Abstract
The ectoderm gives rise not only to the skin but also to the entire CNS. This common embryonic lineage suggests that some molecular isoforms might serve analogous functions in both tissues. Indeed, not only are laminins important components of dermal adhesion mechanisms, but they also regulate some aspects of synaptic development in both the CNS and the PNS. In the skin, laminins are part of a hemidesmosome complex essential for basal keratinocyte adhesion that includes collagen XVII (BP180) and BPAG1 (dystonin/BP230). Here, we show that CNS neurons also express collagen XVII and BPAG1 and that these molecules are expressed in the adult and developing retina. In the retina, isoforms of collagen XVII and BPAG1 are colocalized with laminins at photoreceptor synapses and around photoreceptor outer segments; both molecules are expressed by rods, whereas cones express collagen XVII but not BPAG1. Moreover, biochemical data demonstrate that collagen XVII complexes with retinal laminins. We propose that collagen XVII and BPAG1 isoforms may help to anchor elements of the rod photoreceptor cytomatrix to the extracellular matrix.
Indexing terms: extracellular matrix, synaptogenesis, photoreceptor, CNS development, hemidesmosomes
Laminins are large heterotrimeric (α/β/γ) secreted glycoproteins that play important structural and signaling functions in a variety of tissues (Colognato and Yurchenco, 2000). Five α, three β, and three γ chains are known, giving rise to 45 possible laminins, 15 of which have been reported (Libby et al., 2000). In the nervous system, laminins are essential in axon growth and myelination (Fernandez-Valle et al., 1994; Grimpe et al., 2002), neuromuscular junction formation and stabilization (Noakes et al., 1995; Patton et al., 2001), and Schwann cell development (Anton et al., 1994; Patton et al., 1998). The altered expression of laminin chains leads to various human neuropathologies (Olson and Walsh, 2002). Specifically, perturbations in the α2 chain induce severe congenital muscular dystrophy associated with demyelination (Helbling-Leclerc et al., 1995), and reduced β2 chain expression is seen in Walker-Warburg syndrome, a cerebroocular dysplasia (Wewer et al., 1995). Finally, experimentally induced mutations in CNS laminins or laminin receptors also produce profound defects in cortical morphogenesis (Georges-Labouesse et al., 1998; Halfter et al., 2002; Moore et al., 2002).
Laminins containing the β2 chain appear to be key molecules in the induction of synaptic differentiation in both the peripheral nervous system (PNS) and the CNS (Noakes et al., 1995; Soussand et al., 2001). In the retina, mice lacking the laminin β2 chain have shortened outer segments and poorly formed, improperly functioning rod synapses in the outer plexiform layer (OPL; Libby et al., 1999). The abnormal synapse formation coincides with an aberrant electroretinogram, reflecting neurotransmission deficiencies that are similar to defects observed in muscular dystrophy (Libby et al., 1999). Two laminins recently isolated from the CNS, laminins 14 (α4β2γ3) and 15 (α5β2γ3), may stabilize retinal synapses (Libby et al., 2000) analogously to the role of laminins 4, 9, and 11 at the neuromuscular junction (Noakes et al., 1995).
Understanding the roles of laminins in synaptic development ultimately requires the identification of laminin receptor molecules at the synapse. Such potential receptors include dystroglycan and collagen XVII (the 180-kDa bullous pemphigoid antigen also known as BP180 or BPAG2), two receptors for extracellular matrix (ECM) components that are well characterized at the neuromuscular junction and in skin hemidesmosomes, respectively (Yang et al., 1995; Jones et al., 1998). In the retina, dystroglycan expression lags behind laminin expression and photoreceptor morphogenesis; consequently, it is unlikely to be the primary laminin receptor in photoreceptor morphogenesis (Libby et al., 2000). Here, we have investigated whether dermal adhesion serves as a model for synaptic laminin receptors.
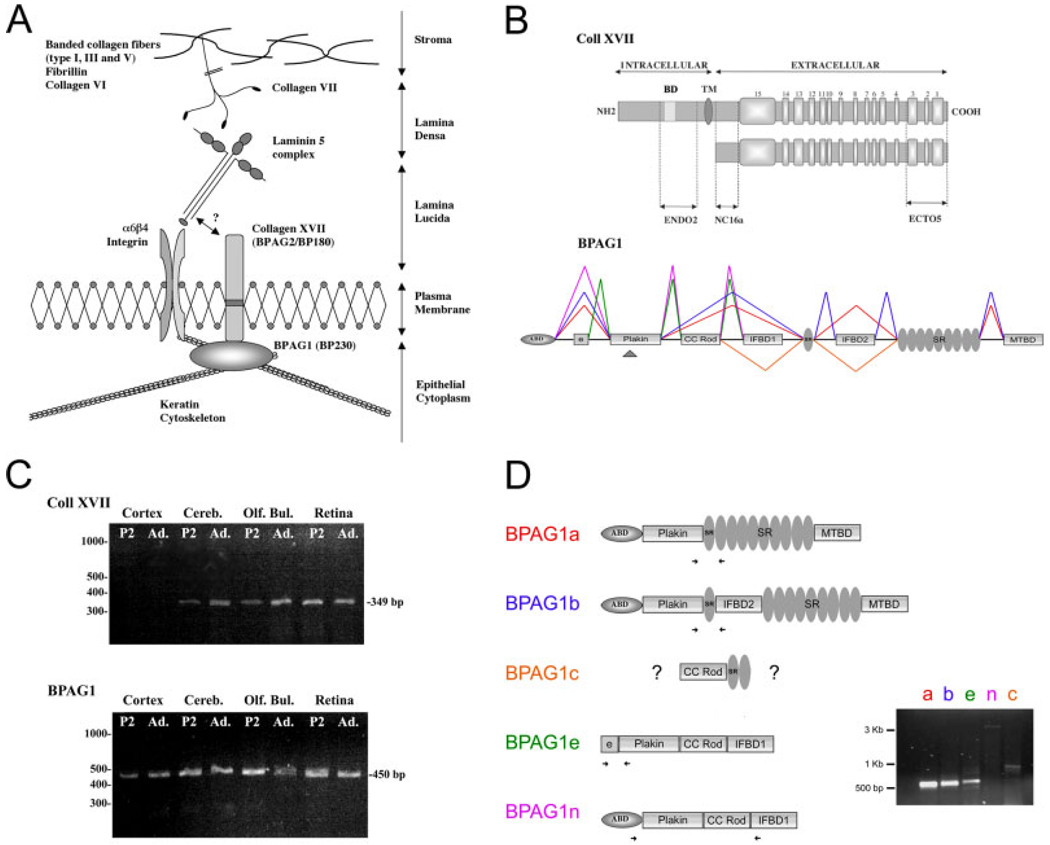
Several laminin isoforms anchor the basal surface of keratinocytes to the basement membrane ECM (Fig. 1A). Dermal adhesion is dependent on an ECM containing laminins, transmembrane receptors for these laminins (integrins and collagen XVII), and elements of the cytomatrix (such as BPAG1), thereby coupling the ECM to the cytoskeleton (for review see Gumbiner, 1996). The α6β4 integrin complex is essential for hemidesmosome formation (Niessen et al., 1994), and mice lacking β4 or α6 integrin chains have skin-blistering phenotypes that mimic human disease (Van der Neut et al., 1996; Georges-Labouesse et al., 1996). Collagen XVII is also believed to bind dermal laminins, thereby participating in hemidesmosome assembly and stability via cytoplasmic interactions with integrins and the epidermal isoforms of BPAG1 (BPAG1e; Hopkinson et al., 1995; Schaapveld et al., 1998; Hopkinson and Jones, 2000). A subpopulation of patients with blistering diseases exhibit mutations in the gene for collagen XVII (COL17A1; McGrath et al., 1995), and some bullous pemphigoid autoimmune disorders target collagen XVII or BPAG1 (Labib et al., 1986).
Fig. 1.
Collagen XVII and BPAG1. A: Hemidesmosome structures in the epithelium. The laminin binding molecules at the dermal–epidermal junction include the integrins α6β4 and collagen XVII (collagen XVII is also known as BPAG2 and BP180). A direct interaction has been reported between laminin 5 and the integrin chains and is suspected for collagen XVII (Niessen et al., 1994). The transmembrane collagen XVII is anchored to the keratin cytoskeleton via the plakin family member BPAG1e. Collagen XVII and integrins are also known to interact directly (Hopkinson et al., 1995; Schaapveld et al., 1998). A laminin complex present in the lamina densa includes laminins 5 α3β3γ2, 6 α3β1γ1, and 7 α3β2γ1 and is coupled to basement membrane components via collagen VII. B: Schematic representation of collagen XVII and BPAG1. Top: Full-length collagen XVII is a transmembrane protein with an amino-terminal intracellular domain, a transmembrane domain (TM), and a carboxy-terminal extracellular domain. The ectodomain consists of 15 collagenous domains (light gray boxes) separated by noncollagenous domains (dark gray). A shedding of the molecule occurs in the NC16a region of the molecule (modified from Schumann et al., 2000). The domains containing the epitopes of the three antibodies used in this study are shown. Bottom: BPAG1 gene structure. The different isoforms generated by alternative splicing are indicated; cassettes encode: an actin binding domain (ABD); plakin domain with collagen XVII binding site (arrowhead); a coiled-coil rod domain (CC ROD); intermediate filament-binding domains (IFBD-1 and -2); spectrin repeats (SR); and, finally, a microtubule binding domain (MTBD) modified from Leung et al. (2001). C: Expression of collagen XVII and BPAG1 mRNA in the CNS. Collagen XVII (top) and BPAG1 (bottom) transcripts were amplified from the indicated mouse CNS areas (Cereb, cerebellum; Olf. Bul., olfactory bulb). Collagen XVII and BPAG1 appear to be cotranscribed in all regions tested, except for the cortex. D: Multiple BPAG1 mRNA isoforms are present in the retina. Domain structures of the four known isoforms (1a, 1b, 1e, 1n) of BPAG are shown. Specific primers for each isoform were designed and are indicated by the arrows on each domain structure; all four primer sets resulted in PCR products indicating that transcripts were made. In addition, a fifth product was found that contained both the CC-rod domain and the spectrin repeats. This novel isoform is tentatively called BPAG1c. A full-length clone of BPAG1c has not been generated.
Two neuronal isoforms of BPAG1 (BPAG1n and −1a) have been reported and are essential for the organization of the neuronal cytoskeleton (Bernier et al., 1995; Yang et al., 1999; Leung et al., 2001). Despite its role in BPAG1 function, neither the expression nor the role of collagen XVII has been studied in the nervous system. In the present investigation, we examined the spatial expression and putative function of collagen XVII along with BPAG1 isoforms in the retina. Both molecules are expressed by rod photoreceptors, and both are present in the outer segment and synaptic regions; in addition, collagen XVII binds β2-containing laminins, so we hypothesize that collagen XVII stabilizes photoreceptor morphogenesis, specifically synaptogenesis and disk morphogenesis.
MATERIALS AND METHODS
All procedures involving animals were approved by Tufts University Animal Care and Use Committee (IACUC) and were in accordance with the National Institutes of Health Guide for the Care and Use of Animals, the policies of the Society for Neuroscience, and the Association for Research in Vision and Ophthalmology. Bovine eyes were purchased from a local supplier. Rats were killed by exposure to CO2.
Reverse transcriptase-polymerase chain reaction
Total RNA was isolated from postnatal day 2 (P2) and adult mouse tissues by using the RNeasy kit (Qiagen, Valencia, CA) as previously described (Koch et al., 2000). mRNA was reverse transcribed with an RT-PCR kit (Clontech Laboratories Inc., Palo Alto, CA) primed with random hexamers per the manufacturer’s recommendations. The cDNA was amplified with the specific primers: 1) collagen XVII forward: 5′-GGGAACAAAGGAGAGAAAGG-3′; reverse: 5′-GCTGGGCTAAATGCTAAAGA-3′ (Genbank No. L08407) and 2) BPAG1 forward: 5′-AGCGAATCTCCTTGCTGAAA-3′ and reverse 5′-TCTGGGGGCACATTATATCC-3′ (Genbank No. AF115383). Thermocycler protocol was: 94°C, 2 minutes; 35 cycles of 94°C, 30 seconds; 54°C, 30 seconds; 72°C, 1 minute; and 10 minutes at 72°C. Reaction products were analyzed on a 1% agarose gel.
