Summary
Increased incidence of sexually transmitted diseases (STD) and radical social changes have taken place at the same time in Estonia.
Our aim was to study the trends in syphilis incidence, selected sociodemographic factors and health status indicators during the economic transition.
Associations were estimated by the ordinary least square regression method for change in and actual values of syphilis and tuberculosis incidence rate (IR), percentage of non-ethnic Estonians and urban population, homicides rate, unemployment rate and, birth rate. The analysis was performed by counties at three different time periods.
Syphilis IR significantly correlated with the proportion of non-ethnic Estonians, urban population, tuberculosis IR and birth rate. Change of syphilis IR correlated significantly with concurrent changes in unemployment rate and tuberculosis IR.
Our findings support the theory that syphilis is a social disease, thus emphasizing the importance of social factors in the occurrence of STDs.
Keywords: syphilis, STD, tuberculosis, urban population, birth rate, unemployment
Introduction
Sexually transmitted diseases (STD) have been named ‘social diseases’ euphemistically, referring to social factors as important determinants of risk and case distribution of the diseases involving human sexual activity1. At the population level, the dynamics of the epidemic are governed by the prevalence of the infection itself as well as by other characteristics of the population2: any observed pattern of the incidences of STDs is influenced by a complex of social, behavioural and biological factors. Risk factors have been described at individual as well as community level. While the individual level factors are more or less under the control of a person (partner choice, frequency of partner change, use of condoms), community level factors are usually not led by the person — they are present in the society in which the individual lives3. Studies on sociophysical factors of community syphilis rates have revealed associations between sociogeographic (urban areas)4–8, sociodemographic (nationality/race relations, rate of violent crime, birth rate), economic (unemployment rate) factors4–6,9, and the prevalence of the disease in the population4,5,8.
Public order and public health rely on the stability of personal, domestic and intercommunity networks. Social disintegration, i.e. the disruption of such networks, might lead to increased violence, sexual and substance abuse, and criminality in the community9,10, increasing the overall levels of morbidity and mortality. Lower living standards, rising unemployment, uncertainty and other types of social dislocation may produce stress and induce increases in unhealthy behaviour (e.g. non-discriminating sexual partner recruitment, consumption of alcohol and drugs)11. In addition to STDs, social disintegration has demonstrated to exacerbate epidemics of several other infectious diseases, including tuberculosis12,13.
The monitoring of STDs in Estonia dates back to the 1920s. Since 1950s, the surveillance of STDs has been based on the mandatory universal notification of new cases identified by physicians to the central institution, the Health Protection Inspectorate. The detailed description of syphilis incidence rates in Estonia is given elsewhere14. In 1930s, the estimated syphilis incidence rate was approximately 100/100,000. The reported peak in syphilis incidence occurred at the end of 1940s, being 149/100,000 in 194915. During this period, Estonia lost one-fourth of its native population through World War II, mass deportations, executions and emigration due to annexation by the Soviet Union in 1940, which was followed by massive immigration from Russia. Following the stabilization of post-war migration and the economic situation in the Soviet Union, introduction of penicillin for treatment, disease control and partner notification system, syphilis incidence rate decreased to less than 7/100,000 by 1959, and remained at this low level until 197014,15. Another increase of syphilis rate occurred in 1970s, and the highest levels were reached by 1976 (42/100,000)15. Whether it was a marker of change in sexual behaviour (‘sexual revolution’) or a powerful policy of forced migration within the Soviet Union, remains unclear. As a result of almost repressive measures of partner notification and compulsory treatment, the incidence of syphilis decreased to 8/100,000 in 198215. Estonia regained its independence 1991 and the incidence of syphilis escalated in early 1990s, peaking at 76/100,000 in 1998. Similar epidemic has been documented in the other former republics of the Soviet Union14–17.
According to the extent of the socioeconomic changes and their intensity, the post-communist transition in Estonia has been divided into the following periods: (1) 1987–91 — liberation and political breakthrough, (2) 1991–94 — restoration of independent state and radical political reforms, and (3) 1995–present — emergence of a stable democratic system, economic and cultural stabilization18. The present paper aims to study the associations between the incidence rate of syphilis and sociodemographic factors in the unique situation when short-term and radical changes occurred during transition from a communist regime to a democratic society.
