000, Ramamirtham et al., 2007, Smith & Hung, 1999, Smith et al., 1999b) |
| Monocular, Full-field Form Deprivation | Three different strength diffuser lenses were employed | 36 (I = 35, C = 1) | (Qiao, et al., 2001, Smith & Hung, 1999, Smith & Hung, 2000, Smith et al., 1994, Smith et al., 1999b, Smith et al., 2002a) |
| Binocular Peripheral Form Deprivation | Diffuser lenses with 4 – 8 mm central aperture | 12 (I = 0, C = 12) | (Smith et al., 2005) |
| Monocular and Binocular Cylindrical Lenses | +1.50 – 3.00 D x 45, 90, 135 or 180 | 37 (I = 37, C = 0) | (Kee et al., 2005, Kee et al., 2004, Kee et al., 2002) |
| Binocular Soft Contact Lenses | Plano to −3 D | 18 (I = 1, C = 17) | (Hung & Smith, 1996) |
I: Indian-derived Monkeys; C: Chinese-derived Monkeys
It is important to note that the subject population included both Chinese- and Indian-derived subspecies of rhesus monkeys. Because there are significant differences in the ocular dimensions between normal Chinese- and Indian-derived rhesus monkeys (Qiao-Grider et al., 2007a), the data for the 56 Chinese-derived monkeys and 154 Indian-derived monkeys were analyzed separately. Twelve Indian-derived monkeys and 6 Chinese-derived monkeys that were reared with unrestricted vision were used to define the normal range of refractive errors.
Biometric Measurements
Cross-sectional data on refractive error, corneal power, axial dimensions and the optical properties of the crystalline lens were obtained from both eyes of all subjects at or near the end of the experimental treatment period (mean = 135 ± 13 days for Indian-derived monkeys and 143 ± 14 days for Chinese-derived monkeys). To make these measurements, the monkeys were anesthetized (intramuscular injection: ketamine hydrochloride, 15–20 mg/kg and acepromazine maleate, 0.15–0.2 mg/kg; topical: 1–2 drops of 0.5% tetracaine hydrochloride) and cycloplegia was induced using 1% tropicamide that was topically instilled into the eyes 20–30 minutes before any measurements that were potentially influenced by the state of accommodation.
The spherical-equivalent, spectacle-plane refractive correction for each eye was measured along the pupillary axis by two experienced investigators using a streak retinoscope and averaged (Harris, 1988). We have previously shown that our retinoscopy results are strongly correlated with autorefractor measures of refractive errors in monkeys (Smith & Hung, 1999) and highly repeatable (Hung et al., 2008). The anterior radius of curvature of the cornea was measured with a hand-held keratometer (Alcon Auto-keratometer; Alcon Systems Inc, St Louis, MO) and/or a videotopographer (EyeSys 2000; EyeSys technologies Inc, Houston, TX). The two instruments provided repeatable and comparable measures of corneal curvature in infant monkeys (Kee et al., 2002). Corneal power was calculated by assuming that the cornea was a single, spherical refracting surface separating air from a media with a refractive index of 1.3375.
The axial dimensions of the eye, including anterior chamber and vitreous chamber depths, lens thickness, and the sum of these, axial length, were measured by A-scan ultrasonography using an instrument with a focused, 7-MHz transducer (Image 2000, Mentor, Norwell, MA). The reported axial dimensions represent the average of 10 individual readings.
The curvatures of the anterior and posterior lens surfaces were measured by video phakometry (Mutti et al., 1992). Specifically, the equivalent radii of curvature for the anterior and posterior surfaces were derived by measuring the apparent sizes of Purkinje Images I, III, and IV produced by the collimated light from two point sources that were optically imaged at infinity. Data were obtained for the 45, 90, and 135 deg meridians and then averaged. At least 2 clear frames were measured for each image. The equivalent radii for the lens surfaces were determined by comparing the sizes of the Purkinje images to the catoptric images obtained from a series of precision ball bearings (Mutti et al., 1992). With knowledge of the eye’s refractive error, corneal power, and axial dimensions, the anterior radius of curvature for the crystalline lens was calculated by paraxial ray tracing. For the posterior lens surface, an iterative procedure was used to find the posterior lens radius of curvature and the equivalent refractive index of the lens that produced agreement between the measured refractive error and the refractive error calculated from ocular component values. The equivalent lens power was then calculated using the thick lens formula (Keating, 2002).
Data Analysis
When comparing several means where there was only one way to classify the populations of interest (such as refractive error), we employed a one-way analysis of variance (ANOVA) (Moore & McCabe, 1993a). One way ANOVAs were used to compare the mean values for each ocular component between the different refractive-error groups (i.e., hyperopic, emmetropic and myopic groups) for the right and left eyes. Simple linear regression analysis was used when studying the relationship between a response variable and one or more explanatory variables (Moore & McCabe, 1993c). In the present study, simple correlation coefficients between each ocular component and refractive error were calculated for each refractive-error group for each subspecies of monkeys. Two sample t-tests were used to compare the mean values between groups. There is no evidence that the right and left eyes respond differently to the same treatment regimen; multi-linear regression analyses using the left and right eyes as dummy variables indicated that there were no significant differences between the results for the right and left eyes (p = 0.128 to 0.934). As a result, the left and right eye data were pooled together in regression and correlation analyses. The primary reason for presenting both left and right eye data was that doing so provided an opportunity to examine a relatively rare resource in more detail and to enhance the likelihood that we would detect subtle changes. In anisometropic monkeys, where the left and right eye samples were of equal size and the units in the two samples were matched, the data from the Indian- and Chinese-derived monkeys were pooled and paired t-tests were employed to examine the interocular differences in ocular parameters (Moore & McCabe, 1993b). All statistical tests were performed using Minitab software (Release 12.21, Minitab Inc, State College, PA).
Results
Refractive Error Distributions
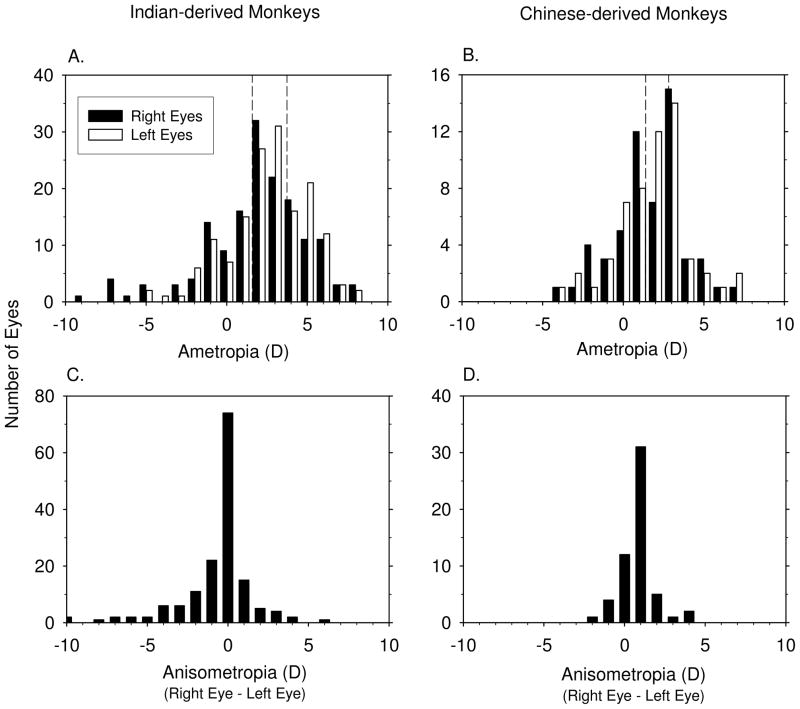
The frequency distributions of spherical-equivalent refractive corrections for the right and left eyes of the Indian- (135 ± 13 days ) and Chinese-derived monkeys (143 ± 14 days ) obtained near the end of the treatment period for the lens-reared monkeys are shown in Figures 1A & B. The different treatment regimens resulted in a wide range of predominately hyperopic refractive errors within each group (+8.25 to −8.50 D for Indian-derived monkeys, +7.00 to −4.00 D for Chinese-derived monkeys). For analysis purposes, “emmetropia” within each subspecies was defined as refractive errors that fell within ±1 SD of the mean refractive error for the normal eyes in each group (the vertical dashed lines in each plot). For the Indian-derived monkeys, the emmetropic range extended from +1.58 to +3.73 D; the range was +1.40 to +2.81 D for the Chinese-derived monkeys. Because the distribution of refractive errors in normal monkeys is leptokurtic, these limits included 15 of the 18 normal monkeys. For analysis purposes, experimental eyes that had refractive errors that fell above and below these ranges were classified as hyperopic and myopic, respectively.
Figure 1.
Frequency distributions for spherical-equivalent refractive error and anisometropia for Indian-derived (left) and Chinese-derived monkeys (right) obtained at the end of the treatment period for the treated monkeys and at equivalent ages for control monkeys. A & B. Refractive errors for right (black bar) and left eyes (white bar), the dashed lines in A & B represent ± 1 standard deviations from the mean refractive errors for the control monkeys. C & D. Anisometropia (right eye – left eye).
The two eyes of the normal control monkeys were very well matched; the average absolute amount of anisometropia was 0.14 ± 0.07 D and 0.23 ± 0.29 D for the Indian- and Chinese-derived controls, respectively. However, the experimental animals exhibited much wider ranges of anisometropias (Figures 1C & D), which provided additional within animal comparisons of the ocular changes associated with ametropias.
Comparisons of Ocular Components between Hyperopic, Emmetropic and Myopic Monkeys
Tables 2 and 3 include the mean (± SD) refractive errors and the ocular component values obtained at ages corresponding to the end of the experimental treatment period for the Indian- and Chinese-derived monkeys, respectively. Each table shows the data for the right and left eyes of the hyperopic, emmetropic, and myopic subgroups and the results of the one-way ANOVAs. Not surprisingly, because each subgroup was defined by their refractive errors, the ANOVAs showed that there were significant differences in the mean refractive errors. More importantly, there were adequate numbers of subjects in each subgroup to support our analyses (Indian-derived right eyes: 47 hyperopic, 53 emmetropic and 54 myopic; Indian-derived left eyes: 53 hyperopic, 59 emmetropic and 42 myopic; Chinese-derived right eyes: 14 hyperopic, 16 emmetropic and 26 myopic; Chinese-derived left eyes: 18 hyperopic, 17 emmetropic and 21 myopic).
Table 2.
