Abstract
Methamphetamine (METH) users showed structural and chemical abnormalities on magnetic resonance (MRI) studies, particularly in the frontal and basal ganglia brain regions. Diffusion tensor imaging (DTI) may provide further insights regarding the microstructural changes in METH users. We investigated diffusion tensor measures in frontal white matter and basal ganglia of 30 adult METH users and 30 control subjects using a 3 T MR scanner. Compared with healthy control subjects, METH users showed lower fractional anisotropy (FA) in right frontal white matter, and higher apparent diffusion coefficient (ADC) in left caudate and bilateral putamen. Higher left putamen ADC was associated with earlier initiation of METH use, greater daily amounts, and a higher cumulative lifetime dose. Similarly, higher right putamen ADC was associated with greater daily amounts and a higher cumulative lifetime dose. The lower FA in the right frontal white matter suggests axonal injury in these METH users. The higher ADC in the basal ganglia suggests greater inflammation or less myelination in these brain regions of those with younger age of first METH use and greater METH usage.
Keywords: MRI, Diffusion tensor imaging, Frontal lobe, Basal ganglia, Apparent diffusion coefficient
1. Introduction
Methamphetamine (METH) is a highly addictive psychostimulant that has been widely abused by many individuals in Asian countries and has become a major public health concern in the United States (Elkashef et al., 2007; Iritani et al., 2007). Methamphetamine abuse is greatest in the Western regions of the United States and represents 1.6% of the population aged 12 years or older, as compared with 0.3 to 0.7% in other regions (SAMHSA, 2007). In the United States, the highest prevalence (2.2%) is reported in the Asian Pacific Islander population, while the lowest prevalence (0.1%) is observed in African Americans. Understanding the biological effects of the drug on the brain may provide the basis for future studies to determine possible genetic influences on addiction to methamphetamine.
Magnetic resonance (MR) techniques have been applied to provide in vivo biological information in human METH users. Structural MR imaging of adult METH users demonstrated abnormalities in the frontal lobe, including lower gray matter density or volumes (Thompson et al., 2004; Kim et al., 2006), larger white matter volumes (Thompson et al., 2004), and more white matter hyperintensities (Bae et al., 2006). Moreover, larger volumes were observed in the parietal cortex (Jernigan et al., 2005) and striatal regions of METH users (Chang et al., 2005a; Jernigan et al., 2005). Whether these enlarged brain regions reflect inflammatory changes and the smaller brain regions reflect brain cell loss is unclear. Proton MR spectroscopy (1H MRS) studies reported decreased neuronal marker N-acetylaspartate (NA) or NA-to-total creatine (tCR) ratios (NA/tCR) in frontal regions (Ernst et al., 2000; Sekine et al., 2002; Salo et al., 2007) and striatum (Ernst et al., 2000; Sekine et al., 2002; Chang et al., 2005b), which suggest neuronal injury or loss in chronic METH abusers. Furthermore, increased concentrations of the glial markers choline (CHO), CHO/CR and myoinositol (MI) were found in the frontal lobes, which would suggest neuroinflammation in chronic METH users (Ernst et al., 2000; Nordahl et al., 2002; Chang et al., 2005b; Sung et al., 2007). These MR studies collectively demonstrate that chronic METH abuse damages neural systems in the frontal cortex and striatal brain regions (Chang et al., 2007; Berman et al., 2008).
MR diffusion tensor imaging (DTI) is a sensitive technique that might detect microstructural changes associated with axonal or myelin loss or increased extracellular water accumulation most likely associated with increased inflammation. DTI measures the Brownian (random) movement of water molecules within tissues and provides parameters of the diffusion tensor, including, fractional anisotropy (FA) and the eigenvalue diffusion coefficient for each direction, which are averaged to yield the mean apparent diffusion coefficient (ADC).
In white matter tissue, FA describes the organization or integrity of white matter fiber bundles while ADC describes the three-dimensional mobility of water in the brain tissue (Le Bihan et al., 2001). The direction of water diffusion along the longitudinal axis of the axon (axial diffusion) and the perpendicular axis of the myelinated fibers (radial diffusion) are characterized by the corresponding eigenvalues (Alexander et al., 2007; Pfefferbaum et al., 2008). Damage to the axon terminals is reflected in lower axial diffusion values while damage to myelin would be reflected in higher radial diffusion values with minimal influence to axial diffusion (Alexander et al., 2007). Furthermore, individuals with neuronal and axonal injury or increased water content such as that associated with inflammation would show higher ADC (Irwan et al., 2005; Alexander et al., 2007) and lower FA (Tang et al., 2007).
Previous DTI studies of human METH abusers reported lower FA in the frontal white matter (Chung et al., 2007), and lower FA and axial diffusion values, as well as higher radial diffusion values in the genu of the corpus callosum (Kim et al., 2009; Salo et al., 2009). These findings suggest injury to the frontal and callosal white matter from chronic METH abuse. However, the basal ganglia, brain regions often affected by METH, were not evaluated in these studies. The purpose of the current investigation is to examine whether the diffusion tensor properties are abnormal in various white matter and subcortical brain regions, including the frontal white matter and basal ganglia structures, in a cohort of METH abusers and healthy comparison subjects. We hypothesized that chronic METH users would demonstrate diffusion tensor abnormalities, with higher diffusion and lower FA, in the frontal white matter and the striatal regions.