Material and methods
The study was designed to evaluate the association between syphilis incidence rates, sociodemographic factors and health status indicators during the economic transition. The character and scope of social changes allowed for the categorization of transitional process in Estonia into three periods. Data from the years 1991 (the final year of period I), 1994 (the final year of period II), and 1999 (the last reported year of period III) were selected for statistical analysis (Figure 1). The Statistical Office of Estonia provided these data on county level. The Estonian administrative division consists of 15 counties; the smallest having a population of approximately 12,000 and the largest 535,000 inhabitants.
Figure 1.
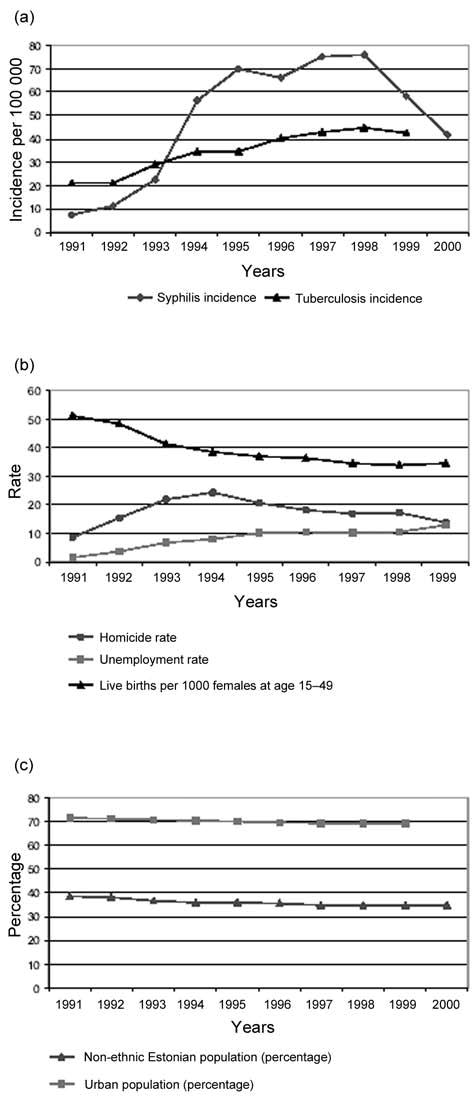
Selected sociodemographic factors and health status indicators, 1991–2000
Syphilis and tuberculosis
The data on syphilis and tuberculosis incidences were obtained from the national reportable infectious disease case surveillance register; syphilis and tuberculosis cases are reported to the County Health Protection Bureaus, which send monthly reports to the Health Protection Inspectorate. Syphilis and tuberculosis rates for the counties were calculated using the population count estimates from official statistics (1991–99) as the population denominator for the percentages and rates.
Sociodemography
Based on earlier studies on sociophysical factors influencing community syphilis rate, and the available information on sociodemographic characteristics and health indicators on county level, we chose the following characteristics for correlation analyses:
(1) percentage of the non-ethnic Estonians in the population, (2) percentage of urban population, (3) rate of homicides per 100,000, (4) unemployment rate per 100, and (5) number of live births per 1000 females at age 15–49.
Statistical analyses
The association between each socio-demographic factor and syphilis rate from periods I, II and III; as well as the association between the change (in percent) in each sociodemographic factor and change (in percent) in incidence of syphilis from period I to II, and from period II to III were assessed by estimation of regression coefficients and the explained variance values estimated by the ordinary least square regression. The associations were calculated for all the 15 counties for the three periods. Five percent significance level was chosen for the P value of the regression coefficient.
Results
Overall syphilis incidence rate, selected sociodemographic indicators in 1991, 1994, and 1999
Syphilis incidence increased from 7/100,000 during the first period (1991) to 58/100,000 during the third period (1999) (Table 1). There were variations of county level syphilis incidence rates, with the highest values recorded in Narva, the third largest city situated in the North-East of Estonia, near Estonia’s border with the Russian Federation (148/100,000 in 1999) and Tallinn, the capital of Estonia (100/100,000 in 1994), (Figure 2). The observed overall change in syphilis rates between periods I and II was 664%, compared with 3.4% difference between periods II and III. More detailed analysis on county level revealed similar changes in syphilis rates between periods I and II, and there was only one county out of 15 where syphilis rate remained unchanged (Figure 2).
