Abstract
The report by Perico et al that a dual arginine vasopressin (AVP) V2 and V1a receptor antagonist lowers blood pressure, proteinuria and glomerulosclerosis in 5/6 nephrectomized rats points to a potential therapeutic value of AVP receptor antagonists in the treatment of chronic kidney disease (CKD). A large body of evidence suggests that AVP likely contributes to CKD progression by its V2 and V1a-mediated effects on renal hemodynamics, blood pressure, and mesangial and/or epithelial cells. The relative contribution of V2 and V1a receptors to CKD progression and potential usefulness of V2 and V1a receptor antagonists for its treatment are not well defined.
In this issue of Kidney International, Perico et al report that treatment with RWJ-676070, a dual V1a and V2 arginine vasopressin (AVP) receptor antagonist (V1/V2RA), initiated 3 weeks after 5/6 nephrectomy, significantly lowers blood pressure, proteinuria and glomerulosclerosis in rats.1 Combined treatment with RWJ-676070 and an ACE inhibitor (ACEI) or an angiotensin II type 1 receptor blocker (ARB) has effects on proteinuria, renal function and structure that are numerically, but not significantly higher than those of an ACEI or an ARB alone. The authors suggest that non-peptide AVP receptor antagonists could be renoprotective in patients with proteinuric chronic kidney disease (CKD).
The identification of a common pathway of progressive renal damage, regardless of the initiating injury, achieved by research in animal models of non-diabetic (i.e. five-sixths nephrectomy) and diabetic CKD (i.e. streptozotocin-induced diabetes mellitus) has been one of the major achievements in Nephrology.2 This pathway includes reductions in afferent and to a lesser degree efferent arteriolar tone; increases in single nephron perfusion, glomerular capillary hydraulic pressure, and filtration rate; resetting of tubuloglomerular feedback allowing persistent glomerular hyperfiltration, and failure of autoregulation exposing glomerular capillaries to systemic hypertension. The superiority of ACEI and ARB in treating glomerular capillary hypertension, compared to antihypertensive agents that mainly dilate preglomerular vessels or activate the renin-angiotensin system, has established the central role of angiotensin II in this pathway. In addition, angiotensin II exerts non-hemodynamic effects on vascular smooth muscle, endothelial and mesangial cells, podocytes, tubular epithelial and interstitial cells that contribute to CKD progression.
Although underecognized, a large body of evidence suggests that AVP contributes to non-diabetic and diabetic CKD progression. Plasma AVP levels are increased in animal models and in patients with non-diabetic CKD, in animal models of streptozotocin-induced and genetic diabetes mellitus and in patients with type I and type II diabetes mellitus.3, 4 Plasma levels of copeptin, a surrogate marker derived from the C-terminal portion of the AVP precursor, are inversely correlated with GFR5. Suppression of AVP by increasing water ingestion reduces blood pressure, proteinuria, renal hypertrophy, glomerulosclerosis, and tubulointerstitial fibrosis in 5/6 nephrectomized rats.6, 7 Compensatory renal hypertrophy and CKD progression following 5/6 nephrectomy are attenuated in Brattleboro rats which cannot secrete AVP, although a study of shorter duration (3 versus 13 weeks) did not detect this effect.8, 9 Brattleboro rats with diabetes mellitus exhibit no or markedly reduced glomerular hyperfiltration, albuminuria, and renal hypertrophy compared to wild-type controls.4 These observations seem to contradict a post hoc analysis of the Modification of Diet in Renal Disease (MDRD) study where an association between high urine volumes and rates of GFR decline where thought to reflect a deleterious effect of increased water intake on disease progression.10 However, it is impossible to conclude from this analysis whether high urine flow rate was a cause or a consequence of GFR decline or whether another independent factor influenced the two variables simultaneously. Furthermore, this association is not unexpected since defective urine concentrating capacity is a manifestation of CKD. In the study by Perico et al, urine output more than doubled in the 5/6 nephrectomized compared to control rats and did not increase further following V1a/V2RA administration, possibly due to AVP resistant downregulation of aquaporin-2 and -3 as well as downregulation of aquaporin-1.11
Like angiotensin II, AVP has effects on glomerular hemodynamics, arterial blood pressure, and non-hemodynamic renal mechanisms. AVP acts on three G protein-coupled receptors: V2 (cAMP second messenger) and V1a and V1b (also called V3) (calcium second messenger). In the kidney, V2 receptors are found in the medullary thick ascending limb of Henle (TAL), macula densa, connecting tubule, and cortical and medullary collecting duct, and to a lesser extent in cortical TAL and distal convoluted tubule (Figure 1).12 Contrary to previous belief, a recent comparative study has shown similar patterns of V2 receptor expression in rat, mouse and human TAL and collecting duct and of AVP-dependent NaK2Cl cotransporter phosphorylation in TAL cells from rats and rabbits.12 V1a receptors are found in the renal vasculature from the interlobular arteries to the efferent arterioles and vasa recta, mesangial cells, macula densa, collecting duct principal and alpha intercalated cells13, 14. The localization and function of V1b receptors in the kidney, possibly in the inner medullary collecting duct, are not well characterized.