For the analysis of BPAG isoforms, the following protocol was used. RNA was isolated from P2–P5 mouse retina with Trizol (Invitrogen, Carlsbad, CA) per the manufacturer’s instructions. RNA was DNase treated by using DNA-Free (Ambion, Austin, TX) per the manufacturer’s instructions. cDNA was synthesized with SuperScript II (Invitrogen, Carlsbad, CA) per the manufacturer’s instructions. BPAG1 isoform-specific primers for RT-PCR were as follows.
For both 1a and 1b isoforms: 5′-ATTCAAGAGTTCATGGACCTACGGACAC-3′ was used as forward primer with specific reverse primers as follows: 1aR 5′-TAATTAGGCGGTTTTCAGTCTGGGTGAG-3′ and 1bR 5′-CAATAAGGCCTCTTAAAACTGCCTGAAA-3′. For 1c isoform forward primer was 5′-AGATAAAGGAACATGAGCACCAGT-3′ 1bR primer was used for the reverse direction. For isoform 1e forward primer was 5′-ATGAGAATCTCCTGTCCGTTCATT-3′ and reverse 1e-R 5′-CTGATATCATGAGCTTGGTCTGTT-3′. For the 1n isoform 5′-CAAGGTTATCATCCGAACGACAT-3′ was used in the forward direction and 5′-CCCGTGCTCAGAATTCTCTTTAG-3′ in the reverse. The PCR conditions were as follows: 94°C, 2 minutes; 35 cycles of 94°C, 30 seconds; 62°C, 30 seconds; 72°C, 30 seconds (2 minutes for 1n isoform); 72°C, 10 minutes.
Protein extraction
Adult bovine retina, pigmented epithelium, and optic nerve; a rat Müller cell line (RMC-1); and primary human keratinocyte cells were homogenized in 10 vol extraction buffer containing 150 mM NaCl, 10 mM EDTA, 1% protease inhibitor (Sigma, St. Louis, MO), 1 mM phenylmethylsulfonyl fluoride, and 25 mM Tris-HCl, pH 7.4. After centrifugation at 1,000g for 10 minutes, supernatants were recovered and centrifuged for an additional 30 minutes at 17,000g, and the final enriched membrane fraction pellet was resuspended in extraction buffer. Protein concentration was determined with a BCA assay (Pierce, Rockford, IL) per the manufacturer’s instructions.
Immunoprecipitations
Immunoprecipitations were performed by using a previously described method (Claudepierre et al., 2000). Briefly, bovine retinae and RMC-1 cell lines were extracted in 10 vol extraction buffer (as daescribed above) with the addition of 1% Triton X-100. After a rapid centrifugation at 1,000g for 5 minutes to remove cell debris, the protein lysate was incubated with protein G Sepharex beads for 60 minutes and then centrifuged at 3,000g for 5 minutes. The supernatants were incubated overnight with primary antibody at 4°C. Protein G Sepharex beads were added, and the mix was incubated for 30–60 minutes and then spun at 3,000g for 5 minutes (this fraction is labeled P in all figures). The remaining supernatant is used to test the efficiency of the immunoprecipitation (labeled S in all figures). Negative controls were performed by omitting the primary antibody (labeled –Ab in all figures).
Synaptosome preparation
Synaptosomes were prepared according to Dunkley et al. (1986). Rat retinae were homogenized in a chilled solution of 0.32 M sucrose, 0.25 mM DTT, 1 mM EDTA, pH 7.4. After centrifugation at 1,000g for 10 minutes, 2 ml of the supernatant was overlaid on a discontinuous Percoll gradient [consisting of 2 ml each of 23%, 15%, 10%, and 3% Percoll (v/v) in the homogenizing solution]. After centrifugation at 32,000g, the 10–15% and 15–23% interfaces were collected; pooled together; and washed twice in 132 mM NaCl, 4.8 mM KCl, 2.4 mM MgSO4, 10 mM glucose, and 10 mM HEPES, pH 7.4; and, finally, the pellet was resuspended in the same buffer.
β2 Laminin affinity column
Proteins from mature bovine cerebellum were extracted in 2 M urea, 0.5 M NaCl, 10 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 50 mM Tris-HCl, pH 7.8, and stirred for 24 hours at 4°C. Cerebellum extract was cleared by centrifugation at 30,000g for 60 minutes, dialyzed in 0.5 M NaCl and 50 mM Tris-HCl, pH 7.8, and then cleared by centrifugation at 100,000g for 60 minutes. Tissue extract was then loaded over a gelatin Sepharose column (Pharmacia, Piscataway, NJ) with immobilized D5 monoclonal antibody directed against β2 laminin (Hunter et al., 1989). Unbound material was washed off the column with 0.5 M NaCl, 5 mM CaCl2, and 5 mM MgCl2 in 50 mM Tris-HCl, pH 7.4, and specifically bound proteins were eluted by using 0.1 M acetic acid followed by 2 M urea and 0.154 M NaCl in 0.1 M Tris-HCl, pH 7.8. Eluate fractions were collected, absorbance was measured at 280 nm, and the peak fractions were identified. Peak fractions of the eluate were collected and assayed for collagen XVII presence by protein transfer blotting.
Collagenase digestion
Enriched membrane fractions were made from confluent keratinocytes or Müller cells (above) and were placed in extraction buffer containing: 10 mM EDTA, 0.1 M NaCl, 1% Nonidet P-40, 1 mM Pefablock, 14 µg/ml chymostatin, 7 µg/ml antipain, 7 µg/ml leupeptin, 14 µg/ml pepstatin in 25 mM Tris-HCl, pH 7.4. Protein was precipitated from extracts by the addition of 100% ethanol (1:1 v/v) overnight at −20°C and centrifuged at 14,000 rpm for 30 minutes at 4°C. Pellets were resuspended in 100 µl collagenase buffer: 0.9% NaCl, 10 mM CaCl2, 1 mM Pefablock (Merck, Darmstadt Germany), 1 mM N-ethylmaleimide (NEM), and 20 mM Tris-HCl, pH 7.4. The samples were divided into equal fractions and incubated at 37°C for 2 hours; 2 units of collagenase (Advanced Biofactures, Lynbrook, NY) were added to one sample, and the other served as control. The collagenase digestion was stopped by the addition of 3 µl of 0.5 M EDTA.
Protein transfer blots
Twenty micrograms of each protein extract preparation were resolved with 7.5% (5% in Fig. 2E) gel electrophoresis (SDS-PAGE) and then electrotransferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). The efficiency of protein transfer was assessed by Ponceau S staining of the membrane and Coomassie brilliant blue staining of the gel. Blots were blocked with 1% bovine serum albumin (BSA) and 3% nonfat dry milk in phosphate-buffered saline (PBS; 137 mM NaCl, 2.68 mM KCl, 10 mM Na2HPO4, 1.76 mM KH2PO4, pH 7.4) and incubated overnight at 4°C with primary antibodies diluted in 0.5% Tween-20 in PBS. Then, they were washed and incubated for 1 hour at ambient temperature with horseradish peroxidase-conjugated secondary antibodies (Boehringer Mannheim, Indianapolis, IN). Secondary antibodies used were as follows: 1:20,000, anti-rabbit; 1:2,000, anti-mouse. Chemiluminescent detection was performed by using the Super-Signal kit (Pierce, Evansville, IL) and documented on BioMax Light films (Kodak, Rochester, NY).
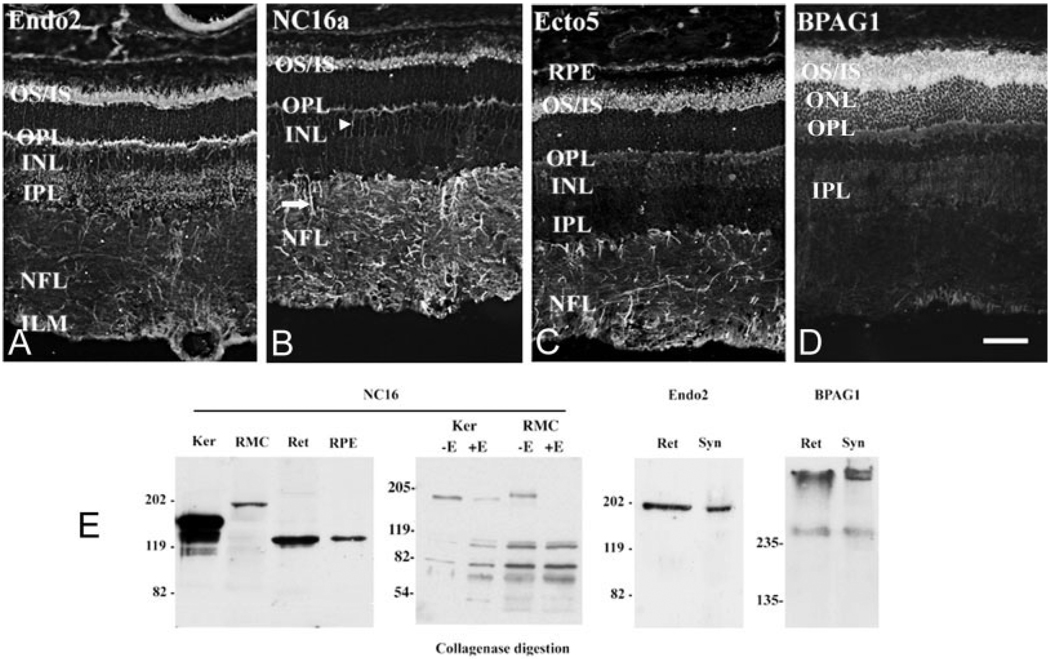
Fig. 2.
Collagen XVII and BPAG1 are expressed in the outer retina. A–C: Collagen XVII IR in retina. Three independent collagen XVII antibody preparations react similarly with the retina; specifically, these antibodies react with the outer segments of photoreceptors, the photoreceptor synaptic layer, the outer plexiform layer (OPL), and glial cell processes. A: The endo2 antibody produces bright immunoreactive signal over the outer and inner segments (OS/IS) and OPL and some faint labeling of the inner plexiform (synaptic) layer (IPL). B: The NC-16a reacts with OS, OPL, and, prominently, processes in the inner nuclear layer (INL; arrowhead) and the nerve fiber layer (NFL; arrow). C: The antibody ecto5 reacts with OS and radial processes and faintly with the OPL. D: BPAG1 IR is confined to the photoreceptor layer. IR is localized in the OS, the cell bodies (ONL), and the OPL. Faint BPAG1 immunoreactivity was observed in the IPL and occasionally in the inner aspect of the NFL, perhaps in the end feet of Müller cells. E: Biochemical confirmation of collagen XVII and BPAG1. Extracts from: keratinocytes (Ker), an immortalized rat Müller cell line (RMC), bovine retina (Ret), and bovine retinal pigmented epithelium (RPE) were assayed by Western blot with NC16a. A protein of 180 kDa, in keratinocytes, or 195 kDa, in RMC-1, was detected. The 135-kDa protein found in retina and RPE likely corresponds to the shed domain. Keratinocyte and RMC extracts were treated with collagenase; the treated fraction (+E) showed increased mobility supporting the authenticity of the antigen as a collagen. Full-length collagen XVII (using Endo2) and BPAG1 were detected in bovine retinal extracts (Ret) and rat retinal synaptosomes (Syn). Two isoforms of BPAG1 were detected, one with an apparent mass of 280 kDa and a larger (>500-kDa) form. Scale bar = 80 µm in D (applies to A–D).