2. Methods
2.1. Participants
More than 100 subjects were screened, but more than 40 subjects did not meet the study criteria and were not included in the analysis. Thirty subjects with a history of methamphetamine (METH) dependence (23 men, 7 women) and 30 age- and sex-matched comparison subjects were studied. All subjects were evaluated with detailed drug use history and psychiatric evaluations during face-to-face interviews by a trained research staff and verified by one of the board-certified clinicians (DA or LC). Cumulative METH use was determined by detailed interviews regarding quantity of METH use per day, number of days per week, and duration of use. The influence of METH use on the subject's life was assessed with the Addiction Severity Index and subjective reports of craving were assessed with the Visual Analog Scale. The structured assessments included the Symptom Checklist-90 (SCL-90) and the Center for Epidemiologic Study-Depression (CES-D) questionnaire to assess psychiatric symptoms. Any subject who reported a psychiatric diagnosis currently or in the past, was receiving or had received psychotropic medication, scored a value greater than 16 on the CES-D or had significant responses on the SCL-90 was further evaluated with an abbreviated psychiatric assessment by the clinician in order to clarify the diagnosis and to assess stability or further referrals. Subjects received additional assessments including physical and neurological examinations as well as laboratory screening blood and urine tests.
METH subjects were included in the study only if they fulfilled the following inclusion criteria: 1) men and women of any ethnicity ages ≥18 years old; 2) meeting DSM-IV-TR (American Psychiatric Association, 2000) criteria for a history of METH dependence with average use of at least 0.2 g/day, at least 2 days/week, for at least 6 months; 3) provided a negative urine drug screen for the following drugs of abuse: cocaine, opiates, benzodiazepines, cannabinoids; 4) had no current or history of dependence on other drugs of abuse including alcohol, cocaine, opiates, cannabis, inhalants and barbiturates (but not nicotine); and 5) were willing and able to provide informed consent. Potential participants were excluded from the study if they had 1) co-morbid or history of psychiatric illness (e.g. schizophrenia, major depression unrelated to drug withdrawal), neurological disorder (e.g. multiple sclerosis, Parkinson's Disease, other primary degenerative brain diseases, any brain infections or neoplasms), or major chronic medical illnesses (e.g. diabetes, uncontrolled hypertension, heart disease) that may confound the results or analysis of this study; 2) metallic or electronic devices, or implants (e.g., pacemaker, surgical clips, pumps, etc.); or 3) were pregnant.
Thirty comparison subjects with the same age range and sex proportion (23 men, 7 women) were also studied. These subjects fulfilled the same inclusion and exclusion criteria as the METH users, excluding a history of drug abuse or dependence other than nicotine (including alcohol, cocaine, methamphetamine, opiates, cannabis, inhalants, and barbiturates). All subjects signed an informed consent approved by both the University of Hawaii, Committee on Human Studies and the Queen's Medical Center, Research and Institutional Review Committee.
2.2. Acquisition and processing of diffusion tensor Imaging
MR scans were performed on a 3 T Siemens Trio scanner (Siemens Medical Solutions, Erlangen, Federal Republic of Germany), using the following spin-echo sequences: three structural scans (1) 3-plane localizer, repetition time (RT)/echo time (TE); averages (TR/TE=20/5 ms; 208×256×1 matrix), field of view of 24×24 cm, (2) sagittal high-resolution 3D magnetization-prepared rapid gradient echo (MP-RAGE) (TR/TE/inversion time (TI)=2200/4.91/1000 ms, 208×256×144), (3) Transversal fluid-attenuated inversion recovery (FLAIR) (TR/TE=1000/85 ms, 205×32×28), and two full brain axial sections were acquired with 4 mm slice thickness diffusion scans (1) Transversal DWI (TR/TE=4000/80 ms, 128×128×28 matrix), b =(0,1000) s/mm2, (2) Transversal DTI (TR/TE=3700/88 ms, 128×128×28), b factor=([0,1000] s/mm2, 12 directions). All images from subjects included in this study were reviewed to ensure that there were no structural abnormalities, excess motion, and other artifacts.
2.3. Diffusion image analyses
Diffusion scans were processed using DtiStudio version 2.03 (Jiang et al., 2006). The diffusion tensor yielded eigenvalue—0(λ0), eigenvalue—1 (λ1) and eigenvalue—2 (λ2). Based on the eigenvalues, FA, ADC, axial (longitudinal) diffusion (λ0) and radial (transverse) diffusion ((λ1+λ2) /2) values were measured for each region of interest (ROI). The same ROI was used for all diffusion measurements. First, an ROI was placed to measure the FA value using the axial FA map of the DtiStudio software program. The same ROI was then applied to measure the axial ADC, λ0, λ1 and λ2 maps for each anatomic structure of interest. The variance in the measured values and the partial volume artifacts from surrounding CSF were minimized by cross-referencing the ROI placement with the available coronal and sagittal images. ROIs were drawn on the axial slices in which the respective structure appeared largest and most delineated.
All drawings were completed by investigators blinded to the subjects' status. In most cases, ROIs for the right and left frontal white matter (5×5×2 mm oval), the right and left parietal white matter (5×5×2 mm oval), the genu of the corpus callosum (8×2×2 mm rectangle), and right and left caudate (3.5×3.5×2 mm oval) were drawn on the axial slice where the caudate appeared largest and most delineated. In cases where the boundaries of the genu and frontal white matter were smaller than the size of the ROI, the slice just superior to the caudate's axial slice was used. ROIs for the right and left putamen (4×4×2 mm oval), right and left thalamus (5×5×2 mm oval), and cerebellar vermis (5×5×2 mm oval) were each drawn on the axial slices in which the respective structure appeared largest and most delineated (see Fig. 1).
Fig. 1.
Axial views of regions of interest (ROI) placement on the fractional anisotropy (FA) maps for measurement of FA, ADC and the diffusion eigenvalues. All FA and diffusion values were measured bilaterally in these brain regions, except for the genu of the corpus callosum and the cerebellar vermis.
