Abstract
Following injury or surgical resection, the liver has the remarkable ability to regenerate. Despite over 100 years of research, the trigger of the liver regeneration cascade has only recently been identified. Shear stress-induced nitric oxide (NO), released secondary to a hemodynamic event following partial hepatectomy (PHX), has been implicated as the trigger of the liver regeneration cascade. However, it is also known that prostaglandins (PGs) are released following PHX, and in response to shear stress. Therefore, it is hypothesized that PGs, released secondary to an increase in the blood flow-to-liver mass ratio following PHX, trigger the liver regeneration cascade, and that NO and PGs interact during the triggering event. An index of initiation of the liver regeneration cascade, c-fos mRNA expression 15 min after PHX, has been employed. As expected, c-fos mRNA expression increased 15 min after PHX and this increase was inhibited by the NO synthase antagonist, L-NAME. This inhibition was reversed by the NO donors, SIN-1 and SNAP, and by the PGs, PGE2 and PGI2. Also, the increase in c-fos mRNA expression was inhibited by indomethacin, a cyclooxygen-ase antagonist. This inhibition was also reversed by the NO donors, SIN-1 and SNAP, and by the PGs, PGE2 and PGI2. These results suggest that there is interaction between NO and PGs in triggering the liver regeneration cascade, and that in a situation where either NO or COX is inhibited, provision of excess exogenous NO or PGs can reverse the inhibition. This suggests that exogenous NO and/or PGs may play a role in potentiation of the liver regeneration cascade.
Keywords: c-fos, Protooncogene, Nitric oxide
The liver has remarkable ability to regenerate following surgical resection or chemical damage. Numerous growth factors, cytokines, and other mediators are released following 2/3 partial hepatectomy (PHX), a common model used in the study of liver regeneration [1]. It has been previously hypothesized that numerous of these growth factors and cytokines are the trigger of the liver regeneration cascade, including hepatocyte growth factor (HGF) [2], epidermal growth factor (EGF) [3], and interleukin-6 (IL-6) [4]. However, the concentration of these putative triggering agents does not immediately increase after PHX. One event that is immediate following PHX is a hemodynamic change. The liver does not control inflowing portal venous blood, but must accept the entire outflow from other splanchnic organs, such as the intestine, stomach, spleen, and pancreas. Removal of 2/3 of the liver (PHX) causes a threefold increase in the blood flow-to-liver mass in the remaining 1/3 [5,6]. It is hypothesized that this increase in the blood flow-to-liver mass ratio causes shear stress and release of nitric oxide (NO) and prostaglandins (PGs), which trigger the liver regeneration cascade.
It has been previously demonstrated that an increase in the blood flow-to-liver mass ratio causes an elevation in portal venous pressure (PVP) immediately following PHX [7,8]. This is important because it has been shown that PVP can be used as an index of the amount of shear stress in the liver [9]. Although shear stress has also been recognized by others as essential for liver regeneration to proceed normally [10,11], the mechanism of the triggering event was never determined.
Previous experiments have provided support for the hypothesis that shear stress-induced NO is involved in triggering the liver regeneration cascade [8,12,13]. Briefly, liver mass restoration 48 h after PHX is inhibited by administration of Nω-nitro-L-arginine methyl ester (L-NAME), a nonselective NO synthase antagonist [13,14]. Further, proliferative factors normally detected in the blood 4 h after PHX are inhibited by administration of L-NAME prior to PHX. This inhibition is reversed by administration of L-arginine, the substrate for NOS [13]. The complete inhibition of proliferative factors in response to antagonism of NO synthase implies the inhibition of all of the factors that are generated after PHX that can cause hepatocyte proliferation. For this reason, proliferative factor presence in blood is taken as a powerful tool to indicate whether the liver regeneration cascade has been activated.
Also, an index of initiation of the liver regeneration cascade closer to the actual triggering event was employed. This index is c-fos mRNA expression 15 min after PHX, and it was selected as an index because c-fos mRNA expression was shown to peak 15 min after PHX, and also to increase proportionate to the degree of PHX performed [15]. In addition, c-fos mRNA expression increases in response to shear stress [16], and in proportion to the shear stress applied [17]. NO also stimulates expression of c-fos mRNA [18–21]. Previous experiments have shown that blockade of NO synthase by L-NAME inhibits the increase in c-fos mRNA expression normally observed 15 min after PHX [8]. This inhibition is reversed by the NO donor 3-morpholinosydnonimine (SIN-1). This provided further support for the hypothesis that NO triggers the liver regeneration cascade.
