Abstract
Conclusion
Middle and inner ear interactions in otitis media can lead to cochlear pathology. More severe pathological changes observed in the basal turn of the cochlea are consistent with prevalence of sensorineural hearing loss at higher frequencies in patients with otitis media.
Methods
Of 614 temporal bones with otitis media, 47 with chronic and 35 with purulent otitis media were selected following strict exclusion of subjects with a history of acoustic trauma, head trauma, ototoxic drugs, and other diseases affecting the cochlear labyrinth. Temporal bones with labyrinthine inflammatory changes were further evaluated for loss of hair cells and other histopathologic changes compared to age-matched controls.
Results
In all, 19% of temporal bones with chronic and 9% with purulent otitis media showed labyrinthine inflammatory changes. In chronic otitis media, inflammatory changes were: 56% localized purulent, 22% localized serous, 11% generalized seropurulent, and 11% generalized serous. Inflammatory changes in temporal bones with purulent otitis media included 67% localized purulent and 33% were generalized seropurulent. Pathological findings included: serofibrinous precipitates and inflammatory cells in scala tympani of basal turn and cochlear aqueduct, significant loss of outer and inner hair cells, and significant decrease in area of stria vascularis in the basal turn of the cochlea, as compared to controls.
Keywords: Histopathology, cochlear changes, cochlear turns, otitis media, temporal bone
Introduction
Otitis media occurs as a continuum of related diseases: acute otitis media, recurrent acute otitis media, otitis media with effusion (serous, mucoid, purulent), and chronic otitis media. Otitis media is clinically defined as chronic when middle ear effusion has been present for at least 3 months and is histopathologically characterized by intractable middle ear or mastoid tissue pathology like granulation tissue or cholesteatoma [1]. Inner ear complications accompanied by temporary or permanent hearing loss have been reported in children and adult patients with otitis media [2,3]. The extended high-frequency sensorineural hearing loss (SNHL) commonly seen in these patients strongly indicates structural damage of the basal turn of the cochlea [4–7]. Inflammatory damage of the cochlea and high-frequency SNHL was suggested to arise from passage of toxic substances (bacterial products and inflammatory mediators) from the middle ear through the round window membrane into the inner ear [8].
Loss of outer hair cells (OHCs) and inner hair cells (IHCs) in the cochlear base and other pathological changes in the inner ear have been reported in experimental otitis media in animals [9,10] and in the affected ear compared with the contralateral ear in human temporal bones of patients with unilateral chronic otitis media [11]. Passage of toxic substances through the round window membrane can cause perilymphatic inflammation starting in the cochlear basal turn, which can spread apically resulting in auditory threshold shifts at lower frequencies.
Although various authors have presented the audiological implications of inner ear involvement in otitis media, the exact effects of otitis media on the cochlear pathology and function in humans are not clearly understood. Previous histopathologic studies of human temporal bones have used limited or no exclusion criteria of other diseases that might affect inner ear pathology, leaving questions as to its cause. This study examines the effect of otitis media on inner ear pathology with strict exclusion criteria for subjects with a history of acoustic trauma, head trauma, ototoxic drugs, and other diseases, which can affect the cochlear labyrinth. All human temporal bones were from patients with bilateral otitis media, and cases with inflammatory cell infiltration in the inner ear due to otitis media were also included in this study, in contrast to our previous report of human temporal bones with unilateral chronic otitis media [11].
Material and methods
All temporal bones used in this study were previously removed at autopsy, fixed in formalin solution, decalcified, and embedded in celloidin. Temporal bones were serially sectioned in the horizontal plane at a thickness of 20 μm. Every 10th section was stained with hematoxylin and eosin (H&E) and mounted on a glass slide for light microscopic observation.
Of the 614 bones with otitis media, 47 (30 cases) with chronic otitis media and 35 (21 cases) with purulent otitis media were selected for this study. Subjects with a history of acoustic trauma, head trauma, ototoxic drugs, cholesteatoma, otosclerosis, otological surgery, leukemia, and other otological or systemic diseases affecting the labyrinth were excluded. Chronic otitis media was defined as inflammation of the middle ear cleft with pathological changes including granulation tissue, cholesterol granuloma, bony changes, fibrosis, and tympano-sclerosis. Purulent otitis media was defined as inflammation with purulent effusion and edematous subepithelial spaces infiltrated with inflammatory cells, mainly polymorphonuclear leukocytes. Inflammation limited to the area of scala tympani adjacent to the round window membrane and cochlear aqueduct area was classified as localized, while inflammatory changes throughout the labyrinth were classified as generalized. Patterns of labyrinthine inflammation were classified as localized purulent, localized serous, generalized seropurulent, or generalized serous.
