Abstract
Objective
To assess the short-term effects of extended-release niacin (ERN) on endothelial function in HIV-infected patients with low high-density lipoprotein-cholesterol (HDL-c) levels.
Methods
Randomized controlled study to determine the short-term effects of ERN on endothelial function, measured by flow-mediated vasodilation (FMD) of the brachial artery, in HIV-infected adults with low HDL-c. Participants on stable HAART with fasting HDL-c less than 40 mg/dl and low-density lipoprotein-cholesterol less than 130 mg/dl were randomized to ERN or control arms. ERN treatment started at 500 mg/night and titrated to 1500 mg/night for 12 weeks. Controls received the same follow-up but were not given ERN (no placebo). Participants were excluded if they had a history of cardiac disease, uncontrolled hypertension, diabetes mellitus, or were on lipid-low-ering medications such as statins and fibrates. Change in FMD was compared between arms with respect to baseline HDL-c.
Results
Nineteen participants were enrolled: 89% men, median age 50 years, 53% white/non-Hispanic, median CD4 cell count 493 cells/μl, and 95% of them had HIV RNA below 50 copies/ml. Participants receiving ERN had a median HDL-c (interquartile range) increase of 3.0 mg/dl (0.75 to 5.0) compared with −1.0 mg/dl in controls (−6.0 to 2.5), a P value is equal to 0.04. The median change in FMD was 0.91% (−2.95 to 2.21) for ERN and −0.48% (−2.65 to 0.98) for controls (P=0.67). However, end of study FMD for ERN was significantly different from controls after adjusting for baseline differences in FMD and HDL-c, 6.36% (95% confidence interval 4.85–7.87) and 2.73% (95% confidence interval 0.95–4.51) respectively, a P value is equal to 0.048.
Conclusion
This pilot study demonstrated that short-term niacin therapy could improve endothelial function in HIV-infected patients with low HDL-c.
Keywords: endothelial function, flow-mediated vasodilation, niacin
Introduction
The incidence of myocardial infarction (MI) in HIV-infected individuals has been increasing. Although much of the coronary artery disease (CAD) risk in the HIV population has been attributed to elevated levels of low-density lipoprotein-cholesterol (LDL-c) and hypertriglyceridemia, low levels of high-density lipoprotein-cholesterol (HDL-c) also contribute to CAD risk. In the general population, low serum levels of HDL-c are associated with increased risk for MI, restenosis after angioplasty, sudden cardiac death, and stroke [1-3]. The primary mechanism by which HDL-c exerts its atheroprotective effect is believed to be reverse cholesterol transport; however, HDL-c also has antioxidant effects [4]. Additionally, HDL-c has direct arterial effects that help preserve endothelial function. Endothelial dysfunction is an early phenomenon in atherosclerosis that precedes structural changes of the arterial wall and clinical manifestations of CAD [5]. This protective effect of HDL-c on endothelium-dependent vasoreactivity may depend on the binding of HDL-c to scavenger receptor class B type I and subsequent stimulation of nitric oxide formation [6]. HDL-c activates both extracellular signal-regulated kinase 1/2 and Akt, resulting in enhanced stability of endothelial nitric oxide synthase [7].
In dyslipidemic HIV-infected patients on stable anti-retroviral therapy (ART), low levels of HDL-c have been associated with endothelial dysfunction [8]. Moreover, use of statins in HIV-infected patients on ART improves endothelial function [9]. We hypothesized that increases in HDL-c associated with the use of niacin also would improve endothelial function in HIV-infected individuals. Therefore, we conducted a pilot study to assess the effects of niacin on endothelial function in HIV-infected patients with low HDL-c levels.
Methods
This was a randomized controlled study evaluating endothelial function, measured by flow-mediated vasodilation (FMD) of the brachial artery, in HIV-1-infected adults with low HDL-c before and after 12 weeks of treatment with extended-release niacin (ERN). Participants randomized to the treatment arm received ERN (Niaspan; Abbott Laboratories, Abbott Park, Illinois, USA) starting at 500 mg per night and titrated to a maximum tolerated dose (not exceeding 1500 mg per night) over 8 weeks. Participants in the control arm received the same follow-up as the treatment arm but were not be given ERN (no placebo) and were instructed to not take any supplemental niacin. CD4 cell count, HIV RNA viral load, FMD, lipid parameters, insulin sensitivity, and C-reactive protein were obtained at baseline and after 12 weeks for both control and treatment arms.