Table 1.
Syphilis and tuberculosis incidence rates (per 100,000) and selected sociodemographic characteristics by time periods in Estonia (at national level)
| Period | Syphilis incidence rate | Non-Estonian population | Urban population | Homicide rate (per 100,000) | Unemployment rate | TB* incidence rate | Live births rate (per 1000 females at age 15–49) |
|---|---|---|---|---|---|---|---|
| I | 7.4 | 38% | 71% | 8.7 | 1.5 | 21.5 | 51.0 |
| II | 56.5 | 36% | 70% | 24.2 | 7.9 | 34.6 | 38.6 |
| III | 58.4 | 35% | 69% | 13.8 | 12.8 | 42.3 | 34.5 |
TB=tuberculosis
Figure 2.
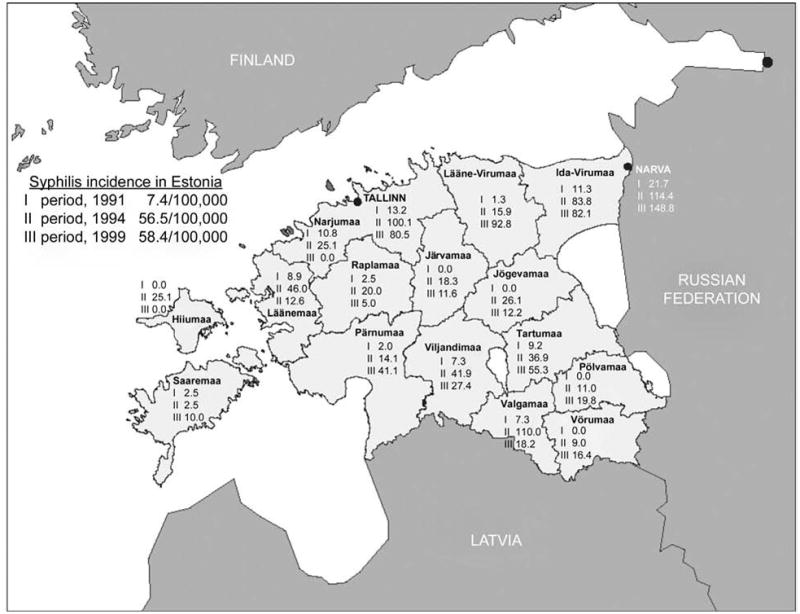
Syphilis incidence rate per 100,000 in counties and selected towns by time periods in Estonia
In 1990s, the ethnic composition of the Estonian population remained unchanged, with ethnic Estonians making up about two-thirds of the total population. Also the proportion of urban (~70%) and rural (~30%) population in Estonia remained unchanged in the 1990s (Figure 1, Table 1).
The number of live births continued to decrease in Estonia during the period under observation. The number of live births per 100,000 females (aged 15–49) decreased from 51 in year 1991 to 39 and 35 in years 1994 and 1999 respectively (Table 1).
An increase in homicide rate was observed in Estonia during 1990s (Table 1). During period I, total homicide rate was 9/100,000, in period II there was 365 homicides, which gives the rate of 24/100,000. During the third period, homicide rate was less than 14/100,000. The areas of the highest crime levels were Tallinn, Narva and Ida-Virumaa County.
Unemployment has increased constantly throughout the periods, from 1.5% in period I and 8% in period II to 13% in 1999 (Table 1).
The incidence of tuberculosis rose from 22/100,000 in the first period to 42/100,000 in the third period. The majority of the infected were men (approximately 70%), the age group 35–55 being the most affected (Table 1).
Association between sociodemographic factors and syphilis rates
Statistically significant positive correlations were found between syphilis incidence rate and the percentage of non-Estonian population (Pearson’s r=0.509), urban population (Pearson’s r=0.524), and tuberculosis incidence (Pearson’s r=0.444), which explains 26%, 28%, and 20% of the variation in the syphilis incidence rates across the counties during the three periods, respectively. A statistically significant negative correlation was found between syphilis incidence rate and the birth rate (Pearson’s r=−0.501), which explains 25% of the variance. All analyses on associations were performed at county level, and we found no correlation between syphilis incidence rate and homicide rate or unemployment rate (Table 2).