Intrarater reliabilities of the manual ROI analysis method were calculated for FA, ADC and axial and radial diffusion values in all ROIs examined. The operator repeated the manual ROI placements on the FA maps and measured the FA, ADC and eigenvalue diffusion values from the single ROI from a random series of 10 scans. Mean intraclass correlation coefficients (ICC) ranged from r=0.41 to r=0.99.
2.4. Statistical analyses
Statistical analyses were performed using StatView 5.0.1 (SAS Institute Inc., Cary, NC). Repeated measures analysis of variance (ANOVA with brain hemisphere as a within-subject measure and drug use as a between-subject measure) and Student t-tests (two-tailed) were performed to determine significance of differences between the DTI measures in METH users (n=30) and non-drug user comparison subjects (n=30). Mean±standard deviations are reported for clinical and imaging measures. To test for possible relationships between drug exposure measures and diffusion measures, linear regression analyses were performed using DTI values as dependent variables, and drug use measures as independent variables. To reduce the number of correlations, only DTI measures that showed significant group differences (METH vs. control) were tested. A type 1 error probability≤0.05 was used to determine significance.
3. Results
3.1. Demographics and clinical data
METH subjects (age 33.3±8.6, range 20-51 years) had similar ages to the control subjects (32.7±9.5, range 20–56 years). The METH users had slightly but significantly lower educational attainment (12.9±1.4, range 11–18 years) compared with controls (14.1±1.6, range 12–17 years), t(58)=2.9, P=0.005.
The primary route of METH use was by smoking of the crystalline form called “ice,” and none of the METH-using subjects took METH intravenously. On average, the 30 METH subjects first used METH at the age of 21.9±6.1 years (median=21 years, range 13–33 years). Although METH usage varied both across and within subjects, the average use by each subject was entered into the calculations. Typically, METH was used 5.3±1.7 days/week (median=5.3 days/week, range: 2–7 days/week) at a dose of 0.71±0.52 g/day (median=0.53 g/day, range: 0.2–2.3 g/day) for a period of 124.2±72.7 months (median=126, range: 10–312 months) and a lifetime METH cumulative dose of 2,367±3,033 g (median=1,336 g, range: 18–14,014 g). METH abusers had last used 140±400 days (median=24 days, range: 0–1,798 days) prior to the study.
3.2. Diffusion tensor analysis
3.2.1. Effect of METH on apparent diffusion coefficient (ADC)
Relative to healthy comparison subjects, METH users had significantly higher ADC values in the putamen (F1, 58=5.7, P=0.02) and a trend toward higher ADC in the caudate (F1, 58=3.6, P=0.06) when the two hemispheres were included in repeated measures ANOVA. However, since slight but significant hemispheric effects were observed in several brain regions, post hoc analyses were performed. On post hoc analyses, METH users showed higher ADC values in the left caudate nucleus (+2.3%, t(58)=−2.1, P=0.04), and bilaterally in the putamen [right: +4.2%, t(58)=−2.2, P=0.03); left: +3.2%, t(58)=−2.0, P=0.05)]; see Fig. 2A and Table 1.
Fig. 2.
Compared with healthy non-drug users, METH users showed higher apparent diffusion coefficients (ADC) in the left caudate nucleus and bilateral putamen (A), and lower fractional anisotropy (FA) in bilateral frontal white matter (B). Error bars represent standard deviations.
Table 1.
Mean DTI values in brain regions of methamphetamine users and healthy comparison subjects.
| Region | Hemi | ICC | Controls (n=30) |
METH users (n=30) |
% Difference |
METH effect |
Hemi effect |
METH× Hemi |
METH vs. controls |
|---|---|---|---|---|---|---|---|---|---|
| P-value | P-value | P-value | P-value | ||||||
| Apparent diffusion coefficient ×10 3 | |||||||||
| Putamen | Right | 0.46 | 7.21±0.64 | 7.51±0.35 | 4.2% | 0.020 | <0.001 | 0.648 | 0.028 |
| Left | 0.72 | 7.58±0.52 | 7.83±0.44 | 3.2% | 0.052 | ||||
| Caudate | Right | 0.64 | 7.59±0.33 | 7.66±0.24 | 0.9% | 0.064 | 0.330 | 0.271 | 0.353 |
| Left | 0.72 | 7.59±0.36 | 7.76±0.28 | 2.3% | 0.044 | ||||
| Thalamus | Right | 0.87 | 8.13±0.33 | 8.13±0.29 | 0.0% | 0.863 | 0.010 | 0.739 | 0.984 |
| Left | 0.93 | 8.06±0.26 | 8.04±0.26 | −0.3% | 0.748 | ||||