Participants were eligible for the study if they were HIV-infected and were at least 18 years of age. Participants must have been on stable HAART for at least 6 months prior to study entry and have a HDL-c below 40 mg/dl and LDL-c below 130 mg/dl. Participants were excluded if they had cardiac disease, uncontrolled hypertension, pregnancy, or diabetes mellitus. Participants were ineligible if they were on nitrates, metformin, or thiazolidinediones. Treatment with lipid-lowering drugs such as statins and fibrates were excluded. A pharmacist prepared a computer-generated single-block randomized treatment list to determine each patient’s treatment assignment. The study pharmacist did not participate in the selection and assessment of patients or the collection of any trial data. This study was approved by the Institutional Review Board of University of Hawaii. All participants provided informed consent.
Endothelial function was assessed by ultrasound brachial artery reactivity testing. Testing was performed in the morning. Participants were required to fast for 12 h. Those who regularly used tobacco products refrained for at least 8 h. After 10-min rest in a temperature-controlled room (70–76°F), the diameter of the right brachial artery and baseline blood flow were measured with a high-resolution linear array vascular ultrasound transducer (Siemens Medical Solutions Inc., Mountain View, California, USA). Increased forearm blood flow was induced by inflating a pneumatic blood pressure tourniquet placed around the widest part of the forearm and inflated to 250 mmHg. This was followed by deflation after 5 min. Repeat brachial artery diameter and blood flow scans were obtained immediately thereafter. Twenty minutes were allowed for vessel recovery and repeat resting brachial artery diameter and blood flow scans were obtained. Sublingual nitroglycerin (400 μg) was administered, and final scans were performed after 3 min. Each study was recorded digitally and sent to the University of Wisconsin Atherosclerosis Imaging Research Program core ultrasound laboratory in Madison, Wisconsin, USA. Brachial artery diameters were measured in triplicate by a single blinded reader [8,10,11].
The primary objective was to assess the change in FMD with respect to 12 weeks of ERN vs. control. The Shapiro–Wilks normality test was used to assess the homogeneity of variances between and within the study arms. Categorical variables were compared using the χ2 test. Continuous variables were analyzed by Wilcoxon rank test. For between-group differences, a two-way analysis of covariance (ANCOVA) for both arms was conducted and controlled for within patient correlation. To control for the effects of baseline FMD and HDL-c, a multivariate ANCOVA was performed. A two-sided probability of P value is less than 0.05 was used to determine statistical significance. All statistical analyses were performed using the JMP statistical program (SAS Institute Inc., Cary, North Carolina, USA).
Results
Baseline characteristics
The demographic and clinical characteristics of the 19 participants are presented in the Table 1. Participants consisted of 17 men and two women, with a median age of 50 years (range 28–65 years). Four were current and seven were former smokers. The median CD4 cell count was 493 cells/μl (range 280–1096). All participants had undetectable HIV viral loads except for one participant with a viral load of 1520 copies/ml. The majority of participants (47%) were on an efavirenz-based regimen, whereas 42% were on a protease inhibitor-based regimen. A nonnucleoside reverse transcriptase inhibitor (NNRTI)-based regimen was used in 60% of the ERN group compared with 44% of controls; a protease inhibitor-based regimen was used in 30% of ERN and 56% of controls. One participant in the ERN group was on an NRTI-only regimen. At baseline, there were differences (P<0.10) between the arms in FMD, nitroglycerin-mediated dilation (NTGMD), and HDL-c.