Temporal bones clearly showing these labyrinthine inflammatory changes were further studied for inner ear pathology in basal, middle, and apical cochlear turns. The following parameters were assessed: 1) OHC loss, 2) IHC loss, 3) changes in stria vascularis area, and 4) changes in spiral ligament area. All inner ear findings were compared to age-matched controls. Morphometric measurements were made for areas of spiral ligament and stria vascularis. The calculations were made in all turns of the cochlea at mid-modiolar level and two adjacent sections. The image was obtained with a charge-coupled device camera that was connected to a personal computer. The calibrated image was acquired at an original magnification of ×4 for spiral ligament and ×20 for the stria vascularis. The areas of spiral ligament and stria vascularis were quantified by determining areas of their cut surfaces with the aid of a computer. Measurements were made using commercially available image analysis software (Image-Pro Plus, version 3.0, Media Cybernetics, Silver Springs MD, USA). Any observed cystic-like structures in the stria vascularis were excluded from the total area of the stria vascularis. OHCs and IHCs were investigated under light microscopy at the magnification of ×100 and loss of hair cells was calculated for each temporal bone in a section-wise manner. Percentage of hair cell loss was evaluated using the following formula: % of hair cell loss (OHC or IHC) = the number of absent hair cells/the total number of hair cells possible in that turn × 100.
The sections in which the hair cells could not be counted – because of cutting angle, processing artifacts, or post-mortem changes – were excluded [12]. All inner ear findings were compared to age-matched controls. Temporal bone slides were reviewed for histopathologic changes by light microscopy in a blinded fashion. To emphasize the internal validity of the study, both test and retest examinations were conducted by multiple researchers to prevent possible observer bias. Mann-Whitney rank sum test was performed to calculate significant differences in hair cell loss and areas of stria vascularis and spiral ligament between diseased and control temporal bones. Significant difference was defined as p < 0.05.
Results
In chronic otitis media, inflammatory changes were: 56% localized purulent, 22% localized serous, 11% generalized seropurulent, and 11% generalized serous; and 19% (nine temporal bones) showed labyrinthine inflammatory changes. Inflammatory changes in temporal bones with purulent otitis media included 67% localized purulent and 33% were generalized seropurulent; 9% (three temporal bones) showed labyrinthine inflammatory changes. Pathological findings in temporal bones with labyrinthine changes included inflammatory changes of the round window membranes with serofibrinous precipitates and inflammatory cells in the adjacent scala tympani of the basal turn and the cochlear aqueduct (Figure 1), loss of OHCs and IHCs, and changes in the area of stria vascularis.
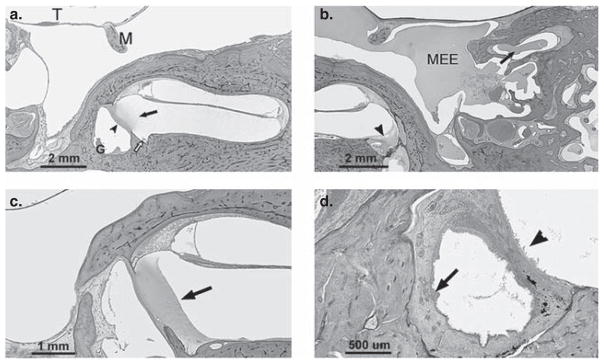
Figure 1.
(a) Section of temporal bone showing inflammation extending into the scala tympani (arrow) via an inflamed round window membrane (arrowhead). There is granulation tissue (G) in the round window niche and inflammatory cells in the cochlear aqueduct area (open arrow). (b) Section showing effusion in the middle ear cavity (MEE) and within the mastoid air cells (arrow). Effusion is also seen in the round window niche and in the perilymphatic space of the cochlea (arrowhead) across the round window membrane. (c) Section showing serofibrinous precipitate (arrow) in the scala tympani (basal turn) adjacent to the round window membrane. The round window membrane appears partly obliterated by the precipitate. (d) Section showing marked thickening and infiltration of the round window niche mucosa by inflammatory cells (arrow). Inflammatory cells are seen lining the perilymphatic space (arrowhead) of the inner ear adjacent to the round window membrane. Staining with H&E. T, tympanic membrane; M, malleus.
Twelve temporal bones showing obvious labyrinthine inflammatory changes were selected for analysis of histpathologic changes in all three cochlear turns compared to age-matched control temporal bones. Loss of OHCs and IHCs was found to vary for different cochlear turns (Fig. 2a and b). Loss of both OHCs and IHCs was significant only in the basal turn (OHCs, p = 0.016; IHCs, p = 0.032). Mean loss of OHCs was 39.3% for diseased temporal bones compared with 9.7% for controls, and IHC loss for diseased temporal bones was 22.0% compared with 2.8% for controls. In the middle turns, there was no significant loss of OHCs and IHCs in diseased and control temporal bones (OHCs, p = 0.095; IHCs, p = 0.095). In the apical turns, insignificant loss or no loss of OHCs or IHCs was observed in diseased compared to control temporal bones (OHCs, p = 0.310; IHCs, p = 1.000).