One Way ANOVA Comparing Hyperopic, Emmetropic and Myopic Monkeys: Indian-derived Monkeys (Mean ± Standard Deviation)
| Groups | Eyes | Refractive Error | Corneal Power | Anterior Chamber Depth |
Lens Thickness | Vitreous Chamber Depth |
Axial Length | Anterior Lens Radius of Curvature | Posterior Lens Radius of Curvature | Equivalent Lens Power |
|---|---|---|---|---|---|---|---|---|---|---|
| Hyperopic Group | OD | 5.27 ± 1.19 | 55.86 ± 1.84 | 2.86 ± 0.34 | 3.54 ± 0.10 | 9.43 ± 0.40 | 15.82 ± 0.49 | 6.88 ± 0.55 | 4.70 ± 0.40 | 47.62 ± 6.94 |
| OS | 5.20 ± 1.05 | 56.18 ± 1.72 | 2.95 ± 0.27 | 3.53 ± 0.11 | 9.36 ± 0.38 | 15.86 ± 0.42 | 6.89 ± 0.64 | 4.74 ± 0.26 | 51.47 ± 2.50 | |
| Emmetropic Group | OD | 2.56 ± 0.52 | 56.19 ± 1.70 | 2.94 ± 0.32 | 3.53 ± 0.12 | 9.80 ± 0.39 | 16.28 ± 0.47 | 6.63 ± 0.61 | 4.86 ± 0.38 | 50.44 ± 3.25 |
| OS | 2.60 ± 0.58 | 56.06 ± 1.49 | 2.91 ± 0.27 | 3.53 ± 0.15 | 9.86 ± 0.36 | 16.29 ± 0.43 | 6.66 ± 0.48 | 4.91 ± 0.35 | 49.47 ± 2.98 | |
| Myopic Group | OD | −1.23 ± 2.56 | 55.98 ± 1.40 | 3.01 ± 0.31 | 3.59 ± 0.14 | 10.37 ± 0.53 | 17.00 ± 0.55 | 6.61 ± 0.51 | 4.76 ± 0.32 | 49.49 ± 3.37 |
| OS | −0.54 ± 1.62 | 55.66 ± 1.40 | 3.13 ± 0.37 | 3.60 ± 0.15 | 10.32 ± 0.43 | 17.06 ± 0.57 | 6.62 ± 0.47 | 4.70 ± 0.33 | 48.48 ± 3.82 | |
| ANOVA p value | OD | < 0.001 | 0.584 | 0.084 | 0.055 | < 0.001 | < 0.001 | 0.362 | 0.42 | 0.186 |
| OS | < 0.001 | 0.246 | 0.002 | 0.030 | < 0.001 | < 0.001 | 0.349 | 0.083 | 0.053 | |
| Confidence Interval of the Differences Between Emmetropic vs. Hyperopic | OD | −3.51 to −1.91* | −0.45 to 1.12 | −0.07 to 0.24 | −0.07 to 0.05 | 0.15 to 0.59* | 0.21 to 0.71* | −0.72 to 0.23 | −0.15 to 0.47 | −0.82 to 6.47 |
| OS | −3.09 to −2.10* | −0.81 to 0.58 | −0.18 to 0.10 | −0.06 to 0.06 | 0.31 to 0.67* | 0.21 to 0.65* | −0.66 to 0.21 | −0.11 to 0.44 | −4.76 to 0.76 | |
| Confidence Interval of the Differences Between Emmetropic vs. Myopic | OD | 3.02 to 4.55* | −0.53 to 0.97 | −0.21 to 0.09 | −0.11 to 0.003 | −0.79 to −0.37* | −0.96 to −0.48* | −0.37 to 0.41 | −0.16 to 0.34 | −2.00 to 3.91 |
| OS | 2.61 to 3.66* | −0.34 to 1.13 | −0.37 to −0.07* | −0.13 to 0.00 | −0.65 to −0.27* | −1.00 to −0.54* | −0.30 to 0.40 | −0.02 to 0.43 | −1.25 to 3.23 | |
| Confidence Interval of the Differences Between Hyperopic vs. Myopic | OD | 5.70 to 7.29* | −0.90 to 0.66 | −0.30 to 0.01 | −0.11 to 0.01 | −1.16 to −0.73* | −1.43 to −0.94* | −0.20 to 0.74 | −0.37 to 0.24 | −5.47 to 1.73 |
| OS | 5.20 to 6.27* | −0.24 to 1.27 | −0.33 to −0.02* | −0.14 to 0.00 | −1.15 to −0.76* | −1.44 to −0.97* | −0.19 to 0.72 | −0.25 to 0.33 | 0.10 to 5.88* |
The differences between groups are statistically significant at α = 0.05 family error rate. Tukey’s Test
Table 3.
One Way ANOVA Comparing Hyperopic, Emmetropic and Myopic Monkeys: Chinese-derived Monkeys (Mean ± Standard Deviation)
| Groups | Eyes | Refractive Error | Corneal Power | Anterior Chamber Depth |
Lens Thickness | Vitreous Chamber Depth |
Axial Length | Anterior Lens Radius of Curvature | Posterior Lens Radius of Curvature | Equivalent Lens Power |
|---|---|---|---|---|---|---|---|---|---|---|
| Hyperopic Group | OD | 4.15 ± 1.25 | 54.66 ± 1.16 | 3.27 ± 0.24 | 3.93 ± 0.08 | 9.30 ± 0.36 | 16.51 ± 0.48 | 5.90 ± 0.13 | 4.36 ± 0.15 | 43.27 ± 2.86 |
| OS | 4.07 ± 1.36 | 54.16 ± 1.35 | 3.42 ± 0.38 | 3.94 ± 0.11 | 9.38 ± 0.46 | 16.67 ± 0.65 | 5.48 ± 0.35 | 4.07 ± 0.28 | 46.07 ± 14.10 | |
| Emmetropic Group | OD | 2.44 ± 0.29 | 54.35 ± 1.60 | 3.43 ± 0.21 | 3.95 ± 0.09 | 9.74 ± 0.39 | 17.13 ± 0.27 | 5.93 ± 0.56 | 4.61 ± 0.68 | 45.46 ± 6.13 |
| OS | 2.13 ± 0.38 | 53.85 ± 1.72 | 3.55 ± 0.25 | 3.94 ± 0.12 | 9.90 ± 0.29 | 17.39 ± 0.44 | 5.77 ± 0.62 | 4.51 ± 0.65 | 46.14 ± 5.24 | |
| Myopic Group | OD | −0.17 ± 1.51 | 54.54 ± 1.73 | 3.51 ± 0.25 | 3.93 ± 0.16 | 10.18 ± 0.47 | 17.65± 0.56 | 6.26 ± 0.57 | 4.69 ± 0.71 | 45.12 ± 3.63 |
| OS | −0.38 ± 1.45 | 54.80 ± 1.67 | 3.52 ± 0.22 | 3.89 ± 0.20 | 10.09 ± 0.47 | 17.53 ± 0.61 | 6.44 ± 0.39 | 4.96 ± 0.67 | 47.75 ± 4.19 | |
| ANOVA p value | OD | < 0.001 | 0.864 | 0.046 | 0.903 | < 0.001 | < 0.001 | 0.305 | 0.677 | 0.726 |
| OS | < 0.001 | 0.189 | 0.470 | 0.666 | < 0.001 | 0.001 | 0.004 | 0.045 | 0.881 | |
| Confidence Interval of the Differences Between Emmetropic vs. Hyperopic | OD | −2.78 to −0.63* | −1.69 to 1.08 | −0.10 to 0.41 | −0.12 to 0.16 | −0.02 to 0.91 | 0.10 to 1.14* | −0.78 to 0.83 | −0.75 to 1.26 | −4.91 to 9.29 |
| OS | −2.92 to −1.96* | −1.60 to 1.00 | −0.14 to 0.40 | −0.16 to 0.16 | 0.11 to 0.94* | 0.16 to 1.29* | −0.39 to 0.98 | −0.41 to 1.29 | −10.67 to 10.80 | |
| Confidence Interval of the Differences Between Emmetropic vs. Myopic | OD | 1.68 to 3.54* | −1.39 to 1.11 | −0.29 to 0.12 | −0.09 to 0.13 | −0.82 to −0.07* | −0.94 to −0.10* | −0.95 to 0.28 | −0.84 to 0.68 | −5.04 to 5.74 |
| OS | 1.56 to 3.44* | −2.18 to 0.32 | −0.24 to 0.05 | −0.10 to 0.18 | −0.55 to 0.19 | −0.65 to 0.37 | −1.25 to −0.09* | −1.17 to 0.27 | −10.69 to 7.45 | |
| Confidence Interval of the Differences Between Hyperopic vs. Myopic | OD | 3.34 to 5.29* | −1.14 to 1.38 | −0.47 to −0.01* | −0.10 to 0.19 | −1.30 to −0.47* | −1.60 to −0.68* | −1.13 to 0.41 | −1.30 to 0.62 | −8.62 to 4.93 |
| OS | 3.51 to 5.37* | −1.86 to 0.62 | −0.36 to 0.15 | −0.36 to 0.11 | −1.09 to −0.33* | −1.39 to −0.35* | −1.65 to 0.27 | −1.74 to −0.04* | −12.41 to 9.05 |
The differences between groups are statistically significant at α = 0.05 family error rate. Tukey’s Test
For both the Indian- and Chinese-derived populations, the most consistent result from the ANOVA analyses were that there were significant differences in vitreous chamber depth and axial length between the refractive-error subgroups. For the Indian-derived animals, the confidence intervals for all of the between refractive-error subgroup comparisons for vitreous chamber depth and axial length did not include zero, i.e. there were significant differences in axial length and vitreous chamber depth between these subgroups. For the Chinese-derived animals, the myopic eyes had longer vitreous chambers and axial lengths than the hyperopic (both left and right eyes) and emmetropic eyes (right eyes only). There were also significant differences in axial length and vitreous chamber depth (3 out of 4 possible comparisons) between the emmetropic and hyperopic eyes.
There were no systematic differences in corneal power or lens equivalent power between the refractive-error subgroups. However, there were some anterior segment differences. Altogether 6 of the possible 60 comparisons of anterior segment components between refractive error subgroups had confidence intervals that excluded zero. These significant comparisons indicated that, in comparison to hyperopic or emmetropic eyes, myopic eyes tended to have longer anterior chamber depths (3 of 12 comparisons), longer lens radii (2 of 24 comparisons), and higher equivalent lens powers (1 of 12 comparisons).
Simple Correlation Coefficients between Refractive Errors and Ocular Components
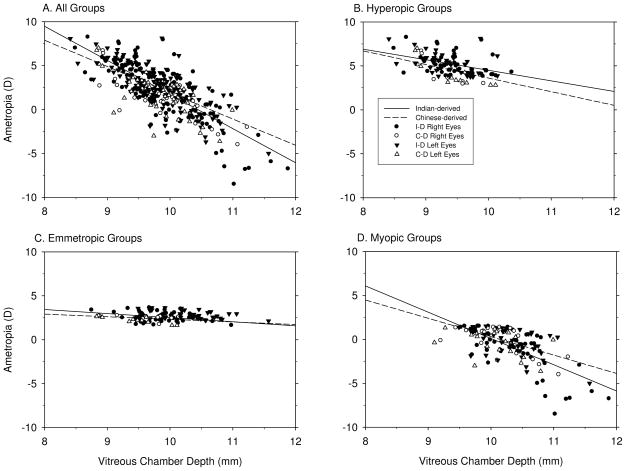
Tables 4 shows simple correlation matrices that were calculated for the data from both eyes of the Indian- and Chinese-derived monkeys. Correlation coefficients are shown for the hyperopic, emmetropic and myopic subgroups and for all of the refractive-error subgroups combined. Only two ocular component values, vitreous chamber depth and axial length, were consistently correlated with the eye’s refractive error. For both Indian- and Chinese-derived monkeys, refractive error became more myopic/less hyperopic with increases in vitreous chamber depth (Figure 2) and axial length. By inspection, the correlations tended to be the strongest in the combined and myopic data sets, weaker in the hyperopic subgroups, and weakest or non-existent in the emmetropic subgroups. In order to test whether the correlations between refractive error and vitreous chamber depth were different between the different refractive-error subgroups, multi-linear regression analyses using the different refractive groups as dummy variables were performed, which indicated that in Chinese-derived (p ≤ 0.001 for both the slope and intercept), but not Indian-derived monkeys (p = 0.128 for intercept, p = 0.508 for slope), there were significant differences in the correlations between refractive error and vitreous chamber depth between the different refractive-error groups. When the data for the Indian-derived and Chinese-derived monkeys were combined, there were also significant differences in the vitreous chamber depth versus refractive error correlations between the different refractive-error subgroups (p < 0.001 for both the slope and intercept). However, one needs to be cautious when comparing the correlation coefficients for refractive error between these different refractive groups because the absolute ranges of refractive errors in the emmetropic, hyperopic and myopic groups were quite different. No other ocular components, in either the Indian or Chinese-derived data set, were significantly correlated with refractive error
Table 4.