Rabbit polyclonal collagen XVII antibodies were previously described (Schumann et al., 2000) and used as follows: Endo2 at 1:1,000, NC16a and Ecto5 at 1:3,000. The mouse monoclonal 10C5 antibody recognizes both skin and neuronal isoforms of BPAG1 (Dalpe et al., 1998); it was a generous gift from Dr. J.C. Jones (Northwestern University Medical School, Chicago, IL) and was used at 1:10.
Immunohistochemistry
Bovine and rat retinae were processed and sectioned as previously described (Libby et al., 2000). Nonspecific binding was blocked with a preincubation in 3% normal goat serum in PBS for 30–45 minutes at room temperature. Sections were rinsed and incubated in primary antibody overnight at 4°C. Antibodies used were collagen XVII (1:300), BPAG1 (10C5; mouse monoclonal, 1:5), SV2 (mouse monoclonal, 1:250), PSD95 (mouse monoclonal, 1:500; ABR, Golden, CO), vimentin (V9; mouse monoclonal, 1:500; Sigma, St Louis, MO), kinesin II (mouse monoclonal, 1:250; Covance Inc., Princeton, NJ), and GP1 (guinea pig polyclonal, anti-β2 laminin; 1:300; Hunter et al., 1992). Peanut lectin (PNA; 1:300; Molecular Probes, Eugene, OR) was used as a cone marker. After primary incubation, sections were rinsed three times in PBS and incubated with secondary antibodies (1:500). Anti-rabbit secondaries were conjugated to Alexa 488 and anti-mouse to Alexa 568 or 488, and anti-guinea pig was coupled to Alexa 568. PNA was directly conjugated to Alexa 488 and kinesin II was directly coupled to the fluorochrome Alexa 660 per the manufacturer’s protocol (Molecular Probes). Widefield microscopic images were captured with a Nikon E800 microscope and a Spot-RT camera (Diagnostics Instruments, Sterling Heights, MI) with the native software or with an Orca ER camera (Hamamatsu Ltd.) and the OpenLab digital image software (Improvision Ltd., Lexington, MA). Confocal images were captured on a Leica TCS SP2 in the Tufts University Integrated Confocal Facility. Images were imported into Adobe Photoshop 7.0, cropped, grouped, and labeled for publication. Only contrast and brightness adjustments were made.
RESULTS
Structure of collagen XVII
Collagen XVII forms an integral portion of the dermal anchoring complex, participating in the coupling of the ECM containing laminins and collagens to the cytoskeleton via cytomatrix proteins, including BPAG1 (Fig. 1A). The structural domains of collagen XVII (Li et al., 1993; Schumann et al., 2000) and BPAG1 (Leung et al., 2001) are shown in Figure 1B. The C-terminal extracellular domain of collagen XVII is part of the anchoring filament extending to the lamina densa (cf. Fig. 1A; Matsunaga et al., 1997), whereas the noncollagenous domain (NC16A) is reported to interact with the ectodomain of the α6 integrin subunit (Hopkinson et al., 1995). Intracellularly, distinct regions of the amino-terminal segment of collagen XVII bind to α4 integrin and BPAG1 (both neural and epithelial isoforms; Aho and Uitto, 1998; Hopkinson and Jones, 2000; Koster et al., 2003). A soluble collagen XVII fragment of 120 kDa can be generated by proteolytic cleavage of the full-length transmembrane molecule in the NC16a domain (Fig. 1B, Schäcke et al., 1998, Franzke et al., 2002). Three different collagen XVII antibodies were used in these studies; they recognize the following domains: ecto5, C-terminus; NC16a, juxtamembranous noncollagenous domain; endo2, N-terminus (Fig. 1B). All three recognize full-length collagen XVII and cleaved products containing their epitopes. BPAG1 transcripts are alternatively spliced in a variety of different isoforms; all of the reported isoforms contain the plakin domain. Several of the reported neural isoforms contain actin binding domains (ABD) and microtubular binding domains (MTBD), suggesting a role for BPAG1 in binding to the cytoskeleton.
Collagen XVII and BPAG1 are expressed in the CNS
Oligonucleotide primers specific to collagen XVII and the plakin domain of BPAG1 (Brown et al., 1995; Leung et al., 2001) were used to detect expression of these molecules in several regions of the CNS, including the retina, at postnatal day 2 (P2) and in the adult (older than P25; Fig. 1C). BPAG1 isoforms and collagen XVII were coexpressed in the brain cerebellum (Cereb) and olfactory bulb (Olf. Bul.; Fig. 1C). In contrast, in the forebrain (cortex), only BPAG1 isoforms were expressed; no collagen XVII was observed. We noted no major difference between these two ages in the regions surveyed.
Both collagen XVII and BPAG1 isoforms were expressed in retina (Fig. 1C). Although it has been long known that mutations in the BPAG1 gene result in selective neuronal degeneration (Benier et al., 1995), these data are the first to demonstrate the expression of collagen XVII RNA along with BPAG1, its canonical intracellular ligand, in the CNS. To determine which isoforms of BPAG1 are expressed in the retina, we employed a PCR approach. Primers were used that were specific to the known isoforms (arrows in Fig. 1D); all the known isoforms are found in retina. A fragment of a novel BPAG1 isoform was also identified, tentatively named BPAG1c. This isoform contains the coiled-coil domain as well as several spectrin repeats; however, unlike other isoforms, it does not contain either the IFBD2 of the IFBD1 domain. Whether this is a new member of the family or another related molecule awaits the isolation a full-length clone of this molecule, which is ongoing.
Collagen XVII and BPAG1 localization in the adult bovine retina
Collagen XVII immunoreactivity was detected in the neural retina by using three anticollagen XVII antibodies. All three give the same basic pattern and suggest that photoreceptors and Müller cells express collagen XVII (Fig. 2A–C). All three antibodies labeled photoreceptor outer segments (OS) and the OPL, although the labeling of the OPL by the endo2 and NC16a antibodies was slightly stronger than that by the ecto5 antibody (Fig. 2). Together these data suggest localization of collagen XVII to the photoreceptor cell layer. These data are the first to demonstrate the presence of collagen XVII protein in the CNS. Additional data (in situ hybridization and the loss of collagen XVII protein during rd-induced retinal degeneration) support photoreceptor-specific expression of collagen XVII (data not shown). Punctate collagen XVII immunoreactivity was also observed in the second synaptic layer of the retina [the inner plexiform layer (IPL); Fig. 2A] as well as in fibers traversing the retinal inner nuclear layer (INL) and nerve fiber layer (NFL). The latter immunoreactivity most likely represents labeling of the Müller glial cell and is particularly prominent with the NC16a and the ecto5 antibodies (Fig. 2B,C). Minor variations aside, all three antibodies demonstrate that collagen XVII is expressed by photoreceptors and glial cells.
In contrast, BPAG1 immunoreactivity was largely restricted to photoreceptor terminals in the OPL and, very prominently, photoreceptor inner and outer segments (Fig. 2D). Weak staining was detected in the IPL and in the innermost aspect of the retina, perhaps in Müller cell end feet. Taken together, our results suggest that collagen XVII and BPAG1 are coexpressed in the neural retina and colocalize in the synaptic regions in the OPL and at the interface between the photoreceptor and the interphotoreceptor matrix.
Expression of collagen XVII and BPAG1 proteins in retinal compartments
Biochemical studies confirm the presence of authentic collagen XVII in retina and Müller cells (Fig. 2E). Extracts of keratinocytes (Ker; Fig. 2E) contain a protein band with an apparent mobility of 180 kDa corresponding to full-length collagen XVII as well as two proteins with increased mobility, one that corresponds to the shed domain of the molecule (~130 kDa) and a second, apparent degradation product (less than 110 kDa; NC16; Fig. 2E). Extracts prepared from an immortalized line of rat Müller cells (RMC) contained a major band with an apparent mobility of 195 kDa. In extracts prepared from retina (Ret) and retinal pigmented epithelium (RPE), full-length collagen XVII was barely detectable, whereas the shed domain (130-kDa band) was prominent, indicating that much of the collagen XVII in these preparations was cleaved. In contrast, in other preparations of retina, probed with an antibody against the intracellular epitope that does not recognize the shed domain, full-length collagen XVII was evident (Endo2; Fig. 2E). Keratinocyte and RMC-1 extracts were treated with collagenase and digested products evaluated by Western blot. After enzyme treatment, the amount of full-length collagen XVII is dramatically reduced (Ker) or completely absent (RMC), which is accompanied by an increase in lower molecular weight species, consistent with collagenase digestion.
To determine whether collagen XVII and BPAG1 proteins are coexpressed and whether these molecules are present in the synaptic regions of the CNS, we prepared a synaptosome fraction of the retina. Full-length collagen XVII was obtained from both whole retinal homogenates (Ret) and rat retinal synaptosomes (Syn; Fig. 2E). Similarly, BPAG1 was present in both whole homogenate and synaptosomes. Two species of BPAG1 are present. One band (~280 kDa) is consistent with the reported size of BPAG1n (Yang et al., 1996; Dalpe et al., 1998). The other high-molecular-weight protein (above 500 kDa) may represent either the novel BPAG1c isoform (see Fig. 1) or other known large isoforms, BPAG1a and BPAG1b (Leung et al., 2001; Okumura et al., 2002). In contrast, although Müller cells express collagen XVII, they apparently do not synthesize BPAG1 (data not shown).