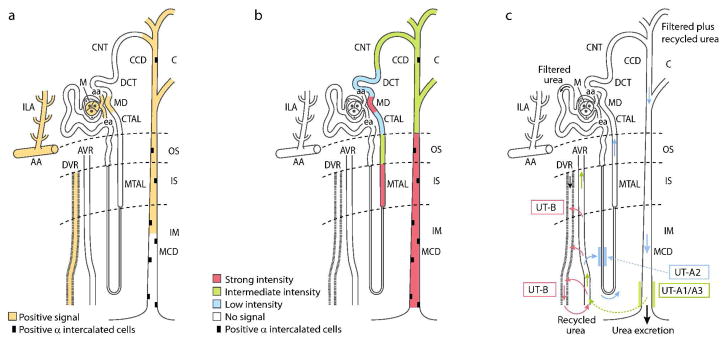
Figure 1.
A) Segmental distribution of AVP V1a receptor (adapted from references 13, 14 and 31). AVP, acting on V1a receptors in the macula densa, regulates renin secretion; acting on V1a receptors in the vasa recta, reduces blood flow to the inner medulla and minimizes solute escape from the medullary interstitium; and, acting on V1a receptors on the luminal side of collecting duct principal cells, stimulates synthesis of prostaglandins that attenuate V2-mediated antidiuretic action and inhibit sodium transport. B) Segmental distribution of AVP V2 receptor (adapted from reference 12). AVP acting on V2 receptors contribute to urinary concentration by inserting AQP-2 into the apical cell membrane of collecting duct principal cells within minutes and up-regulating AQP-2 gene expression on a longer-term; activating UT-A1 and UT-A3 in the terminal part of the inner medullary collecting duct; stimulating ENaC sodium transport in cortical and outer medullary collecting ducts, and increasing Na-K-2Cl cotransporter expression and sodium reabsorption in TAL. C) Vascular and tubular routes of urea recycling within the kidney. Only a long loop of Henle is depicted for simplicity. Urea delivery to the inner medulla and transit in ascending vasa recta are shown in green. The pathways allowing urea to return to the inner medulla are indicated by red arrows for the vascular route and by blue arrows for the tubular route (adapted from reference 38).38 C, cortex; OS, outer stripe of the outer medulla; IS, inner stripe of the outer medulla; IM, inner medulla; AA, arcuate artery; ILA, interlobular artery; aa, afferent arteriole; ea, efferent arteriole; M, mesangium; MTAL, medullary thick ascending limb of Henle; CTAL, cortical thick ascending limb of Henle; MD, macula densa; DCT, distal convoluted tubule; CNT, connecting tubule; CCD, cortical collecting duct; MCD, medullary collecting duct; DVR, descending vasa recta; AVR, ascending vasa recta; UT-A1/3, vasopressin-regulated urea transporters A1/3; UT-B, urea transporter B; UT-A2, urea transporter A-2.