| Frontal WM | Right | 0.89 | 7.85±0.35 | 7.95±0.32 | 1.2% | 0.397 | 0.030 | 0.657 | 0.275 |
| Left | 0.99 | 7.78±0.43 | 7.83±0.43 | 0.8% | 0.600 | ||||
| Parietal WM | Right | 0.91 | 8.36±0.48 | 8.35±0.49 | −0.2% | 0.891 | 0.093 | 0.664 | 0.912 |
| Left | 0.93 | 8.22±0.52 | 8.26±0.50 | 0.5% | 0.737 | ||||
| Genu CC | Medial | 0.98 | 8.14±0.37 | 8.20±0.37 | 0.7% | n.a. | n.a. | n.a. | 0.560 |
| Cerebellar vermis | Medial | 0.81 | 8.40±0.58 | 8.43±0.82 | 0.3% | n.a. | n.a. | n.a. | 0.898 |
| Fractional anisotropy | |||||||||
| Putamen | Right | 0.94 | 0.165±0.025 | 0.174±0.023 | 5.5% | 0.198 | 0.009 | 0.470 | 0.151 |
| Left | 0.93 | 0.158±0.026 | 0.163±0.020 | 2.7% | 0.465 | ||||
| Caudate | Right | 0.77 | 0.168±0.027 | 0.166±0.025 | −1.4% | 0.841 | 0.010 | 0.266 | 0.730 |
| Left | 0.58 | 0.173±0.033 | 0.178±0.028 | 2.9% | 0.532 | ||||
| Thalamus | Right | 0.94 | 0.267±0.026 | 0.277±0.025 | 3.6% | 0.313 | 0.006 | 0.267 | 0.148 |
| Left | 0.83 | 0.260±0.025 | 0.261±0.024 | 0.3% | 0.917 | ||||
| Frontal WM | Right | 0.94 | 0.406±0.043 | 0.362±0.032 | −10.8% | 0.002 | 0.931 | 0.162 | <0.001 |
| Left | 0.98 | 0.397±0.061 | 0.370±0.049 | −6.9% | 0.061 | ||||
| Parietal WM | Right | 0.94 | 0.385±0.055 | 0.410±0.052 | 6.4% | 0.430 | 0.267 | 0.079 | 0.079 |
| Left | 0.93 | 0.410±0.056 | 0.404±0.064 | −1.4% | 0.715 | ||||
| Genu CC | Medial | 0.99 | 0.782±0.041 | 0.782±0.046 | −0.1% | n.a. | n.a. | n.a. | 0.953 |
| Cerebellar vermis | Medial | 0.82 | 0.205±0.031 | 0.214±0.031 | 4.1% | n.a. | n.a. | n.a. | 0.303 |
| Axial diffusivity ×10−4 | |||||||||
| Putamen | Right | 0.63 | 8.47±0.74 | 8.77±0.57 | 3.5% | 0.043 | <0.001 | 0.828 | 0.077 |
| Left | 0.80 | 8.83±0.61 | 9.10±0.51 | 3.1% | 0.063 | ||||
| Caudate | Right | 0.84 | 8.94±0.43 | 8.96±0.34 | 0.2% | 0.030 | 0.016 | 0.015 | 0.871 |
| Left | 0.79 | 8.94±0.44 | 9.31±051 | 4.1% | 0.004 | ||||
| Thalamus | Right | 0.93 | 10.36±0.41 | 10.40±0.34 | 0.4% | 0.877 | 0.001 | 0.490 | 0.639 |
| Left | 0.82 | 10.21±0.30 | 10.19±0.38 | −0.2% | 0.810 | ||||
| Frontal WM | Right | 0.91 | 11.39±0.74 | 11.24±0.91 | −1.3% | 0.538 | 0.063 | 0.723 | 0.484 |
| Left | 0.86 | 11.16±0.79 | 11.08±0.83 | −0.7% | 0.714 | ||||
| Parietal WM | Right | 0.92 | 11.97±0.89 | 12.19±0.81 | 1.8% | 0.531 | 0.604 | 0.397 | 0.312 |
| Left | 0.93 | 12.01±0.97 | 12.02±0.79 | 0.1% | 0.957 | ||||
| Genu CC | Medial | 0.98 | 17.72±0.97 | 17.83±0.98 | 0.6% | n.a. | n.a. | n.a. | 0.663 |
| Cerebellar vermis | Medial | 0.83 | 10.20±0.64 | 10.30±0.84 | 1.0% | n.a. | n.a. | n.a. | 0.616 |
| Radial diffusivity ×10−4 | |||||||||
| Putamen | Right | 0.41 | 6.58±0.62 | 6.81±0.47 | 3.5% | 0.054 | <0.001 | 0.998 | 0.108 |
| Left | 0.71 | 6.96±0.50 | 7.19±0.42 | 3.3% | 0.058 | ||||
| Caudate | Right | 0.97 | 6.97±0.40 | 6.98±0.32 | 0.1% | 0.112 | 0.324 | 0.075 | 0.926 |
| Left | 0.89 | 6.91±0.40 | 7.17±0.54 | 3.8% | 0.037 | ||||
| Thalamus | Right | 0.86 | 7.02±0.36 | 7.00±0.32 | −0.3% | 0.769 | 0.387 | 0.996 | 0.789 |
| Left | 0.93 | 6.99±0.31 | 6.96±0.38 | −0.4% | 0.788 | ||||
| Frontal WM | Right | 0.95 | 6.11±0.47 | 6.32±0.42 | 3.4% | 0.126 | 0.389 | 0.704 | 0.072 |
| Left | 0.92 | 6.08±0.58 | 6.25±0.59 | 2.8% | 0.268 | ||||
| Parietal WM | Right | 0.95 | 6.56±0.55 | 6.43±0.55 | −2.0% | 0.776 | 0.076 | 0.200 | 0.347 |
| Left | 0.95 | 6.32±0.55 | 6.39±0.63 | 1.1% | 0.687 | ||||
| Genu CC | Medial | 0.99 | 3.43±0.63 | 3.38±0.59 | −1.5% | n.a. | n.a. | n.a. | 0.790 |
| Cerebellar vermis | Medial | 0.82 | 7.50±0.59 | 7.49±0.84 | −0.1% | n.a. | n.a. | n.a. | 0.940 |
DTI data values in bold reflect a type 1 error probability ≤ 0.05 used to determine significance.
3.2.2. Effect of METH on fractional anisotropy (FA)
Hemispheric differences were observed in the putamen, caudate and thalamus; but these subcortical regions did not show a METH effect. However, in the frontal white matter, METH users showed lower FA values compared with non-users both on repeated measures ANOVA, when the two hemispheres were included (F1,58=10.7, P=0.002), and on post hoc analyses bilaterally (right hemisphere: −10.8%, t(58)=4.4, P=<0.0001; left hemisphere: −6.9%, t(58)=1.9, P=0.06); Fig. 2B, Table 1. Neither hemispheric nor METH effects were observed in the cerebellar vermis, the genu of the corpus callosum, or the parietal white matter (Table 1).