Collagen XVII is expressed by Müller cells in discrete subcellular compartments
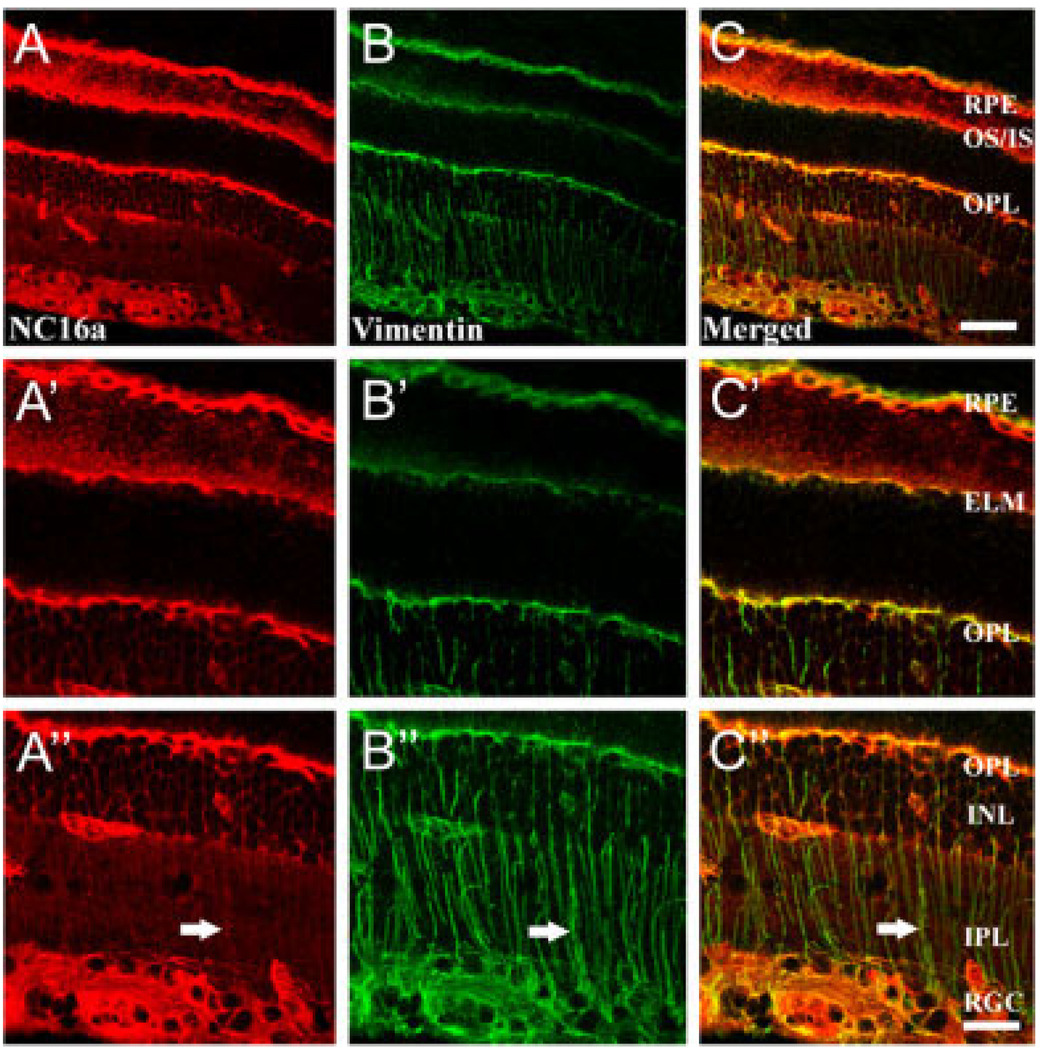
We critically tested whether Müller cells express collagen XVII by employing a specific marker of Müller cells in the retina, vimentin, together with the collagen XVII antibody, NC16a. Simultaneous localization of collagen XVII and vimentin demonstrate that vimentin has a more restricted distribution, exhibiting a typical Müller cell pattern of expression (Fig. 3A–C). Higher power images (Fig. 3A′–C″) reveal some of the subcellular details of the Müller cell. Vimentin immunoreactivity is present in the lateral expansions of the Müller cell in the OPL and in the apical processes at the external limiting membrane (ELM; Fig. 3B′), where the outer segments (OS) of the photoreceptors arise. The terminal expansions (“end feet”) of the Müller cell, surrounding retinal ganglion cells (RGC) at the vitreal border, are also labeled (Fig. 3B″). Vimentin immunoreactivity is present also in the retinal pigmented epithelium (RPE; Fig. 3B,B′). Collagen XVII and vimentin immunoreactivities are codistributed in several subcellular locations: viz. the basal side of the RPE, at the apical processes of the Müller cell, at the horizontal processes of the Müller cells in the OPL, and at the Müller cell end feet (Fig. 3A–C). Little collagen XVII immunoreactivity is present in the radial processes of the Müller cells as they traverse the IPL and INL (Fig. 3A″–C″). BPAG1 immunoreactivity did not colocalize with Müller cell markers. Therefore, we conclude that Müller cells express collagen XVII specifically at expansions of the cell: their end feet, horizontal processes in the OPL, and apical ends at the ELM. These are the points of contact between the Müller cell and the ECM (Libby et al., 2000) and suggest that Müller cells may use collagen XVII as ECM receptors, as epithelial cells do.
Fig. 3.
Collagen XVII is expressed by Müller cells. Images of bovine retina (A–C) with selected regions of the same images at higher power of outer (A′–C′) and inner (A″–C″) retina. Collagen XVII IR (NC16a; A) was colocalized with a marker of the Müller cell (vimentin; B) in bovine retina. Collagen XVII is strongly expressed in the retinal ganglion cell (RGC) layer, outer plexiform layer (OPL), outer segments (OS), retinal pigmented epithelium (RPE), and capillary beds. The merged confocal images (C) demonstrate overlap in the distribution of collagen XVII and vimentin immunoreactivity, particularly in the RGC layer, in the OPL, at the external limiting membrane (ELM), and in the RPE. Scale bar = 90 µm in C (applies to A–C); 45 µm in C″ (applies to A′–C′,A″–C″).
Collagen XVII and BPAG1 are expressed by photoreceptors and near their synapses
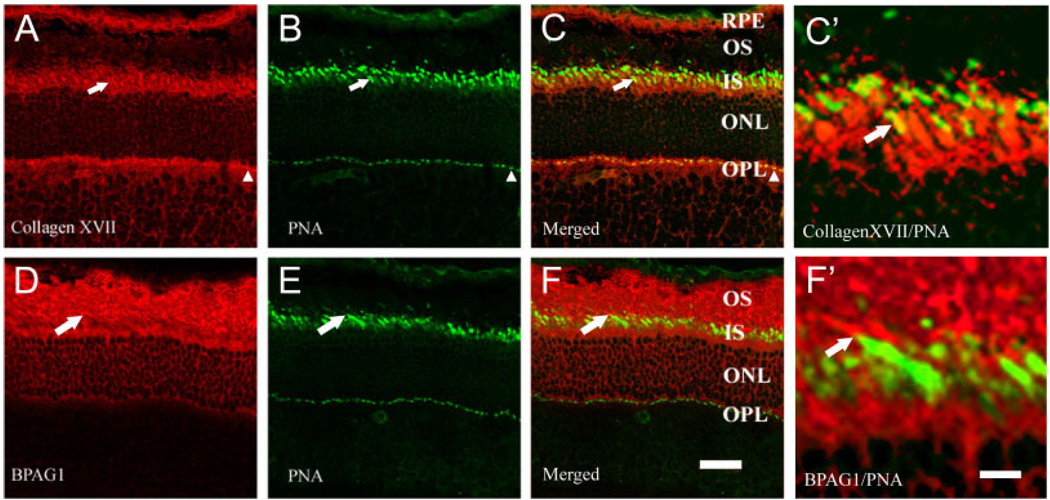
Because collagen XVII immunoreactivity is found in the OPL and over inner and outer segments, we determined whether photoreceptors express collagen XVII by using several photoreceptor markers. Collagen XVII and BPAG1 are both present in the photoreceptor layer of the retina (Fig. 4), and both colocalized with rhodopsin, a marker of rods, and recoverin, a marker of rods and cones (data not shown). Collagen XVII colocalized with PNA binding, a marker of cones; specifically, collagen XVII (Fig. 4A) was present surrounding all PNA-labeled inner segments and synaptic terminals (Fig. 4B), and they are often codistributed (Fig. 4C,C′). In contrast, BPAG1 (Fig. 4D) is associated with neither cone terminals nor cone inner segments (Fig. 4E,F,F′). Taken together, these data support the presence of collagen XVII and BPAG1 in rod photoreceptors, but collagen XVII alone in cones.
Fig. 4.
Collagen XVII and BPAG1 are expressed by photoreceptors. Collagen XVII and BPAG1 were colocalized with the cone marker PNA. A–C: Confocal images show collagen XVII (A) and PNA (B) overlapping in cone photoreceptors. These two overlap (C) both at cone inner segments (arrows) and at cone terminals (arrowheads). D–F: BPAG1 (D) is present in the photoreceptor layer but does not overlap with PNA (E,F), indicating expression of BPAG1 in rod photoreceptors, but not in cones. C′,F′: Higher power images from the regions of cone outer segments indicated with arrows in C and F. Collagen XVII colocalizes with PNA (yellow), whereas BPAG1 and PNA are distinct (red and green). Scale bars = 20 µm in F (applies to A–F); 40 µm in F′ (applies to C′,F′).
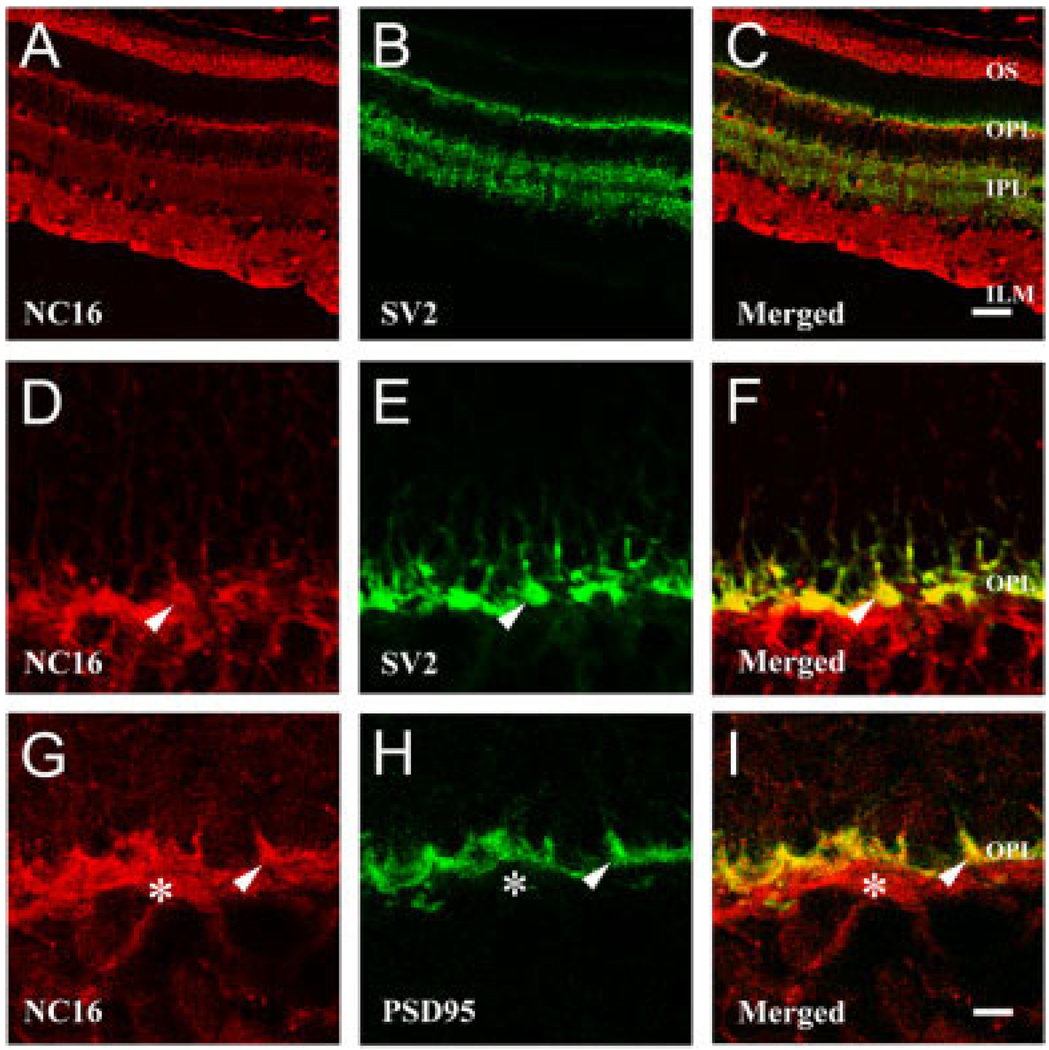
Next, we analyzed expression of these molecules in photoreceptor synaptic regions by comparing collagen XVII (Fig. 5A,D,G) with two synaptic markers, SV2 (Fig. 5B,E) and PSD95 (Fig. 5H), both of which are present in the presynaptic (photoreceptor) terminal. SV2 is expressed in both the OPL and the IPL; its expression colocalizes with that of collagen XVII only in the OPL (Fig. 5C). Higher power images demonstrate that there is extensive colocalization of collagen XVII and SV2 (Fig. 5F) as well as of collagen XVII and PSD 95 (Fig. 5I) in the OPL. Because SV2 and PSD95 are expressed by the photoreceptor terminals and not postsynaptic elements, these observations support photoreceptor expression of collagen XVII. Both rod and cone terminals are labeled by SV2; some cone terminals are particularly clear in Figure 5D–F because of their large size (arrowheads, Fig. 5). In the OPL, the collagen XVII present below the PSD-95-labeled terminals may represent label in the lateral expansion processes of the Müller cell (asterisks, Fig. 5G–I). Nonetheless, the presynaptic and outer segment expression of collagen XVII is certainly consistent with a photoreceptor expression of the molecule.
Fig. 5.