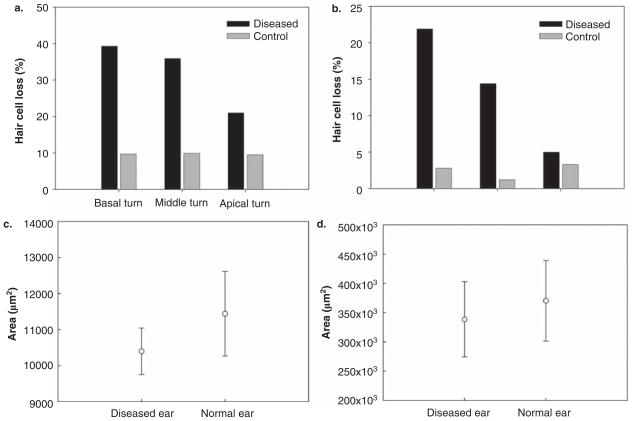
Figure 2.
(a) Histogram comparing mean loss of outer hair cells (OHCs) in diseased and control bones in different cochlear turns. There is a significantly higher percentage of loss in the basal turn of diseased bones as compared with controls. (b) Histogram comparing mean loss of inner hair cells (IHCs) in diseased and control bones. There is a significantly higher percentage of loss in the basal turn of diseased bones as compared with controls. (c) Histogram comparing the areas of stria vascularis in diseased and control temporal bones in the basal turn. The area of stria vascularis in diseased bones is significantly less compared with normal controls. (d) Histogram comparing the areas of spiral ligament in diseased and control bones in the basal turn. There is no significant difference in the area of spiral ligament between diseased and control bones.
The area (mean ± SD) of stria vascularis in the basal turns were 10 398 ± 646 μm2 for diseased temporal bones and 11 440 ± 1172 μm2 for controls. The areas of the stria vascularis in temporal bones with labyrinthine inflammatory changes was significantly decreased as compared with controls (p = 0.042) (Fig. 2c). The areas for spiral ligament in the basal turns were 338 581 ± 64 194 μm2 and 370 182 ± 68 783 μm2 for diseased and control temporal bones, respectively. There was no significant difference (p = 0.331) in spiral ligament areas between diseased and control temporal bones (Fig. 2d).
Discussion
The middle–inner ear interaction that occurs in otitis media has been an area of interest for many researchers. It has been observed that children who have recovered from otitis media have significantly poorer hearing in extended high frequencies as compared with their counterparts without otitis media [6]. Pathological changes in temporal bones and high-frequency SNHL in animals and patients with otitis media strongly suggest preferential involvement of the cochlear basal turn [2,9–11]. Numerous data indicate that bacterial components and inflammatory mediators produced in the middle ear pass through the round window membrane into the inner ear, altering expression of other proteins [13,14], including K+, Na+, and other ion channels and transporters localized in the stria vascularis and spiral ligament, resulting in disturbance of ion homeostasis, which in turn can cause injury of various types of cochlear cells, including hair cells, and result in SNHL [8,15,16].
In the present study, about 19% of temporal bones with chronic otitis media and about 9% with purulent otitis media had inflammatory changes of the round window membranes, with inflammation of the adjacent scala tympani and pathology of the basal turns of the cochlea. (Figure 1). Since we excluded subjects with a history of acoustic trauma, head trauma, ototoxic drugs, cholesteatoma, otosclerosis, otological surgery, leukemia, and other otological or systemic diseases affecting the labyrinth, it can be concluded that our findings of significant loss of OHCs or IHCs and decrease in area of stria vascularis in the basal turn of affected temporal bones as compared to age-matched controls are results of labyrinthine pathological changes secondary to otitis media. In our previous study of human temporal bones with unilateral chronic otitis media, we also found significant changes in OHCs and IHCs and area of stria vascularis in the basal cochlear turn [11], indicating the vulnerability of these cochlear structures in the process of developing of otitis media. Our findings provide details of significant structural damage in the basal cochlear turn, which can be associated with high-frequency SNHL previously described in patients with otitis media [4–8]. These findings are important, because a number of studies have shown that the degree of SNHL can be increased with the duration and extent of pathological changes in the middle ear [2,17].