Table 2.
Association between syphilis incidence rate and sociodemographic factors: explained variance, correlation and regression coefficients (analysis at county level)
| Explained variance | Regression coefficient | P value of regression coefficient | Correlation coefficient | |
|---|---|---|---|---|
| Non-Estonian population (percentage) | 0.259 | 0.738 | 0.000 | 0.509 |
| Urban population (percentage) | 0.275 | 0.841 | 0.000 | 0.524 |
| Homicide rate (per 100,000) | 0.095 | −0.632 | 0.118 | −0.308 |
| Unemployment rate (percentage) | 0.001 | −0.002 | 0.909 | −0.022 |
| Live births (per 1000 females, age 15–49) | 0.251 | −0.012 | 0.000 | −0.501 |
| Tuberculosis incidence rate (per 100,000) | 0.197 | 0.009 | 0.002 | 0.444 |
Association between the change in sociodemographic factors and the change in the incidence of syphilis from period I to II, and from period II to III
When data were analysed at county level, the statistically significant positive correlation was found between the changes in syphilis incidence rate and the changes in unemployment rate (Pearson’s r=0.630) and tuberculosis incidence rate (Pearson’s r=0.588), which explains 40% and 35% of the variation in the syphilis incidence rates across the time periods, respectively. Associations between changes in syphilis incidence and the other four sociodemographic factors were not identified (Table 3).
Table 3.
Association between change in syphilis rate (percent) and change in socio-demographic factors (percent) between period I and II, and between II and III: explained variance, correlation and regression coefficients (analysis at county level)
| Explained variance | Regression coefficient | P value of regression coefficient | Correlation coefficient | |
|---|---|---|---|---|
| Change in non-Estonian population | 0.002 | 1.79 | 0.836 | 0.045 |
| Change in urban population | 0.097 | −80.27 | 0.138 | −0.312 |
| Change in homicide rate | 0.009 | 0.267 | 0.753 | 0.093 |
| Change in unemployment rate | 0.397 | 1.62 | 0.009 | 0.630 |
| Change in live births | 0.003 | −2.38 | 0.785 | −0.059 |
| Change in tuberculosis incidence rate | 0.346 | 3.64 | 0.002 | 0.588 |
Discussion
The rise in the syphilis incidence rate in Estonia coincidences with the major societal changes throughout the last century, including the period of World War II, and the transition period in the 1990s. We conducted an ecological study to investigate the correlation between syphilis incidence rate and selected sociodemographic factors, such as ethnic composition (percentage of the non-ethnic Estonians), percentage of urban population, homicide rate, birth and unemployment rates in the 15 Estonian counties during the past decade.
Socioeconomic and health indicators used in our analysis did not change linearly throughout the 1990s but showed rather remarkable difference on national level, and even more on county level. To avoid their aggregation in one variable we preferred to stratify our data, and to take into account the extent and intensity of these changes we used the post-communist transition periods presented by Lauristin et al.18. The first period of post communist transition, (1987–1991, Stage I) is characterized by strong political mobilization and restoration of civil society, rapid economic decline and hyperinflation. The second period, (1991–1994, Stage II) involved rapid change in the economy —shock therapy was applied, followed by monetary reform and extensive privatization of industry and agriculture. Poor/rich opposition became apparent, regional differences in living standard increased and unemployment grew gradually. During the last period (Stage III, from 1995 onwards) the economy and banking system stabilized and inflation decreased, followed by a slow growth in living standards18. In the long run, the transition is expected to lead to improvement in health status through long-term increases in real income, more effective approaches to disease prevention, healthier lifestyle, and improved regulation of environmental and occupational risks11.
The reported incidence of syphilis increased dramatically from the first to the second period, and remained stable between the second and the third period. It is important to note that mechanism of reporting the new cases did not change. Regardless of large county level differences in actual syphilis rates in Estonia, and somewhat unevenly distributed access to specialized STD care across the country, similar changes in the syphilis rates on county level were observed, and there was only one county out of 15 where syphilis rates remained unchanged. The reported incidence of other bacterial STD (gonorrhoea, chlamydiosis) started to decline already in 1994–199514,15,19 after escalating simultaneously with syphilis rates at the beginning of 1990s. According to many earlier interpretations, decline in STDs incidence reflects the fact that peak values among the socially disadvantaged and at risk have been achieved, in addition to the changes in care-seeking patterns14,16,20. This reversal trend may also be attributed to the fact that new and potent medicines and treatment schedules used in developed countries were introduced in Estonia. Nonetheless, the socioeconomic stabilization of the community is to be considered14.