Simple Correlation Coefficients between Ocular Components in Refractive Error Subgroups of Chinese- and Indian-derived Monkeys
| Groups | Ocular Component | Refractive Error | Corneal Power | Anterior Chamber Depth | Lens Thickness | Vitreous Chamber Depth | |
|---|---|---|---|---|---|---|---|
| Hyperopic Group | Corneal Power | Indian | −0.091 | ||||
| Chinese | −0.054 | ||||||
| Anterior Chamber Depth | Indian | 0.154 | −0.171 | ||||
| Chinese | −0.217 | −0.308 | |||||
| Lens Thickness | Indian | 0.198 | −0.021 | 0.205* | |||
| Chinese | −0.315 | −0.194 | 0.111 | ||||
| Vitreous Chamber Depth | Indian | −0.413*** | −0.698*** | −0.135 | −0.283* | ||
| Chinese | −0.520** | −0.199 | 0.278 | −0.315 | |||
| Axial Length | Indian | −0.223* | −0.700*** | 0.592*** | 0.110 | 0.680*** | |
| Chinese | −0.683*** | −0.380 | 0.596** | 0.462* | 0.652*** | ||
| Emmetropic Group | Corneal Power | Indian | 0.122 | ||||
| Chinese | −0.024 | ||||||
| Anterior Chamber Depth | Indian | 0.046 | 0.080 | ||||
| Chinese | −0.313 | −0.255 | |||||
| Lens Thickness | Indian | −0.181 | −0.270** | 0.409*** | |||
| Chinese | −0.014 | −0.372* | −0.095 | ||||
| Vitreous Chamber Depth | Indian | −0.312*** | −0.813*** | −0.340*** | −0.046 | ||
| Chinese | −0.302 | −0.431* | 0.170 | −0.5** | |||
| Axial Length | Indian | −0.277** | −0.708*** | 0.511*** | 0.529*** | 0.591*** | |
| Chinese | −0.419* | −0.804*** | 0.453* | 0.259 | 0.648*** | ||
| Myopic Group | Corneal Power | Indian | −0.106 | ||||
| Chinese | −0.068 | ||||||
| Anterior Chamber Depth | Indian | 0.133 | −0.060 | ||||
| Chinese | 0.229 | −0.143 | |||||
| Lens Thickness | Indian | 0.154 | −0.324*** | 0.067 | |||
| Chinese | −0.249 | 0.069 | 0.055 | ||||
| Vitreous Chamber Depth | Indian | −0.671*** | −0.382*** | −0.067 | −0.188 | ||
| Chinese | −0.615*** | −0.589*** | −0.107 | −0.186 | |||
| Axial Length | Indian | −0.436*** | −0.418*** | 0.558*** | 0.102 | 0.731*** | |
| Chinese | −0.611*** | −0.589*** | 0.260 | 0.231 | 0.853*** | ||
| All | Corneal Power | Indian | 0.041 | ||||
| Chinese | −0.093 | ||||||
| Anterior Chamber Depth | Indian | −0.114 | −0.067 | ||||
| Chinese | −0.187 | −0.188 | |||||
| Lens Thickness | Indian | −0.112 | −0.227*** | 0.254*** | |||
| Chinese | −0.151 | −0.174 | 0.041 | ||||
| Vitreous Chamber Depth | Indian | −0.777*** | −0.504*** | 0.011 | 0.007 | ||
| Chinese | −0.681*** | −0.336*** | 0.181 | −0.242* | |||
| Axial Length | Indian | −0.727*** | −0.48*** | 0.526*** | 0.307*** | 0.828*** | |
| Chinese | −0.730*** | −0.437*** | 0.469*** | 0.289** | 0.817*** |
p ≤ 0.05
p ≤ 0.01
p ≤ 0.001
Figure 2.
Simple correlation analyses between refractive error and vitreous chamber depth for all eyes (A), hyperopic eyes (B), emmetropic eyes (C) and myopic eyes (D). The filled and open circles represent individual Indian-derived right and left eyes, respectively. The filled and open triangles represent individual Chinese-derived right and left eyes, respectively. The correlations between refractive error and vitreous chamber depth are represented by the solid (Indian-derived monkeys) and dashed lines (Chinese-derived monkeys). Note that the slopes of the regression lines for emmetropic eyes were flatter than those for the myopic, hyperopic or combined subject groups.
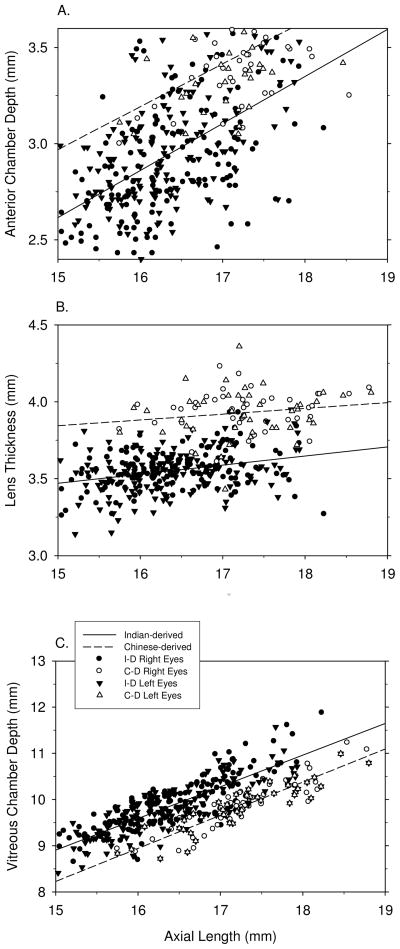
The three ocular components that contribute to axial length (i.e., anterior chamber depth, lens thickness and vitreous chamber depth) showed relatively consistent positive correlations with axial length (Figure 3). For vitreous chamber depth, the highest correlations with axial length were found in the combined data set for all of the refractive-error subgroups, however, all 8 possible correlations were highly significant and the correlation coefficients were higher than 0.591 for all refractive-error subgroups (Table 4). Anterior chamber depth was positively and significantly correlated with axial length in 7 of 8 subgroup analyses; however, in each case the correlation coefficients were smaller than those for the vitreous chamber. Lens thickness was positively correlated with axial length in only 4 of the 8 possible subgroup analyses. Given that the vitreous chamber is the only axial component significantly correlated with refractive error and that only some subgroups showed significant differences in anterior chamber depth or lens thickness between myopes and hyperopes (Table 2 & 3), it is reasonable to expect that vitreous chamber depth would contribute more to variations in total axial length than anterior chamber depth or lens thickness.
Figure 3.
Simple correlation analyses between ocular components (A. anterior chamber depth, B. lens thickness, C. vitreous chamber depth) and axial length. For details, see Figure 2.
Anterior chamber depth was significantly correlated with vitreous chamber depth in 1 of the possible 2 emmetropic eye correlations. However, there were no significant correlations between anterior chamber depth and vitreous chamber depth in either the myopic, hyperopic or combined refractive-error subgroups, i.e. these two components develop in an independent manner when ametropia develops. On the other hand, lens thickness tended to be negatively correlated with vitreous chamber depth, but this relationship reached statistical significance in only 3 of 8 possible comparisons, i.e. lens thickness is largely independent of vitreous chamber depth. There was no clear cut relationship between anterior chamber depth and lens thickness. Although there was a significant positive correlation between lens thickness and anterior chamber depth in 3 of the 8 possible comparisons, the correlation coefficients were relatively low.
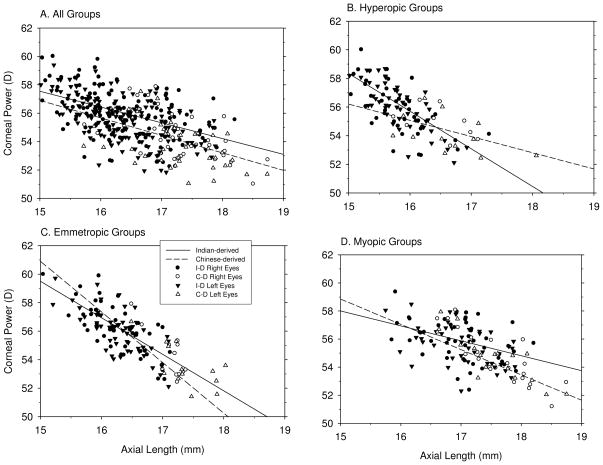
Corneal power was consistently negatively correlated with the axial components, especially vitreous chamber depth and total axial length, i.e., the longer the eye, the lower the corneal power (Figure 4). These correlations tended to be highest in the emmetropic subgroups (Table 4); probably reflecting the fact that the cornea is the dominant optical component and that in emmetropic eyes there is a clear cut relationship between total refracting power and axial length. However, significant correlations between corneal power and axial length were found in all three refractive-error subgroups and in the combined data (7 out of 8 possible comparisons, Table 4). To compare the correlations between the different refractive-error subgroups, multi-linear regression analyses using the different refractive groups as dummy variables indicated that in Indian-derived (p ≤ 0.001 for both the slope and intercept) but not Chinese-derived monkeys (p = 0.05 for intercept, p = 0.10 for slope), there were significant differences in the correlations between corneal power and axial length for the different refractive-error subgroups. Moreover, when the data for the Indian- and Chinese-derived monkeys were combined, there were significant differences in the correlations between the different refractive-error subgroup (p < 0.001 for both the slope and intercept), which indicates that the ratio between corneal power and axial length is likely to be different between hyperopic, emmetropic, and myopic monkeys.
Figure 4.
Simple correlation analyses between corneal power and axial length in all eyes (A), hyperopic eyes (B), emmetropic eyes (C) and myopic eyes (D). For details, see Figure 2.