Table 1. Baseline characteristics according to assignment arm.
| Control | Extended-release niacin | P | |
|---|---|---|---|
| N | 9 | 10 | |
| Age (years) | 50.2 (38.7, 56.3) | 52.6 (42.2, 59.4) | 0.60 |
| Ethnicity, whites/others | 5/4 | 5/5 | 0.22 |
| Sex, male/female | 7/2 | 10/0 | 0.21 |
| HR (pulse/min) | 56 0.0 (44.0, 60.0) | 56.0 (51.0, 63.5) | 0.63 |
| BMI (kg/m2) | 25.7 (23.6, 29.7) | 24.3 (22.9, 26.1) | 0.24 |
| SBP (mmHg) | 118.0 (117.0, 131.0) | 123.5 (110.8, 128.5) | 0.64 |
| DBP (mmHg) | 74.0 (71.0, 76.0) | 76.0 (68.3, 81.3) | 0.95 |
| CD4 cells (cells/μl) | 493.0 (412.0, 611.0) | 474.0 (369.0, 531.0) | 0.49 |
| HIV RNA, n (% undetectable) | 9 (100%) | 9 (90%) | 0.64 |
| Baseline | |||
| Total cholesterol (mg/dl) | 180.0 (157.5, 210.0) | 181.0 (142.3, 193.5) | 0.78 |
| HDL-c (mg/dl) | 34.0 (25.0, 37.0) | 38.5 (34.3, 41.0) | 0.07 |
| LDL-c (mg/dl) | 115.0 (98.5, 144.0) | 118.5 (75.0, 126.5) | 0.65 |
| Triglycerides (mg/dl) | 145.0 (94.0, 207.0) | 166.5 (89.0, 203.0) | 0.97 |
| Fasting glucose (mg/dl) | 88.0 (86.5, 96.0) | 92.0 (86.5, 102.5) | 0.62 |
| Fasting insulin (μUI/ml) | 7.0 (5.1, 19.2) | 5.9 (2.8, 11.5) | 0.31 |
| CRP (mg/l) | 0.7 (0.4, 2.6) | 0.9 (0.5, 2.8) | 0.56 |
| HOMA-IR | 1.50 (1.10, 4.75) | 1.49 (0.64, 2.71) | 0.31 |
| Baseline diameter (mm) | 0.51 (0.45, 0.52) | 0.43 (0.40, 0.49) | 0.13 |
| FMD (%) | 3.31 (1.58, 4.26) | 4.99 (4.05, 9.21) | 0.04 |
| NTGMD (%) | 7.38 (4.59, 7.83) | 11.87 (6.35, 14.30) | 0.07 |
| End of study | |||
| Total cholesterol (mg/dl) | 167.0 (137.0, 192.0) | 169.5 (154.5, 197.8) | 0.87 |
| HDL-c (mg/dl) | 27.0 (24.0, 39.0) | 42.0 (39.3, 43.5) | 0.02 |
| LDL-c (mg/dl) | 105.0 (83.0, 127.0) | 108.5 (88.5, 125.0) | 0.97 |
| Triglyceride (mg/dl) | 117.0 (103.5, 209.5) | 111.5 (88.3, 175.5) | 0.60 |
| Fasting glucose (mg/dl) | 94.0 (85.5, 102.0) | 91.5 (86.0, 94.8) | 0.25 |
| Fasting insulin (μUI/ml) | 9.1 (4.7, 25.9) | 5.8 (4.8, 9.7) | 0.24 |
| CRP (mg/l) | 0.5 (0.3, 3.4) | 1.4 (0.6, 3.7) | 0.24 |
| HOMA-IR | 2.31 (1.01, 6.29) | 1.30 (0.99, 2.18) | 0.22 |
| Baseline diameter (mm) | 0.48 (0.41, 0.52) | 0.44 (0.41, 0.52) | 0.65 |
| FMD (%) | 3.25 (1.95, 4.85) | 4.51 (4.46, 8.32) | 0.09 |
| NTGMD (%) | 7.87 (6.22, 10.63) | 12.09 (6.46, 15.26) | 0.23 |
Continuous variables shown as median (1st, 3rd quartile), compared by Wilcoxon rank nonparametric testing. CRP, C-reactive protein; FMD, flow-mediated vasodilation; HDL-c, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment-insulin resistance; HR, heart rate; LDL-c, low-density lipoprotein cholesterol; NTGMD, nitroglycerin-mediated dilation.