The incidence rate of tuberculosis was positively correlated with syphilis rate both in analyses of the actual disease level and changes across the periods. Presuming that any known similarity exists in transmission of these morphologically distinctive microbes, the reason for such correlation should be identified by studying the community level factors, e.g. social disintegration.
In our study, birth rate showed negative correlation with county syphilis rate, but we found no correlation between the changes in syphilis rate and birth rate across the periods. Similar population downturn has been noted in Eastern European countries and in the Russian Federation21. The decrease in live birth rate in Estonia was steeper during the first half of the 1990s (1st period of observation) but the decline in fertility has slowed down since 1993. These observations conflict with the existing concepts of poverty giving impetus to rapid population growth — an event observed in several regions of the world22,23. It seems, that in the countries with transitional economy, the rate of live birth might be considered as a marker of social (dis)integration instead. Parental ability and willingness to invest in a child should be considered, and Estonia is a country with long-established traditions of legalized voluntary abortion. Although social security and means of private investments are generally absent, children are not considered as the only vital source of labour or probable support for old age. Thus we believe that community level factors influence the incidence of diseases and these factors may predetermine reproductive events in the society.
Although syphilis incidence rate was not correlated with unemployment rate, we do provide support that change in unemployment rate was correlated with the change in syphilis incidence rate. Unemployment, virtually non-existent during the years of Soviet regime, was introduced into Estonia within the past decade. The sectors of economy that have continuously deteriorated after the restoration of independence in Estonia are those sectors which depend on import of raw material from, and export of products into the Russian Federation20. Socioeconomic factors are the most powerful determinants of health status24, and income differentials (rather than income level) predict health status better25. It is possible that deepening problems with unemployment lead to worse health outcome.
Although at the national level the proportion of urban population and non-ethnic population (Table 1) remained relatively stable during the period of observation, we found significant correlations between these factors and their changes, if compared with the syphilis rates at the county level of analysis (Tables 2, 3). Urban residence has been associated with high syphilis rates6, although it may also be a surveillance artefact — better access to medical care in urban areas26 results in higher rates of appropriate diagnosis and reporting of syphilis. Statistically significant correlation between the actual syphilis rate and the proportion of urban population was found, but the change of urban population was not correlated with the change of syphilis rate during that period. This might suggest that inside the urban residence areas other factors such as an internal migration that is not properly reflected in county level statistics play important role in influencing syphilis rate. It has been previously shown that internal migration had mostly occurred in non-registered changes of residence in 15–34 year-old males and 15–29 year-old females27 — the age groups most vulnerable to STD related problems. Syphilis rate was positively associated with the proportion of non-Estonians in the population. The ethnic origin per se cannot be considered a biological risk factor for STD, and the occurrence of high syphilis incidence rate in the county with the largest proportion of non-Estonian population (Ida-Virumaa county), apparently reflects the effects of other socioeconomic and demographic factors (unemployment, geographical location). Considerable territorial differences in health status related to inequalities in the society have been described also in Lithuania and in other post-communist countries28. The geographical proximity of Ida-Virumaa, the county with the highest syphilis levels (Figure 1), to St Petersburg (Russian Federation) — an area with extremely high STD rates (234/100,000 population)29 — could be considered as an important confounding factor for the correlation with ethnic origin. According to a recent study in Estonia, sexual behaviour associated with travelling might contribute to STD rates, thus determining the STD occurrence patterns30.
As a typical ecological study relying on national trend analyses this study lacks information on individual behaviour. Our empirical testing is limited to trends and simple correlations at county level to confirm the expectations expressed by the initial hypothesis. Only aggregated county-level data were used, while relevant data on sexual behaviour and alcohol/substance abuse remained unavailable. Nevertheless, the unique social and political situation along with radical changes in the community during a very short period allows us to look for the community-level indicators to correlate them with the syphilis incidence rate, which varied considerably in space and time, resulting in the social pattern of the disease. The correlation between syphilis rate and community level factors emphasize the importance of establishing close connections between health care sector and other community resources to prevent sexually transmitted infections.
