Abstract
Metabotropic glutamate receptors (mGluRs) mediate a variety of responses to glutamate in the central nervous system. A primary role for group-III mGluRs is to inhibit neurotransmitter release from presynaptic terminals, but the molecular mechanisms that regulate presynaptic trafficking and activity of group-III mGluRs are not well understood. Here, we describe the interaction of mGluR7, a group-III mGluR and presynaptic autoreceptor, with the cytoskeletal protein, alpha tubulin. The mGluR7 carboxy terminal (CT) region was expressed as a GST fusion protein and incubated with rat brain extract to purify potential mGluR7-interacting proteins. These studies yielded a single prominent mGluR7 CT-associated protein of 55 kDa, which subsequent microsequencing analysis revealed to be alpha tubulin. Coimmunoprecipitation assays confirmed that full-length mGluR7 and alpha tubulin interact in rat brain as well as in BHK cells stably expressing mGluR7a, a splice variant of mGluR7. In addition, protein overlay experiments showed that the CT domain of mGluR7a binds specifically to purified tubulin and calmodulin, but not to bovine serum albumin. Further pull-down studies revealed that another splice variant mGluR7b also interacts with alpha tubulin, indicating that the binding region is not localized to the splice-variant regions of either mGluR7a (900–915) or mGluR7b (900–923). Indeed, deletion mutagenesis experiments revealed that the alpha tubulin-binding site is located within amino acids 873–892 of the mGluR7 CT domain, a region known to be important for regulation of mGluR7 trafficking. Interestingly, activation of mGluR7a in cells results in an immediate and significant decrease in alpha tubulin binding. These data suggest that the mGluR7/alpha tubulin interaction may provide a mechanism to control access of the CT domain to regulatory molecules, or alternatively, that this interaction may lead to morphological changes in the presynaptic membrane in response to receptor activation.
Keywords: carboxy terminal domain, mGluR7, protein–protein interaction, regulation, tubulin
Metabotropic glutamate receptors (mGluRs) are G protein-coupled receptors (GPCRs) that are widely distributed throughout the central nervous system and modulate cell excitability and synaptic transmission. There are eight molecular subtypes of mGluRs that are divided into three groups based on their sequence identity, signal transduction, and pharmacological profiles. Group-I mGluRs 1 and 5 couple to Gq to activate phospholipase C and are potently and selectively activated by 3,5-dihydroxyphenylglycine (DHPG). Group-II mGluRs 2 and 3 and group-III mGluRs 4, 6, 7 and 8 couple to Gi/Go to inhibit the activation of adenylate cyclase in heterologous expression systems, and are potently and selectively activated by (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)-glycine (DCG-IV) and S-4-phosphono-2-aminobutyric acid (S-AP4), respectively (for review, see Conn and Pin 1997; Pin et al. 1999).
There are a handful of cytoplasmic proteins known to associate with the intracellular regions of metabotropic glutamate receptors. The mGluR second intracellular loop and carboxy terminal (CT) domains are required to interact with G proteins (Pin et al. 1994). The CT domain of group-I mGluRs can also interact with intracellular protein regulators such as the Homer proteins (Brakeman et al. 1997), which can physically tether these mGluRs to the inositol trisphosphate receptor (Tu et al. 1998). The CT domain of mGluR1 has also been shown to bind to beta tubulin (Ciruela et al. 1999; Ciruela and McIlhinney 2001), while the CT domain of mGluR5 has been shown to bind to calmodulin (Minakami et al. 1997) and the CT domains of both group-I mGluRs have been shown to associate with Siah-1 A, a mammalian homolog of the Drosophila seven in absentia (Ishikawa et al. 1999). The CT domain of mGluR7 has been shown to bind to calmodulin (Nakajima et al. 1999; O'Connor et al. 1999) and PICK1 (Boudin et al. 2000; Dev et al. 2000; El Far et al. 2000). Calmodulin-binding to mGluR7 overlaps the protein kinase C (PKC) phosphorylation site, thus calcium/calmodulin binding to the receptor inhibits PKC phosphorylation of mGluR7 (Nakajima et al. 1999; El Far et al. 2001). Conversely, calmodulin does not bind to phosphorylated mGluR7. PICK1, a PKCα substrate and binding protein interacts with the most distal regions of the CT domain of mGluR7a (Dev et al. 2000). mGluR7a forms a complex with PICK1 and PKCα, and PICK1 can inhibit the PKCα phosphorylation of mGluR7a (Dev et al. 2000). These data suggest that PICK1 and calmodulin can modulate the phosphorylation state of mGluR7a and regulate receptor responses by controlling the desensitization of the receptor.
The studies described here were designed to identify additional proteins that can interact with the CT domain of presynaptic mGluRs. We have identified alpha tubulin as a cellular binding partner of mGluR7. Deletion mutagenesis experiments reveal that the alpha tubulin-binding site lies within amino acids 873–892 in the mGluR7 CT domain. This site overlaps with the region previously identified as an mGluR7 axonal targeting signal (Stowell and Craig 1999), suggesting that the interaction with alpha tubulin may play a role in the subcellular targeting of mGluR7. Interestingly, the interaction between mGluR7a and alpha tubulin is dynamically regulated in that mGluR7a shows a rapid (within one minute) and significant (> 50%) dissociation from alpha tubulin upon receptor activation. These data suggest that the interaction between mGluR7 and alpha tubulin may provide a mechanism to control access of the CT domain to regulatory molecules, or alternatively, that this interaction may contribute to morphological changes in the presynaptic membrane in response to receptor activation.