Acknowledgments
The authors thank Carolyn R. Sutherland for her technical support. This work was supported by NIDCD R01 DC006452, NIDCD 3U24 DC008559-03S109, International Hearing Foundation, Hubbard Foundation, and Starkey Foundation.
References
- 1.Daly KA, Hunter LL, Giebink GS. Chronic otitis media with effusion. Pediatr Rev. 1999;20:85–94. doi: 10.1542/pir.20-3-85. [DOI] [PubMed] [Google Scholar]
- 2.Paparella MM, Oda M, Hiraide F, Brady D. Pathology of sensorineural hearing loss in otitis media. Ann Otol. 1972;81:632–47. doi: 10.1177/000348947208100503. [DOI] [PubMed] [Google Scholar]
- 3.Meyerhoff WL, Kim CS, Paparella MM. Pathology of chronic otitis media. Ann Otol Rhinol Laryngol. 1978;87:749–60. doi: 10.1177/000348947808700602. [DOI] [PubMed] [Google Scholar]
- 4.Margolis RH, Hunter LL, Saupe JR, Giebink GS. Effect of otitis media on extended high frequency hearing in children. Ann Otol Rhinol Laryngol. 1993;102:1–5. doi: 10.1177/000348949310200101. [DOI] [PubMed] [Google Scholar]
- 5.Hunter LL, Margolis RH, Rykken JR, Le CT, Daly KA, Giebink GS. High frequency hearing loss associated with otitis media. Ear Hear. 1996;17:1–11. doi: 10.1097/00003446-199602000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Margolis RH, Saly GL, Hunter L. High-frequency loss and wideband middle ear impedance in children with otitis media histories. Ear Hear. 2000;21:206–11. doi: 10.1097/00003446-200006000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Papp Z, Rezes S, Sziklai I. Sensorineural hearing loss in chronic otitis media. Otol Neurotol. 2003;24:141–4. doi: 10.1097/00129492-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Juhn SK, Jung TTK, Lin J, Rhee CK. Effect of inflammatory mediators on middle ear pathology and inner ear function. Ann N Y Acad Sci. 1997;830:130–42. doi: 10.1111/j.1749-6632.1997.tb51885.x. [DOI] [PubMed] [Google Scholar]
- 9.Schachern PA, Paparella MM, Hybertson R, Sano S, Duvall AJ., 3rd Bacterial tympanogenic labyrinthitis, meningitis and sensorineural damage. Arch Otolaryngol Head Neck Surg. 1992;118:53–7. doi: 10.1001/archotol.1992.01880010057016. [DOI] [PubMed] [Google Scholar]
- 10.Cook RD, Postma DS, Brinson GM, Prazma J, Pillsbury HC. Cytotoxic changes in hair cells secondary to pneumococcal middle-ear infection. J Otolaryngol. 1999;28:325–31. [PubMed] [Google Scholar]
- 11.Cureoglu S, Schachern P, Paparella MM, Lindgren BR. Cochlear changes in chronic otitis media. Laryngoscope. 2004;114:622–6. doi: 10.1097/00005537-200404000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Kariya S, Cureoglu S, Fukushima H, Kusunoki T, Schachern PA, Nishizaki K, et al. Histopathologic changes of contralateral human temporal bone in unilateral Meniere’s disease. Otol Neurotol. 2007;28:1063–8. doi: 10.1097/MAO.0b013e31815a8433. [DOI] [PubMed] [Google Scholar]
- 13.Ghaheri BA, Kemton JB, Pillers DA, Trune DR. Cochlear cytokine gene expression in murine acute otitis media. Laryngoscope. 2007;117:22–9. doi: 10.1097/01.mlg.0000240170.48584.73. [DOI] [PubMed] [Google Scholar]
- 14.Ghaheri BA, Kemton JB, Pillers DA, Trune DR. Cochlear cytokine gene expression in murine chronic otitis media. Otolaryngol Head Neck Surg. 2007;137:332–7. doi: 10.1016/j.otohns.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Ichimiya I, Suzuki M, Hirano T, Mogi G. The influence of pneumococcal otitis media on the cochlear lateral wall. Hear Res. 1999;131:128–34. doi: 10.1016/s0378-5955(99)00025-8. [DOI] [PubMed] [Google Scholar]
- 16.Juhn SK, Jung MK, Hoffman MD, Drew BR, Preciado DA, Sausen NJ, et al. The role of inflammatory mediators in the pathogenesis of otitis media and sequelae. Clin Exp Otorhinolaryngol. 2008;1:117–38. doi: 10.3342/ceo.2008.1.3.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blakley BW, Kim S. Does chronic otitis media cause sensorineural hearing loss? J Otolaryngol. 1998;27:17–20. [PubMed] [Google Scholar]