Analysis of mass data at the individual level of organization does not always lead to understanding of transmission and diseases in the social context31. Addressing both individual and society levels of epidemic allows us to design coherent strategies of public health interventions. The need for effective measures in STD control becomes even more clear in the light of the advent of an HIV/AIDS epidemic in Estonia.
Acknowledgments
The authors would like to thank Marika Kivilaid and Aime Lauk from the Statistical Office of Estonia for their assistance in data collection, and Avo Trumm from the Department of Sociology, Faculty of Social Sciences, University of Tartu, for his advice and useful comments in revision.
This study was supported by a grant No. 3 D43 TW00233 from the Fogarty International Centre.
References
- 1.Potterat JJ, Rothenberg RB, Woodhouse DE, Muth JB, Pratts CI, Fogle JS. Gonorrhea as a social diseases. Sex Transm Dis. 1985;12:25–32. doi: 10.1097/00007435-198501000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Susser M, Susser E. Choosing a future for epidemiology: II. From black box to Chinese boxes and eco-epidemiology. Am J Pub Health. 1996;86:674–7. doi: 10.2105/ajph.86.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiboski S, Padian NS. Population- and individual-based approaches to the design and analysis of epidemiologic studies of sexually transmitted disease transmission. J Infect Dis. 1996;174 (Suppl 2):S188–200. doi: 10.1093/infdis/174.supplement_2.s188. [DOI] [PubMed] [Google Scholar]
- 4.Thomas JC, Clark M, Robinson J, Monnett M, Kilmarx PH, Peterman TA. The social ecology of syphilis. Soc Sci Med. 1999;48:1081–94. doi: 10.1016/s0277-9536(98)00408-0. [DOI] [PubMed] [Google Scholar]
- 5.Aral SO, Holmes KK. Demographic and social correlates of sexually transmitted disease. In: Holmes KK, Mardh PA, Sparling PF, Wiesner PJ, editors. Sexually transmitted diseases. New York: McGraw-Hill; 1990. p. 33. [Google Scholar]
- 6.Kilmarx PH, Zaidi AA, Thomas JC, Nakashima AK, St Louis E, Flock ML. Sociodemographic factors and the variation in syphilis rates among US counties, 1984 through 1993: and ecological analysis. Am J Pub Health. 1997;87:1937–43. doi: 10.2105/ajph.87.12.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice RJ, Roberts PL, Handsfield HH. Sociodemographic distribution of gonorrhea incidence: implications for prevention and behavioral research. Am J Pub Health. 1991;81:1252–8. doi: 10.2105/ajph.81.10.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stokols D. Establishing and maintaining healthy environments: toward a social ecology of health promotion. Am Psychologist. 1992;42:6–22. doi: 10.1037//0003-066x.47.1.6. [DOI] [PubMed] [Google Scholar]
- 9.Wallace R. Urban desertification, public health and public order: planned shrinkage, violent death, substance abuse and AIDS in the Bronx. Soc Sci Med. 1990;31:801–31. doi: 10.1016/0277-9536(90)90175-r. [DOI] [PubMed] [Google Scholar]
- 10.Wallace R, Wallace D. Socioeconomic determinants of health: community marginalisation and the diffusion of disease and disorder in the United States. BMJ. 1997;314:1341–5. doi: 10.1136/bmj.314.7090.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adeyi O, Chellaraj G, Goldstein E, Preker A, Ringold D. Health status during the transition in Central and Eastern Europe: development in reverse? Health Policy Plann. 1997;12:132–45. doi: 10.1093/heapol/12.2.132. [DOI] [PubMed] [Google Scholar]
- 12.Miles TP, McBride D. World War I origins of the syphilis epidemic among 20th century black americans: a bio-historical analysis. Soc Sci Med. 1997;45:61–9. doi: 10.1016/s0277-9536(96)00316-4. [DOI] [PubMed] [Google Scholar]