Experimental procedures
Plasmid DNA constructs and mutagenesis
The mGluR CT domains were amplified by PCR using specific oligonucleotide primers engineered with restriction sites 5′ proximal to the end of the oligomer. For each mGluR construct, the following amino acids were included: mGluR1a, 841–1199; mGluR2, 815–872; mGluR7a, 851–915; mGluR7b, 815–923. The PCR products were digested with EcoR1 and Xho1 (mGluRs 1a and 7b) or Not1 (mGluRs 2 and 7a) and subcloned in-frame into the polylinker region of the glutathione S-transferase gene fusion expression vector pGex-6P-3 (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA). The mGluR2 and mGluR7a constructs were kindly shared by Drs H. Schaffhauser and T. Macek, respectively. The Quik-Change Mutagenesis protocol (Stratagene, La Jolla, CA, USA) was used to generate the deletion mutant mGluR7Δ893 and mGluR7Δ873 by inserting a stop codon into the mGluR7a CT fusion protein sequence. Subcloned DNAs were transformed into BL21 Gold Escherichia coli cells (Stratagene) and plated onto LB/ampicillin agar plates. Single colonies were grown overnight in LB/ampicillin medium, and plasmid DNA was isolated using DNA kits (Qiagen, Valencia, CA, USA). Clones were verified by restriction enzyme analysis and DNA sequence analysis, and the predicted amino acid sequences were determined by computer analysis.
Generation and purification of glutathione S-transferase (GST) fusion proteins
Large-scale preparations of bacterial sonicates for the purification of GST-fusion proteins were performed according to the manufacturers protocol (Pharmacia). In brief, a single colony of E. coli BL21 cells containing the recombinant pGex-6P-3 plasmid was grown overnight and used to inoculate 2xYT medium containing 100 μg/mL ampicillin (1 : 100 dilution). The cells were grown at 30°C with shaking to an A600 of approximately 1, and protein expression was induced by incubation for an additional 3 h in 0.1 mM isopropyl β-D-thiogalactoside (IPTG). The cells were collected by sedimentation, resuspended in phosphate-buffered saline (PBS), sonicated, solubilized in 1 × PBS, 1% Triton X-100, then bound to Glutathione Sepharose 4B. The glutathione-fusion protein matrix was washed three times in 10 bed volumes of 1 × PBS, assayed for protein content, and analyzed by Coomassie gel.
Total brain lysate preparation, pull-down assays, and immunoblots
Frozen rat brains (Sprague–Dawley adult male, ~250 g) were homogenized by 15–20 strokes of a dounce homogenizer in ice-cold 1 × HEN (50 mM HEPES pH 7.4, 10 mM EDTA, 25 mM NaCl) plus Complete™ protease inhibitors (Roche Molecular Biochemicals, Indianapolis, IN, USA). The lysate was solubilized in 1% Triton X-100 with shaking at 4°C for 30 min, then centrifuged at 14 000 g for 15 min at 4°C. The supernatant was transferred to a new tube, a 1 : 100 dilution of glutathione sepharose-4B beads was added for 30 min at 4°C to preclear the lysate, and the sample was again centrifuged at 14 000 g for 15 min at 4°C. The supernatant was then assayed for protein content. Purified GST-fusion proteins linked to glutathione sepharose-4B beads were added to rat brain lysate and incubated for 1.5 h at 4°C with gentle shaking. The beads were centrifuged at 1800 g for 4 min and washed three times in 10 bed volumes of ice cold 1 × PBS, 1% Triton X-100. The samples were brought into sodium dodecyl sulfate (SDS) loading buffer, heated to 55°C for 5 min, centrifuged at 14 000 g and subjected to SDS–polyacrylamide gel electrophoresis. The resultant gels were either analyzed by Coomassie stain or transferred onto polyvinylidene difluoride (PVDF) membranes. Blots were blocked in 1 × Tris-buffered saline (TBS), 5% milk at 4°C for 16 h and incubated with 5 μg/mL anti-alpha tubulin monoclonal antibody (Calbiochem, San Diego, CA, USA). The blots were washed and incubated with goat anti-mouse second antibody conjugated to horse radish peroxidase (HRP) (Bio-Rad Laboratories, Hercules, CA, USA), washed and processed to film by electrochemiluminescence (ECL) (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Coimmunoprecipitation
Cells expressing stably transfected mGluR1a or mGluR7a were plated in poly-D-lysine-coated six-well plates (106 cells/well) and the cultures were maintained at 37°C in 5% CO2 for 5–7 days in Dulbecco's modified Eagle's medium (DMEM) supplemented with penicillin and streptomycin. For activation of the mGluRs, the cells were treated with 2 mM glutamate for 0, 1, 3, 10, 30 or 60 min The cells were rinsed twice in ice-cold 1 × HEN on ice, then scraped and collected into 1 mL of ice-cold 1 × HEN plus Complete™ protease inhibitors (Roche Molecular Biochemicals, Indianapolis, IN, USA). The cells were sonicated (4 Watts, 60 A) until the lysate was cleared, adjusted to 1% Triton X-100, and incubated at 4°C for 1.5 h with gentle shaking. Five per cent (v/v) of a 50% Protein A sepharose slurry was added for 1 h to preclear the lysate. The samples were centrifuged at 14 000 g for 15 min at 4°C and the supernatant collected to a new tube. For coimmunoprecipitation, 4 μg of anti-alpha tubulin was added to 500 μg of total cell lysate and incubated for 1.5 h at 4°C with shaking. The samples were further incubated with 50 μL of a 50% Protein A sepharose slurry for 1 h and centrifuged at 14 000 g for 5 min. The sepharose beads were washed three times in 1 × HEN, resuspended in 1 × SDS loading buffer, subjected to SDS–polyacrylamide gel electrophoresis and transferred to PVDF. The resulting membrane blots were incubated with anti-mGluR1a (Upstate Biotechnology, Lake Placid, NY, USA) or anti-mGluR7a (Bradley et al. 1996) and processed to film as described above.
Protein overlay experiments
Purified alpha/beta tubulin heterodimer (10 μg; Cytoskeleton Inc., Denver, CO, USA) and calmodulin (1 μg; Biogenesis, Kingston, NH, USA) as well as bovine serum albumin (10 μg; Sigma) were separated by SDS–polyacrylamide gel electrophoresis and the proteins were transferred onto PVDF membranes. The resulting blots were incubated in 1 × blocking buffer (20 mM HEPES pH 7.5, 100 mM KCl, 1 mM CaCl2, 5% BSA) plus Complete™ protease inhibitors (Roche) at room temperature for 1.5 h. They were then incubated in blocking buffer plus 10 μg/mL GST or GST-mGluR7a CT fusion protein for 1 h at room temperature, washed 4 times in blocking buffer without BSA, incubated with an anti-GST antibody (1 μg/mL; Stressgen, Collegeville, PA, USA) and processed to film as described above.