3.2.3. Effect of METH on axial diffusivity (λ0)
Relative to healthy comparison subjects, METH users had higher axial diffusion values in the caudate (F1,58=4.9, P=0.03) and putamen (F1,58=4.3, P=0.04) when both hemispheres were included in the repeated measures ANOVA. Hemispheric differences were observed in the putamen, caudate and thalamus. In addition there was a hemisphere by METH use interaction in the caudate (F1,58=6.3, P=0.02), with higher axial diffusion in left (4.1%, t(58)=−3.0, P=0.004) but not right caudate. There were also trends for higher axial diffusion in the putamen in both hemispheres (right: 3.5%, t(58)=−1.8, P=0.08; left: 3.1%, t(58)=−1.9, P=0.06).
3.2.4. Effect of METH on radial diffusivity ((λ1+λ2)/2)
Relative to control subjects, METH users had higher radial diffusion values in the putamen (F1,58=3.9, P=0.05) when both hemispheres were included in the repeated measures ANOVA. Hemispheric differences also were observed in the putamen. In addition there was a trend for a hemisphere by METH use interaction in the caudate (F1,58=3.3, P=0.08), with higher radial diffusion in the left (3.8%, t(58)=−2.1, P=0.04) but not right caudate. There were trends toward higher radial diffusion values in both putamen hemispheres (right: 3.5%, t(58)=−1.6, P=0.1; left: 3.3%, t(58)=−1.9, P=0.06).
3.2.5. Correlations between METH usage and diffusion measures
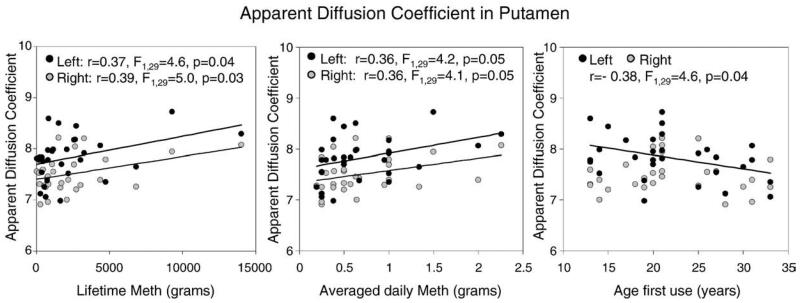
Drug usage parameters did not correlate with frontal white matter FA values in the METH users. However, higher ADC, axial and radial diffusion values in the putamen, but not the caudate, correlated with several aspects of drug use. Higher left putamen ADC and radial diffusion were associated with younger age of first METH use (ADC: r=−0.38, F1,29=4.6, p=0.04; ((λ1 + λ2)/2): r=−0.42, F1,29=6.1, p=0.02), while higher left putamen ADC and axial diffusion were associated with higher average grams of METH used per day (ADC: r=0.36, F1,29=4.2, p=0.05; λ0: r=0.37, F1,29=4.5, p=0.04), and higher cumulative lifetime dose (ADC: r=0.37, F1,29=4.6, p=0.04; λ0: r=0.40, F1,29=5.2, p=0.03). Similarly, higher right putamen ADC was associated with higher average grams of METH used per day (r=0.36, F1,29=4.1, p=0.05), and higher cumulative lifetime dose of METH used (r=0.39, F1,29=5.0, p=0.03); Fig. 3. METH users's subjective reports of craving did not correlate with the abnormal DTI measures or with any of the drug usage measurements.
Fig. 3.
Putamen diffusivity correlated with METH use. Left putamen ADC was higher in participants with younger age of first use, higher average grams of METH used per day, and higher cumulative lifetime dose. Similarly, right putamen ADC was higher in those with higher average grams of METH used per day, and higher cumulative lifetime dose of METH used.
4. Discussion
Higher diffusion in the basal ganglia structures and lower FA in the frontal white matter were observed in a group of chronic METH-dependent individuals. The lower FA in the frontal white matter is consistent with abnormalities previously reported in METH abusers (Kim et al., 2005; Chung et al., 2007), but we did not observe abnormal diffusion and FA values in the genu of the corpus callosum in METH users, as reported previously (Kim et al., 2009; Salo et al., 2009). The novel observation in this study is the higher diffusion in the basal ganglia, which is found in both putamen and caudate in the axial direction, as well as the putamen in the radial direction. Furthermore, our subjects with the higher diffusion in the putamen had earlier age of first METH use, greater daily METH use and higher cumulative lifetime dose of METH. These findings suggest that METH use may lead to greater inflammation or less myelination in the striatal structures, since either condition would be associated with higher diffusion (Harvey et al., 2000; Irwan et al., 2005). The inflammation may also lead to axonal injury that might lead to lower FA in the frontal white matter.
Findings from several prior imaging studies support the possibilities that chronic METH use might be associated with inflammatory changes and axonal damage in the striatal and frontal brain regions. For instance, morphometry studies in METH users demonstrated enlarged striatal structures (Chang et al., 2005a; Jernigan et al., 2005) as well as increased volumes of the frontal white matter (Thompson et al., 2004) and parietal cortex (Jernigan et al., 2005). The enlarged basal ganglia volumes are likely related to the inflammation and increased water content in these regions, which would lead to the higher diffusion. In the frontal white matter, however, we only observed lower FA with no changes in diffusion measures, suggesting axonal damage, which might be a consequence of prior neuroinflammation. Activated microglia or astroglia, signs of neuroinflammation, indeed have been reported in preclinical models after METH administration (Harvey et al., 2000; Thomas et al., 2004; Thomas et al., 2007).