Collagen XVII is expressed in retinal synaptic regions in bovine retina. A–C: Confocal images of immunoreactivity for collagen XVII (NC16; A) and the synaptic vesicle marker (SV2; B) are shown. The two are colocalized only in the OPL (yellow in merged image; C). D–I: Higher power images of the OPL. Collagen XVII immunoreactivity (D,G) was colocalized with two different markers of the photoreceptor synaptic terminal: SV2 (E) and PSD95 (H). There is good colocalization (F,I) of the markers at identifiable terminals, particularly cones (arrowheads); collagen XVII is associated with the entire synaptic bouton. Müller cell expression is likely to account for the extrasynaptic collagen XVII subjacent to the SV2 and PSD95 (asterisks in G–I; compare with Fig. 3). Scale bars = 90 µm in C (applies to A–C); 15 µm in I (applies to D–I).
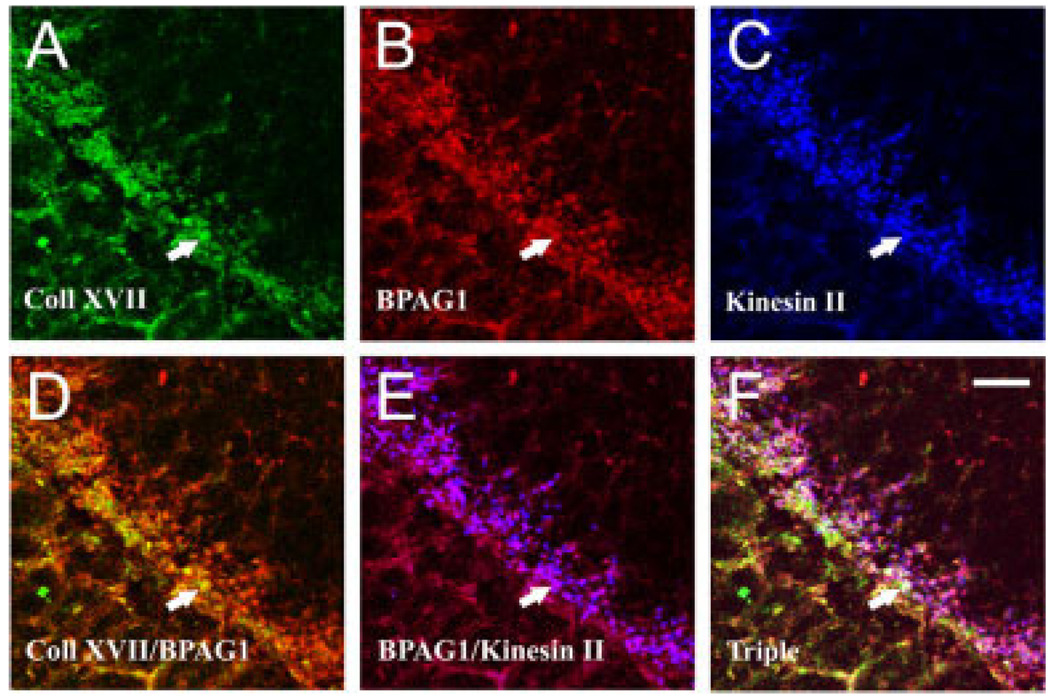
To determine whether collagen XVII and BPAG1 are located near elements of the synaptic transduction cascade, we simultaneously localized BPAG1 and kinesin II by using a direct immunofluorescence technique (see Materials and Methods). In the OPL, collagen XVII (Fig. 6A) and BPAG1 (Fig. 6B) colocalized well (Fig. 6D). Kinesin II is a component of the synaptic ribbon, a docking site for vesicles in the photoreceptor terminal; BPAG1 (Fig. 6B) and kinesin II (Fig. 6C) were closely apposed (Fig. 6E). The triple staining confirmed that collagen XVII, BPAG1, and kinesin II are localized within the same subcellular compartments (Fig. 6F, triple). These anatomical data together with our biochemical data from synaptosomes (Fig. 2) demonstrate that the hemidesmosome components, collagen XVII and BPAG1, are expressed at rod photoreceptor terminals.
Fig. 6.
Collagen XVII and BPAG1 colocalize in photoreceptor terminals. Shown are simultaneous confocal images of collagen XVII (A), BPAG1 (B), and kinesin II (C) in the bovine OPL, which forms a band running at 45°. Single-channel images are given in A–C, and the merged images of the indicated channels are given in D–F. There is strong colocalization of collagen XVII and BPAG1 (yellow in D). Moreover, BPAG1 is colocalized with kinesin II (purple in E), and collagen XVII, BPAG1, and kinesin are closely associated with one another (triple, white in F). Arrows point to the same photoreceptor terminal in all panels. Scale bar = 15 µm in F (applies to A–F).
Developmental expression of collagen XVII and BPAG1 in rat retina
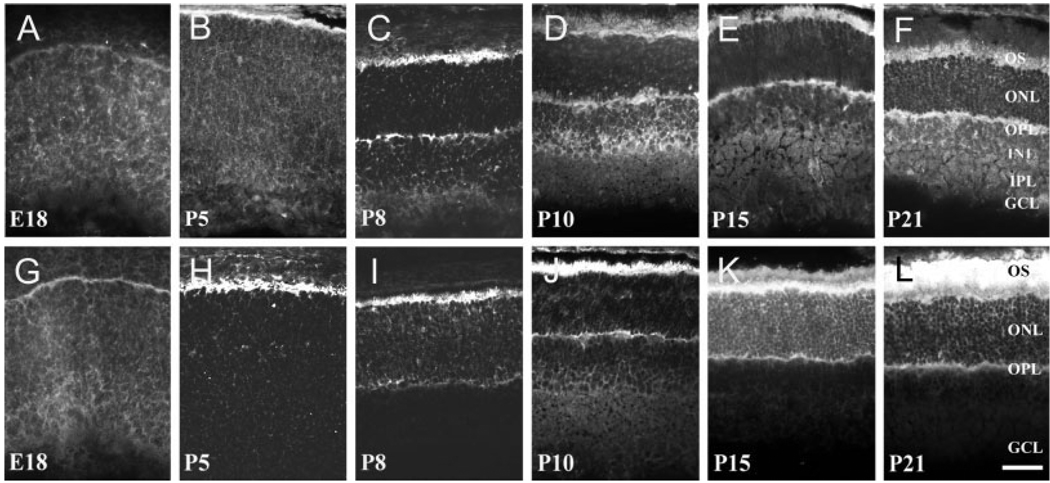
For collagen XVII to serve as a receptor for laminins during development, it should follow a similar time course of expression. Laminin expression is well documented in the developing rat; laminins are expressed first at the apical surface of the undifferentiated neuroepithelium and then become progressively restricted to the interphotoreceptor matrix and synaptic layers (Libby et al., 1996, 2000). At E18, both collagen XVII (Fig. 7A) and BPAG1 (Fig. 7G) were weakly and diffusely expressed throughout the rat retina; some BPAG1 immunoreactivity is seen on the apical surface of the retina (Fig. 7G). At P5, as photoreceptors are undergoing their major morphogenesis, collagen XVII immunoreactivity was present mostly on the apical surface of the retina, the site of rod production (Fig. 7B). At this time, BPAG1 and collagen XVII immunoreactivities colocalize (Fig. 7H). Between P5 and P10, photoreceptors begin to elaborate their synaptic terminals, as an emerging OPL forms. As it does, both collagen XVII and BPAG1 are expressed in both the synaptic layer and the outer segments (Fig. 7C,I). At P10, retinal neurogenesis is nearly complete, and all cell types, including Müller cells, are differentiated and undergoing their final morphogenesis. At this point, the expression of both collagen XVII and BPAG1 was found restricted to the photoreceptor layer of the retina, specifically associated with inner and outer segments and the synaptic layer (Fig. 7D,J). At more mature stages (P15 and P21), BPAG1 immunoreactivity is confined to photoreceptor layer and the OPL (Fig. 7E,F), whereas some weak collagen XVII is seen in processes in the INL and around retinal ganglion cells (Fig. 7K,L). We interpret this staining to be in the Müller cell.
Fig. 7.
Collagen XVII and BPAG1 are expressed in parallel during early retinal development. The time course of collagen XVII (A–F, using endo-2) and BPAG1 (G–L) expression is shown for the rat retina. A–F: In late embryonic development (E18; A), weak and diffuse collagen XVII immunoreactivity (IR) is seen diffusely throughout the neuroepithelium. At P5 (B), collagen XVII is present at the apical surface of the retina around maturing outer segments (OS). At P8 (C), collagen XVII appears in the nascent outer plexiform layer (OPL). At P10 through P21 (D–F), collagen XVII becomes more intense in the OPL and OS, and some labeling of the Müller cells becomes apparent through the inner nuclear layer (INL), inner plexiform layer (IPL), and ganglion cell layer (GCL). G–L: BPAG1 follows a similar pattern of expression during development. It is first diffuse in the embryonic retina (E18, G), with a clear expression in the apical region. At P5 (H), BPAG1 is present in the OS. At P8 (I), prominent IR is present in photoreceptor OS, with diffuse IR in the OPL. From P8 to P21 (J–L), the distribution of BPAG1 IR increases in the OS and in the OPL. The dramatic increase in the expression of BPAG1 in the OS follows the maturation states of these photoreceptor structures. Scale bar = 50 µm in L (applies to A–L).
In summary, the expression of both collagen XVII and BPAG1 by photoreceptors begins early in photoreceptor differentiation and continues as they mature. Moreover, both molecules are expressed at both poles of the cell, the outer segment and synaptic terminal. Although collagen XVII is expressed by Müller glial cells, in vitro and in vivo, no Müller glial expression of a BPAG1 isoform was detected.
β2 Laminin and collagen XVII colocalize and complex with each other
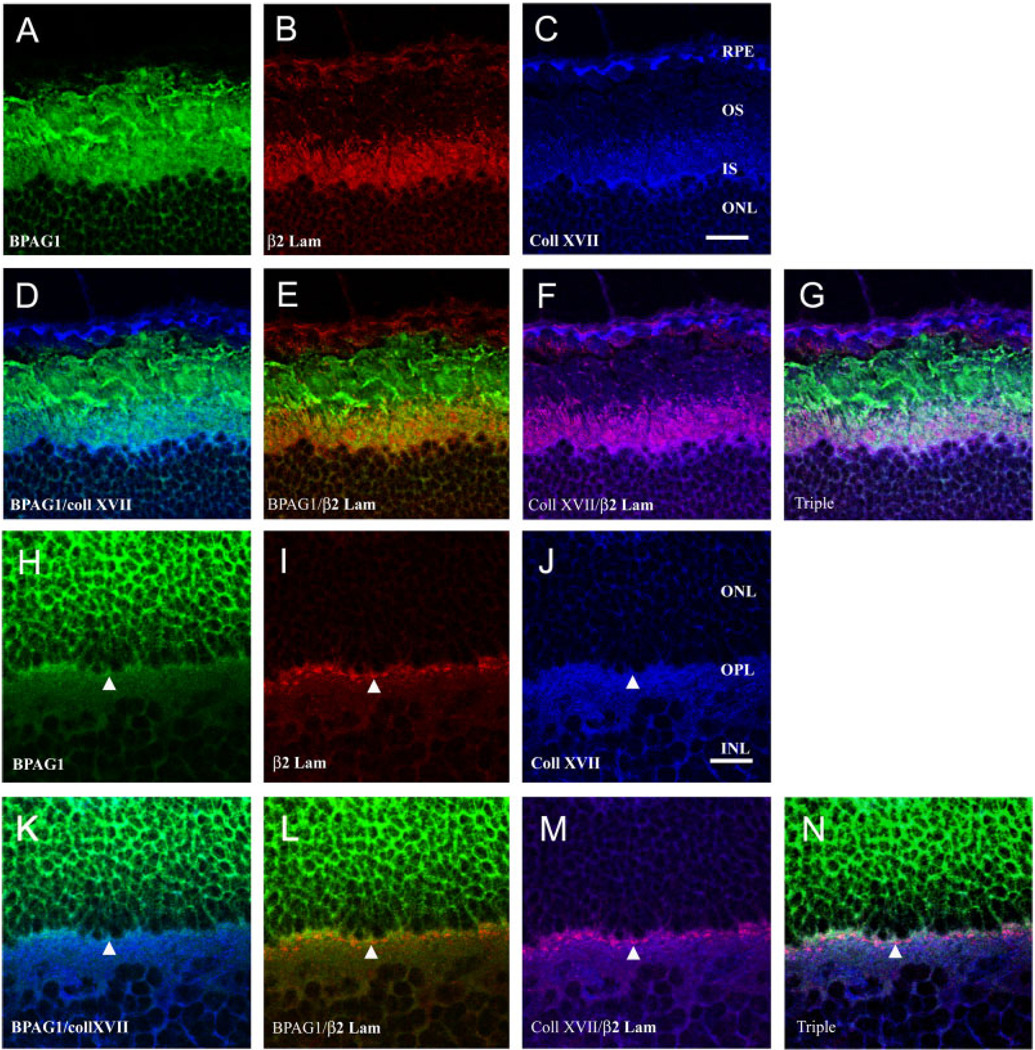
Because the spatial and temporal expression of collagen XVII and BPAG1 both parallel laminin expression in the developing retina, we investigated whether the expressions of collagen XVII and BPAG1 were spatially colocalized with retinal laminins. Adult bovine retinal sections were simultaneously reacted with anti-BPAG1 (Fig. 8A,H), β2 laminin (Fig. 8B,I), and collagen XVII (Fig. 8C,J) antibodies and imaged by confocal microscopy. Collagen XVII and BPAG1 were colocalized in the inner segments (Fig. 8D), where they both overlapped with β2 laminin expression (Fig. 8E,F). It is clear that BPAG1 expression extends farther along the outer segments than either collagen XVII or β2 laminin expression (Fig. 8G). At the photoreceptor synaptic terminal region, all three molecules are coexpressed (Fig. 8K–N).