Although RWJ-676070, the compound used by Perico et al, is a dual V1a/V2RA, it has higher affinity for rat V2 than for V1a receptors (Ki 16 and 86 nM, respectively).15 Previous studies had shown that V2RA or V1aRA or both in combination have a renoprotective effect suggesting that both receptors contribute to CKD progression (Table 1). Nevertheless, the specificity of these antagonists is not absolute. One study showing that the V2 receptor agonist 1-deamino-8-D-arginine AVP (DDAVP), but not AVP, worsens proteinuria and renal insufficiency in 5/6 nephrectomized Brattleboro rats even raised the possibility that V1a effects of AVP could afford a relative protection against deleterious V2 effects. Therefore the relative contribution of V2 and V1a receptors to and potential usefulness of V2 and V1a receptor antagonists for the treatment of CKD remain ill-defined.
Table 1.
Studies ascertaining effects of V2 and/or V1a agonism or antagonism on CKD progression
| Study * | Drug | Class | Animal model | Treatment duration | Outcome |
|---|---|---|---|---|---|
| Okada 1994 | OPC-21268 or OPC-31260 or both | V1aRA, V2RA | DOCA-salt and adryamycin treated rats | 2–6 wks | All reduced BP rise, V1aRA/V2RA ameliorated histology |
| Okada 1995 | OPC-21268 or OPC-31260 or both | V1aRA, V2RA | Uninephrectomized DOCA-salt hypertensive rats | 6–10 wks (starting at surgery or 4 wks later) | V2RA or V1aRA/V2RA reduced BP rise |
| Okada 1995 | OPC-21268 or OPC-31260 or both | V1aRA, V2RA | 5/6 nephrectomized spontaneously hypertensive rats | V1aRA or V1aRA/V2RA reduced BP rise, proteinuria and arteriolosclerosis | |
| Okada 1996 | OPC-21268 or OPC-31260 or both | V1aRA, V2RA | Adryamycin treated rats | 5–7 wks | All reduced proteinuria and histological alterations |
| Kurihara 1996 | OPC-21268 | V1aRA | Uninephrectomized spontaneously hypercholesterolemic rats | 9 wks | V1aRA reduced BP and glomerular sclerosis, and improved renal function |
| Bouby 1999 | DDAVP | V2R agonist | Brattleboro 5/6 nephrectomized rats | 13 weeks | DDAVP increased proteinuria and worsened renal function |
| Naito 2001 | DDAVP and VP-343 | V2R agonist, V2RA | Sprague-Dawley rats | 15 days (VP-343 given only on days 5– 15) | DDAVP induced and VP- 343 prevented hypertrophy, tubular dilatation, interstitial infiltration |
| Fernandes 2002 | DDAVP or SR- 121463 | V2R agonist, V2RA | Uninephrectomized DOCA-salt hypertensive rats | 8 weeks | DDAVP worsened hypertension, albuminuria and histology |
| Bardoux 2003 | SR-121463 | V2RA | Streptozotocin-induced diabetes mellitus | 9 wks | V2RA prevented rise in albuminuria |
| Windt 2006 | YM-218 | V1aRA | 5/6 nephrectomized rats | 8 wks (starting 2 wks post surgery) | V1RA reduced proteinuria and glomerular sclerosis |
| Windt 2006 | YM-218 | V1aRA | 5/6 nephrectomized rats | 4 wks (starting 6 wks post surgery) | No effect |
| Okada 2009 | Tolvaptan | V2RA | Puromycin aminonucleoside nephrosis | 10 days | V2RA reduced proteinuria and kidney weight and improved renal function |
| Perico 2009 | RWJ-676070 | V1a/V2 RA | 5/6 nephrectomized rats | 39 days (starting 3 wks after surgery) | V1a/V2 RA reduced BP rise, proteinuria and glomerular sclerosis |
References available on request
Strong experimental evidence supports the hypothesis that the V2 mediated urine concentrating activity is mainly responsible for the renal hemodynamic effects of AVP (Figure 1).16, 17 A positive correlation between urine osmolality and GFR was observed (only when urine osmolality was equal or above that of plasma) in conscious rats when urine concentrating activity was increased by a constant infusion of DDAVP or reduced by increasing water intake. This is likely due to suppression of tubuloglomerular feedback possibly caused by enhanced urea recycling and/or sodium chloride reabsorption in the TAL