Interocular Differences in Ocular Components in Anisometropic Monkeys
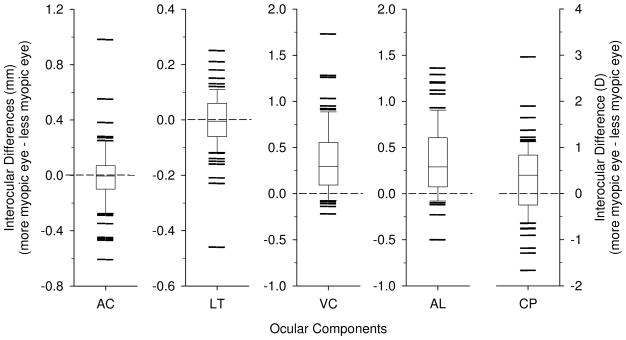
Fifty-four of the Indian-derived monkeys and 13 of the Chinese-derived monkeys had anisometropias larger than 1 D. To examine how the two eyes of anisometropic monkeys differ, the Indian-derived and Chinese-derived data were combined and paired t-tests were used to determine if there were interocular differences in the ocular components. As illustrated in Figure 5, the more myopic/less hyperopic eyes had significantly longer axial lengths (16.89 ± 0.73 mm vs. 16.52 ± 0.72 mm, p < 0.001) than the more hyperopic/less myopic eyes. These longer axial lengths were characterized by longer vitreous chambers (10.16 ± 0.65 mm vs. 9.79 ± 0.53 mm, p < 0.001). The more myopic eyes also had significantly more powerful corneas (55.84 ± 1.77 D vs. 55.48 ± 1.63 D, p < 0.001). No significant interocular differences were found for anterior chamber depth (3.08 ± 0.37 mm vs. 3.09 ± 0.36 mm, p = 0.64) or for any of the lens parameters including lens thickness (3.63 ± 0.21 mm vs. 3.64 ± 0.20 mm, p = 0.64), anterior lens radius (6.665 ± 0.64 mm vs. 6.70 ± 0.51 mm, p = 0.39), posterior lens radius (4.68 ± 0.41 mm vs. 4.73 ± 0.32 mm, p = 0.17), equivalent lens index (1.470 ± 0.024 vs. 1.472 ± 0.017, p = 0.53)and equivalent lens power (46.90 ± 5.47 D vs. 47.77 ± 3.99 D, p = 0.39), i.e. the lens parameters did not show any differential growth during the development of anisometropia.
Figure 5.
Box plots for Interocular differences (more myopic eye – less myopic eye) in ocular components of anisometropic monkeys. The horizontal solid line inside the box represents the median, the bottom and top of the box represent the 25th and 75th percentiles. The whiskers that extend vertically from the top and bottom of the box represent the 90th and 10th percentiles, respectively. Horizontal lines outside of the upper and lower limits represent outliers. Dashed horizontal lines are reference lines at zero differences. AC: anterior chamber depth; LT: lens thickness; VC: vitreous chamber depth; AL: axial length; CP: corneal power.
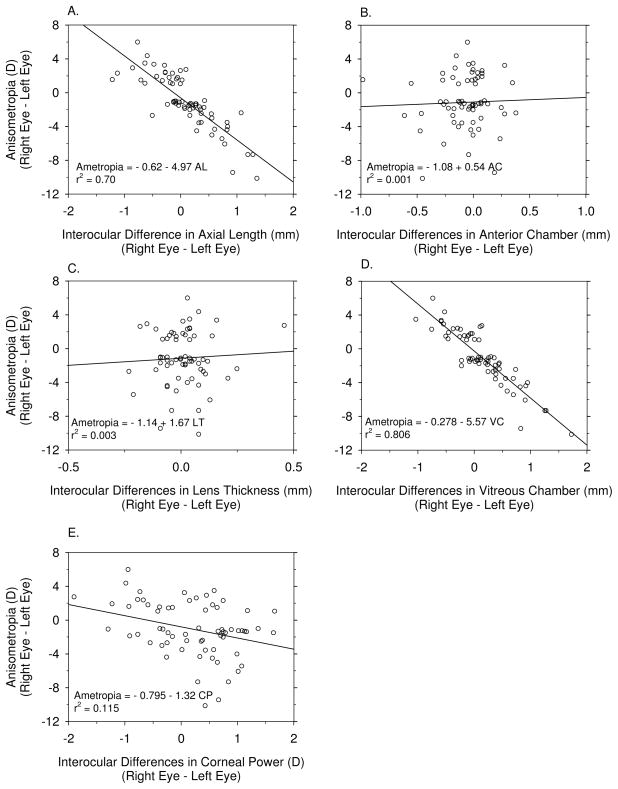
The interocular correlation coefficients for ocular components are shown in Table 5 for the anisometropic monkeys. The significant contributions of axial length and vitreous chamber depth to the interocular differences in refractive error were predominant (Figure 6A & D). The correlation between the interocular differences in corneal power and refractive error was also significant, i.e., the more myopic eyes tended to have steeper corneas (Figure 6E). However, the degree of anisometropia was not significantly correlated with interocular differences in either anterior chamber depth or lens thickness. The interocular differences in axial length were associated with interocular differences in anterior chamber depth and vitreous chamber depth (Table 5). In addition, interocular differences in corneal power were positively correlated with axial length, i.e., the longer the eye, the steeper the cornea. However, interocular differences in lens thickness did not contribute to anisometropia or to interocular differences in axial length (Table 5).
Table 5.
Simple Correlation Coefficients between Interocular Differences in Ocular Components
| Ocular Component | Refractive Error | Corneal Power | Anterior Chamber Depth | Lens Thickness | Vitreous Chamber Depth |
|---|---|---|---|---|---|
| Corneal Power | −0.339** | ||||
| Anterior Chamber Depth | 0.038 | 0.154 | |||
| Lens Thickness | 0.055 | −0.148 | 0.167 | ||
| Vitreous Chamber Depth | −0.898*** | 0.151 | −0.127 | 0.041 | |
| Axial Length | −0.838*** | 0.237* | 0.335** | 0.227 | 0.867*** |
p ≤ 0.05
p ≤ 0.01
p ≤ 0.001
Figure 6.
Simple correlation analyses between the interocular differences in refractive error and various axial dimensions (A. axial length; B. anterior chamber depth; C. lens thickness; D. vitreous chamber depth, and E. corneal power). Open symbols represent individual monkeys and the solid lines represent the regression function for each plot.
Discussion
Our results revealed that the experimentally induced ametropic eyes in infant rhesus monkeys have similar structural characteristics as those observed in naturally occurring ametropias in humans. As in humans, ametropia development in young rhesus monkeys emphasized the dominant role of the eye’s axial dimensions, in particular vitreous chamber depth, with corneal power as a secondary factor. Specifically in young monkeys, the more myopic eyes were characterized with longer axial lengths, longer vitreous chamber depths and steeper corneas with vitreous chamber depth and corneal power accounting for approximately 80% and 10% of the variations in refractive error, respectively. None of the crystalline lens parameters (i.e., lens thickness, anterior and posterior lens radii of curvature, lens index, and lens equivalent power) consistently contributed to the eye’s refractive status.
The Vitreous Chamber Dominates Ametropia Development
In both Indian- and Chinese-derived monkeys, the more myopic eyes had significantly longer vitreous chamber depths. All analyses, including one-way ANOVAs, correlation analyses between ocular components, and paired t-tests and correlation analyses between interocular differences in ocular components indicated that vitreous chamber depth contributed significantly to refractive error in both sub-species. This was, however, more obvious in Indian-derived monkeys than in Chinese-derived monkeys, probably due to the fact that the sample size of the Indian-derived subject group (n = 154) was larger than that for the Chinese-derived group (n = 56). However, it is also possible that there are quantitative differences in the responsiveness between these two subspecies to alterations in visual experience. Unfortunately, we do not have sufficient data obtained from both subspecies under identical rearing conditions to test this hypothesis (e.g., see monocular form deprivation treatment numbers in Table 1).
The consensus that the vitreous chamber acts as the most significant contributor to ametropia development has been well established in human refractive-error studies. From regression analyses (Carroll, 1981; Carroll, 1982; Hirsch, 1947; Stenstrom, 1948; van Alphen, 1961) to between group comparisons (Bullimore et al., 1992; Fledelius, 1995; Grosvenor & Scott, 1991; McBrien & Millodot, 1987), from cross-sectional data (Larsen, 1971a; Larsen, 1971b; Larsen, 1971c; Larsen, 1971d; Stenstrom, 1948) to longitudinal studies (Fledelius, 1982; Garner et al., 2006; Grosvenor & Scott, 1993; Jensen, 1991; Jorge et al., 2007; McBrien & Adams, 1997), and from studies of children (Fledelius, 1982; Fulk et al., 2002; Fulk et al., 2003; Garner et al., 2006; Gwiazda et al., 2003; Larsen, 1971a; Larsen, 1971b; Larsen, 1971c; Larsen, 1971d; Mutti et al., 2005; Saw et al., 2002; Saw et al., 2005a; Saw et al., 2005b; Zadnik et al., 2003) to studies of adults or late-onset ametropias (Bullimore et al., 1992; Fledelius, 1988; Fledelius, 1995; Garner et al., 2004; Garner et al., 2006; Grosvenor & Scott, 1991; Grosvenor & Scott, 1993; Jensen, 1991; Jorge et al., 2007; Mallen et al., 2005; McBrien & Adams, 1997; McBrien & Millodot, 1987; Saw et al., 2002; Saw et al., 2005a; Saw et al., 2005b), more myopic eyes have always been shown to have longer vitreous chambers. There is little axial length data available for human infants at ages that are equivalent to those of our infant monkeys (on average about 14 months of age in humans). However, the available data from human infants also supports the axial nature of refractive errors. For example, Mutti et al. (2005) reported that in young infants 3 to 9 months of age, the reduction in hyperopia associated with emmetropization correlated primarily with axial elongation with little contribution from either the lens or cornea, although both the lens and cornea are growing and losing optical power during this period. Moreover, for anisometropic subjects, refractive-error studies of humans (Logan et al., 2004) and many animals, (e.g., chicken (Hodos et al., 1985; Hodos & Kuenzel, 1984; Wallman et al., 1978; Yinon et al., 1980), tree shrews (Marsh-Tootle & Norton, 1989; Sherman et al., 1977), marmosets (Troilo & Judge, 1993), cats (Gollender et al., 1979; Kirby et al., 1982; Yinon et al., 1984), pigeons (Bagnoli et al., 1985), grey squirrels (McBrien et al., 1993), the American kestrel (Andison et al., 1992), barn owls (Knudsen, 1989), guinea pigs (Howlett & McFadden, 2006), wallabies (Harman et al., 1999), rabbits (Gao et al., 2006) and mice (Tejedor & de la Villa, 2003)) have all reported significant interocular differences in vitreous chamber depth in anisometropic individuals. Nonetheless, the ages of the monkeys used in this study were equivalent to about 1–2 years of age for human infants. Therefore, as discussed below in more detail, caution should be used when attempting to extrapolate these experimental results from infant rhesus monkeys to adolescent humans, i.e., to older ages when myopia most commonly develops in children.