Previous research demonstrated that inhibition of cyclooxygenase (COX) using indomethacin resulted in blockade of proliferating factor production after PHX [22]. However, the PGs involved and the nature of the triggering role were not determined. Although the liver produces various types of PGs, the roles of PGE2 and PGI2 in the liver regeneration cascade were investigated. PGE2 is the most abundant PG produced by the liver [23], and both PGE2 and PGI2 have been shown to be cytoprotective to hepatocytes, and this cytoprotection is abolished by indomethacin [24]. PGE2 and PGI2 levels are increased after PHX [25,26]; and it was demonstrated that COX antagonists inhibit DNA synthesis and delay liver regeneration [27,28]. In addition, PGE2 and PGI2 are released in response to shear stress [29–32].
Interaction between NO and PGs has also been demonstrated in other situations. For example, there is interaction between NO and PG in bone formation. McAllister et al. [33] found that shear stress stimulated NO, PGE2, and PGI2 production, within 1 h of stimulation of osteoclast precursor cells. In addition, shear stress stimulated mouse bone cells to produce NO and PGE2 [34,35]. The increase in NO [34,35] and PGE2 [34] production was inhibited by NOS antagonists, thus suggesting interaction between NO and PGs in stimulation of bone formation.
The goal of this paper was to test the hypothesis that PGs are also involved in triggering the liver regeneration cascade and to investigate the interaction between NO and PGs in this triggering role. The c-fos mRNA expression index is used as a tool to evaluate, in a screening manner, potential therapeutic approaches that may be directed to stimulate liver regeneration. Results indicate that inhibition of COX by indomethacin results in inhibition of c-fos mRNA expression 15 min after PHX. In addition, under conditions where either NO or PG production is blocked, the inhibition of c-fos mRNA expression can be reversed by either PGs or NO donors. This suggests that both NO and PGs are required for c-fos mRNA expression to increase after PHX, and that, in the absence of one, excess exogenous supply of the other can compensate. These findings also suggest that NO and PGs may be potential therapeutic targets for potentiation of the liver regeneration cascade.
Experimental procedures
Animals
Male Sprague–Dawley rats, 250 g, were fed standard laboratory chow ad libitum until the day before the experiment, when they were fasted for 8 h and fed for 2 h prior to experimentation. Animals were treated according to the guidelines of the Canadian Council on Animal Care and all protocols were approved by the Ethics Committee on Animal Care at The University of Manitoba.
Surgical preparation
Male Sprague–Dawley rats were anesthetized using sodium pentobarbital. Tracheotomy was performed, and cannulae were placed in the femoral artery, femoral vein, and portal vein for infusion of drugs and anesthetic. Laparotomy was performed and the animal was allowed to stabilize for 30 min. Drug or saline was then administered, and 2/3 PHX (resection of the left lateral and median lobes of the liver, as described by Higgins and Anderson [36]) or sham procedures were performed. The remnant liver was then removed after 15 min and immediately frozen on dry ice for RNA analysis.
In the first set of experiments, the NOS antagonist, L-NAME, was employed. Rats were divided into the following experimental groups: sham, PHX, sham + L-NAME, PHX +L-NAME, sham+L-NAME + PGE2, PHX+ L-NAME+PGE2, sham+ L-NAME+PGI2, PHX +L-NAME+PGI2, sham+ L-NAME+SIN-1, PHX +L-NAME+SIN-1, sham+L-NAME+s-nitroso-n-acetyl-penicillamine (SNAP), and PHX+L-NAME+SNAP. In addition, a second set of experiments, using the COX antagonist, indomethacin (INDO), included the groups: sham, PHX, sham+INDO, PHX+INDO, sham+ INDO + PGE2, PHX + INDO + PGE2, sham + INDO +PGI2, PHX + INDO+PGI2, sham+INDO+SIN-1, PHX+ INDO +SIN-1, sham + INDO + SNAP, and PHX +INDO+SNAP.