- 13.Wallace R. A synergism of plaques: ‘planned shrinkage’, contagious housing destruction, and AIDS in the Bronx. Envirom Res. 1988;47:1–33. doi: 10.1016/s0013-9351(88)80018-5. [DOI] [PubMed] [Google Scholar]
- 14.Uusküla A, Silm H, Vessin T. Sexually transmitted diseases in Estonia: past and Present. Int J STD AIDS. 1997;8:446–50. doi: 10.1258/0956462971920505. [DOI] [PubMed] [Google Scholar]
- 15.National Board of Health Protection. Communicable diseases statistics in Estonia. National Board of Health Protection; 1998. p. 24.p. 26.p. 30.p. 61. [Google Scholar]
- 16.Waugh MA. Task force for the urgent response to the epidemics of sexually transmitted diseases in Eastern Europe and Central Asia. Int J STD AIDS. 1999;10:60–2. doi: 10.1258/0956462991912962. [DOI] [PubMed] [Google Scholar]
- 17.Gomberg MA, Akovbian VA. Resurgence of sexually transmitted diseases in Russia and eastern Europe. Dermatol Clin. 1998;16:659–62. doi: 10.1016/s0733-8635(05)70029-2. [DOI] [PubMed] [Google Scholar]
- 18.Lauristin M, Vihalemm P. Return to the western world. In: Lauristin M, Vihalemm P, Rosengren E, Weibull, editors. Recent historical developments in Estonia: three stages of transition (1987–1997) Estonia: Tartu University Press; 1997. pp. 79–83. [Google Scholar]
- 19.Health Protection Inspectorate. Reported infectious diseases in Estonia, 1999. Eesti Arst. 2000;4:222–5. [Google Scholar]
- 20.Dehne KL, Pokrovskiy V, Kobyshca Y, Schwartländer B. Update on epidemics of HIV and other sexually transmitted infections in the newly independent states of the former Soviet Union. AIDS. 2000;14(Suppl 3):S75–84. [PubMed] [Google Scholar]
- 21.Krus DJ, Nelsen EA. Changes in crime rates and family related values in selected east European countries. Psychol Rep. 1997;81:747–51. doi: 10.2466/pr0.1997.81.3.747. [DOI] [PubMed] [Google Scholar]
- 22.UNICEF . The state of the world’s children. New York: Oxford University Press; 1994. p. 27. [Google Scholar]
- 23.Kibirige JS. Population growth, poverty and health. Soc Sci Med. 1997;45:247–59. doi: 10.1016/s0277-9536(96)00341-3. [DOI] [PubMed] [Google Scholar]
- 24.World Bank. World development report: investing in health. New York: Oxford University Press; 1993. [Google Scholar]
- 25.Wilkinson RG. Income distribution and life expectancy. BMJ. 1992;304:165–8. doi: 10.1136/bmj.304.6820.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Center for Disease Control and Prevention. 1992 National Health Interview Survey. Healthy People 2000 Review. Washington, DC: US Dept of Health and Human Services; 1994. (PHS 95-1258-1) [Google Scholar]
- 27.Statistical office of Estonia . Internal migration. Population of Estonia 1993. Tallinn: Statistical office of Estonia; 1995. publishing section. [Google Scholar]
- 28.Grabauskas V, Kalediene R. Tackling social inequality through the development of health policy in Lithuania. Scand J Pub Health. 2002;30 (Suppl 59):12–19. [PubMed] [Google Scholar]
- 29.Tichonova L, Borisenko K, Ward H, Meheus A, Gromyko A, Renton A. Epidemics of syphilis in the Russian Federation: trends, origins, and priorities for control. Lancet. 1997;350:210–13. doi: 10.1016/S0140-6736(97)01382-2. [DOI] [PubMed] [Google Scholar]
- 30.Wilson T, Uusküla A, Feldman J, Holman S, DeHovitz J. A case control study of beliefs and behaviors associated with STD occurrence in Estonia. Sex Transm Dis. 2001;28:624–9. doi: 10.1097/00007435-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Susser M, Susser E. Choosing a future for epidemiology: I. eras and paradigms. Am J Pub Health. 1996;86:668–73. doi: 10.2105/ajph.86.5.668. [DOI] [PMC free article] [PubMed] [Google Scholar]