Other Ocular Components Make Minor Contributions to Refractive-Error Development
Our statistical tests failed to reveal a consistent anterior chamber contribution towards ametropia. No significant interocular differences in anterior chamber depth were found in anisometropic monkeys. In simple correlation analyses, none of the analyses showed a significant correlation between refractive error and anterior chamber depth. While the ANOVA tests showed that anterior chamber depth was significantly different between some refractive-error subgroups, only 3 out of 12 possible comparisons showed confidence intervals that excluded zero.
Previous studies in humans have also been ambiguous about the role of the anterior chamber in refractive-error development. For example, using simple regression analyses some researchers have reported a small, but significant, positive contribution of anterior chamber depth to refractive error (e.g., Stenstrom (1948), Hirsch & Weymouth (1947), van Alphen (1961) and Larsen (1971a)). However, others (Grosvenor & Scott, 1993; Scott & Grosvenor, 1993) claimed that anterior chamber depth was not a contributing factor in either youth-onset or adult-onset myopias. Similarly, Bullimore et al. (1992) failed to find a difference in anterior chamber depth between their groups of emmetropes, early-onset myopes and late-onset-myopes. Nonetheless, McBrien and Millodot (1987) found significantly deeper anterior chamber depths in 30 late-onset-myopes compared to emmetropes and McBrien and Adams (1997) also reported significantly deeper anterior chamber depths in youth-onset and adult-onset myopes when compared with emmetropes. However, no changes in anterior chamber depth were detected longitudinally during myopia progression in the same study (McBrien & Adams, 1997).
We failed to find any significant correlations by simple correlation analysis between corneal power and refractive error. Moreover, there were not any significant differences in corneal power between the hyperopic, emmetropic and myopic subgroups in either subspecies of monkeys. Mutti et al. (2005) also failed to detect any correlations between the reduction in hyperopia during emmetropization and changes in corneal power in human infants. For corneal power, statistical significance was only detected by interocular comparisons, which is arguably our most sensitive analysis. Both paired t-tests in anisometropic monkeys as well as simple correlation analyses showed a significant relationship between interocular differences in corneal power and refractive error. However, correlation analysis showed that corneal power only contributed about 10% to the development of anisometropia (r2 = 11.5%).
In human studies, the extent of the contribution of corneal power to refractive error varies depending on the statistical strategy employed. However, sometimes even the same statistical analyses have yielded opposite findings in different populations. For example, Stenstrom (1948) found a weak correlation between corneal power and refractive error using simple correlation analysis. Hirsch & Weymouth (1947), as well as van Alphen (1961), using partial correlations, which boosted the correlation coefficients, all claimed that the corneas were the second most important factor in myopia development. Grosvenor and Scott (1991; 1993) reported strong correlations between corneal power and refractive error with simple correlation analyses as well as with between group comparisons. However, Sorsby et al. (1961) failed to find any differences between emmetropic and low ametropic groups in their cross-sectional studies. In agreement with our study, Fledelius (1988) reported a poor correlation between corneal power and refractive error in a cross-sectional study of 67 eighteen-year-old subjects.
It needs to be emphasized that corneal development can be affected by visual experience. We have previously shown that monkeys that had experimentally induced axial ametropias frequently developed substantial amounts of corneal astigmatism (Kee et al., 2005). The changes in astigmatism were caused by a decrease in the rate of flattening for one of the eye’s principal meridians, which results in an increase in the average corneal power. Human studies have also reported that large astigmatic errors have been associated with large spherical ametropias (Duke-Elder, 1970; Fulton et al., 1982; Haugen et al., 2001; Kaye & Patterson, 1997; Kronfeld & Devney, 1930). This suggests that vision-dependent mechanisms may contribute to corneal changes in humans as well.
Although surface curvature, refractive index and total power are the most relevant lens parameters to refractive error, crystalline lens thickness has been the most common lens parameter measured in refractive-error studies because it is the easiest and most straight forward biometric measurement for the crystalline lens. In this respect, some human studies (Fledelius, 1995; Garner et al., 1992; Zadnik et al., 1995) have reported negative correlations between lens thickness and refractive error, (i.e. more myopic eyes have thinner lenses), other studies reported no correlation (Bullimore et al., 1992; Grosvenor & Scott, 1991; Jensen, 1991; Larsen, 1971b; McBrien & Adams, 1997; McBrien & Millodot, 1987). We did not find consistent contributions from crystalline lens thickness in any of our statistical analyses. Even the interocular differences in anisometropic animals did not reach statistical significance for lens thickness.
Age differences potentially provide a possible explanation for the discrepancy between some human studies and our results in monkeys concerning the contribution of lens thickness. Although a small number of animal studies (Papastergiou et al., 1998; Smith et al., 1999a; Smith et al., 2002b; Troilo et al., 2000; Zhong et al., 2004) have focused on vision-induced refractive errors in adolescent and/or mature animals, none of these studies has systematically examined the role of the lens in the observed refractive-error changes. The great majority of animal studies have involved infants because experimentally induced refractive errors are larger and occur faster in young animals. The monkeys in our study ranged from 104 to 200 days of age, equivalent to about 1 to 2 years of age in humans (Bito et al., 1982; Boothe et al., 1980; Bradley et al., 1999; Kiely et al., 1987; Qiao-Grider et al., 2007b; Smith & Harwerth, 1984; Teller et al., 1978; Torczynski, 1979). On the other hand, the human refractive-error studies that reported lens thinning usually involved children 7- 8 years of age and older (Fledelius, 1995; Garner et al., 1992; Zadnik et al., 1995). It is possible that during the early infantile stage of ocular growth, because the lens is growing so rapidly at these ages that any ocular enlargement that would potentially cause lens thinning in older animals would be compensated by lens growth in the younger animals. Similar to our findings in infant monkeys, Mutti et al. (2005) reported that there was no correlation between the reduction in hyperopia during emmetropization and changes in lens power in human infants.
Another possible explanation would be that vision-dependent mechanisms that influence the eye’s refractive development early in life have no effect on crystalline lens development. Specifically, the animals in our study developed ametropias as a result of an experimental visual intervention. On the other hand, human ametropias may be influenced by more than just visual experience. It is possible that some factors that contribute to human ametropias might work through a different, non-visual mechanism that influences lens development, resulting in thinner lenses in more myopic eyes.
Vision-Induced Ametropias in Monkeys Are Similar to Natural Ametropias in Humans
In humans, it has been reported that in comparison to emmetropic and hyperopic eyes, myopic eyes are more elongated and relatively more prolate in shape with more pronounced axial dimensions than transverse dimensions (Atchison et al., 2004; Atchison et al., 2005; Cheng et al., 1992; Logan et al., 2004; Mutti et al., 2007; Mutti et al., 2000; Schmid, 2003; Wang et al., 1994). Although we do not have data on the transverse dimensions of our animals, inspection of the results of our statistical analyses revealed that the ocular shapes of the ametropic eyes of infant rhesus monkeys, especially the myopic eyes, were different from those of emmetropic eyes. For example, assuming proportional ocular growth, eyes with longer vitreous chamber depths would be expected to have flatter, less powerful corneas. However, paired t-tests indicated that the more myopic eyes of anisometropic monkeys were characterized by longer vitreous chambers and steeper corneas. Additionally, close examination of the correlation analysis between corneal power and axial length (Figure 4) illustrates that while corneal power shows a negative correlation with axial length (i.e., longer eyes tend to have flatter corneas), the slopes for the hyperopic and myopic subgroups were less steep than those for the emmetropic subgroups. That is to say, the corneas of the ametropic eyes were steeper (myopic eyes) or flatter (hyperopic eyes) than one would predict from the emmetropic eye data. In other words, instead of growing in a similar manner as emmetropic eyes, the more myopic eyes have proportionally steeper corneas. Moreover, the correlations between vitreous chamber depth and axial length in ametropic eyes were stronger than in emmetropic eyes (Table 4), i.e., the contribution of vitreous chamber depth was larger in ametropic eyes than that of emmetropic eyes, indicating that the vitreous chamber depths in the more myopic eyes were proportionally longer than those in emmetropic eyes, while those of the more hyperopic eyes were proportionally shorter. MRI imaging in monkeys with experimentally induced ametropias confirm this interpretation (Huang et al., 2009). Similar results have been reported in humans. Koretz et al (1995), using simple correlation analysis in 185 human eyes, also found that the negative correlation between corneal power and axial length became weaker, while the positive correlation between axial length and vitreous chamber became stronger, in ametropic eyes in comparison with emmetropic eyes.
Summary
Evidence from a wide range of animal species indicates that early in life, at ages corresponding to the rapid infantile phase of ocular growth in humans, visual feedback influences the eye’s refractive development (Norton & Siegwart, 1995; Smith, 1998a; Wildsoet, 1997). In this study we used several different types of statistical analyses to determine the ocular structural characteristics of vision-induced refractive errors in infant rhesus monkey. We found that for myopia development, vision-dependent mechanisms resulted in an elongated vitreous chamber and a steeper cornea; however, the development of anterior chamber depth and the crystalline lens were largely unchanged. These results are in agreement with previous studies in humans, suggesting that the refractive errors commonly observed in humans also reflect, at least in part, the activity of vision-dependent mechanisms and that the effects of visual experience mostly affect vitreous chamber growth. This strong agreement in ocular structural features between monkeys and humans further emphasize the applicability of rhesus monkeys in refractive error studies and the extrapolation of the results of experimental data to the understanding of human refractive development.
Acknowledgments
This work was supported by NIH grants EY-03611 and EY-07551 and funds from the Vision CRC in Sydney and the UH Foundation.