Drugs
L-NAME (Sigma) (5 mg/kg, i.v., 0.5 ml bolus infused over 2 min), a non-selective NOS antagonist, was dissolved in saline, and the rat was allowed to stabilize for 10 min following administration. The NO donors, SIN-1 (5 mg/kg) (Alexis Corp.) and SNAP (5 mg/kg) (Sigma), were also dissolved in saline, and a 0.2 ml bolus was infused intraportally over 2 min. Indomethacin (INDO; 7.5 mg/kg) (Sigma) was dissolved in 5% sodium bicarbonate and a 0.2 ml bolus was infused intravenously over 2 min. PGE2 (10 μg/kg) (Sigma) and PGI2 (10 μg/kg) (Sigma) were dissolved in 95% ethanol in a stock solution, and diluted with the appropriate amount of saline to achieve the desired concentration. A 0.1 ml bolus of either PG was infused intraportally over 1 min. PHX or sham procedures were performed immediately following NO donor or PG administration.
RNA isolation and Northern blot analysis
RNA analysis has been previously described [8]. Briefly, total RNA was extracted using a 3 M/6 M lithium chloride/urea solution, centrifuged at 25,000 rpm for 20 min at 4 °C, and total RNA was extracted using phenol/chloroform. The concentration of the RNA was then determined and 20 μg of total RNA was separated by gel electrophoresis under denaturing conditions, transferred to nylon membrane, and fixed by UV cross-linking. The membranes were prehybridized at 42 °C for 3 h in prehybridization buffer, and c-fos mRNA was detected using a 1.8 kb cDNA probe labeled by the random prime method, with α-dCTP 32P. The membranes were hybridized overnight at 42 °C, washed at 65 °C, exposed to film, and the density of the c-fos mRNA band was determined by densitometry. c-Fos mRNA expression is reported relative to 18S rRNA. Results were analyzed using ANOVA, with p < 0.05 deemed significant.
Results
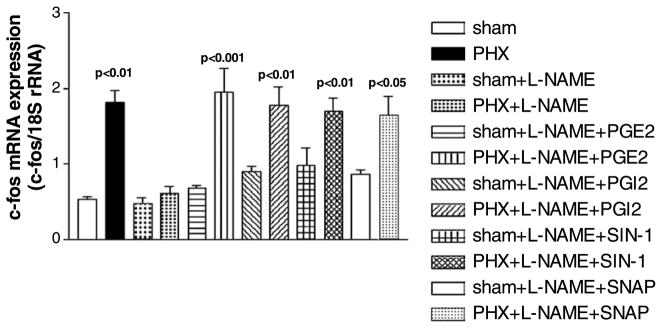
The interaction between NO and PGs in the liver regeneration cascade was investigated using the NOS antagonist, L-NAME, the COX antagonist, INDO, the NO donors, SIN-1 and SNAP, and the PGs, PGE2 and PGI2. The increase in c-fos mRNA expression after PHX (1.82 ± 0.16, n = 5, p < 0.01 vs. sham, n = 5, 0.53 ± 0.03) (Fig. 1) was inhibited by the NOS antagonist, L-NAME (0.62 ± 0.09, n = 5, NS from sham). The inhibition by L-NAME was reversed by the PGs, PGE2 (1.95 ± 0.32, n = 7, p < 0.001 vs. sham) and PGI2 (1.78 ± 0.24, n = 8, p < 0.01 vs. sham), and by the NO donors, SIN-1 (1.70 ± 0.17, p < 0.01 vs. sham, n = 9) and SNAP (1.64 ± 0.26, n = 7, p < 0.05 vs. sham). In addition, L-NAME had no effect on c-fos mRNA expression in normal, non-PHX livers (0.47 ± 0.08, n = 6, NS from sham). Neither PGE2 (0.68 ± 0.04, n = 4), PGI2 (0.90 ± 0.06, n = 5), SIN-1 (0.98 ± 0.24, n = 4) nor SNAP (0.87 ± 0.05, n = 7) administered after L-NAME had any effect on c-fos mRNA expression compared to normal livers in sham rats. Therefore, the NOS antagonist, L-NAME, inhibits c-fos mRNA expression after PHX, and PGE2, PGI2 or the NO donors, SIN-1 or SNAP, reversed the inhibition.
Fig. 1.
c-Fos mRNA expression after PHX and NOS inhibition. The inhibition of c-fos mRNA expression by L-NAME after PHX can be reversed by PGE2, PGI2 or the NO donors, SIN-1 or SNAP (data shown as means ± SEM).