lowering the sodium concentration at the macula densa. These effects that are more marked in juxtamedullary nephrons may be responsible for functional and structural differences between superficial and deep nephrons.18 Urine concentrating activity and GFR (or renal blood flow, RBF) are also correlated in healthy human volunteers during low and high hydration.19 AVP administration to water loaded healthy volunteers induces parallel increases in creatinine clearance and urine osmolality.20 Conversely, administration of V2RA after dehydration induces a fall in creatinine clearance. Compelling arguments have been made linking increased levels of AVP and urine concentrating activity to the increased risk for CKD progression associated with high protein intake, male gender, and black race.21–23
Under physiological conditions, the renal vasculature and total RBF are relatively insensitive to the action of AVP on V1a receptors, possibly due shear stress mediated release of nitric oxide.24 A renal vasoconstrictor response to AVP is observed only with very high local concentrations or in pathological conditions such as congestive heart failure with simultaneous activation of the renin-angiotensin and sympathetic nervous systems. Under physiological conditions, however, AVP has a significant effect on the medullary circulation.25 At physiological concentrations (10−l2 to 10−l1 M) AVP contracts efferent arterioles isolated from rabbit kidneys, while it has no effect on afferent arterioles.26 Development of diabetes insipidus in dogs is associated with efferent arteriolar dilation.27 Administration of a V1 antagonist to euvolemic anaesthetized rats lowers GFR and filtration fraction with no change in RBF, presumably by blocking the effect of the anesthesia-induced AVP elevation on the efferent arterioles.28 Administration of a V1a receptor antagonist to patients with non-insulin dependent diabetes mellitus is also associated with a decrease in GFR and filtration fraction.29
The renal hemodynamic effects of AVP may also be due to its effects on the renin-angiotensin system. AVP could potentially stimulate renin secretion directly via activation of V2 receptors or indirectly through reduction in sodium concentration at the macula densa.12 Indeed, DDAVP-induced rises in urine albumin excretion are accompanied by an increase in plasma renin activity in humans and prevented by ACEI in rats.30 V1a receptors are coexpressed with neuronal nitric oxide synthase and/or cyclic oxygenase-2 in the macula densa and distal tubules and may control renin secretion by stimulation of production of nitric oxide and/or prostaglandin E2.31 Stimulation of renin secretion, along with suppression of tubuloglomerular feedback, may lead to glomerular hyperfiltration, albuminuria, renal hypertrophy, and tubulointerstitial disease.
The effects of AVP on blood pressure are complex. V1a effects on vascular smooth muscle and medullary renal blood flow32 and V2 effects enhancing beta and gamma epithelial sodium channel (EnaC) expression and EnaC function in the cortical collecting duct33 increase blood pressure. On the other hand, at high circulating levels of AVP, V1a receptor activation may have an antihypertensive effect by inducing synthesis of prostaglandins in collecting ducts inhibiting sodium transport33 and V2 receptor activation may induce nitric oxide synthesis in collecting ducts increasing medullary blood flow.32 AVP likely plays a role in salt-sensitive forms of human and experimental hypertension where circulating levels of and sensitivity (e.g. upregulation of V1a receptors in preglomerular vessels or of V2 receptors in collecting ducts or downregulation of nitric oxide synthase) to AVP are increased. These observations are likely relevant to the development of hypertension and progression of CKD.