Footnotes
Proprietary interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andison ME, Sivak JG, Bird DM. The refractive development of the eye of the American kestrel (Falco sparverius): a new avian model. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral physiology. 1992;170(5):565–574. doi: 10.1007/BF00199333. [DOI] [PubMed] [Google Scholar]
- Atchison DA, Jones CE, Schmid KL, Pritchard N, Pope JM, Strugnell WE, Riley RA. Eye shape in emmetropia and myopia. Investigative Ophthalmology & Vision Science. 2004;45(10):3380–3386. doi: 10.1167/iovs.04-0292. [DOI] [PubMed] [Google Scholar]
- Atchison DA, Pritchard N, Schmid KL, Scott DH, Jones CE, Pope JM. Shape of the retinal surface in emmetropia and myopia. Investigative Ophthalmology & Vision Science. 2005;46(8):2698–2707. doi: 10.1167/iovs.04-1506. [DOI] [PubMed] [Google Scholar]
- Bagnoli P, Porciatti V, Francesconi W. Retinal and tectal responses to alternating gratings are unaffected by monocular deprivation in pigeons. Brain Research. 1985;338(2):341–345. doi: 10.1016/0006-8993(85)90165-9. [DOI] [PubMed] [Google Scholar]
- Bito LZ, DeRousseau CJ, Kaufman PL, Bito JW. Age-dependent loss of accommodative amplitude in rhesus monkeys: an animal model for presbyopia. Investigative Ophthalmology & Vision Science. 1982;23(1):23–31. [PubMed] [Google Scholar]
- Boothe RG, Williams RA, Kiorpes L, Teller DY. Development of contrast sensitivity in infant Macaca nemestrina monkeys. Science. 1980;208(4449):1290–1292. doi: 10.1126/science.6769162. [DOI] [PubMed] [Google Scholar]
- Bradley DV, Fernandes A, Lynn M, Tigges M, Boothe RG. Emmetropization in the rhesus monkey (Macaca mulatta): birth to young adulthood. Investigative Ophthalmology & Vision Science. 1999;40(1):214–229. [PubMed] [Google Scholar]
- Bullimore MA, Gilmartin B, Royston JM. Steady-state accommodation and ocular biometry in late-onset myopia. Documenta Ophthalmologica. Advances in Ophthalmology. 1992;80(2):143–155. doi: 10.1007/BF00161240. [DOI] [PubMed] [Google Scholar]
- Carroll JP. Regression curves for the optical parameters of the eye. American Journal of Optometry and Physiological Optics. 1981;58(4):314–323. doi: 10.1097/00006324-198104000-00010. [DOI] [PubMed] [Google Scholar]
- Carroll JP. Component and correlation ametropia. American Journal of Optometry and Physiological Optics. 1982;59(1):28–33. doi: 10.1097/00006324-198201000-00005. [DOI] [PubMed] [Google Scholar]
- Cheng HM, Singh OS, Kwong KK, Xiong J, Woods BT, Brady TJ. Shape of the myopic eye as seen with high-resolution magnetic resonance imaging. Optometry and Vision Science. 1992;69(9):698–701. doi: 10.1097/00006324-199209000-00005. [DOI] [PubMed] [Google Scholar]
- Duke-Elder E. Anomalies of refraction. In: Duke-Elder E, editor. System of Ophthalmology. London: C.V. Mosby Company; 1970. pp. 274–292. [Google Scholar]
- Fledelius HC. Ophthalmic changes from age of 10 to 18 years. A longitudinal study of sequels to low birth weight. IV. Ultrasound oculometry of vitreous and axial length. Acta Ophthalmologica. 1982;60(3):403–411. doi: 10.1111/j.1755-3768.1982.tb03031.x. [DOI] [PubMed] [Google Scholar]
- Fledelius HC. Corneal curvature radius. Oculometric considerations with reference to age and refractive change. Acta Ophthalmologica. Supplement. 1988;185:74–77. doi: 10.1111/j.1755-3768.1988.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Fledelius HC. Adult onset myopia--oculometric features. Acta Ophthalmologica Scandinavica. 1995;73(5):397–401. doi: 10.1111/j.1600-0420.1995.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Fulk GW, Cyert LA, Parker DA. Seasonal variation in myopia progression and ocular elongation. Optometry and Vision Science. 2002;79(1):46–51. doi: 10.1097/00006324-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Fulk GW, Cyert LA, Parker DE, West RW. The effect of changing from glasses to soft contact lenses on myopia progression in adolescents. Ophthalmic & Physiological Optics. 2003;23(1):71–77. doi: 10.1046/j.1475-1313.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- Fulton AB, Hansen RM, Petersen RA. The relation of myopia and astigmatism in developing eyes. Ophthalmology. 1982;89(4):298–302. doi: 10.1016/s0161-6420(82)34788-0. [DOI] [PubMed] [Google Scholar]
- Gao Q, Liu Q, Ma P, Zhong X, Wu J, Ge J. Effects of direct intravitreal dopamine injections on the development of lid-suture induced myopia in rabbits. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2006;244(10):1329–1335. doi: 10.1007/s00417-006-0254-1. [DOI] [PubMed] [Google Scholar]
- Garner LF, Stewart AW, Kinnear RF, Frith MJ. The Nepal longitudinal study: predicting myopia from the rate of increase in vitreous chamber depth. Optometry and Vision Science. 2004;81(1):44–48. doi: 10.1097/00006324-200401000-00009. [DOI] [PubMed] [Google Scholar]
- Garner LF, Stewart AW, Owens H, Kinnear RF, Frith MJ. The Nepal Longitudinal Study: biometric characteristics of developing eyes. Optometry and Vision Science. 2006;83(5):274–280. doi: 10.1097/01.opx.0000215251.27409.16. [DOI] [PubMed] [Google Scholar]
- Garner LF, Yap M, Scott R. Crystalline lens power in myopia. Optometry and Vision Science. 1992;69(11):863–865. doi: 10.1097/00006324-199211000-00005. [DOI] [PubMed] [Google Scholar]
- Gollender M, Thorn F, Erickson P. Development of axial ocular dimensions following eyelid suture in the cat. Vision Research. 1979;19(2):221–223. doi: 10.1016/0042-6989(79)90053-1. [DOI] [PubMed] [Google Scholar]
- Goss DA, Cox VD, Herrin-Lawson GA, Nielsen ED, Dolton WA. Refractive error, axial length, and height as a function of age in young myopes. Optometry and Vision Science. 1990;67(5):332–338. doi: 10.1097/00006324-199005000-00006. [DOI] [PubMed] [Google Scholar]
- Goss DA, Erickson P. Meridional corneal components of myopia progression in young adults and children. American Journal of Optometry and Physiological Optics. 1987;64(7):475–481. doi: 10.1097/00006324-198707000-00001. [DOI] [PubMed] [Google Scholar]
- Graham B, Judge SJ. The effects of spectacle wear in infancy on eye growth and refractive error in the marmoset (Callithrix jacchus) Vision Research. 1999;39(2):189–206. doi: 10.1016/s0042-6989(98)00189-8. [DOI] [PubMed] [Google Scholar]
- Greene PR. Optical constants and dimensions for the myopic, hyperopic and normal rhesus eye. Experimental Eye Research. 1990;51(4):351–359. doi: 10.1016/0014-4835(90)90148-n. [DOI] [PubMed] [Google Scholar]
- Grosvenor T, Scott R. Comparison of refractive components in youth-onset and early adult-onset myopia. Optometry and Vision Science. 1991;68(3):204–209. doi: 10.1097/00006324-199103000-00008. [DOI] [PubMed] [Google Scholar]
- Grosvenor T, Scott R. Three-year changes in refraction and its components in youth-onset and early adult-onset myopia. Optometry and Vision Science. 1993;70(8):677–683. doi: 10.1097/00006324-199308000-00017. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Hyman L, Hussein M, Everett D, Norton TT, Kurtz D, Leske MC, Manny R, Marsh-Tootle W, Scheiman M. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Investigative Ophthalmology & Vision Science. 2003;44(4):1492–1500. doi: 10.1167/iovs.02-0816. [DOI] [PubMed] [Google Scholar]
- Harman AM, Hoskins R, Beazley LD. Experimental eye enlargement in mature animals changes the retinal pigment epithelium. Visual Neuroscience. 1999;16(4):619–628. doi: 10.1017/s0952523899164022. [DOI] [PubMed] [Google Scholar]
- Harris WF. Algebra of sphero-cylinders and refractive errors, and their means, variance, and standard deviation. American Journal of Optometry and Physiological Optics. 1988;65(10):794–802. doi: 10.1097/00006324-198810000-00003. [DOI] [PubMed] [Google Scholar]
- Haugen OH, Hovding G, Eide GE. Biometric measurements of the eyes in teenagers and young adults with Down syndrome. Acta Ophthalmologica Scandinavica. 2001;79(6):616–625. doi: 10.1034/j.1600-0420.2001.790613.x. [DOI] [PubMed] [Google Scholar]
- Hirsch HE. Cytological Phenomena and Sex in Hypomyces Solani F. Cucurbitae. Proceedings of the National Academy of Sciences of the United States of America. 1947;33(9):268–270. doi: 10.1073/pnas.33.9.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch MJ, Weymouth FW. Notes on ametropia-a further analysis of Stenstrom’s data. American Journal of Optometry and Archives of American Academy of Optometry. 1947;24:601–608. doi: 10.1097/00006324-194712000-00005. [DOI] [PubMed] [Google Scholar]
- Hodos W, Fitzke FW, Hayes BP, Holden AL. Experimental myopia in chicks: ocular refraction by electroretinography. Investigative Ophthalmology & Vision Science. 1985;26(10):1423–1430. [PubMed] [Google Scholar]
- Hodos W, Kuenzel WJ. Retinal-image degradation produces ocular enlargement in chicks. Investigative Ophthalmology & Vision Science. 1984;25(6):652–659. [PubMed] [Google Scholar]
- Hofstetter HW. Emmetropization--biological process or mathematical artifact? American Journal of Optometry and Archives of American Academy of Optometry. 1969;46(6):447–450. [PubMed] [Google Scholar]
- Howlett MH, McFadden SA. Form-deprivation myopia in the guinea pig (Cavia porcellus) Vision Research. 2006;46(1–2):267–283. doi: 10.1016/j.visres.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Huang J, Hung LF, Ramamirtham R, Blasdel TL, Humbird TL, Bockhorst KH, Smith EL., 3rd Effects of form deprivation on peripheral refractions and ocular shape in infant rhesus monkeys (Macaca mulatta) Investigative Ophthalmology & Vision Science. 2009;50(9):4033–4044. doi: 10.1167/iovs.08-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung LF, Crawford ML, Smith EL., III Spectacle lenses alter eye growth and the refractive status of young monkeys. Nature Medicine. 1995;1(8):761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- Hung LF, Huang J, Qiao-Grider Y, Blasdel TL, Humbird TL, Bockhorst KH, Smith EL., 3rd Optical Defocus Regulates Refractive Development in Primates via Local Retinal Mechanisms. Investigative Ophthalmology & Vision Science. 2009;50 E-Abstract 3934. [Google Scholar]
- Hung LF, Ramamirtham R, Huang J, Qiao-Grider Y, Smith EL. Peripheral refraction in normal infant rhesus monkeys. Investigative Ophthalmology & Vision Science. 2008 doi: 10.1167/iovs.07-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung LF, Smith EL., 3rd Extended-wear, soft, contact lenses produce hyperopia in young monkeys. Optometry and Vision Science. 1996;73(9):579–584. doi: 10.1097/00006324-199609000-00003. [DOI] [PubMed] [Google Scholar]
- Hung LF, Wallman J, Smith EL., III Vision-dependent changes in the choroidal thickness of macaque monkeys. Investigative Ophthalmology & Vision Science. 2000;41(6):1259–1269. [PubMed] [Google Scholar]