Results
Identification of a 55-kDa protein, alpha tubulin, that interacts with the CT domain of mGluR7
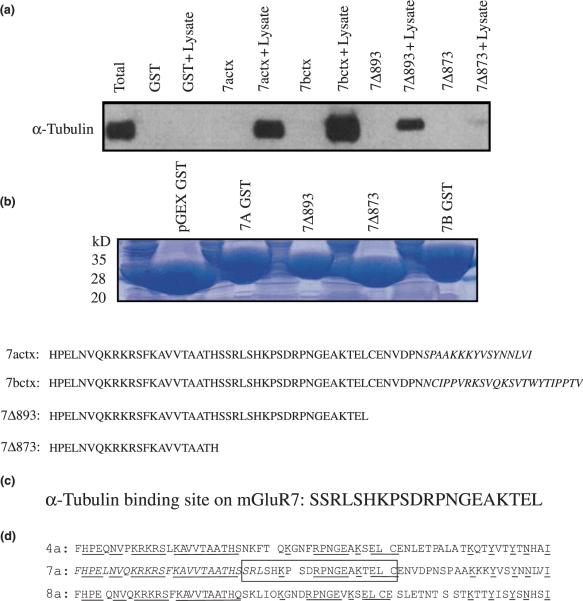
The goal of this study was to identify novel proteins that interact with the CT domain of presynaptic mGluRs. Thus the CT domains of mGluRs 2a and 7a were expressed as GST-fusion proteins and linked to GST-4B sepharose beads. These GST-fusion proteins were then incubated with total rat brain lysate, using GST alone as a negative control. The GST-fusion protein-sepharose bead complexes were washed extensively to remove proteins that bind non-specifically to GST, and specifically bound proteins were eluted by the addition of SDS sample buffer and separated by SDS–polyacrylamide gel electrophoresis. The resulting gels were stained with Coomassie blue and analyzed for proteins that appeared to uniquely interact with GST-mGluR fusion proteins. We found that the GST-mGluR7a fusion protein bound specifically to a prominent protein band of approximately 55 kDa (Fig. 1). The 55 kDa protein band was excised from the polyacrylamide gel and subjected to microchemical analysis. The isolated band was digested with Lys-C proteinase and the peptides were purified by HPLC. The peptide sequences were analyzed by MALDI-TOF mass spectrometry and revealed a peptide sequence `VGINYQPPTVVP' (Val-Gly-Iso-Asn-Try-Gln-Pro-Pro-Thr-Val-Val-Pro) that corresponds to amino acids 353–364 of alpha tubulin. Although the alpha and beta tubulin monomers are approximately 40% identical, this sequence is unique for alpha tubulin and shares virtually no overlap with beta tubulin. These data suggest that the cytoskeletal protein alpha tubulin binds specifically to the CT domain of mGluR7a.
Fig. 1.
Alpha tubulin binds to the GST-mGluR7a CT domain. An SDS-polyacrylamide gel stained with Coomassie blue shows that a 55-kDa rat brain protein associates with the GST-mGluR7a fusion protein. Neither GST alone nor the GST-mGluR2a fusion protein bound to any proteins detectable under these conditions. The protein band associating with the GST-mGluR7a fusion protein was excised from the gel and subjected to microchemical analysis. Peptide sequence analysis combined with MALDI-TOF mass spectrometry revealed a peptide sequence `Val-Gly-Iso-Asn-Try-Gln-Pro-Pro-Thr-Val-Val-Pro' that corresponds to the amino terminal sequence of alpha tubulin. This sequence is unique to alpha tubulin, does not share any identity with beta tubulin, and did not correspond to any other known protein in a BLAST search.
Alpha tubulin and mGluR7 coimmunoprecipitate
To ascertain whether the interaction between mGluR7 and alpha tubulin occurs in vivo, we performed coimmunoprecipitation experiments using lysates from either total rat brain or BHK cells stably expressing mGluR7a. Lysates were incubated with an antibody to alpha tubulin to immunoprecipitate alpha tubulin. Protein A sepharose beads selectively precipitated the protein complexes and these were subsequently separated by SDS–polyacrylamide gel electrophoresis and immunoblotted with anti-mGluR7a. The results show that alpha tubulin coimmunoprecipitates mGluR7a from rat brain lysates (Fig. 2a) as well as from mGluR7-expressing BHK cells (Fig. 2b) and support the hypothesis that these proteins specifically interact in vivo. While there appears to be more mGluR7a immunoprecipitated from the mGluR7-expressing BHK cells than from total rat brain lysates this is most likely due to differences in the expression of mGluR7 protein in each preparation. The coimmunoprecipitation experiments were also analyzed with antibodies to mGluR1a and mGluR2/3. The results from these experiments (data not shown) reveal that alpha tubulin coimmunoprecipitates with mGluR1a and may reflect a tubulin association via the interaction between beta tubulin and mGluR1a (Ciruela et al. 1999; Ciruela and McIlhinney 2001). However, alpha tubulin did not coimmunoprecipitate with mGluR2/3, consistent with the lack of alpha tubulin binding to the GST-mGluR2a CT fusion protein (Fig. 1).
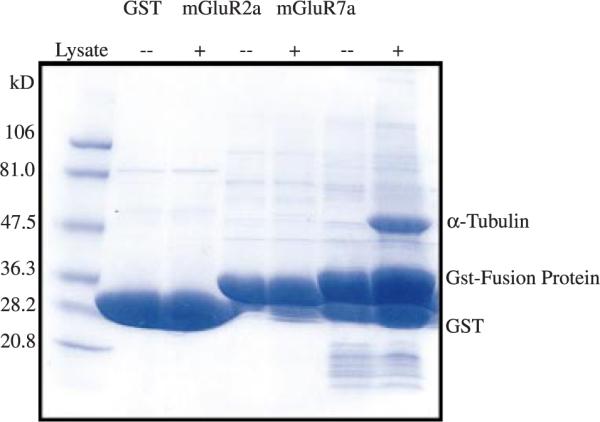
Fig. 2.