Fig. 8.
Collagen XVII and β2 laminin chain colocalize in the retina. Colocalization of collagen XVII, BPAG2, and β2-containing laminins by confocal microscopy suggests that these molecules may form a complex at photoreceptor terminals and around photoreceptor inner and outer segments. A–G: Images of the outer retina, near the photoreceptor outer segments; single-channel images for given proteins are shown in A–C, and the indicated merged images are shown in D–G. H–N: Images of the photoreceptor synaptic regions in the OPL; single-channel images for given proteins are shown in H–J and the indicated merged images are shown in K–N. Colocalization of red and green leads to orange/yellow, of blue and red to lavender/purple, and of all three to purple/white. There is strong colocalization of all three markers over the proximal portion of the inner and outer segments (G), and all three proteins are near one another in the OPL (N). Scale bars = 20 µm in C (applies to A–G), J (applies to H–N).
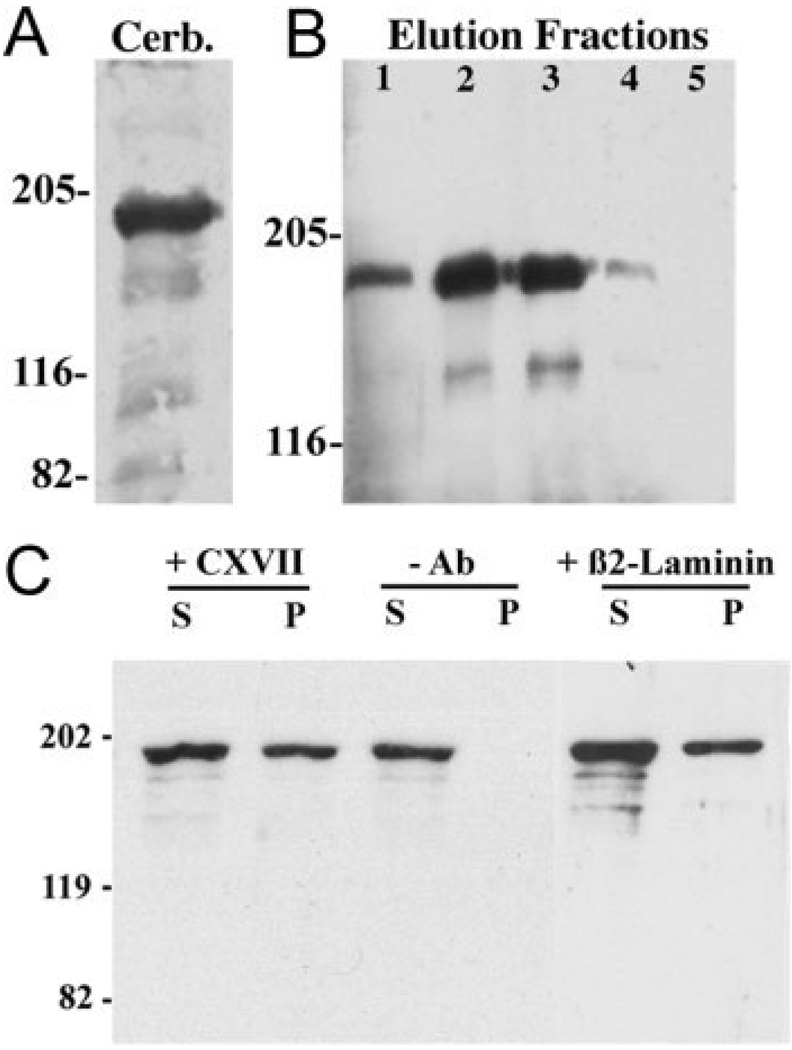
Next, we used a solid-phase assay system to assess whether CNS-derived laminins complex with collagen XVII. Three such laminins (α3β2γ3, α4β2γ3, and α5β2γ3) have been isolated from the CNS (Libby et al., 2000); our strategy was to immobilize these CNS laminins by using an immunoaffinity column directed against the β2 chain and then assay for the presence of bound collagen XVII. To have enough material, we started with bovine cerebellum; as with retina, bovine cerebellum extracts contain full-length collagen XVII and a weak band corresponding to the shed domain (Fig. 9A). Collagen XVII was recovered from these columns, and its release peaked in fractions 2 and 3 (Fig. 9B); the dominant form recovered was the full-length molecule. In these two fractions, the release of laminin β2 chain was maximal (data not shown). There was a direct correlation between the recovery of collagen XVII and the release of laminin β2 chain from the affinity column, clearly indicating an interaction between β2-containing laminins and collagen XVII.
Fig. 9.
Collagen XVII binds to β2-containing laminins in the CNS. A: Protein transfer blot of collagen XVII (using NC16a) demonstrating full-length collagen XVII in bovine cerebellum extract (Cerb.). B: Collagen XVII (full-length and shed ectodomain) is found in elution fractions 1–4 from an anti-β2 column (see text). Collagen XVII eluted in the same fraction as the laminin β2 chain, suggesting that collagen XVII is complexed with β2-containing laminins. C: Immunoprecipitations from RMC-1 extracts were performed with either collagen XVII (NC16a; +CXVII) or laminin β2 (5F11; + β2 laminin) antibodies and revealed with the collagen antibody. Full-length collagen XVII (195 kDa) is present in supernatant (S) fractions under all conditions. Collagen XVII is absent from the pellet (P) fraction in the absence of precipitating antibody (–Ab) but is present in the pellet fraction both when precipitated with anticollagen XVII antibody and when precipitated with anti-β2 laminin antibody. These results indicate that laminins and collagen XVII form a complex.
It is reasonable to extrapolate from our results with an extract from the cerebellum that collagen XVII and β2-containing laminins interact in all areas of the CNS where they are coexpressed. Nevertheless, to determine whether collagen XVII and β2-containing laminins can interact in retinal cells, we tested for this interaction in a retinal cell line. With the NC16a antibody, we performed coimmunoprecipitations of collagen XVII and β2 laminin from a rat Müller cell line (Fig. 9C). Full-length collagen XVII is present in both supernatant and pellet fractions (Fig. 9C, +CXVII), but, when the antibody was omitted (–Ab), collagen XVII was detected only in the supernatant. In parallel, complexes including β2 chain-containing laminins were immunoprecipitated with an anti-β2 chain antibody (Fig. 9C, +β2 laminin), then probed with the collagen XVII antibody. Collagen XVII was present in both supernatant (S) and pellet (P) fractions. Therefore, collagen XVII can be coimmunoprecipitated with β2-containing laminins. Together these data suggest that collagen XVII and CNS-derived laminins complex with each other.
DISCUSSION
In the present study, we demonstrate the coexpression of collagen XVII and BPAG1 in CNS areas such as the retina, cerebellum, and olfactory bulb. In retina, both molecules are colocalized at the rod photoreceptor outer segments and synaptic regions, but only collagen XVII is expressed in cone photoreceptors. Moreover, the temporal and spatial expression of collagen XVII and BPAG1 both parallel that of retinal laminins and, in adult retina, colocalize with β2-containing laminins in the synaptic layer (OPL) and around the inner segments and proximal outer segments. Finally, biochemical data suggest that collagen XVII and β2-containing laminins complex with each other in both cerebellum and retinal cell lines. The likely laminins involved are the recently isolated CNS laminins 13 α3β2γ3, 14 α4β2γ3, and 15 α5β2γ3 (cerebellum; Champliaud et al., unpublished observations; Libby et al., 2000). Such β2-containing laminins appear to be key ECM molecules in CNS stabilization (Libby et al., 1999; Soussand et al., 2001). We hypothesize that CNS laminins (e.g., laminins 13, 14, and 15) bind to an anchoring complex composed of the transmembrane collagen XVII and the cytoskeletal-associated protein BPAG1. Moreover, we theorize that a neural-specific complex anchors the presynaptic terminals to underlying ECM molecules to stabilize the synapse, much as the skin-specific complex of laminins, collagen XVII, and BPAG1 acts to stabilize the dermal–epidermal junction.
Stabilization of the presynaptic element
Both collagen XVII and BPAG1 are expressed in the rod terminal but near the vesicle docking site, i.e., the kinesin-containing ribbon. We have shown that the transmembrane collagen XVII complexes with laminins isolated from brain and retina. We tentatively propose that collagen XVII binds cytoplasmically with BPAG1 and thereby anchors the presynaptic cytomatrix to laminins in the ECM.
The integrity of the photoreceptor synapse appears to be dependent on the presence of β2-containing laminins, because genetic ablation of Lamb2 (the β2 gene) results in dysmorphogensis of the photoreceptor terminal (Libby et al., 1999). Other CNS synapses are affected similarly (Egles et al., unpublished data), as are peripheral synapses (Noakes et al., 1995; Patton et al., 1998). These data suggest that a transmembrane receptor for laminins exists and confers this stabilizing function. Collagen XVII appears to be a good candidate for this receptor. Its temporal and spatial expression parallels that of laminins (see above; see also Libby et al., 1997, 2000), and the molecule has the required molecular affinities; i.e., binding to elements of the ECM and the cytomatrix.
Although the interaction (binding) between collagen XVII and laminins in the CNS has been documented with two biochemical approaches (above), an interaction between collagen XVII and BPAG1 has not been confirmed. However, collagen XVII is thought to bind the epidermally derived form of BPAG1, BPAG1e, via the respective amino-terminal domains (Hopkinson and Jones, 2000; Koster et al., 2003). Although the first 56 amino acids of the neural isoforms of BPAG1 contain a unique ABD domain, Sonnenberg and colleagues have shown that the collagen XVII binding motif within the plakin domain is conserved between neural and epidermal isoforms of BPAG1 (Koster et al., 2003). Thus, based on the available evidence, collagen XVII and BPAG1 isoforms should complex in the CNS as they do in the skin.