- Irving EL, Callender MG, Sivak JG. Inducing ametropias in hatchling chicks by defocus--aperture effects and cylindrical lenses. Vision Research. 1995;35(9):1165–1174. doi: 10.1016/0042-6989(94)00235-e. [DOI] [PubMed] [Google Scholar]
- Jensen H. Myopia progression in young school children. A prospective study of myopia progression and the effect of a trial with bifocal lenses and beta blocker eye drops. Acta Ophthalmologica Supplement. 1991;(200):1–79. [PubMed] [Google Scholar]
- Jiang L, Schaeffel F, Zhou X, Zhang S, Jin X, Pan M, Ye L, Wu X, Huang Q, Lu F, Qu J. Spontaneous axial myopia and emmetropization in a strain of wild-type guinea pig (Cavia porcellus) Investigative Ophthalmology & Vision Science. 2009;50(3):1013–1019. doi: 10.1167/iovs.08-2463. [DOI] [PubMed] [Google Scholar]
- Jones LA, Mitchell GL, Mutti DO, Hayes JR, Moeschberger ML, Zadnik K. Comparison of ocular component growth curves among refractive error groups in children. Investigative Ophthalmology & Vision Science. 2005;46(7):2317–2327. doi: 10.1167/iovs.04-0945. [DOI] [PubMed] [Google Scholar]
- Jorge J, Almeida JB, Parafita MA. Refractive, biometric and topographic changes among Portuguese university science students: a 3-year longitudinal study. Ophthalmic & Physiological Optics. 2007;27(3):287–294. doi: 10.1111/j.1475-1313.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- Kaye SB, Patterson A. Association between total astigmatism and myopia. Journal of Cataract and Refractive Surgery. 1997;23(10):1496–1502. doi: 10.1016/s0886-3350(97)80020-x. [DOI] [PubMed] [Google Scholar]
- Keating MP. Geometric, physical, and visual optics. Boston: Butterworth-Heinemann; 2002. The Gauss System; pp. 295–322. [Google Scholar]
- Kee CS, Hung LF, Qiao-Grider Y, Ramamirtham R, Smith EL., 3rd Astigmatism in monkeys with experimentally induced myopia or hyperopia. Optometry and Vision Science. 2005;82(4):248–260. doi: 10.1097/01.opx.0000159357.61498.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee CS, Hung LF, Qiao-Grider Y, Ramamirtham R, Winawer J, Wallman J, Smith EL., 3rd Temporal constraints on experimental emmetropization in infant monkeys. Investigative Ophthalmology & Vision Science. 2007;48(3):957–962. doi: 10.1167/iovs.06-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee CS, Hung LF, Qiao-Grider Y, Roorda A, Smith EL., 3rd Effects of optically imposed astigmatism on emmetropization in infant monkeys. Investigative Ophthalmology & Vision Science. 2004;45(6):1647–1659. doi: 10.1167/iovs.03-0841. [DOI] [PubMed] [Google Scholar]
- Kee CS, Hung LF, Qiao Y, Habib A, Smith EL., III Prevalence of astigmatism in infant monkeys. Vision Research. 2002;42(11):1349–1359. doi: 10.1016/s0042-6989(02)00060-3. [DOI] [PubMed] [Google Scholar]
- Kee CS, Hung LF, Qiao Y, Smith EL., 3rd Astigmatism in infant monkeys reared with cylindrical lenses. Vision Research. 2003;43(26):2721–2739. doi: 10.1016/s0042-6989(03)00469-3. [DOI] [PubMed] [Google Scholar]
- Kee CS, Marzani D, Wallman J. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Investigative Ophthalmology & Vision Science. 2001;42(3):575–583. [PubMed] [Google Scholar]
- Kiely P, Crewther S, Nathan J, Brennan N, Efron N, Madigan M. A comparison of ocular development of the cynomolgus monkey and man. Clinical Vision Sciences. 1987;1:269–280. [Google Scholar]
- Kirby AW, Sutton L, Weiss H. Elongation of cat eyes following neonatal lid suture. Investigative Ophthalmology & Vision Science. 1982;22(2):274–277. [PubMed] [Google Scholar]
- Knudsen EI. Fused binocular vision is required for development of proper eye alignment in barn owls. Visual Neuroscience. 1989;2(1):35–40. doi: 10.1017/s0952523800004302. [DOI] [PubMed] [Google Scholar]
- Koretz JF, Rogot A, Kaufman PL. Physiological strategies for emmetropia. Trans Am Ophthalmol Soc. 1995;93:105–118. Discussion 118–122. [PMC free article] [PubMed] [Google Scholar]
- Kronfeld P, Devney C. The frequency of astigmatism. Archives of Ophthalmology. 1930;4:873–884. [Google Scholar]
- Larsen JS. The sagittal growth of the eye. 1. Ultrasonic measurement of the depth of the anterior chamber from birth to puberty. Acta Ophthalmologica. 1971a;49(2):239–262. doi: 10.1111/j.1755-3768.1971.tb00949.x. [DOI] [PubMed] [Google Scholar]
- Larsen JS. The sagittal growth of the eye. 3. Ultrasonic measurement of the posterior segment (axial length of the vitreous) from birth to puberty. Acta Ophthalmologica. 1971b;49(3):441–453. doi: 10.1111/j.1755-3768.1971.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Larsen JS. The sagittal growth of the eye. II. Ultrasonic measurement of the axial diameter of the lens and the anterior segment from birth to puberty. Acta Ophthalmologica. 1971c;49(3):427–440. doi: 10.1111/j.1755-3768.1971.tb00968.x. [DOI] [PubMed] [Google Scholar]
- Larsen JS. The sagittal growth of the eye. IV. Ultrasonic measurement of the axial length of the eye from birth to puberty. Acta Ophthalmologica. 1971d;49(6):873–886. doi: 10.1111/j.1755-3768.1971.tb05939.x. [DOI] [PubMed] [Google Scholar]
- Lauber JK. Light-induced avian glaucoma as an animal model for human primary glaucoma. Journal of Ocular Pharmacology. 1987;3(1):77–100. doi: 10.1089/jop.1987.3.77. [DOI] [PubMed] [Google Scholar]
- Lauber JK, McGinnis J. Eye lesions in domestic fowl reared under continuous light. Vision Research. 1966;6(12):619–626. doi: 10.1016/0042-6989(66)90073-3. [DOI] [PubMed] [Google Scholar]
- Li T, Troilo D, Glasser A, Howland HC. Constant light produces severe corneal flattening and hyperopia in chickens. Vision Research. 1995;35(9):1203–1209. doi: 10.1016/0042-6989(94)00231-a. [DOI] [PubMed] [Google Scholar]
- Logan NS, Gilmartin B, Wildsoet CF, Dunne MC. Posterior retinal contour in adult human anisomyopia. Investigative Ophthalmology & Vision Science. 2004;45(7):2152–2162. doi: 10.1167/iovs.03-0875. [DOI] [PubMed] [Google Scholar]
- Mallen EA, Gammoh Y, Al-Bdour M, Sayegh FN. Refractive error and ocular biometry in Jordanian adults. Ophthalmic & Physiological Optics. 2005;25(4):302–309. doi: 10.1111/j.1475-1313.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- Marsh-Tootle WL, Norton TT. Refractive and structural measures of lid-suture myopia in tree shrew. Investigative Ophthalmology & Vision Science. 1989;30(10):2245–2257. [PubMed] [Google Scholar]
- Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB. Cycloplegic refractions in healthy children aged 1 through 48 months. Archives of Ophthalmology. 2001;119(11):1625–1628. doi: 10.1001/archopht.119.11.1625. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Adams DW. A longitudinal investigation of adult-onset and adult-progression of myopia in an occupational group. Refractive and biometric findings. Investigative Ophthalmology & Vision Science. 1997;38(2):321–333. [PubMed] [Google Scholar]
- McBrien NA, Millodot M. A biometric investigation of late onset myopic eyes. Acta Ophthalmologica. 1987;65(4):461–468. doi: 10.1111/j.1755-3768.1987.tb07024.x. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Moghaddam HO, New R, Williams LR. Experimental myopia in a diurnal mammal (Sciurus carolinensis) with no accommodative ability. The Journal of physiology. 1993;469:427–441. doi: 10.1113/jphysiol.1993.sp019821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBrien NA, Norton TT. The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri) Vision Research. 1992;32(5):843–852. doi: 10.1016/0042-6989(92)90027-g. [DOI] [PubMed] [Google Scholar]
- Moore D, McCabe G. Introduction to the practice of statistics. New York: WH Freeman and Company; 1993a. Analysis of variance; pp. 712–789. [Google Scholar]
- Moore D, McCabe G. Introduction to the practice of statistics. New York: WH Freeman and Company; 1993b. Inference for distributions; pp. 498–573. [Google Scholar]
- Moore D, McCabe G. Introduction to the practice of statistics. New York: WH Freeman and Company; 1993c. Inference for regression; pp. 636–711. [Google Scholar]
- Mutti DO, Hayes JR, Mitchell GL, Jones LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Twelker JD, Zadnik K. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Investigative Ophthalmology & Vision Science. 2007;48(6):2510–2519. doi: 10.1167/iovs.06-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, Moeschberger ML, Zadnik K. Axial Growth and Changes in Lenticular and Corneal Power during Emmetropization in Infants. Investigative Ophthalmology & Vision Science. 2005;46(9):3074–3080. doi: 10.1167/iovs.04-1040. [DOI] [PubMed] [Google Scholar]
- Mutti DO, Sholtz RI, Friedman NE, Zadnik K. Peripheral refraction and ocular shape in children. Investigative Ophthalmology & Vision Science. 2000;41(5):1022–1030. [PubMed] [Google Scholar]
- Mutti DO, Zadnik K, Adams AJ. A video technique for phakometry of the human crystalline lens. Investigative Ophthalmology & Vision Science. 1992;33(5):1771–1782. [PubMed] [Google Scholar]
- Mutti DO, Zadnik K, Fusaro RE, Friedman NE, Sholtz RI, Adams AJ. Optical and structural development of the crystalline lens in childhood. Investigative Ophthalmology & Vision Science. 1998;39(1):120–133. [PubMed] [Google Scholar]
- Norton TT, Siegwart JT., Jr Animal models of emmetropization: matching axial length to the focal plane. Journal of the American Optometry Association. 1995;66(7):405–414. [PubMed] [Google Scholar]
- Norton TT, Siegwart JT, Jr, Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Investigative Ophthalmology & Vision Science. 2006;47(11):4687–4699. doi: 10.1167/iovs.05-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papastergiou GI, Schmid GF, Laties AM, Pendrak K, Lin T, Stone RA. Induction of axial eye elongation and myopic refractive shift in one- year-old chickens. Vision Research. 1998;38(12):1883–1888. doi: 10.1016/s0042-6989(97)00347-7. [DOI] [PubMed] [Google Scholar]
- Parssinen TO. Corneal refraction and topography in school myopia. The Clao Journal. 1993;19(1):69–72. doi: 10.1097/00140068-199301000-00013. [DOI] [PubMed] [Google Scholar]
- Priolo S, Sivak JG, Kuszak JR, Irving EL. Effects of experimentally induced ametropia on the morphology and optical quality of the avian crystalline lens. Investigative Ophthalmology & Vision Science. 2000;41(11):3516–3522. [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL., 3rd Recovery from form-deprivation myopia in rhesus monkeys. Investigative Ophthalmology & Vision Science. 2004;45(10):3361–3372. doi: 10.1167/iovs.04-0080. [DOI] [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL., 3rd A comparison of refractive development between two subspecies of infant rhesus monkeys (Macaca mulatta) Vision Research. 2007a;47(12):1668–1681. doi: 10.1016/j.visres.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL., 3rd Normal ocular development in young rhesus monkeys (Macaca mulatta) Vision Research. 2007b;47(11):1424–1444. doi: 10.1016/j.visres.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamirtham R, Kee CS, Hung LF, Qiao-Grider Y, Huang J, Roorda A, Smith EL., 3rd Wave aberrations in rhesus monkeys with vision-induced ametropias. Vision Research. 2007;47(21):2751–2766. doi: 10.1016/j.visres.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamirtham R, Kee CS, Hung LF, Qiao-Grider Y, Roorda A, Smith EL., 3rd Monochromatic ocular wave aberrations in young monkeys. Vision Research. 2006;46(21):3616–3633. doi: 10.1016/j.visres.2006.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw SM, Carkeet A, Chia KS, Stone RA, Tan DT. Component dependent risk factors for ocular parameters in Singapore Chinese children. Ophthalmology. 2002;109(11):2065–2071. doi: 10.1016/s0161-6420(02)01220-4. [DOI] [PubMed] [Google Scholar]