Alpha tubulin and mGluR7a receptors can be coimmunoprecipitated. An immunoblot showing that native mGluR7a receptors coimmunoprecipitate with alpha tubulin when lysates from either total rat brain (a) or mGluR7a-expressing BHK cells (b) are incubated with anti-alpha tubulin polyclonal antibody (Biogenesis) to immunoprecipitate alpha tubulin. The protein complexes were subjected to SDS gel electrophoresis and immunoblotted with anti-mGluR7a.
The mGluR7 CT domain binds directly to alpha tubulin
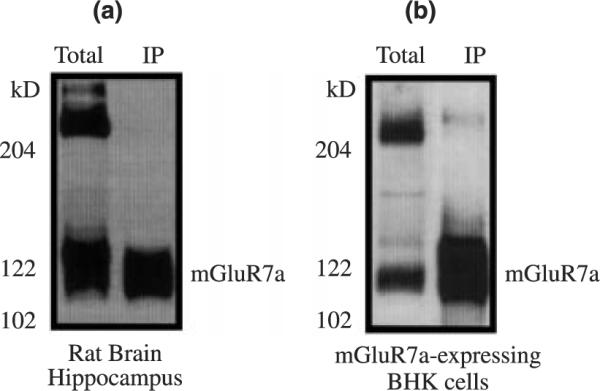
We have shown that the GST-mGluR7a CT fusion protein binds to alpha tubulin in pull-down assays using total rat brain homogenate, and that these proteins coimmunoprecipitate from rat brain and heterologous expression systems. However, this approach is limited in that alpha tubulin may interact with mGluR7 via an intermediate protein that links mGluR7a to alpha tubulin. Thus we used protein overlay experiments to determine if the alpha tubulin binding is direct. Purified tubulin was immobilized onto PDVF membrane and overlaid with GST alone or GST-mGluR7 fusion protein. Calmodulin, a previously reported mGluR7 binding partner (Nakajima et al. 1999; O'Connor et al. 1999), was used as a positive control while bovine serum albumin was used as a negative control. The results show that the GST-mGluR7a CT fusion protein binds specifically to tubulin (Tub, 10 μg; 60 kDa) and calmodulin (CaM, 1 μg; 17 kDa), but not to bovine serum albumin (BSA, 10 μg; 66 kDa) (Fig. 3a). Control experiments show that the GST fusion protein alone (1 μg; 27 kDa) is recognized by the anti-GST antibody but does not bind to tubulin or calmodulin (Fig. 3b). Thus these experiments prove that tubulin directly binds to the CT domain of mGluR7a and that this interaction is not dependent on an intermediate binding protein.
Fig. 3.
Alpha tubulin binds directly to mGluR7a. Protein overlay blots probed with anti-GST antibody show that the GST-mGluR7a CT fusion protein binds specifically to purified tubulin (Tub, 10 μg; 60 kDa) and calmodulin (CaM, 1 μg; 17 kDa), but not to bovine serum albumin (BSA, 10 μg; 66 kDa) (a). Control experiments show that the GST fusion protein (1 μg; 27 kDa) does not bind to tubulin or calmodulin, but is specifically recognized by the anti-GST antibody (b).
Identification of the alpha tubulin-binding region of mGluR7
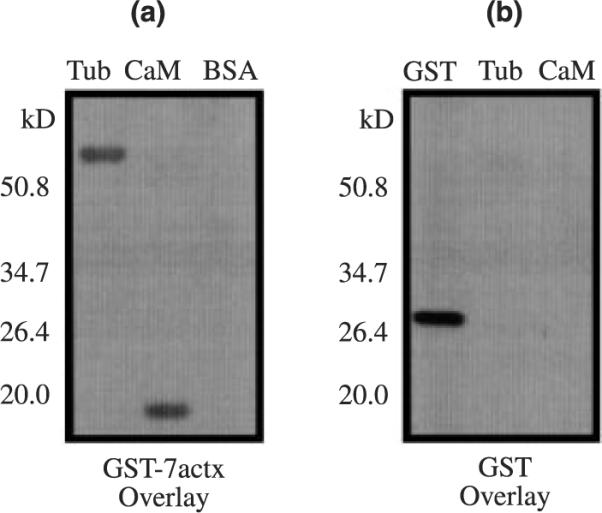
To determine the region of the mGluR7 CT domain that directly binds alpha tubulin, pull-down assays were used to study the binding of alpha tubulin to mGluR7b CT fusion protein and mGluR7 CT deletion mutant proteins. The GST-mGluR7b and GST-mGluR7 CT deletion mutant proteins were incubated with total rat brain homogenate and analyzed for their ability to bind to alpha tubulin. The results from these experiments show that both mGluR7a (residues 851–915) and mGluR7b (residues 851–923) bind to alpha tubulin. Thus, the terminal 15 or 23 amino acids, respectively, located in the extreme CT domains of the receptors are not involved in binding to alpha tubulin (Fig. 4a). The deletion mutant mGluR7Δ893 (expressing residues 851–892) also interacts with alpha tubulin, and the binding is not disrupted until the deletion mutant mGluR7Δ873 (expressing residues 851–872) is used. A Coomassie stained 4–20% SDS–polyacrylamide gel shows that each of the mGluR7-GST fusion proteins used in the pull-down assay were expressed in equal amounts (Fig. 4b). These results indicate that alpha tubulin binds to a region of the mGluR7 CT domain between amino acid residues 873 and 892 (Fig. 4c). The CT domain of the group-III mGluRs is depicted; the conserved amino acid residues are underlined and the alpha tubulin-binding site is boxed, the calmodulin/Gβγ-binding site and PKA/PKC phosphorylation sites are italicized (Fig. 4d).
Fig. 4.