Furthermore, in positron emission tomography (PET) studies with a radiotracer for activated microglia, ([11C](R)-PK11195), chronic METH users abstinent for over 2 years showed significantly higher ([11C](R)-PK11195) binding in the midbrain, striatum, thalamus, orbitofrontal cortex and insular cortex compared with age-, gender-and education-matched healthy subjects (Sekine et al., 2008). The activated glia may express excess inflammatory cytokines and chemokines that may lead to (nerve terminal) axonal damage (Thomas et al., 2004; Thomas and Kuhn, 2005), which in turn would lead to lower FA in the white matter in our METH users.
Evidence for glial activation, with elevated myoinositol or choline, and decreased neuronal marker N-acetylaspartate (NA), also were observed in the frontal white matter and striatum on proton MR spectroscopy studies of METH users (Ernst et al., 2000; Nordahl et al., 2002; Chang et al., 2005b). Our findings of lower FA in the frontal white matter are consistent with the prior DTI study in METH users in Korea (Chung et al., 2007), despite the fact that the Korean subjects first used the drug at a slightly older age (average age 24 years vs. 21 years in our subjects), had a lower average duration of use (70 months vs. 120 months in our subjects) and lower cumulative lifetime usage (~400 g vs. 1300 g in our subjects), and all used METH intravenously as compared with our subjects, who all smoked the drug.
The lack of diffusion abnormalities in the frontal white matter of our METH users may be related to the simultaneous increase in glial cellularity or cell volumes and increased water content associated with axonal damage or myelin degradation. The increased cell volumes, often observed with the neuroinflammatory response of glial activation, may lead to decreased diffusion while increased axonal damage or myelin degradation may lead to increased diffusion, which might have led to opposite effects on the diffusion, yielding a net effect of minimal or no significant differences in the diffusion eigenvalues, but the brain volume still might be enlarged due to these inflammatory processes.
An automated morphometry study of chronic METH users indeed demonstrated the increased white matter volume, suggesting increased inflammation, in chronic METH users (Thompson et al., 2004). The larger white matter in METH users also makes it less likely that lower FA in the frontal white matter is due to myelin degradation or axonal degradation, especially since we did not observe abnormal radial or axial diffusion in the white matter. Brain tissue inflammation would be represented as lower FA while cellular loss would be reflected in higher diffusion eigenvalues (Alexander et al., 2007). Therefore, the lower FA observed in frontal white matter in METH users may reflect inflammation without significant cellular degradation. Since these studies were performed in different subject groups, future studies using combined MRI, MRS and DTI techniques that evaluate the same brain regions in the same individuals are needed to further evaluate these relationships.
In contrast to the lower FA in the frontal white matter, there were no significant differences in FA between the two groups in the striatum, suggesting no apparent changes in the fiber coherence in these structures of our adult METH users, despite well-documented findings from preclinical and postmortem studies that demonstrated METH-mediated dopaminergic and serotonergic terminal injury in these regions (Wilson et al., 1996; Moszczynska et al., 2004; Yamamoto and Bankson, 2005; Fleckenstein et al., 2007). Similarly, chronic METH users also showed decreased dopaminergic and serotonergic transporters on PET studies, although some of these changes were found to be reversible with prolonged abstinence (Volkow et al., 2001a). In addition, studies of human METH users demonstrated possible neuronal loss or dysfunction in the striata, with decreased neuronal marker NA on MRS (Ernst et al., 2000; Chang et al., 2005b) and decreased glucose metabolism on PET (Volkow et al., 2001b). Furthermore, prior reports found that longer duration of METH use was associated with smaller basal ganglia volumes (Chang et al., 2005a), lower total creatine concentrations (Sekine et al., 2002), lower dopamine transporter density (Sekine et al., 2001; Volkow et al., 2001a; Volkow et al., 2001c), and lower serotonin receptor density in the basal ganglia (Sekine et al., 2006).
Therefore, it was somewhat surprising that our METH subjects showed normal FA values in the striata, but they did show higher ADC, radial and axial diffusion values in the left caudate nucleus and bilateral putamen. These findings suggest increased water content and diffusion in the basal ganglia, which also may be related to inflammatory processes or less myelination. The increased diffusion and water content may explain the enlarged striatal volumes observed on MRIs of chronic METH users (Chang et al., 2005a; Jernigan et al., 2005). Our METH subjects who had greater usage of METH (lifetime dose and average daily use) and earlier age of onset of usage of the drug had higher diffusion in the basal ganglia. These findings further suggest that greater METH usage may lead to greater neuroinflammation, and that those who use the drug at an earlier age may show even stronger neuroinflammatory response or may have had altered brain development with less myelination and more interstitial space in the striatum, as suggested in those observed in older healthy subjects (Pfefferbaum et al., 2008). It is possible that the neuroinflammatory response, indicated by higher diffusion without lower FA, reflects the early compensatory or adaptive changes in the brain. Further prolonged usage of METH might lead to additional neurotoxicity and lower FA.
An alternate interpretation to explain the relatively normal FA in the striatal brain regions of METH subjects may be the opposing effects of tissue degradation in the basal ganglia (leading to higher FA) and neuroinflammation (with lower FA). This interpretation is consistent with recent studies of older healthy subjects showing that age-related tissue degradation in the basal ganglia is associated with higher FA, as well as higher axial diffusion, which may be related to the increased iron content (Bhagat and Beaulieu 2004; Pfefferbaum et al., 2008).