All participants achieved the titrated dose of ERN of 1500 mg daily. Only one participant receiving ERN developed serious flushing (grade 2), which was attributed to the participant accidentally taking twice the prescribed dose. This flushing resolved after the ERN dose was reduced. There were no grade 3 or higher adverse events during the study in either group.
After 12 weeks, participants receiving ERN had a median HDL-c (interquartile range) increase of 3.0 mg/dl (0.75 to 5.0) compared with −1.0 mg/dl in controls (−6.0 to 2.5), P value is equal to 0.04. There was no significant change in brachial diameters; the median change in participants receiving ERN was 0.00 mm (−0.01 to 0.03) compared with −0.01 mm in controls (−0.02 to 0.02), P value is equal to 0.56. The median change in FMD from baseline to week 12 was 0.91% (−2.95 to 2.21) for those on ERN vs. −0.48% (−2.65 to 0.98) for controls (P=0.67). However, after adjusting for baseline differences in FMD and HDL-c, there was a statistically significant increase in FMD in participants receiving niacin (P=0.048, see Fig. 1). Participants with low HDL-c had a more dramatic improvement in FMD with niacin. In participants with HDL-c 35 mg/dl or less, the adjusted end of study FMD was 8.4% with 95% confidence interval (CI) (6.7 to 10.1) for the niacin group and 4.4% with 95% CI (3.18 to 5.68) for the control group (P = 0.01). There were no differences in NTGMD between the two arms, even after adjusting for baseline NTGMD and HDL-c.
Fig. 1. Flow-mediated vasodilation improved with niacin.
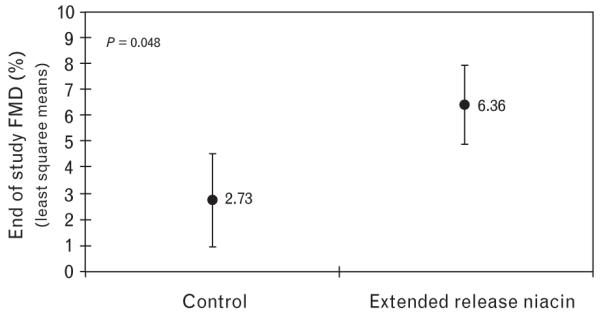
Analysis of covariance demonstrates end of study mean FMD in the niacin-treated individuals compared with control, adjusted for baseline FMD and baseline HDL with 95% CI. CI, confidence interval; FMD, flow-mediated vasodilation; HDL, high-density lipoprotein.
Discussion
In this pilot study, we demonstrated that HIV-infected patients on stable ART with low HDL-c had improved brachial artery FMD after 12 weeks of ERN. The improvement in FMD was more significant in patients with low baseline HDL-c. In individuals with HIV, low HDL-c levels increase risk of future CAD [12-14]. Changes in HDL-c with ART also seem to affect short-term CAD [15]. Our study is consistent with recent studies on individuals without HIV infection, showing that interventions targeted at raising HDL-c levels may have vascular benefits. Indeed, two studies [16,17] have shown that in individuals with low HDL-c, use of ERN can improve FMD. Similarly, ERN has been shown to significantly regress carotid intima–media thickness [18]. Our study is the first to determine the effect of niacin on endothelial function in HIV-infected individuals. This is a clinically relevant finding, as many patients with HIV and dyslipidemia have low HDL-c, and the effects of raising HDL-c on endothelial function in patients with HIV have not been investigated previously. Also, niacin’s effects on lipids in patients with HIV seem to be less than observed in the general population [19].
Niacin can inhibit vascular inflammation by decreasing endothelial reactive oxygen species production and subsequent LDL-c oxidation and inflammatory cytokine production, key events involved in atherogenesis. Potential mechanisms of the vascular protective effects of HDL-c have been described [20]. HDL-c enhances reverse cholesterol transport, promoting the efflux of cholesterol from atherosclerotic plaque rupture, thus promoting stabilization [21]; HDL-c and its associated proteins attenuate the formation of the highly atherogenic oxidized LDL-c species previously shown to impair endothelial function [22]; HDL-c stabilizes prostacyclin, an important vasodilator and platelet inhibitor [23]; and HDL-c may augment the ability of the endothelium to produce nitric oxide [16]. In addition to the protective effects of HDL-c, niacin helps increase LDL-c particle size, which also may improve endothelial function. Our finding supports the paradigm that niacin-induced increases in HDL-c contribute to improvements in endothelial function in participants with low HDL-c. Of interest, in ACTG study A5152s, positive correlations were observed between ART-associated increases in HDL-c, large HDL-c particles, and FMD (personal communication).