Characterization of the alpha tubulin-binding site on mGluR7. An immunoblot shows that both mGluR7a and mGluR7b CT fusion proteins bind to tubulin and suggests that the splice variant regions (15 and 23 terminal amino acids, respectively) are not necessary for alpha tubulin-binding (a). Deletion mutagenesis experiments show that the deletion mutant mGluR7Δ893 (expressing residues 851–892) binds to alpha tubulin while the deletion mutant mGluR7Δ873 (expressing residues 851–872) does not (a). A Coomassie stained 4–20% SDS–polyacrylamide gel shows that each of the GST fusion proteins used in the pull-down assay were expressed in equal amounts (b). The results indicate that alpha tubulin binds to a region of the mGluR7 CT domain between amino acid residues 873 and 892 (c). The group-III CT domains are depicted; the conserved amino acid residues are underlined, the alpha tubulin-binding site is boxed, the calmodulin/Gβγ-binding site and PKA/PKC phosphorylation sites are italicized (d).
Regulation of alpha tubulin binding by mGluR7 activation
To determine whether the interaction between mGluR7 and alpha tubulin is dynamically regulated, we analyzed the protein interaction at various times after receptor activation. mGluR-expressing BHK cells were grown to 80% confluency, and the cells were incubated in the presence of 2 mM glutamate for 0–60 min. The cells were collected, lysed, and immunoprecipitated with antibody to alpha tubulin. The immunoprecipitation blots were incubated with antibodies to mGluR7a as well as to mGluR1a as earlier coimmunoprecipitation experiments showed that mGluR1a also binds alpha tubulin. The results from these experiments show that alpha tubulin dissociates from mGluR7a within one minute of receptor activation (Fig. 5a). Densitometry analysis of the immunoblots using NIH Image 1.62 measure a 58% decrease in the mGluR7a association with alpha tubulin within one minute and a maximal decrease in binding (72%) at 10 min of glutamate application. However, there is no significant decrease in the binding of mGluR1a and alpha tubulin (Fig. 5b). Taken together, these results suggest that there is a specific and dynamic regulation of the interaction between alpha tubulin and mGluR7.
Fig. 5.
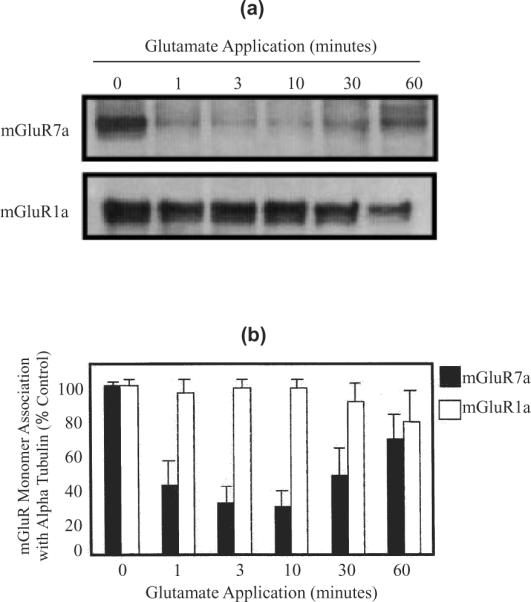
mGluR7a receptor activation regulates the alpha tubulin interaction. Immunoblots show that the monomeric form of mGluR7a receptors coimmunoprecipitate with alpha tubulin when mGluR7a-expressing BHK cell lysate is incubated with anti-alpha tubulin polyclonal antibody. However, mGluR7a binding to alpha tubulin is significantly reduced when the cells are incubated with 2 mM glutamate (a). In contrast, identical blots probed with an anti-mGluR1a antibody reveal that the regulation of alpha tubulin-binding is specific for mGluR7a. Bar graph shows the densitometry analysis of representative immunoblots using NIH Image 1.62. There is a 58% decrease in the mGluR7a association with alpha tubulin within one minute and a maximal decrease in binding (72%) at 10 min of glutamate application. However, there is no significant decrease in the binding of mGluR1a and alpha tubulin (b). These results suggest that there is a specific and dynamic regulation of the interaction between alpha tubulin and mGluR7. n = 3 for each experiment, error bars show the standard deviation.
Discussion
Previous studies have identified calmodulin and PICK1 as proteins that interact with the CT domain of mGluR7 (for review see Dev et al. 2001). Our results demonstrate that in addition to these proteins, alpha tubulin binds to the CT domain of mGluR7. The mGluR7a interaction with alpha tubulin was originally detected by Coomassie stain of an SDS–polyacrylamide gel analyzing proteins that interacted with the GST-mGluR7a CT fusion protein. Although PICK1 and calmodulin associate with mGluR7a, they are not expressed as abundantly as tubulin and thus were not readily detected by this method. However, in protein overlay experiments the GST-mGluR7a CT fusion protein bound to both purified tubulin and calmodulin, but not to bovine serum albumin, indicating that the binding of mGluR7 to these regulatory molecules is direct. Molecular analysis revealed that the binding of mGluR7 to alpha tubulin occurs in a region located between the calmodulin and PICK1 binding domains, slightly overlaps with the calmodulin binding domain, and also overlaps with a central region that has been identified as necessary for axonal targeting (Stowell and Craig 1999). Coimmunoprecipitation studies show that mGluR7 associates with alpha tubulin both in native brain tissue and in BHK cells stably transfected with mGluR7a. Interestingly, while both the monomer and dimer forms of mGluR7 are detected in total rat brain or mGluR7a-BHK cellular lysate, only the monomer form of mGluR7 is coimmunoprecipitated with alpha tubulin. This may suggest that alpha tubulin specifically interacts with the monomeric form of mGluR7, as the immunoprecipitation protocol does not disrupt the detection of both monomer and dimer forms of mGluR7 when it is immunoprecipitated. Finally, the alpha tubulin/mGluR7 interaction is dynamically regulated by receptor activation. This may be a potential mechanism to control the access of regulatory molecules such as calmodulin, PKC or PICK1 to the CT domain of the receptor.