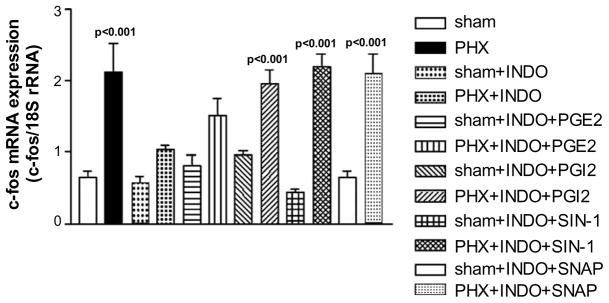
c-Fos mRNA expression increased after PHX (2.12 ± 0.40, n = 5, p < 0.001 vs sham (0.65 ± 0.09)), which was inhibited by INDO, a COX antagonist (1.03 ± 0.07, n = 7, NS vs. sham) (Fig. 2). The inhibition tended to be reversed by PGE2 (1.52 ± 0.23, n = 6), although the trend was not significantly different from sham, and fully reversed by PGI2 (1.96 ± 0.18, n = 6, p < 0.001 vs. sham). The NO donors, SIN-1 and SNAP, also reversed the inhibition of c-fos mRNA expression after INDO (2.20 ± 0.17, n = 5, p < 0.001 and 2.09 ± 0.28, n = 6, p < 0.001, respectively). c-Fos mRNA expression in the normal, non-PHX liver was neither affected by INDO (0.58 ± 0.08, n = 5, NS from sham) nor by INDO followed by PGE2 (0.81 ± 0.15, n = 4), PGI2 (0.96 ± 0.06, n = 6), SIN-1 (0.44 ± 0.04, n = 6), or SNAP (0.66 ± 0.09, n = 6). The increase in c-fos mRNA expression was not affected by the ethanol-saline vehicle in which PGE2 and PGI2 were dissolved (data not shown). Thus, inhibition of PG production by COX inhibits c-fos mRNA expression, an index of the initiation of the liver regeneration cascade, and this inhibition can be reversed by administration of either PGI2 or a NO donor.
Fig. 2.
c-Fos mRNA expression after PHX and COX inhibition. c-Fos mRNA expression increases after PHX, and this increase is blocked by INDO, a COX antagonist. The inhibition is reversed by PGI2, or the NO donors, SIN-1 or SNAP. In addition, these results also provide evidence that there is interaction between NO and PGs in triggering the liver regeneration cascade (data shown as means ± SEM).
Discussion
Prostaglandins and the liver regeneration cascade
The inhibition of the increase in c-fos mRNA expression after PHX by L-NAME (a NOS antagonist) is reversed by PGE2, PGI2 or a NO donor (SIN-1 or SNAP). Also, the increase in c-fos mRNA expression is inhibited by INDO (a COX antagonist). This inhibition is reversed by PGI2 or the NO donors, SIN-1 or SNAP. Thus, under conditions where either NO or PG production is blocked, the inhibition of c-fos mRNA expression can be reversed by either PGs or NO donors. This suggests that both NO and PGs are required for c-fos mRNA expression to increase after PHX, and that, in the absence of one, excess exogenous supply of the other can compensate.
Previous experiments suggested an interaction between NO and PGs in triggering the liver regeneration cascade, using proliferative factor production after PHX as an index of initiation of the liver regeneration cascade. Briefly, proliferative factors include all the growth factors, cytokines, etc., produced after PHX that comprise the liver regeneration cascade. Proliferating factor production was found to increase, peaking 4 h after PHX [14]. This increase was inhibited by L-NAME and the inhibition was partially reversed by L-arginine, the substrate for NOS [13]. Administration of indomethacin also inhibits proliferating factor production 4 h after PHX [22], suggesting that both NO and PGs play a role in triggering the liver regeneration cascade. In the case of both indices, c-fos mRNA expression and proliferating factor production, it appears that inhibition of NO or PG synthesis completely blocks the index, which indicates that NO and PG production appear to have an important role in the liver regeneration cascade.
An additional index of initiation of the liver regeneration cascade, liver weight restoration after PHX, was also investigated [13]. Inhibition of NOS caused a 30% reduction in liver weight restoration 48 h after PHX, and was also associated with a high mortality rate that was found not to be due to L-NAME itself [22]. Blockade of NOS prior to PHX was also shown to inhibit DNA synthesis in vivo [37]. Other studies have also implicated PGs in liver weight restoration [38]. Inhibition of COX by indomethacin causes a decrease in DNA synthesis and delays liver regeneration after PHX [27,28]. Thus, both NO and PGs are an integral part of both the triggering and propagation mechanisms of the liver regeneration cascade.