DTI has also been applied to study other stimulant users. Similar to our METH users, cocaine users showed lower FA in inferior frontal white matter (Lim et al., 2002; Lim et al., 2008) and anterior regions (genu and rostral body) of the corpus callosum (Moeller et al., 2005), especially in the radial direction, suggesting altered or damaged myelin (Moeller et al., 2007a). However, MDMA users had significantly lower axial diffusion in the rostral body of the anterior corpus callosum, suggesting axonal damage rather than disruption in the myelin (Moeller et al., 2007b).
Several issues should be considered for future studies. First, possible sex differences in the neuroinflammatory response to METH may exist. We did not evaluate sex differences because of the relatively small number of female participants, although we matched for the gender proportion between the METH-using and healthy control groups. Second, tobacco use may be a potential confound on the evaluation of brain diffusion differences. Recent MR studies reported that tobacco use was associated with decreased gray matter volumes in multiple cortical and subcortical brain regions, including the cerebellum (Brody et al., 2004; Gallinat et al., 2006), increased perfusion in the left frontal brain regions (Rose et al., 2003), and increased fractional anisotropy in the anterior cortical white matter in adolescent cigarette smokers (Jacobsen et al., 2007). More of our METH users than controls were also tobacco smokers; therefore, some of the DTI differences observed may be related to tobacco instead. Future studies should include an additional group of tobacco-smoking control subjects. Third, we had the lowest intraclass correlation in the putamen. This might have resulted from partial volume artifacts from adjacent white matter or cerebrospinal fluid that might have been included in the ROI. Although we checked each ROI placement against the corresponding slices on the structural images, future studies that include co-registered structural images with the DTI should improve the ROI placement and minimize the variance between subjects. Another possible reason for the lower reproducibility in this brain region could be the higher iron deposition in this region of the brain (Pfefferbaum et al., 2008). Finally, our diffusion tensor data were acquired using 12 co-linear directions; future studies with a higher number of diffusion MR scanning directions may provide further resolution for detecting possible differences in FA in the striatum.
In summary, adult METH abusers demonstrate microstructure abnormalities as measured by lower FA values in the bilateral frontal white matter, and higher axial and radial diffusion values in the bilateral striatal brain regions. The higher diffusion values in the basal ganglia were greater in those individuals with younger age of first METH use, and those with more daily drug use and greater total lifetime exposure to METH. Therefore, our diffusion tensor data further support the hypothesis that METH use is associated with neuronal damage from inflammatory processes in the frontal lobe and possibly both inflammation and neurodegeneration in the striatum. Since the majority of these adult METH users report the onset of their drug use during adolescence, future DTI studies in teenage METH users are necessary to determine whether METH affects white matter integrity during this highly vulnerable period of brain development.
Acknowledgments
This work was supported in part by funds from the National Institutes of Health [K01-DA021203-CC, K24-DA16170-LC; K02-DA16991-TE and U54-039406-DA & CC, P20 RR11091-10 & 2 G12 RR003061-21 (NCRR)], and Office of National Drug Control Policy. We are grateful to the research subjects who participated in this study. We also thank D. Ramones and K. Taketa for participant evaluation and data entry and R. Yakupov and K. Yue for technical assistance.
Footnotes
The authors report no competing interests.
References
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press; Washington, DC: 2000. [Google Scholar]
- Bae SC, Lyoo IK, Sung YH, Yoo J, Chung A, Yoon SJ, Kim DJ, Hwang J, Kim SJ, Renshaw PF. Increased white matter hyperintensities in male methamphetamine abusers. Drug and Alcohol Dependence. 2006;81:83–88. doi: 10.1016/j.drugalcdep.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Berman S, O'Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Annals of the New York Academy of Sciences. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat YA, Beaulieu C. Diffusion anisotropy in subcortical white matter and cortical gray matter: changes with aging and the role of CSF-suppression. Journal of Magnetic Resonance Imaging. 2004;20:216–227. doi: 10.1002/jmri.20102. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biological Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biological Psychiatry. 2005a;57:967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. American Journal of Psychiatry. 2005b;162:361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chung A, Lyoo IK, Kim SJ, Hwang J, Bae SC, Sung YH, Sim ME, Song IC, Kim J, Chang KH, Renshaw PF. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. International Journal of Neuropsychopharmacology. 2007;10:765–775. doi: 10.1017/S1461145706007395. [DOI] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2007;33:1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: a 1H MRS study. Neurology. 2000;54:1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annual Review of Pharmacology and Toxicology. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedtgen M. Smoking and structuralbrain deficits: a volumetric MR investigation. European Journal of Neuroscience. 2006;24:1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- Harvey DC, Lacan G, Tanious SP, Melega WP. Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra cell loss. Brain Research. 2000;871:259–270. doi: 10.1016/s0006-8993(00)02439-2. [DOI] [PubMed] [Google Scholar]
- Iritani BJ, Hallfors DD, Bauer DJ. Crystal methamphetamine use among young adults in the USA. Addiction. 2007;102:1102–1113. doi: 10.1111/j.1360-0443.2007.01847.x. [DOI] [PubMed] [Google Scholar]