Non-hemodynamic AVP effects on mesangial cell proliferation and hypertrophy, production of type I and IV collagen and fibronectin, and inhibition of the synthesis of matrix metalloproteinase (MMP)-2, may also contribute to the development of glomerulosclerosis and CKD progression.34, 35 The proliferative effect of AVP -induced cAMP accumulation on polycystic kidney disease (PKD)-derived epithelial cells, which is linked to alterations in intracellular calcium and opposite to the inhibitory effect observed in wild-type cells, has been the basis for preclinical and currently active clinical trials for PKD.36 A recent study has shown that elevated circulating AVP sustained (by prolonged osmotic stimulation or continuous infusion) for at least three days, induces a proliferative response in cells expressing V2 receptors (TAL and collecting duct) which is blocked by V2RA but not by V1a or V1b RA, suggesting that prolonged stimulation can convert these cells to a cAMP-dependent proliferative phenotype.37
In summary, there is ample evidence that AVP contributes to CKD progression. To what extent, if any, vasopressin receptor antagonists may be a valuable addition to the current treatment of CKD deserves attention.
Acknowledgments
Acknowledgments including Disclosure of Financial Interests
Dr. Torres is the principal investigator for clinical trials of tolvaptan in ADPKD sponsored by Otsuka Corp.
References
- 1.Perico N, Coja C, Corna D, et al. V1/V2 vasopressin receptor antagonism potentiates renoprotection of ras inhibition in rats with renal mass reduction. Kidney Int. 2009 doi: 10.1038/ki.2009.267. In press. [DOI] [PubMed] [Google Scholar]
- 2.Brenner BM. AMGEN International Prize: the history and future of renoprotection. Kidney Int. 2003;64:1163–1168. doi: 10.1046/j.1523-1755.2003.00249.x. [DOI] [PubMed] [Google Scholar]
- 3.Bardoux P, Bruneval P, Heudes D, et al. Diabetes-induced albuminuria: role of antidiuretic hormone as revealed by chronic V2 receptor antagonism in rats. Nephrol Dial Transplant. 2003;18:1755–1763. doi: 10.1093/ndt/gfg277. [DOI] [PubMed] [Google Scholar]
- 4.Bardoux P, Martin H, Ahloulay M, et al. Vasopressin contributes to hyperfiltration, albuminuria, and renal hypertrophy in diabetes mellitus: study in vasopressin-deficient Brattleboro rats. Proc Natl Acad Sci U S A. 1999;96:10397–10402. doi: 10.1073/pnas.96.18.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhandari SS, Loke I, Davies JE, et al. Gender and renal function influence plasma levels of copeptin in healthy individuals. Clin Sci (Lond) 2009;116:257–263. doi: 10.1042/CS20080140. [DOI] [PubMed] [Google Scholar]
- 6.Bouby N, Bachmann S, Bichet D, et al. Effect of water intake on the progression of chronic renal failure in the 5/6 nephrectomized rat. Am J Physiol. 1990;258:F973–979. doi: 10.1152/ajprenal.1990.258.4.F973. [DOI] [PubMed] [Google Scholar]
- 7.Sugiura T, Yamauchi A, Kitamura H, et al. High water intake ameliorates tubulointerstitial injury in rats with subtotal nephrectomy: possible role of TGF-beta. Kidney Int. 1999;55:1800–1810. doi: 10.1046/j.1523-1755.1999.00443.x. [DOI] [PubMed] [Google Scholar]
- 8.Bouby N, Hassler C, Bankir L. Contribution of vasopressin to progression of chronic renal failure: study in Brattleboro rats. Life Sci. 1999;65:991–1004. doi: 10.1016/s0024-3205(99)00330-6. [DOI] [PubMed] [Google Scholar]