Mechanism of NO and PG release in the liver regeneration cascade
As discussed above, the liver does not control inflowing blood from the intestine and must accept all the portal venous inflow. Removal of 2/3 of the liver tissue results in a threefold increase in the blood flow-to-liver mass ratio in the remaining 1/3 of the liver [5], and evidence is presented that this increase in blood flow causes shear stress (reflected as an immediate increase in PVP), and the release of NO, which thereby triggers the liver regeneration cascade.
It is also hypothesized that PG production is a result of the same shear stress-dependent mechanism that causes NO release. PGE2 and PGI2 are known to increase after PHX [25–27], with PGI2 increasing threefold immediately after PHX [26]. NO and PGI2 are vaso-active mediators, released in response to an increase in flow, and thereby shear stress, in blood vessels [30,39]. Also, NO, PGI2, and PGE2 are released in response to shear stress applied in vitro [29–32,40], and release of these mediators was shown to be proportionate to the amount of shear stress applied [30,39]. PVP, an index of shear stress in the liver, increases after PHX [8], indicating that shear stress is increased in the liver, along with the increase in the blood flow-to-liver mass ratio.
Interaction between NO and PGs
NO production causes an increase in cGMP, which stimulates c-fos mRNA expression in fibroblasts [18] and PC12 cells [41]. PG production causes an increase in cAMP, which thereby causes an increase in c-fos mRNA expression in macrophages [42] and osteocytes [43]. Thus, an increase in either cGMP or cAMP stimulates c-fos mRNA expression, and after PHX, both of these second messengers may be required to stimulate c-fos mRNA expression. However, in the absence of NO, and therefore cGMP, it is possible that administration of excess exogenous PGs caused an increase in the amount of cAMP, thereby stimulating c-fos mRNA expression. Similarly, in the case of COX inhibition, the provision of excess exogenous NO could cause an increase in cGMP, thereby stimulating c-fos mRNA expression after PHX. Thus, in this manner, excess amounts of NO or PGs could compensate for the absence of actions of the other in situations of either NOS or COX inhibition.
There are several examples of interaction between NO and PGs in other tissues. For example, under normal, non-shear stress conditions, NO stimulates PG production in endothelial cells in vitro. Endothelial NO production stimulates PGI2 production, possibly by directly activating COX, and inhibition of NOS and guanylate cyclase (GC) reduced levels of PGI2 [44]. However, under conditions of shear stress, inhibition of NOS or GC resulted in an increase in PGI2 production in endothelial and smooth muscle cells undergoing shear stress [45,46]. Thus, when NOS was inhibited, in vitro endothelial cell production of PGI2 increases and the excess amount of this PG could compensate for the absent effects of NO. In addition, shear conditions caused an increase in eNOS expression and activity in endothelial cells, which was potentiated by the COX antagonist, indomethacin [47]. Gastric eNOS is also upregulated in conditions of decreased PGE2 production and COX inhibition [48]. This suggests that in the absence of PGs, endothelial cell NO production increases and the excess amount of NO could compensate for the lack of effects of PGs. Thus, c-fos mRNA expression could be restimulated under conditions of NOS inhibition after PHX, by excess amounts of PGs, or under conditions of COX inhibition after PHX, by excess amounts of NO.
However, the results from these studies suggest that endogenous production of either NO or PGs cannot compensate for the loss of the other, although provision of excess exogenous NO or PGs does result in a compensatory effect. Reversal of the inhibition of c-fos mRNA expression in either case occurs only when adequate levels of exogenous NO or PGs are provided. It may be possible, however, that the compensatory mechanism takes some time to upregulate, and therefore c-fos mRNA expression is merely delayed after PHX. This seems unlikely, though, since c-fos mRNA expression after PHX is still inhibited by L-NAME at 60 min after PHX [8]. Thus, NO and PGs seem not to compensate for the absence of the other endogenously, at least with regard to c-fos mRNA expression, and/or within the limited time frame examined after PHX. However, this situation would seem to provide an excellent therapeutic opportunity by administration of exogenous NO donors or the PGs, PGE2 or PGI2, to stimulate the liver regeneration cascade. As an initial screening tool the expression of c-fos is straightforward and can direct development of therapeutics which then must also be confirmed to not only activate the regeneration cascade but also result in the ultimate goal of stimulating the end target, liver mass restoration.