In addition, other cytoplasmic ligands for collagen XVII have been identified within the retina. P120ctn catenin is an essential molecule for keratinocyte adhesion and has been suggested to interact with collagen XVII (Aho et al., 1999). P120ctn catenin is expressed in both cerebellum and retina. Its distribution in the retina—in the synaptic layers of the retina and at the outer limiting membrane (Golenhofen and Drenckhahn, 2000)—overlaps that shown above for collagen XVII.
Thus, several elements of keratinocyte adhesion are expressed in the retina at the photoreceptor synapse. It seems reasonable to propose that these molecules are important in synaptic adhesion or stability. It should be mentioned that two forms of collagen XVII might coexist in the OPL, a soluble form released from the membrane and the full-length transmembrane form. Both these forms of collagen XVII contain the ectodomain and, thereby, the putative laminin binding domain. Although the role of the soluble form of the collagen XVII is not known with certainty, it might act as a dominant negative by binding competitively with laminins 14 and 15. In consideration of the function of β2-containing laminins in synapse stability (Libby et al., 1997), the regulation in the release of the shed form of collagen XVII might play a dynamic role in synapse remodeling and plasticity. In this regard, the regulatory function of matrix metalloproteases or other proteases should be investigated.
Collagen XVII and BPAG1 in photoreceptor outer segments
The striking expression of BPAG1 isoforms in rod photoreceptor outer segments might be associated with a role for this molecule in adhesion between the neural retina and the RPE. The rod photoreceptor outer segment is an elongated membrane-enclosed stack of membranous discs, whereas the cone outer segment is a single continuous, but invaginating, membrane. Outer segments and RPE interaction via the interphotoreceptor matrix molecules are essential to prevent retinal detachment and for the constant renewal of the outer segments (Lazarus and Hageman, 1992; Miceli and Newsome, 1996). In rods, there is considerable turnover of the outer segments, with new discs generated by invaginations of the membrane at the base of the outer segment and older disc material removed by phagocytic action of the RPE. It is possible that the differential expression of BPAG1 in these two cell types reflects a role of BPAG1 in outer segment structural stability or synthesis. This hypothesis is compatible with the specific cytoskeletal composition of rod outer segments (Eckmiller, 2000). The dysmorphisms in outer segment formation in the β2-deficient mice suggest a role for laminin 14 and 15 in their morphogenesis (Libby et al., 1999). Taken together with these observations, the data suggest the existence of a macromolecular complex bridging the outer segment membrane to the RPE via the ECM laminins. This complex might include laminins 14 and 15, anchored at the outer segment surface by collagen XVII, which in turn is bound to the rod photoreceptor cytoskeleton via BPAG1 isoforms.
Is BPAG1 a key molecule in retinal development?
BPAG1 might be essential for maintaining photoreceptor cytoskeleton integrity as suggested by the perturbations observed in dystonia musculorum mice (Duchen and Strich, 1964). These mice have a hereditary neurodegenerative disease, leading to an ataxia, which is caused by the absence or the reduction of the BPAG1 (dystonin) protein in neurons (Bernier et al., 1995). The affected neurons exhibit intermediate filament accumulation and perturbed microtubules (Dalpe et al., 1998; Yang et al., 1999). Additional splice variants of BPAG1 containing a microtubule-binding domain have recently been described for the CNS (Leung et al., 2001). The BPAG1 isoforms expressed in the CNS, therefore, have the ability to bind to all three classes of cytoskeletal filaments in neurons. The dystroglycan/dystrophin complex is likely to be an additional anchoring mechanism for β2-containing laminins (Claudepierre et al., 1999; Dalloz et al., 2001). However, the temporal expression of the dystroglycan/dystrophin complex lags laminin expression and therefore may be important in later stages of stabilization and OPL formation. Members of the new plakin family, the microtubule actin cross-linking family (MACF), have recently been cloned, and their sequences reflect a hybrid of both dystrophin and plectin (such as BPAG1) families (Gong et al., 2001). Consequently, if MACFs are expressed in the retina, they may participate with either collagen XVII or dystroglycan in a macromolecular anchoring complex in photoreceptor cells.
Consistently with a role for BPAG1 in synapse formation, the BPAG1 promoter is regulated differentially by homeoproteins Hoxc-8 and Engrailed (Mainguy et al., 2000). Engrailed has been implicated in numerous developmental events, including retinal axon guidance and targeting (Friedman and O’Leary, 1996). Furthermore, in an extensive analysis of candidate retinal disease genes, BPAG1 isoforms have been detected in early embryonic and adult mouse retina (see supplementary Tables S2 and S8 in Blackshaw et al., 2001).
In the present study, we showed for the first time synaptic (and extrasynaptic) expression of a transmembrane collagen molecule—collagen XVII—in the CNS. Moreover, this molecule is expressed together with BPAG1, in rod photoreceptor synaptic regions and outer segments. Taken together, these data suggest the existence of a hemidesmosome-like structure that may participate in the formation or stabilization of OPL synapses. It is tempting to speculate that defects in the composition of these structures may be the cause of some of the neurological symptoms observed in bullous pemphigoid patients (Foureur et al., 2001). Indeed, other studies (Hashimoto et al., 2002) have demonstrated a novel shed portion of a transmembrane collagen is associated with amyloid plaques in Alzheimer’s disease. Thus, transmembrane collagens and the cytomatrix molecules to which they couple are likely to be important in the biology and pathology of the CNS.
ACKNOWLEDGMENTS
Core facilities used in these studies were supported, in part, by NIH grant P30EY13078 (Tufts Center For Vision Research). W.J.B. and L.B.-T. acknowledge the support of Dr. Robert E. Burgeson during their sabbatical leaves in his laboratory. The authors thank Dr. J.C. Jones (Northwestern University Medical School, Chicago, IL) for providing us with mouse monoclonal 10C5.
Grant sponsor: National Institutes of Health; Grant number: R01EY12676 (to W.J.B.); Grant number: R01NS39502 (to W.J.B.); Grant number: R01EY12037 (to D.D.H.); Grant number: DFG SFB293-B3 (to L.B.-T.).
LITERATURE CITED
- Aho S, Uitto J. Direct interaction between the intracellular domains of bullous pemphigoid antigen 2 (BP180) and β4 integrin, hemidesmosomal components of basal keratinocytes. Biochem Biophys Res Commun. 1998;243:694–699. doi: 10.1006/bbrc.1998.8162. [DOI] [PubMed] [Google Scholar]
- Aho S, Rothenberger K, Uitto J. Human p120ctn catenin: tissue-specific expression of isoforms and molecular interactions with BP180/type XVII collagen. J Cell Biochem. 1999;73:390–399. [PubMed] [Google Scholar]
- Anton ES, Sandrock AW, Jr, Matthew WD. Merosin promotes neurite growth and Schwann cell migration in vitro and nerve regeneration in vivo: evidence using an antibody to merosin, ARM-1. Dev Biol. 1994;164:133–146. doi: 10.1006/dbio.1994.1186. [DOI] [PubMed] [Google Scholar]
- Bernier G, Brown A, Dalpe G, De Repentigny Y, Mathieu M, Kothary R. Dystonin expression in the developing nervous system predominates in the neurons that degenerate in dystonia musculorum mutant mice. Mol Cell Neurosci. 1995;6:509–520. doi: 10.1006/mcne.1995.0003. [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Fraioli RE, Furukawa T, Cepko CL. Comprehensive analysis of photoreceptor gene expression and the identification of candidate retinal disease genes. Cell. 2001;107:579–589. doi: 10.1016/s0092-8674(01)00574-8. [DOI] [PubMed] [Google Scholar]
- Brown A, Bernier G, Mathieu M, Rossant J, Kothary R. The mouse dystonia musculorum gene is a neural isoform of bullous pemphigoid antigen 1. Nat Genet. 1995;10:301–306. doi: 10.1038/ng0795-301. [DOI] [PubMed] [Google Scholar]
- Claudepierre T, Rodius F, Frasson M, Fontaine V, Picaud S, Dreyfus H, Mornet D, Rendon A. Differential distribution of dystrophins in rat retina. Invest Ophthalmol Vis Sci. 1999;40:1520–1529. [PubMed] [Google Scholar]
- Claudepierre T, Dalloz C, Mornet D, Matsumura K, Sahel J, Rendon A. Characterization of the intermolecular associations of the dystrophin-associated glycoprotein complex in retinal Muller glial cells. J Cell Sci. 2000;113:3409–3417. doi: 10.1242/jcs.113.19.3409. [DOI] [PubMed] [Google Scholar]
- Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Dalloz C, Claudepierre T, Rodius F, Mornet D, Sahel J, Rendon A. Differential distribution of the members of the dystrophin glycoprotein complex in mouse retina: effect of the mdx(3Cv) mutation. Mol Cell Neurosci. 2001;17:908–920. doi: 10.1006/mcne.2001.0978. [DOI] [PubMed] [Google Scholar]
- Dalpe G, Leclerc N, Vallee A, Messer A, Mathieu M, De Repentigny Y, Kothary R. Dystonin is essential for maintaining neuronal cytoskeleton organization. Mol Cell Neurosci. 1998;10:243–257. doi: 10.1006/mcne.1997.0660. [DOI] [PubMed] [Google Scholar]
- Duchen LW, Strich SJ. Clinical and pathological studies of an hereditary neuropathy in mice (dystonia musculorum) Brain. 1963;87:367–378. doi: 10.1093/brain/87.2.367. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Jarvie PE, Heath JW, Kidd GJ, Rostas JA. A rapid method for isolation of synaptosomes on Percoll gradients. Brain Res. 1986;372:115–129. doi: 10.1016/0006-8993(86)91464-2. [DOI] [PubMed] [Google Scholar]
- Eckmiller MS. Microtubules in a rod-specific cytoskeleton associated with outer segment incisures. Vis Neurosci. 2000;17:711–722. doi: 10.1017/s0952523800175054. [DOI] [PubMed] [Google Scholar]
- Favre B, Fontao L, Koster J, Shafaatian R, Jaunin F, Saurat JH, Sonnenberg A, Borradori L. The hemidesmosomal protein bullous pemphigoid antigen 1 and the integrin β4 subunit bind to ERBIN. Molecular cloning of multiple alternative splice variants of ERBIN and analysis of their tissue expression. J Biol Chem. 2001;276:32427–32436. doi: 10.1074/jbc.M011005200. [DOI] [PubMed] [Google Scholar]
- Fernandez-Valle C, Gwynn L, Wood PM, Carbonetto S, Bunge MB. Anti-beta 1 integrin antibody inhibits Schwann cell myelination. J Neurobiol. 1994;25:1207–1226. doi: 10.1002/neu.480251004. [DOI] [PubMed] [Google Scholar]
- Foureur N, Descamps V, Lebrun-Vignes B, Picard-Dahan C, Grossin M, Belaich S, Crickx B. Bullous pemphigoid in a leg affected with hemiparesia: a possible relation of neurological diseases with bullous pemphigoid? Eur J Dermatol. 2001;11:230–233. [PubMed] [Google Scholar]