Activation of mGluRs is known to mediate changes in the cytoskeletal architecture. For example, stimulation of mGluR1 causes an increase in actin stress fiber formation that is prevented by calmodulin inhibitors, suggesting that calmodulin signaling plays an important role in cellular function of mGluR1 (Shinohara et al. 2001). In addition, activation of mGluRs can induce a rapid and transient translocation of tubulin from the cytosol to the plasma membrane (Ciruela and McIlhinney 2001) and also can inhibit microtubule formation (Huang and Hampson 2000). Thus there may be a role for the interaction between mGluR7 and alpha tubulin in regulating cytoskeletal changes in the presynaptic axon terminal mediated by activation of presynaptic mGluRs. Several lines of evidence suggest an important role for tubulin in regulation of presynaptic terminals. Early reports showed that tubulin and actin are major components of presynaptic dense projections (Mushynski et al. 1978). In addition, Gozes and Littauer (1979) showed that alpha tubulin is significantly enriched in purified synaptosomes relative to whole brain, and that the ratio of alpha tubulin to beta tubulin is 1.6 : 1 in synaptosomes, as compared with a ratio of 1 : 1 in whole brain and in almost all other tissues. These data reveal that while alpha tubulin is an abundant protein throughout the brain, it is particularly concentrated in presynaptic regions. Ultrastructural studies of synaptosomes reveal the presence of a microtubular coil in the presynaptic nerve terminals, while immunocytochemical studies demonstrate the presence of alpha and beta tubulin subunits in this region (Cumming et al. 1983b) as well as alpha tubulin staining in axonal subpopulations (Cumming et al. 1983a). In addition, G protein-coupled receptor kinases bind and phosphorylate tubulin and may provide an important regulatory link between the cytoskeleton and G protein-coupled receptors (Carman et al. 1998; Haga et al. 1998; Pitcher et al. 1998).
There is growing evidence that membrane-associated tubulin may influence ligand–receptor interaction, receptor × G protein coupling, and G protein-effector coupling for many G protein-coupled receptors (for review see Ravindra 1997). Tubulin binds specifically to the G proteins Gs and Gi1 and leads to the regulation of G protein-activated signaling molecules via a GTP transfer (Popova et al. 1994, 1997, 2000). Tubulin can also contribute to cell signaling through physical forces; for example, GTP binding to tubulin may lead to conformational changes in receptors or G proteins which may in turn regulate agonist binding to the receptor. Finally, regulation of receptor interactions with tubulin may provide a link between the cytoskeleton and the modulation of signal transduction events. For example, changes in dendritic spine morphology are observed following glutamate stimulation of neurons, conceivably due to direct and indirect interactions between cytoskeletal proteins and glutamate receptors on postsynaptic membranes (for review see van Rossum and Hanisch 1999).
It has previously been reported that mGluR1 can associate directly with beta tubulin (Ciruela et al. 1999; Ciruela and McIlhinney 2001). These studies also show that mGluR2, mGluR3 and mGluR4 do not detectably associate with beta tubulin. These findings are consistent with our pull-down experiments, which demonstrated a lack of alpha tubulin interaction with the CT domain of mGluR2. Direct association with tubulin therefore is a property of mGluR1 and mGluR7 but is clearly not a property of all metabotropic glutamate receptors. The CT of mGluR1 and mGluR7 show no obvious sequence similarity, suggesting that these two receptors bind to beta and alpha tubulin, respectively, in completely distinct manners. This possibility is supported by the fact that alpha tubulins share only 36–42% amino acid identity with beta tubulins (for a review see Oakley 2000). Thus based on their divergent primary sequences they are likely to be able to preferentially and independently associate with other proteins. In addition, studies show that there is differential binding of the tubulin monomers to other proteins. For example, the interaction between tubulin and elongation factor 1a, a highly conserved protein involved in translation that is also a microtubule-associated protein, revealed that beta tubulin but not alpha tubulin interacts with EF-1a in GST fusion pull-down assays (Nakazawa et al. 1999). The idea that the mGluR7/tubulin interaction is fundamentally different from the mGluR1/tubulin interaction also gains support from our observations that the mGluR7/tubulin interaction is profoundly inhibited by agonist activation of mGluR7, while the mGluR1/tubulin interaction is not significantly altered by agonist stimulation. The independent regulation of mGluR/alpha tubulin interactions may lie in the different roles that they serve on the pre- and postsynaptic membranes. For example, mGluR1 is rapidly internalized (within 10 min) upon receptor activation, a mechanism of receptor regulation that may serve to protect neurons during an excessive or prolonged presence of agonist (Doherty et al. 1999). More recent studies have shown that agonist activation of mGluR1 in BHK cells leads to the clustering of the receptor and a reorganization of beta tubulin, with some of the clusters corresponding to the intracellular accumulation of tubulin suggesting that the two proteins are closely associated at the plasma membrane (Ciruela and McIlhinney 2001). Interestingly, agonist activation of the receptor resulted in a small but transient increase in the membrane association of beta tubulin similar to that reported by Popova and Rasenick (2000) for agonist activation of muscarinic acetylcholine receptors. The effect of mGluR1 activation on its direct interaction with beta tubulin, however, has not been addressed.
The CT domain within the group-III mGluRs is highly conserved, but is significantly different from the CT domains of the group-I and -II mGluRs. Thus while the alpha tubulin-binding site of mGluR7 is conserved between the group-III mGluRs, there is virtually no homology with CT domain of group-I and -II mGluRs. Within the CT domain of the group-III mGluRs share 52% amino acid identity overall however, the region most proximal to the seventh transmembrane domain is 87% conserved, the middle region containing the alpha tubulin-binding site is 45% conserved, and the region most distal to seventh transmembrane domain is the most divergent with only 30% conserved amino acids. (Fig. 4d). The alpha tubulin-binding site is also in close proximity to the calmodulin binding domain (O'Connor et al. 1999), the Gβγ binding domain (El Far et al. 2001), as well as the protein kinase A (Cai et al. 2001) and protein kinase C phosphorylation sites (Airas et al. 2001). Interestingly, calcium activates an endogenous tubulin kinase system in presynaptic nerve terminal fractions prepared from rat brain, and this kinase system is modulated by calmodulin (Burke and DeLorenzo 1981; Burke and DeLorenzo 1982). Calmodulin-regulated phosphorylation of tubulin causes marked alterations in tubulin, and tubulin kinase may play a major role in converting calcium signals into motor forces at the synapse (DeLorenzo 1982). Interestingly, calmodulin binding to the mGluR7 CT domain inhibits phosphorylation by PKC (Nakajima et al. 1999). Furthermore, calcium/calmodulin binding is required to release G protein βγ subunits from the CT domain of group-III mGluRs and the subsequent G protein-mediated modulation of mGluR7-induced currents (O'Connor et al. 1999). Taken together these results suggest that the interactions between mGluR7 and alpha tubulin, as well as regulatory molecules such as calmodulin, PKC and PICK1 may act in concert to modulate cytoskeletal components that in turn lead to morphological changes in the presynaptic membrane in response to receptor activation.