Acknowledgments
The authors acknowledge Dallas Legare for surgical technical assistance. This work was funded by an operating grant from the Canadian Institutes of Health Research. J.M.S.S. was funded by a Canadian Institutes of Health Research Studentship.
References
- 1.Michalopolous GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 2.Mars WM, Liu ML, Kitson RP, Goldfarb RH, Gabauer MK, Michalopoulos GK. Immediate early detection of urokinase receptor after partial hepatectomy and its implications for initiation of liver regeneration. Hepatology. 1995;21:1695–1701. [PubMed] [Google Scholar]
- 3.Mullhaupt B, Feren A, Fodor E, Jones A. Liver expression of epidermal growth factor RNA. J Biol Chem. 1994;269:19667–19670. [PubMed] [Google Scholar]
- 4.Rai RM, Loffreda S, Karp CL, Yang SQ, Lin HZ, Diehl AM. Kupffer cell depletion abolishes induction of interleukin-10 and permits sustained overexpression of tumor necrosis factor alpha messenger RNA in the regenerating rat liver. Hepatology. 1997;25:889–895. doi: 10.1002/hep.510250417. [DOI] [PubMed] [Google Scholar]
- 5.Rice GC, Leiberman DP, Mathie RT, Ryan CJ, Harper AM, Blumgart LH. Liver tissue blood flow measured by 85Kr clearance in the anaesthetized rat before and after partial hepatectomy. Br J Exp Pathol. 1997;58:243–250. [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn D, van Hoorn-Hickman R, Terblanche J. Liver blood flow after partial hepatectomy in the pig. J Surg Res. 1984;37:290–294. doi: 10.1016/0022-4804(84)90191-4. [DOI] [PubMed] [Google Scholar]
- 7.Um SH, Nishida O, Tokubayashi M, Kimura F, Takimoto Y, Yoshioka H, Inque R, Kita T. Hemodynamic changes after ligation of a major branch of the portal vein in rats: comparison with rats with portal vein constriction. Hepatology. 1994;19:202–209. [PubMed] [Google Scholar]
- 8.Schoen JM, Wang HH, Minuk GY, Lautt WW. Shear stress-induced nitric oxide release triggers the liver regeneration cascade. Nitric Oxide. 2001;5:453–464. doi: 10.1006/niox.2001.0373. [DOI] [PubMed] [Google Scholar]
- 9.Macedo MP, Lautt WW. Shear-induced modulation of vaso-constriction in the hepatic artery and portal vein by nitric oxide. Am J Physiol. 1998;274:G253–G260. doi: 10.1152/ajpgi.1998.274.2.G253. [DOI] [PubMed] [Google Scholar]
- 10.Sato Y, Koyama S, Tsukada K, Hatakeyama K. Acute portal hypertension reflecting shear stress as a trigger of liver regeneration following partial hepatectomy. Jpn J Surg. 1997;27:518–526. doi: 10.1007/BF02385805. [DOI] [PubMed] [Google Scholar]
- 11.Sato Y, Tsukada K, Hatakeyama K. Role of shear stress and immune responses in liver regeneration after a partial hepatectomy. Jpn J Surg. 1999;29:1–9. doi: 10.1007/BF02482962. [DOI] [PubMed] [Google Scholar]
- 12.Wang HH, Lautt WW. Does nitric oxide (NO) trigger liver regeneration? Proc West Pharmacol Soc. 1997;40:17–18. [PubMed] [Google Scholar]
- 13.Wang HH, Lautt WW. Evidence of nitric oxide, a flow-dependent factor, being a trigger of liver regeneration in rats. Can J Physiol Pharmacol. 1998;76:1072–1079. doi: 10.1139/cjpp-76-12-1072. [DOI] [PubMed] [Google Scholar]
- 14.Wang HH, Lautt WW. Hepatocyte primary culture bioassay: a simplified tool to assess the initiation of the liver regeneration cascade. J Pharmacol Toxicol Meth. 1997;38:141–150. doi: 10.1016/s1056-8719(97)00079-8. [DOI] [PubMed] [Google Scholar]