- Franzke CW, Tasanen K, Schacke H, Zhou Z, Tryggvason K, Mauch C, Zigrino P, Sunnarborg S, Lee DC, Fahrenholz F, Bruckner-Tuderman L. Transmembrane collagen XVII, an epithelial adhesion protein, is shed from the cell surface by ADAMs. EMBO J. 2002;21:5026–5035. doi: 10.1093/emboj/cdf532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman GC, O’Leary DD. Retroviral misexpression of engrailed genes in the chick optic tectum perturbs the topographic targeting of retinal axons. J Neurosci. 1996;16:5498–5509. doi: 10.1523/JNEUROSCI.16-17-05498.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E, Mark M, Messaddeq N, Gansmuller A. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr Biol. 1998;8:983–986. doi: 10.1016/s0960-9822(98)70402-6. [DOI] [PubMed] [Google Scholar]
- Golenhofen N, Drenckhahn D. The catenin, p120ctn, is a common membrane-associated protein in various epithelial and non-epithelial cells and tissues. Histochem Cell Biol. 2000;114:147–155. doi: 10.1007/s004180000175. [DOI] [PubMed] [Google Scholar]
- Gong TW, Besirli CG, Lomax MI. MACF1 gene structure: a hybrid of plectin and dystrophin. Mamm Genome. 2001;12:852–861. doi: 10.1007/s00335-001-3037-3. [DOI] [PubMed] [Google Scholar]
- Grimpe B, Dong S, Doller C, Temple K, Malouf AT, Silver J. The critical role of basement membrane-independent laminin γ1 chain during axon regeneration in the CNS. J Neurosci. 2002;22:3144–3460. doi: 10.1523/JNEUROSCI.22-08-03144.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Halfter W, Dong S, Yip YP, Willem M, Mayer U. A critical function of the pial basement membrane in cortical histogenesis. J Neurosci. 2002;22:6029–6040. doi: 10.1523/JNEUROSCI.22-14-06029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Wakabayashi T, Watanabe A, Kowa H, Hosoda R, Nakamura A, Kanazawa I, Arai T, Takio K, Mann DM, Iwatsubo T. CLAC: a novel Alzheimer amyloid plaque component derived from a transmembrane precursor, CLAC-P/collagen type XXV. EMBO J. 2002;21:1524–1534. doi: 10.1093/emboj/21.7.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbling-Leclerc A, Zhang X, Topaloglu H, Cruaud C, Tesson F, Weissenbach J, Tome FM, Schwartz K, Fardeau M, Tryggvason K, Guicheney P. Mutations in the laminin α2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy. Nat Genet. 1995;11:216–218. doi: 10.1038/ng1095-216. [DOI] [PubMed] [Google Scholar]
- Hopkinson SB, Jones JC. The N terminus of the transmembrane protein BP180 interacts with the N-terminal domain of BP230, thereby mediating keratin cytoskeleton anchorage to the cell surface at the site of the hemidesmosome. Mol Biol Cell. 2000;11:277–286. doi: 10.1091/mbc.11.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkinson SB, Baker SE, Jones JC. Molecular genetic studies of a human epidermal autoantigen (the 180-kD bullous pemphigoid antigen/BP180): identification of functionally important sequences within the BP180 molecule and evidence for an interaction between BP180 and α6 integrin. J Cell Biol. 1995;130:117–125. doi: 10.1083/jcb.130.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DD, Shah V, Merlie JP, Sanes JR. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature. 1989;338:229–234. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Murphy MD, Olsson CV, Brunken WJ. S-laminin expression in adult and developing retinae: a potential cue for photoreceptor morphogenesis. Neuron. 1992;8:399–413. doi: 10.1016/0896-6273(92)90269-j. [DOI] [PubMed] [Google Scholar]
- Jones JC, Hopkinson SB, Goldfinger LE. Structure and assembly of hemidesmosomes. Bioessays. 1998;20:488–494. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Koch M, Olson PF, Albus A, Jin W, Hunter DD, Brunken WJ, Burgeson RE, Champliaud MF. Characterization and expression of the laminin γ3 chain: a novel, non-basement membrane-associated, laminin chain. J Cell Biol. 1999;145:605–618. doi: 10.1083/jcb.145.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster J, Geerts D, Favre B, Borradori L, Sonnenberg A. Analysis of the interactions between BP180, BP230, plectin and the integrin α6β4 important for hemidesmosome assembly. J Cell Sci. 2003;116:387–399. doi: 10.1242/jcs.00241. [DOI] [PubMed] [Google Scholar]
- Labib RS, Anhalt GJ, Patel HP, Mutasim DF, Diaz LA. Molecular heterogeneity of the bullous pemphigoid antigens as detected by immunoblotting. J Immunol. 1986;136:1231–1235. [PubMed] [Google Scholar]
- Lazarus HS, Hageman GS. Xyloside-induced disruption of interphotoreceptor matrix proteoglycans results in retinal detachment. Invest Ophthalmol Vis Sci. 1992;33:364–376. [PubMed] [Google Scholar]
- Leung CL, Zheng M, Prater SM, Liem RK. The BPAG1 locus: Alternative splicing produces multiple isoforms with distinct cytoskeletal linker domains, including predominant isoforms in neurons and muscles. J Cell Biol. 2001;154:691–697. doi: 10.1083/jcb.200012098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Tamai K, Tan EM, Uitto J. Cloning of type XVII collagen: Complementary and genomic DNA sequences of mouse 180-kilodalton bullous pemphigoid antigen (BPAG2) predict an interrupted collagenous domain, a transmembrane segment, and unusual features in the 5′-end of the gene and the 3′-untranslated region of the mRNA. J Biol Chem. 1993;268:8825–8834. [PubMed] [Google Scholar]
- Libby RT, Hunter DD, Brunken WJ. Developmental expression of laminin β2 in the rat retina. Invest Ophthal Vis Sci. 1996;37:1651–1661. [PubMed] [Google Scholar]
- Libby RT, Xu Y, Selfors LM, Brunken WJ, Hunter DD. Identification of the cellular source of laminin β2 in adult and developing vertebrate retinae. J Comp Neurol. 1997;389:655–667. doi: 10.1002/(sici)1096-9861(19971229)389:4<655::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Libby RT, Lavallee CR, Balkema GW, Brunken WJ, Hunter DD. Disruption of laminin β2 chain production causes alterations in morphology and function in the CNS. J Neurosci. 1999;19:9399–9411. doi: 10.1523/JNEUROSCI.19-21-09399.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby RT, Champliaud MF, Claudepierre T, Xu Y, Gibbons EP, Koch M, Burgeson RE, Hunter DD, Brunken WJ. Laminin expression in adult and developing retinae: evidence of two novel CNS laminins. J Neurosci. 2000;20:6517–6128. doi: 10.1523/JNEUROSCI.20-17-06517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainguy G, Montesinos ML, Lesaffre B, Zevnik B, Karasawa M, Kothary R, Wurst W, Prochiantz A, Volovitch M. An induction gene trap for identifying a homeoprotein-regulated locus. Nat Biotechnol. 2000;18:746–749. doi: 10.1038/77312. [DOI] [PubMed] [Google Scholar]
- Masunaga T, Shimizu H, Yee C, Borradori L, Lazarova Z, Nishikawa T, Yancey KB. The extracellular domain of BPAG2 localizes to anchoring filaments and its carboxyl terminus extends to the lamina densa of normal human epidermal basement membrane. J Invest Dermatol. 1997;109:200–206. doi: 10.1111/1523-1747.ep12319337. [DOI] [PubMed] [Google Scholar]
- McGrath JA, Gatalica B, Christiano AM, Li K, Owaribe K, McMillan JR, Eady RA, Uitto J. Mutations in the 180-kD bullous pemphigoid antigen (BPAG2), a hemidesmosomal transmembrane collagen (COL17A1), in generalized atrophic benign epidermolysis bullosa. Nat Genet. 1995;11:83–86. doi: 10.1038/ng0995-83. [DOI] [PubMed] [Google Scholar]
- Miceli MV, Newsome DA. Effects of extracellular matrix and Bruch’s membrane on retinal outer segment phagocytosis by cultured human retinal pigment epithelium. Curr Eye Res. 1996;15:17–26. doi: 10.3109/02713689609017607. [DOI] [PubMed] [Google Scholar]
- Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, Hoshi T, Campbell KP. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418:422–425. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- Niessen CM, Hogervorst F, Jaspars LH, de Melker AA, Delwel GO, Hulsman EH, Kuikman I, Sonnenberg A. The alpha 6 beta 4 integrin is a receptor for both laminin and kalinin. Exp Cell Res. 1994;211:360–367. doi: 10.1006/excr.1994.1099. [DOI] [PubMed] [Google Scholar]
- Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin β2. Nature. 1995;374:258–262. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- Okumura M, Yamakawa H, Ohara O, Owaribe K. Novel alternative splicings of BPAG1 (bullous pemphigoid antigen 1) including the domain structure closely related to MACF (microtubule actin cross-linking factor) J Biol Chem. 2002;277:6682–6687. doi: 10.1074/jbc.M109209200. [DOI] [PubMed] [Google Scholar]
- Olson EC, Walsh CA. Smooth, rough and upside-down neocortical development. Curr Opin Genet Dev. 2002;12:320–327. doi: 10.1016/s0959-437x(02)00305-2. [DOI] [PubMed] [Google Scholar]
- Patton BL, Chiu AY, Sanes JR. Synaptic laminin prevents glial entry into the synaptic cleft. Nature. 1998;393:698–701. doi: 10.1038/31502. [DOI] [PubMed] [Google Scholar]
- Patton BL, Cunningham JM, Thyboll J, Kortesmaa J, Westerblad H, Edstrom L, Tryggvason K, Sanes JR. Properly formed but improperly localized synaptic specializations in the absence of laminin alpha4. Nat Neurosci. 2001;4:597–604. doi: 10.1038/88414. [DOI] [PubMed] [Google Scholar]
- Schaapveld RQ, Borradori L, Geerts D, van Leusden MR, Kuikman I, Nievers MG, Niessen CM, Steenbergen RD, Snijders PJ, Sonnenberg A. Hemidesmosome formation is initiated by the beta4 integrin subunit, requires complex formation of beta4 and HD1/plectin, and involves a direct interaction between beta4 and the bullous pemphigoid antigen 180. J Cell Biol. 1998;142:271–284. doi: 10.1083/jcb.142.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäcke H, Schumann H, Hammami-Hauasli N, Raghunath M, Bruckner-Tuderman L. Two forms of collagen XVII in keratinocytes. A full-length transmembrane protein and a soluble ectodomain. J Biol Chem. 1998;273:25937–25943. doi: 10.1074/jbc.273.40.25937. [DOI] [PubMed] [Google Scholar]
- Schumann H, Baetge J, Tasanen K, Wojnarowska F, Schacke H, Zillikens D, Bruckner-Tuderman L. The shed ectodomain of collagen XVII/BP180 is targeted by autoantibodies in different blistering skin diseases. Am J Pathol. 2000;156:685–695. doi: 10.1016/S0002-9440(10)64772-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussand J, Jahke R, Simon-Assmann P, Stoeckel ME, Schimchowitsch S. Tenascin and laminin function in target recognition and central synaptic differentiation. Neuroreport. 2001;12:1073–1076. doi: 10.1097/00001756-200104170-00041. [DOI] [PubMed] [Google Scholar]
- Van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat. Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- Wewer UM, Durkin ME, Zhang X, Laursen H, Nielsen NH, Towfighi J, Engvall E, Albrechtsen R. Laminin β2 chain and adhalin deficiency in the skeletal muscle of Walker-Warburg syndrome (cerebroocular dysplasia-muscular dystrophy) Neurology. 1995;45:2099–2101. doi: 10.1212/wnl.45.11.2099. [DOI] [PubMed] [Google Scholar]
- Yang B, Jung D, Motto D, Meyer J, Koretzky G, Campbell KP. SH3 domain-mediated interaction of dystroglycan and Grb2. J Biol Chem. 1995;270:11711–11714. doi: 10.1074/jbc.270.20.11711. [DOI] [PubMed] [Google Scholar]
- Yang Y, Dowling J, Yu QC, Kouklis P, Cleveland DW, Fuchs E. An essential cytoskeletal linker protein connecting actin microfilaments to intermediate filaments. Cell. 1996;86:655–665. doi: 10.1016/s0092-8674(00)80138-5. [DOI] [PubMed] [Google Scholar]
- Yang Y, Bauer C, Strasser G, Wollman R, Julien JP, Fuchs E. Integrators of the cytoskeleton that stabilize microtubules. Cell. 1999;98:229–238. doi: 10.1016/s0092-8674(00)81017-x. [DOI] [PubMed] [Google Scholar]