There are several limitations to our study. First, this was an unblinded pilot study with a small sample size and its results require verification, as well as evaluation of the mechanism for these observations. Second, the increase in HDL-c was less than that would be predicted based on data in non-HIV-infected cohorts [16], although this study was of short duration (all treated participants were at the maximal daily dose of 1500 mg of ERN for only 4 weeks), and niacin’s beneficial effects on HDL-c levels can continue to increase up to 36 weeks after maximum dose titration [24].
In summary, this pilot study demonstrated that short-term niacin therapy could improve endothelial function in HIV-infected individuals on stable ART who have low HDL-c.
Acknowledgements
This work was supported in part by the Department of Defense Telemedicine and Advanced Technology Research Center, proposal no., 06187003 and a Research Centers in Minority Institutions award, P20RR011091, from the National Center for Research Resources, National Institutes of Health. D.C.C. was supported in part by grants through the National Institutes of Health, Department of Health and Human Services, USA, NHLBI #K23 HL088981, and NCRR R25 RR019321.
We thank the study participants for generously donating their time and effort. We also acknowledge the contributions of the following individuals: Claudia E. Korcarz, Nancy Hanks, James Davis, Pearl Whittaker, and Chad Okabayashi.
Footnotes
Clinical trials #NCT 00986986.
References
- 1.Weverling-Rijnsburger AW, Jonkers IJ, van Exel E, Gussekloo J, Westendorp RG. High-density vs low-density lipoprotein cholesterol as the risk factor for coronary artery disease and stroke in old age. Arch Intern Med. 2003;163:1549–1554. doi: 10.1001/archinte.163.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Shah PK, Amin J. Low high density lipoprotein level is associated with increased restenosis rate after coronary angioplasty. Circulation. 1992;85:1279–1285. doi: 10.1161/01.cir.85.4.1279. [DOI] [PubMed] [Google Scholar]
- 3.Burke AP, Farb A, Malcom GT, Liang Y, Smialek JE, Virmani R. Plaque rupture and sudden death related to exertion in men with coronary artery disease. JAMA. 1999;281:921–926. doi: 10.1001/jama.281.10.921. [DOI] [PubMed] [Google Scholar]
- 4.von Eckardstein A, Hersberger M, Rohrer L. Current understanding of the metabolism and biological actions of HDL. Curr Opin Clin Nutr Metab Care. 2005;8:147–152. doi: 10.1097/00075197-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 6.Mineo C, Shaul PW. HDL stimulation of endothelial nitric oxide synthase: a novel mechanism of HDL action. Trends Cardiovasc Med. 2003;13:226–231. doi: 10.1016/s1050-1738(03)00098-7. [DOI] [PubMed] [Google Scholar]
- 7.Ramet ME, Ramet M, Lu Q, Nickerson M, Savolainen MJ, Malzone A, Karas RH. High-density lipoprotein increases the abundance of eNOS protein in human vascular endothelial cells by increasing its half-life. J Am Coll Cardiol. 2003;41:2288–2297. doi: 10.1016/s0735-1097(03)00481-9. [DOI] [PubMed] [Google Scholar]
- 8.Stein JH, Klein MA, Bellehumeur JL, McBride PE, Wiebe DA, Otvos JD, Sosman JM. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation. 2001;104:257–262. doi: 10.1161/01.cir.104.3.257. [DOI] [PubMed] [Google Scholar]