- Saw SM, Chua WH, Gazzard G, Koh D, Tan DT, Stone RA. Eye growth changes in myopic children in Singapore. The British Journal of Ophthalmology. 2005a;89(11):1489–1494. doi: 10.1136/bjo.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw SM, Tong L, Chua WH, Chia KS, Koh D, Tan DT, Katz J. Incidence and progression of myopia in Singaporean school children. Investigative Ophthalmology & Vision Science. 2005b;46(1):51–57. doi: 10.1167/iovs.04-0565. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Research. 1988;28(5):639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Schmid GF. Variability of retinal steepness at the posterior pole in children 7–15 years of age. Current Eye Research. 2003;27(1):61–68. doi: 10.1076/ceyr.27.2.61.15454. [DOI] [PubMed] [Google Scholar]
- Scott R, Grosvenor T. Structural model for emmetropic and myopic eyes. Ophthalmic & Physiological Optics. 1993;13(1):41–47. doi: 10.1111/j.1475-1313.1993.tb00424.x. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis) Brain Research. 1977;124(1):154–157. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. The susceptible period for deprivation-induced myopia in tree shrew. Vision Research. 1998;38(22):3505–3515. doi: 10.1016/s0042-6989(98)00053-4. [DOI] [PubMed] [Google Scholar]
- Smith EL, 3rd, Bradley DV, Fernandes A, Hung LF, Boothe RG. Continuous ambient lighting and eye growth in primates. Investigative Ophthalmology & Vision Science. 2001;42(6):1146–1152. [PubMed] [Google Scholar]
- Smith EL, 3rd, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Research. 2009a;49(19):2386–2392. doi: 10.1016/j.visres.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, 3rd, Hung LF, Kee CS, Qiao-Grider Y, Ramamirtham R. Continuous ambient lighting and lens compensation in infant monkeys. Optometry and Vision Science. 2003;80(5):374–382. doi: 10.1097/00006324-200305000-00012. [DOI] [PubMed] [Google Scholar]
- Smith EL, 3rd, Kee CS, Ramamirtham R, Qiao-Grider Y, Hung LF. Peripheral vision can influence eye growth and refractive development in infant monkeys. Investigative Ophthalmology & Vision Science. 2005;46(11):3965–3972. doi: 10.1167/iovs.05-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, 3rd, Ramamirtham R, Qiao-Grider Y, Hung LF, Huang J, Kee CS, Coats D, Paysse E. Effects of foveal ablation on emmetropization and form-deprivation myopia. Investigative Ophthalmology & Vision Science. 2007;48(9):3914–3922. doi: 10.1167/iovs.06-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, Huang J, Hung LF, Blasdel TL, Humbird TL, Bockhorst KH. Hemi-Retinal Form Deprivation: Evidence for Local Control of Eye Growth and Refractive Development in Infant Monkeys. Investigative Ophthalmology & Vision Science. 2009b doi: 10.1167/iovs.08-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL., III . Environmentally induced refractive errors in animals. In: Rosenfield M, Gilmartin B, editors. Myopia and Nearwork. Oxford, UK: Butterworth-Heinemann; 1998a. pp. 57–90. [Google Scholar]
- Smith EL., III Spectacle lenses and emmetropization: the role of optical defocus in regulating ocular development. Optometry and Vision Science. 1998b;75(6):388–398. doi: 10.1097/00006324-199806000-00023. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Bradley DV, Fernandes A, Boothe RG. Form deprivation myopia in adolescent monkeys. Optometry and Vision Science. 1999a;76(6):428–432. doi: 10.1097/00006324-199906000-00023. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Harwerth RS. Behavioral measurements of accommodative amplitude in rhesus monkeys. Vision Research. 1984;24(12):1821–1827. doi: 10.1016/0042-6989(84)90013-0. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Harwerth RS, Crawford ML, von Noorden GK. Observations on the effects of form deprivation on the refractive status of the monkey. Investigative Ophthalmology & Vision Science. 1987;28(8):1236–1245. [PubMed] [Google Scholar]
- Smith EL, III, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Research. 1999;39(8):1415–1435. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Hung LF. Form-deprivation myopia in monkeys is a graded phenomenon. Vision Research. 2000;40(4):371–381. doi: 10.1016/s0042-6989(99)00184-4. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Hung LF, Harwerth RS. Effects of optically induced blur on the refractive status of young monkeys. Vision Research. 1994;34(3):293–301. doi: 10.1016/0042-6989(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Hung LF, Harwerth RS. Developmental visual system anomalies and the limits of emmetropization. Ophthalmic & Physiological Optics. 1999b;19(2):90–102. [PubMed] [Google Scholar]
- Smith EL, III, Hung LF, Harwerth RS. The degree of image degradation and the depth of amblyopia. Investigative Ophthalmology & Vision Science. 2000;41(12):3775–3781. [PubMed] [Google Scholar]
- Smith EL, III, Hung LF, Kee CS, Qiao Y. Effects of brief periods of unrestricted vision on the development of form-deprivation myopia in monkeys. Investigative Ophthalmology & Vision Science. 2002a;43(2):291–299. [PubMed] [Google Scholar]
- Smith EL, III, Zhong X, Nie H, Ge J. Compensation for Hyperopic Anisometropia in Adolescent Monkeys. Optometry and Vision Science. 2002b;79(Suppl):196. doi: 10.1167/iovs.04-0226. [DOI] [PubMed] [Google Scholar]
- Sorsby A, Benjamin B, Davey J, Sheridan M, Tanner J. Emmetropia and its aberrations. Special Report Series (Medical Research Council (Great Britain)) 1957;11(293):1–69. [PubMed] [Google Scholar]
- Sorsby A, Benjamin B, Sheridan M, Stone J, Leary GA. Refraction and its components during the growth of the eye from the age of three. Medical Research Council Memorandum. 1961;301(Special):1–67. [PubMed] [Google Scholar]
- Sorsby A, Leary GA, Fraser GR. Family studies on ocular refraction and its components. Journal of Medical Genetics. 1966;3(4):269–273. doi: 10.1136/jmg.3.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenstrom S. Investigation of the variation and the correlation of the optical elements of human eyes. American Journal of Optometry and Archives of American Academy of Optometry. 1948;25(10):496–504. doi: 10.1097/00006324-194810000-00006. [DOI] [PubMed] [Google Scholar]
- Tejedor J, de la Villa P. Refractive changes induced by form deprivation in the mouse eye. Investigative Ophthalmology & Vision Science. 2003;44(1):32–36. doi: 10.1167/iovs.01-1171. [DOI] [PubMed] [Google Scholar]
- Teller DY, Regal DM, Videen TO, Pulos E. Development of visual acuity in infant monkeys (Macaca nemestrina) during the early postnatal weeks. Vision Research. 1978;18(5):561–566. doi: 10.1016/0042-6989(78)90203-1. [DOI] [PubMed] [Google Scholar]
- Tigges M, Tigges J, Fernandes A, Eggers HM, Gammon JA. Postnatal axial eye elongation in normal and visually deprived rhesus monkeys. Investigative Ophthalmology & Vision Science. 1990;31(6):1035–1046. [PubMed] [Google Scholar]
- Torczynski E. Survey of Changes in the Eye. In: Bowden DM, editor. Aging in Nonhuman Primates. New York: Van Nostrand Reinhold; 1979. pp. 143–157. [Google Scholar]
- Troilo D, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus) Vision Research. 1993;33(10):1311–1324. doi: 10.1016/0042-6989(93)90039-y. [DOI] [PubMed] [Google Scholar]
- Troilo D, Li T, Glasser A, Howland HC. Differences in eye growth and the response to visual deprivation in different strains of chicken. Vision Research. 1995;35(9):1211–1216. doi: 10.1016/0042-6989(94)00230-j. [DOI] [PubMed] [Google Scholar]
- Troilo D, Nickla DL, Wildsoet CF. Form deprivation myopia in mature common marmosets (Callithrix jacchus) Investigative Ophthalmology & Vision Science. 2000;41(8):2043–2049. [PubMed] [Google Scholar]
- van Alphen G. On emmetropia and ametropia. Optica Acta. 1961;142(Suppl):1–92. [PubMed] [Google Scholar]
- Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization. Vision Research. 1987;27(7):1139–1163. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201(4362):1249–1251. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- Wang FR, Zhou XD, Zhou SZ. [A CT study of the relation between ocular axial biometry and refraction] Zhonghua Yan Ke Za Zhi. 1994;30(1):39–40. [PubMed] [Google Scholar]
- Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266(5597):66–68. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- Wildsoet C. Structural correlates of myopia. In: Rosenfield M, Gilmartin B, editors. Myopia and Nearwork. Boston: Butterworth-Heinemann; 1998. pp. 31–56. [Google Scholar]
- Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Research. 1995;35(9):1175–1194. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- Wildsoet CF. Active emmetropization--evidence for its existence and ramifications for clinical practice. Ophthalmic & Physiological Optics. 1997;17(4):279–290. [PubMed] [Google Scholar]
- Yinon U, Koslowe KC, Rassin MI. The optical effects of eyelid closure on the eyes of kittens reared in light and dark. Current Eye Research. 1984;3(3):431–439. doi: 10.3109/02713688408997230. [DOI] [PubMed] [Google Scholar]
- Yinon U, Rose L, Shapiro A. Myopia in the eye of developing chicks following monocular and binocular lid closure. Vision Research. 1980;20(2):137–141. doi: 10.1016/0042-6989(80)90155-8. [DOI] [PubMed] [Google Scholar]
- Zadnik K, Manny RE, Yu JA, Mitchell GL, Cotter SA, Quiralte JC, Shipp M, Friedman NE, Kleinstein RN, Walker TW, Jones LA, Moeschberger ML, Mutti DO. Ocular component data in schoolchildren as a function of age and gender. Optometry and Vision Science. 2003;80(3):226–236. doi: 10.1097/00006324-200303000-00012. [DOI] [PubMed] [Google Scholar]
- Zadnik K, Mutti DO, Fusaro RE, Adams AJ. Longitudinal evidence of crystalline lens thinning in children. Investigative Ophthalmology & Vision Science. 1995;36(8):1581–1587. [PubMed] [Google Scholar]
- Zhong X, Ge J, Nie H, Smith EL., 3rd Compensation for experimentally induced hyperopic anisometropia in adolescent monkeys. Investigative Ophthalmology & Vision Science. 2004;45(10):3373–3379. doi: 10.1167/iovs.04-0226. [DOI] [PubMed] [Google Scholar]