Acknowledgements
We thank Ms. Nancy Fox-Ciliax for her technical assistance with cell culture. This work was supported by the NIMH grant R01-521635 and the Alzheimer's Association grant PRG98021 (JAS), and the NIH grants NS313373, NS34876, NS36755 (PJC).
Abbreviations used
- CT
carboxy terminal
- DCG-IV
(2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine
- DHPG
3,5-dihydroxyphenylglycine
- DMEM
Dulbecco's modified Eagle's medium
- ECL
enhanced chemiluminescence
- GPCR
G protein-coupled receptor
- GST
glutathione S-transferase
- HRP
horse radish peroxidase
- IPTG
isopropyl β-D-thiogalactoside
- mGluR
metabotropic glutamate receptors
- PBS
phosphate-buffered saline
- PKC
protein kinase C
- PVDF
polyvinylidene difluoride
- S-AP4
S-4-phosphono-2-aminobutyric acid
- SDS
sodium dodecyl sulfate
- TBS
Tris-buffered saline
References
- Airas JM, Betz H, El Far O. PKC phosphorylation of a conserved serine residue in the C-terminus of group III metabotropic glutamate receptors inhibits calmodulin binding. FEBS Lett. 2001;494:60–63. doi: 10.1016/s0014-5793(01)02311-0. [DOI] [PubMed] [Google Scholar]
- Boudin H, Doan A, Xia J, Shigemoto R, Huganir RL, Worley P, Craig AM. Presynaptic clustering of mGluR7a requires the PICK1 PDZ domain-binding site. Neuron. 2000;28:485–497. doi: 10.1016/s0896-6273(00)00127-6. [DOI] [PubMed] [Google Scholar]
- Bradley SR, Levey AI, Hersch SM, Conn PJ. Immunocytochemical localization of group III metabotropic glutamate receptors in the hippocampus with subtype-specific antibodies. J. Neurosci. 1996;16:2044–2056. doi: 10.1523/JNEUROSCI.16-06-02044.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Burke BE, DeLorenzo RJ. Ca2+- and calmodulin-stimulated endogenous phosphorylation of neurotubulin. Proc. Natl Acad. Sci. USA. 1981;78:991–995. doi: 10.1073/pnas.78.2.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke BE, DeLorenzo RJ. Ca2+ and calmodulin-regulated endogenous tubulin kinase activity in presynaptic nerve terminal preparations. Brain Res. 1982;236:393–415. doi: 10.1016/0006-8993(82)90724-7. [DOI] [PubMed] [Google Scholar]
- Cai Z, Saugstad JA, Sorensen SD, Ciombor KJ, Zhang C, Schaffhauser H, Hubalek F, Pohl J, Duvoisin RM, Conn PJ. Cyclic AMP-dependent protein kinase phosphorylates group III metabotropic glutamate receptors and inhibits their function as presynaptic receptors. J. Neurochem. 2001;78:756–766. doi: 10.1046/j.1471-4159.2001.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman CV, Som T, Kim CM, Benovic JL. Binding and phosphorylation of tubulin by G protein-coupled receptor kinases. J. Biol. Chem. 1998;273:20308–20316. doi: 10.1074/jbc.273.32.20308. [DOI] [PubMed] [Google Scholar]
- Ciruela F, McIlhinney RA. Metabotropic glutamate receptor type 1alpha and tubulin assemble into dynamic interacting complexes. J. Neurochem. 2001;76:750–757. doi: 10.1046/j.1471-4159.2001.00099.x. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Robbins MJ, Willis AC, McIlhinney RA. Interactions of the C terminus of metabotropic glutamate receptor type 1alpha with rat brain proteins: evidence for a direct interaction with tubulin. J. Neurochem. 1999;72:346–354. [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Cumming R, Burgoyne RD, Lytton NA. Axonal subpopulations in the central nervous system demonstrated using monoclonal antibodies against alpha-tubulin. Eur. J. Cell Biol. 1983a;31:241–248. [PubMed] [Google Scholar]
- Cumming R, Burgoyne RD, Lytton NA, Gray EG. Immunocytochemical evidence for tubulin in the presynaptic terminal of synaptosomes. Neurosci. Lett. 1983b;37:215–220. doi: 10.1016/0304-3940(83)90433-0. [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ. Calmodulin in neurotransmitter release and synaptic function. Fed. Proc. 1982;41:2265–2272. [PubMed] [Google Scholar]
- Dev KK, Nakajima Y, Kitano J, Braithwaite SP, Henley JM, Nakanishi S. PICK1 interacts with and regulates PKC phosphorylation of mGLUR7. J. Neurosci. 2000;20:7252–7257. doi: 10.1523/JNEUROSCI.20-19-07252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev KK, Nakanishi S, Henley JM. Regulation of mGlu(7) receptors by proteins that interact with the intracellular C-terminus. Trends Pharmacol. Sci. 2001;22:355–361. doi: 10.1016/s0165-6147(00)01684-9. [DOI] [PubMed] [Google Scholar]
- Doherty AJ, Coutinho V, Collingridge GL, Henley JM. Rapid internalization and surface expression of a functional, fluorescently tagged G protein-couple glutamate receptor. Biochem. J. 1999;341:415–422. [PMC free article] [PubMed] [Google Scholar]
- El Far O, Airas J, Wischmeyer E, Nehring RB, Karschin A, Betz H. Interaction of the C-terminal tail region of the metabotropic glutamate receptor 7 with the protein kinase C substrate PICK1. Eur. J. Neurosci. 2000;12:4215–4221. doi: 10.1046/j.1460-9568.2000.01309.x. [DOI] [PubMed] [Google Scholar]
- El Far O, Bofill-Cardona E, Airas JM, O'Connor V, Boehm S, Freissmuth M, Nanoff C, Betz H. Mapping of calmodulin and Gbetagamma binding domains within the C-terminal region of the metabotropic glutamate receptor 7A. J. Biol. Chem. 2001;276:30662–30669. doi: 10.1074/jbc.M102573200. [DOI] [PubMed] [Google Scholar]