- 9.Hurlimann D, Chenevard R, Ruschitzka F, Flepp M, Enseleit F, Béchir M, et al. Effects of statins on endothelial function and lipid profile in HIV infected persons receiving protease inhibitor-containing antiretroviral combination therapy: a randomised double blind crossover trial. Heart. 2006;92:110–112. doi: 10.1136/hrt.2004.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein JH, Merwood MA, Bellehumeur JL, Aeschlimann S, Korcarz C, Underbakke G, et al. Effects of pravastatin on lipoproteins and endothelial function in patients receiving human immunodeficiency virus protease inhibitors. Am Heart J. 2004;147:713. doi: 10.1016/j.ahj.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Torriani FJ, Komarow L, Parker RA, Cotter BR, Currier JS, Dubé MP, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: the ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52:569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friis-Moller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 13.Glass T, Ungsedhapand C, Wolbers M, Weber R, Vernazza PL, Rickenbach M, et al. Prevalence of risk factors for cardiovascular disease in HIV-infected patients over time: the Swiss HIV Cohort Study. HIV Med. 2006;7:404–410. doi: 10.1111/j.1468-1293.2006.00400.x. [DOI] [PubMed] [Google Scholar]
- 14.Holmberg SD, Moorman AC, Williamson JM, Tong TC, Ward DJ, Wood KC, et al. HIV Outpatient Study (HOPS) investigators Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet. 2002;360:1747–1748. doi: 10.1016/S0140-6736(02)11672-2. [DOI] [PubMed] [Google Scholar]
- 15.Duprez DA, Kuller LH, Tracy R, Otvos J, Cooper DA, Hoy J, et al. INSIGHT SMART Study Group Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis. 2009;207:524–529. doi: 10.1016/j.atherosclerosis.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuvin JT, Ramet ME, Patel AR, Pandian NG, Mendelsohn ME, Karas RH. A novel mechanism for the beneficial vascular effects of high-density lipoprotein cholesterol: enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am Heart J. 2002;144:165–172. doi: 10.1067/mhj.2002.123145. [DOI] [PubMed] [Google Scholar]
- 17.Warnholtz A, Wild P, Ostad MA, Elsner V, Steiber F, Schinzel R, et al. Effects of oral niacin on endothelial dysfunction in patients with coronary artery disease: results of the randomized, double-blind, placebo-controlled INEF study. Atherosclerosis. 2009;204:216–221. doi: 10.1016/j.atherosclerosis.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Taylor AJ, Villines TC, Stanek EJ, Devine PJ, Griffen L, Miller M, et al. Extended-release niacin or ezetimibe and carotid intimamedia thickness. N Engl J Med. 2009;361:2113–2122. doi: 10.1056/NEJMoa0907569. [DOI] [PubMed] [Google Scholar]
- 19.Dube MP, Wu JW, Aberg JA, Deeg MA, Alston-Smith BL, McGovern ME, et al. Safety and efficacy of extended-release niacin for the treatment of dyslipidaemia in patients with HIV infection: AIDS Clinical Trials Group Study A5148. Antivir Ther. 2006;11:1081–1089. [PMC free article] [PubMed] [Google Scholar]
- 20.Ganji SH, Qin S, Zhang L, Kamanna VS, Kashyap ML. Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis. 2009;202:68–75. doi: 10.1016/j.atherosclerosis.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 21.Reichl D, Miller NE. Pathophysiology of reverse cholesterol transport. Insights from inherited disorders of lipoprotein metabolism. Arteriosclerosis. 1989;9:785–797. doi: 10.1161/01.atv.9.6.785. [DOI] [PubMed] [Google Scholar]
- 22.Parthasarathy S, Barnett J, Fong LG. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta. 1990;1044:275–283. doi: 10.1016/0005-2760(90)90314-n. [DOI] [PubMed] [Google Scholar]
- 23.Kawai C. Pathogenesis of acute myocardial infarction. Novel regulatory systems of bioactive substances in the vessel wall. Circulation. 1994;90:1033–1043. doi: 10.1161/01.cir.90.2.1033. [DOI] [PubMed] [Google Scholar]
- 24.Kashyap ML, McGovern ME, Berra K, Guyton JR, Kwiterovich PO, Harper WL, et al. Long-term safety and efficacy of a once-daily niacin/lovastatin formulation for patients with dyslipidemia. Am J Cardiol. 2002;89:672–678. doi: 10.1016/s0002-9149(01)02338-4. [DOI] [PubMed] [Google Scholar]