- 9.Brooks DP, Solleveld HA, Contino LC. Vasopressin and the pathogenesis of chronic renal failure. Br J Pharmacol. 1990;100:79–82. doi: 10.1111/j.1476-5381.1990.tb12055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hebert LA, Greene T, Levey A, et al. High urine volume and low urine osmolality are risk factors for faster progression of renal disease. Am J Kidney Dis. 2003;41:962–971. doi: 10.1016/s0272-6386(03)00193-8. [DOI] [PubMed] [Google Scholar]
- 11.Kwon TH, Frokiaer J, Knepper MA, et al. Reduced AQP1, -2, and -3 levels in kidneys of rats with CRF induced by surgical reduction in renal mass. Am J Physiol. 1998;275:F724–741. doi: 10.1152/ajprenal.1998.275.5.F724. [DOI] [PubMed] [Google Scholar]
- 12.Carmosino M, Brooks HL, Cai Q, et al. Axial heterogeneity of vasopressin-receptor subtypes along the human and mouse collecting duct. Am J Physiol Renal Physiol. 2007;292:F351–360. doi: 10.1152/ajprenal.00049.2006. [DOI] [PubMed] [Google Scholar]
- 13.Mutig K, Paliege A, Kahl T, et al. Vasopressin V2 receptor expression along rat, mouse, and human renal epithelia with focus on TAL. Am J Physiol Renal Physiol. 2007;293:F1166–F1177. doi: 10.1152/ajprenal.00196.2007. [DOI] [PubMed] [Google Scholar]
- 14.Terada Y, Tomita K, Nonoguchi H, et al. Different localization and regulation of two types of vasopressin receptor messenger RNA in microdissected rat nephron segments using reverse transcription polymerase chain reaction. J Clin Invest. 1993;92:2339–2345. doi: 10.1172/JCI116838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunnet JW, Wines P, Xiang M, et al. Pharmacological characterization of RWJ-676070, a dual vasopressin V(1A)/V(2) receptor antagonist. Eur J Pharmacol. 2008;590:333–342. doi: 10.1016/j.ejphar.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Bankir L. Antidiuretic action of vasopressin: quantitative aspects and interaction between V1a and V2 receptor-mediated effects. Cardiovasc Res. 2001;51:372–390. doi: 10.1016/s0008-6363(01)00328-5. [DOI] [PubMed] [Google Scholar]
- 17.Bouby N, Ahloulay M, Nsegbe E, et al. Vasopressin increases glomerular filtration rate in conscious rats through its antidiuretic action. J Am Soc Nephrol. 1996;7:842–851. doi: 10.1681/ASN.V76842. [DOI] [PubMed] [Google Scholar]
- 18.Trinh-Trang-Tan MM, Bouby N, Doute M, et al. Effect of long- and short-term antidiuretic hormone availability on internephron heterogeneity in the adult rat. Am J Physiol. 1984;246:F879–888. doi: 10.1152/ajprenal.1984.246.6.F879. [DOI] [PubMed] [Google Scholar]
- 19.Anastasio P, Cirillo M, Spitali L, et al. Level of hydration and renal function in healthy humans. Kidney Int. 2001;60:748–756. doi: 10.1046/j.1523-1755.2001.060002748.x. [DOI] [PubMed] [Google Scholar]
- 20.Andersen LJ, Andersen JL, Schutten HJ, et al. Antidiuretic effect of subnormal levels of arginine vasopressin in normal humans. Am J Physiol. 1990;259:R53–60. doi: 10.1152/ajpregu.1990.259.1.R53. [DOI] [PubMed] [Google Scholar]
- 21.Bankir L, Bouby N, Trinh-Trang-Tan MM, et al. Direct and indirect cost of urea excretion. Kidney Int. 1996;49:1598–1607. doi: 10.1038/ki.1996.232. [DOI] [PubMed] [Google Scholar]
- 22.Bankir L, Perucca J, Weinberger MH. Ethnic differences in urine concentration: possible relationship to blood pressure. Clin J Am Soc Nephrol. 2007;2:304–312. doi: 10.2215/CJN.03401006. [DOI] [PubMed] [Google Scholar]
- 23.Perucca J, Bouby N, Valeix P, et al. Sex difference in urine concentration across differing ages, sodium intake, and level of kidney disease. Am J Physiol Regul Integr Comp Physiol. 2007;292:R700–705. doi: 10.1152/ajpregu.00500.2006. [DOI] [PubMed] [Google Scholar]
- 24.Loichot C, Krieger JP, De Jong W, et al. Shear stress modulates vasopressin-induced renal vasoconstriction in rats. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:555–561. doi: 10.1007/s00210-002-0638-7. [DOI] [PubMed] [Google Scholar]