- Irwan R, Sijens PE, Potze JH, Oudkerk M. Correlation of proton MR spectroscopy and diffusion tensor imaging. Magnetic Resonance Imaging. 2005;23:851–858. doi: 10.1016/j.mri.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Picciotto MR, Heath CJ, Frost SJ, Tsou KA, Dwan RA, Jackowski MP, Constable RT, Mencl WE. Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. Journal of Neuroscience. 2007;27:13491–13498. doi: 10.1523/JNEUROSCI.2402-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. American Journal of Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Computer Methods and Programs in Biomedicine. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Hwang J, Sung YH, Lee HY, Lee DS, Jeong DU, Renshaw PF. Frontal glucose hypometabolism in abstinent methamphetamine users. Neuropsychopharmacology. 2005;30:1383–1391. doi: 10.1038/sj.npp.1300699. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon Sung Y, Kim J, Kwon DH, Chang KH, Renshaw PF. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. International Journal of Neuropsychopharmacology. 2006;9:221–228. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- Kim IS, Kim YT, Song HJ, Lee JJ, Kwon DH, Lee HJ, Kim MN, Yoo DS, Chang Y. Reduced corpus callosum white matter microstructural integrity revealed by diffusion tensor eigenvalues in abstinent methamphetamine addicts. Neurotoxicology. 2009;30:209–213. doi: 10.1016/j.neuro.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. Journal of Magnetic Resonance Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biological Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, Silverman AB, Rotrosen JP. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug and Alcohol Dependence. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Valdes I, Lai LY, Swann AC, Narayana PA. Diffusion tensor imaging eigenvalues: preliminary evidence for altered myelin in cocaine dependence. Psychiatry Research: Neuroimaging. 2007a;154:253–258. doi: 10.1016/j.pscychresns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Lane SD, Buzby M, Swann AC, Hasan KM, Kramer LA, Narayana PA. Diffusion tensor imaging in MDMA users and controls: association with decision making. American Journal of Drug and Alcohol Abuse. 2007b;33:777–789. doi: 10.1080/00952990701651564. [DOI] [PubMed] [Google Scholar]
- Moszczynska A, Fitzmaurice P, Ang L, Kalasinsky KS, Schmunk GA, Peretti FJ, Aiken SS, Wickham DJ, Kish SJ. Why is parkinsonism not a feature of human methamphetamine users? Brain. 2004;127:363–370. doi: 10.1093/brain/awh046. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Possin K, Gibson DR, Flynn N, Leamon M, Galloway GP, Pfefferbaum A, Spielman DM, Adalsteinsson E, Sullivan EV. Low Nacetyl-aspartate and high choline in the anterior cingulum of recently abstinent methamphetamine-dependent subjects: a preliminary proton MRS study. Psychiatry Research: Neuroimaging. 2002;116:43–52. doi: 10.1016/s0925-4927(02)00088-4. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV. Diffusion tensor imaging of deep gray matter brain structures: effects of age and iron concentration. Neurobiology of Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.04.013. doi:10.1016/J.Neurobiolaging.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Mathew RJ, London ED, Hawk TC, Turkington TG, Coleman RE. PET studies of the influences of nicotine on neural systems in cigarette smokers. American Journal of Psychiatry. 2003;160:323–333. doi: 10.1176/appi.ajp.160.2.323. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Natsuaki Y, Leamon MH, Galloway GP, Waters C, Moore CD, Buonocore MH. Attentional control and brain metabolite levels in methamphetamine abusers. Biological Psychiatry. 2007;61:1272–1280. doi: 10.1016/j.biopsych.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Buonocore MH, Natsuaki Y, Waters C, Moore CD, Galloway GP, Leamon MH. Cognitive control and white matter callosal microstructure in methamphetamine-dependent subjects: a diffusion tensor imaging study. Biological Psychiatry. 2009;65:122–128. doi: 10.1016/j.biopsych.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. American Journal of Psychiatry. 2001;158:1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Minabe Y, Kawai M, Suzuki K, Iyo M, Isoda H, Sakahara H, Ashby CR, Jr., Takei N, Mori N. Metabolite alterations in basal ganglia associated with methamphetamine-related psychiatric symptoms. A proton MRS study. Neuropsychopharmacology. 2002;27:453–461. doi: 10.1016/S0893-133X(02)00321-4. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, Okada H, Minabe Y, Suzuki K, Iwata Y, Tsuchiya KJ, Tsukada H, Iyo M, Mori N. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Archives of General Psychiatry. 2006;63:90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. Journal of Neuroscience. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2006 national survey on drug use and health: national findings. Office of Applied Studies, Substance Abuse and Mental Health Services Administration; Rockville, MD: 2007. (DHHS Publication No. SMA 07-4293, NSDUH Series H-32). [Google Scholar]
- Sung YH, Cho SC, Hwang J, Kim SJ, Kim H, Bae S, Kim N, Chang KH, Daniels M, Renshaw PF, Lyoo IK. Relationship between N-acetyl-aspartate in gray and white matter of abstinent methamphetamine abusers and their history of drug abuse: a proton magnetic resonance spectroscopy study. Drug and Alcohol Dependence. 2007;88:28–35. doi: 10.1016/j.drugalcdep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Tang CY, Friedman J, Shungu D, Chang L, Ernst T, Stewart D, Hajianpour A, Carpenter D, Ng J, Mao X, Hof PR, Buchsbaum MS, Davis K, Gorman JM. Correlations between diffusion tensor imaging (DTI) and magnetic resonance spectroscopy (1H MRS) in schizophrenic patients and normal controls. BioMed Central Psychiatry. 2007;7:25. doi: 10.1186/1471-244X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. Attenuated microglial activation mediates tolerance to the neurotoxic effects of methamphetamine. Journal of Neurochemistry. 2005;92:790–797. doi: 10.1111/j.1471-4159.2004.02906.x. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. Journal of Pharmacology And Experimental Therapeutics. 2004;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. Journal of Neurochemistry. 2007;105:605–616. doi: 10.1111/j.1471-4159.2007.05155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. Journal of Neuroscience. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. Journal of Neuroscience. 2001a;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Wong C, Logan J. Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. American Journal of Psychiatry. 2001b;158:383–389. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. American Journal of Psychiatry. 2001c;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nature Medicine. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Bankson MG. Amphetamine neurotoxicity: cause and consequence of oxidative stress. Critical Reviews in Neurobiology. 2005;17:87–117. doi: 10.1615/critrevneurobiol.v17.i2.30. [DOI] [PubMed] [Google Scholar]