- Gozes I, Littauer UZ. The alpha-subunit of tubulin is preferentially associated with brain presynaptic membrane. FEBS Lett. 1979;99:86–90. doi: 10.1016/0014-5793(79)80255-0. [DOI] [PubMed] [Google Scholar]
- Haga K, Ogawa H, Haga T, Murofushi H. GTP-binding-protein-coupled receptor kinase 2 (GRK2) binds and phosphorylates tubulin. Eur. J. Biochem. 1998;255:363–368. doi: 10.1046/j.1432-1327.1998.2550363.x. [DOI] [PubMed] [Google Scholar]
- Huang XP, Hampson DR. Inhibition of microtubule formation by metabotropic glutamate receptors. J. Neurochem. 2000;74:104–113. doi: 10.1046/j.1471-4159.2000.0740104.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Nash SR, Nishimune A, Neki A, Kaneko S, Nakanishi S. Competitive interaction of seven in absentia homolog-1A and Ca2+/calmodulin with the cytoplasmic tail of group 1 metabotropic glutamate receptors. Genes Cells. 1999;4:381–390. doi: 10.1046/j.1365-2443.1999.00269.x. [DOI] [PubMed] [Google Scholar]
- Minakami R, Jinnai N, Sugiyama H. Phosphorylation and calmodulin binding of the metabotropic glutamate receptor subtype 5 (mGluR5) are antagonistic in vitro. J. Biol. Chem. 1997;272:20291–20298. doi: 10.1074/jbc.272.32.20291. [DOI] [PubMed] [Google Scholar]
- Mushynski WE, Glen S, Therien HM. Actin-like and tubulin-like proteins in synaptic junctional complexes. Can. J. Biochem. 1978;56:820–830. doi: 10.1139/o78-125. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Yamamoto T, Nakayama T, Nakanishi S. A relationship between protein kinase C phosphorylation and calmodulin binding to the metabotropic glutamate receptor subtype 7. J. Biol. Chem. 1999;274:27573–27577. doi: 10.1074/jbc.274.39.27573. [DOI] [PubMed] [Google Scholar]
- Nakazawa M, Moreira D, Laurent J, Le Guyader H, Fukami Y, Ito K. Biochemical analysis of the interaction between elongation factor 1alpha and alpha/beta-tubulins from a ciliate Tetrahymena pyriformis. FEBS Lett. 1999;453:29–34. doi: 10.1016/s0014-5793(99)00692-4. [DOI] [PubMed] [Google Scholar]
- O'Connor V, El Far O, Bofill-Cardona E, Nanoff C, Freissmuth M, Karschin A, Airas JM, Betz H, Boehm S. Calmodulin dependence of presynaptic metabotropic glutamate receptor signaling. Science. 1999;286:1180–1184. doi: 10.1126/science.286.5442.1180. [DOI] [PubMed] [Google Scholar]
- Oakley BR. An abundance of tubulins. Trends Cell Biol. 2000;10:537–542. doi: 10.1016/s0962-8924(00)01857-2. [DOI] [PubMed] [Google Scholar]
- Pin JP, Joly C, Heinemann SF, Bockaert J. Domains involved in the specificity of G protein activation in phospholipase C-coupled metabotropic glutamate receptors. EMBO J. 1994;13:342–348. doi: 10.1002/j.1460-2075.1994.tb06267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, De Colle C, Bessis AS, Acher F. New perspectives for the development of selective metabotropic glutamate receptor ligands. Eur. J. Pharmacol. 1999;375:277–294. doi: 10.1016/s0014-2999(99)00258-7. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Hall RA, Daaka Y, Zhang J, Ferguson SS, Hester S, Miller S, Caron MG, Lefkowitz RJ, Barak LS. The G protein-coupled receptor kinase 2 is a microtubule-associated protein kinase that phosphorylates tubulin. J. Biol. Chem. 1998;273:12316–12324. doi: 10.1074/jbc.273.20.12316. [DOI] [PubMed] [Google Scholar]
- Popova J, Rasenick MM. Muscarinic receptor activation promotes the membrane association of tubulin for the regulation of Gq-mediated phospholipase C1 signaling. J. Neurosci. 2000;20:2774–2782. doi: 10.1523/JNEUROSCI.20-08-02774.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova JS, Johnson GL, Rasenick MM. Chimeric G alpha s/G alpha i2 proteins define domains on G alpha s that interact with tubulin for beta-adrenergic activation of adenylyl cyclase. J. Biol. Chem. 1994;269:21748–21754. [PubMed] [Google Scholar]
- Popova JS, Garrison JC, Rhee SG, Rasenick MM. Tubulin, Gq, and phosphatidylinositol 4,5-bisphosphate interact to regulate phospholipase Cbeta1 signaling. J. Biol. Chem. 1997;272:6760–6765. doi: 10.1074/jbc.272.10.6760. [DOI] [PubMed] [Google Scholar]
- Ravindra R. Is signal transduction modulated by an interaction between heterotrimeric G-proteins and tubulin? Endocrine. 1997;7:127–143. doi: 10.1007/BF02778134. [DOI] [PubMed] [Google Scholar]
- van Rossum D, Hanisch UK. Cytoskeletal dynamics in dendritic spines: direct modulation by glutamate receptors? Trends Neurosci. 1999;22:290–295. doi: 10.1016/s0166-2236(99)01404-6. [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Nakajima Y, Nakanishi S. Glutamate induces focal adhesion kinase tyrosine phosphorylation and actin rearrangement in heterologous mGluR1-expressing CHO cells via calcium/calmodulin signaling. J. Neurochem. 2001;78:365–373. doi: 10.1046/j.1471-4159.2001.00415.x. [DOI] [PubMed] [Google Scholar]
- Stowell JN, Craig AM. Axon/dendrite targeting of metabotropic glutamate receptors by their cytoplasmic carboxy-terminal domains. Neuron. 1999;22:525–536. doi: 10.1016/s0896-6273(00)80707-2. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]