- 25.Cowley AW., Jr Control of the renal medullary circulation by vasopressin V1 and V2 receptors in the rat. Exp Physiol. 2000;85(Spec No):223S–231S. doi: 10.1111/j.1469-445x.2000.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 26.Edwards RM, Trizna W, Kinter LB. Renal microvascular effects of vasopressin and vasopressin antagonists. Am J Physiol. 1989;256:F274–278. doi: 10.1152/ajprenal.1989.256.2.F274. [DOI] [PubMed] [Google Scholar]
- 27.Fisher RD, Grunfeld JP, Barger AC. Intrarenal distribution of blood flow in diabetes insipidus: role of ADH. Am J Physiol. 1970;219:1348–1358. doi: 10.1152/ajplegacy.1970.219.5.1348. [DOI] [PubMed] [Google Scholar]
- 28.Davis JM, Schnermann J. The effect of antidiuretic hormone on the distribution of nephron filtration rates in rats with hereditary diabetes insipidus. Pflugers Arch. 1971;330:323–334. doi: 10.1007/BF00588584. [DOI] [PubMed] [Google Scholar]
- 29.Yamada K, Nakano H, Nishimura M, et al. Effect of AVP.V1-receptor antagonist on urinary albumin excretion and renal hemodynamics in NIDDM nephropathy: role of AVP. V1-receptor. J Diabetes Complications. 1995;9:326–329. doi: 10.1016/1056-8727(95)80033-b. [DOI] [PubMed] [Google Scholar]
- 30.Bardoux P, Bichet DG, Martin H, et al. Vasopressin increases urinary albumin excretion in rats and humans: involvement of V2 receptors and the renin-angiotensin system. Nephrol Dial Transplant. 2003;18:497–506. doi: 10.1093/ndt/18.3.497. [DOI] [PubMed] [Google Scholar]
- 31.Aoyagi T, Izumi Y, Hiroyama M, et al. Vasopressin regulates the renin-angiotensin-aldosterone system via V1a receptors in macula densa cells. Am J Physiol Renal Physiol. 2008;295:F100–107. doi: 10.1152/ajprenal.00088.2008. [DOI] [PubMed] [Google Scholar]
- 32.Perucca J, Bichet DG, Bardoux P, et al. Sodium excretion in response to vasopressin and selective vasopressin receptor antagonists. J Am Soc Nephrol. 2008;19:1721–1731. doi: 10.1681/ASN.2008010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Connor PM, Cowley AW., Jr Vasopressin-induced nitric oxide production in rat inner medullary collecting duct is dependent on V2 receptor activation of the phosphoinositide pathway. Am J Physiol Renal Physiol. 2007;293:F526–532. doi: 10.1152/ajprenal.00052.2007. [DOI] [PubMed] [Google Scholar]
- 34.Tahara A, Tsukada J, Tomura Y, et al. Effect of YM218, a nonpeptide vasopressin V(1A) receptor-selective antagonist, on rat mesangial cell hyperplasia and hypertrophy. Vascular pharmacology. 2007;46:463–469. doi: 10.1016/j.vph.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Tahara A, Tsukada J, Tomura Y, et al. Vasopressin increases type IV collagen production through the induction of transforming growth factor-beta secretion in rat mesangial cells. Pharmacol Res. 2008;57:142–150. doi: 10.1016/j.phrs.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Torres VE. Role of vasopressin antagonists. Clin J Am Soc Nephrol. 2008;3:1212–1218. doi: 10.2215/CJN.05281107. [DOI] [PubMed] [Google Scholar]
- 37.Alonso G, Galibert E, Boulay V, et al. Sustained elevated levels of circulating vasopressin selectively stimulate the proliferation of kidney tubular cells via the activation of V2 receptors. Endocrinology. 2009;150:239–250. doi: 10.1210/en.2008-0068. [DOI] [PubMed] [Google Scholar]
- 38.Yang B, Bankir L. Urea and urine concentrating ability: new insights from studies in mice. Am J Physiol Renal Physiol. 2005;288:F881–896. doi: 10.1152/ajprenal.00367.2004. [DOI] [PubMed] [Google Scholar]