- 15.Moser MJ, Gong Y, Zhang MN, Johnston J, Lipschitz J, Kneteman NM, Minuk GY. Immediate-early oncogene expression and liver function following varying extents of partial hepatectomy in the rat. Dig Dis Sci. 2001;46:907–914. doi: 10.1023/a:1010791915733. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh H-J, Li N-Q, Frangos JA. Pulsatile and steady flow induces c-fos expression in human endothelial cells. J Cell Physiol. 1993;154:143–151. doi: 10.1002/jcp.1041540118. [DOI] [PubMed] [Google Scholar]
- 17.Ranjan V, Diamond SL. Fluid shear stress induces synthesis and nuclear localization of c-fos in cultured human endothelial cells. Biochem Biophys Res Commun. 1993;196:79–84. doi: 10.1006/bbrc.1993.2218. [DOI] [PubMed] [Google Scholar]
- 18.Haby C, Lisovoski F, Aunis D, Zwiller J. Stimulation of the cyclic GMP pathway by NO induces expression of the immediate early genes c-fos and junB in PC12 cells. J Neurochem. 1994;62:496–501. doi: 10.1046/j.1471-4159.1994.62020496.x. [DOI] [PubMed] [Google Scholar]
- 19.Morris BJ. Stimulation of immediate early gene expression in striatal neurons by nitric oxide. J Biol Chem. 1995;270:24740–24744. [PubMed] [Google Scholar]
- 20.Ohki K, Yoshida K, Hagiwara M, Harada T, Takamura M, Ohashi T, Matsuda H, Imaki J. Nitric oxide induces c-fos gene expression via cyclic AMP response element binding protein (CREB) phosphorylation in rat retinal pigment epithelium. Brain Res. 1995;696:140–144. doi: 10.1016/0006-8993(95)00914-c. [DOI] [PubMed] [Google Scholar]
- 21.Pilz RB, Suhasini M, Idriss S, Meinkoth JL, Boss GR. Nitric oxide and cGMP analogs activate transcription from AP-1-responsive promoters in mammalian cells. FASEB J. 1995;9:552–558. doi: 10.1096/fasebj.9.7.7737465. [DOI] [PubMed] [Google Scholar]
- 22.Wang HH. PhD Thesis. University of Manitoba; 1997. Evidence of nitric oxide being the initial trigger of the liver regeneration cascade. [Google Scholar]
- 23.Wernze H, Tittor W, Goerig M. Release of prostanoids into the portal and hepatic vein in patients with chronic liver disease. Hepatology. 1986;6:911–916. doi: 10.1002/hep.1840060517. [DOI] [PubMed] [Google Scholar]
- 24.Guarner F, Fremont-Smith M, Prieto J. Cytoprotective effect of prostaglandins on isolated rat liver cells. Liver. 1985;5:35–39. doi: 10.1111/j.1600-0676.1985.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 25.Tsujii H, Okamoto Y, Kikuchi E, Matsumoto M, Nakano H. Prostaglandin E2 and rat liver regeneration. Gastroenterology. 1993;105:495–499. doi: 10.1016/0016-5085(93)90725-r. [DOI] [PubMed] [Google Scholar]
- 26.Lai OF, Chow PK, Tan S, Song IC, Soo KC, Aw SE, Yu WK, Fook-Chong SM, Satchithanantham S, Chan ST. Changes in prostaglandin and nitric oxide levels in the hyperdynamic circulation following liver resection. J Gastroenterol Hepatol. 2000;15:895–901. doi: 10.1046/j.1440-1746.2000.02295.x. [DOI] [PubMed] [Google Scholar]
- 27.MacManus JP, Braceland BM. A connection between the production of prostaglandins during liver regeneration and the DNA synthetic response. Prostaglandins. 1976;11:609–620. doi: 10.1016/0090-6980(76)90064-2. [DOI] [PubMed] [Google Scholar]
- 28.Besse T, de Hemptinne B, Kabeya V, Lambotte L. Stimulation of liver regeneration by prostacyclin. Transpl Proc. 1991;23:542–544. [PubMed] [Google Scholar]
- 29.Frangos JA, Eskin SG, McIntyre LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 30.Grabowski EF, Jaffe EA, Weksler BB. Prostacyclin production by cultured endothelial cell monolayers exposed to step increases in shear stress. J Lab Clin Med. 1985;105:36–43. [PubMed] [Google Scholar]
- 31.Shah V, Haddad FG, Garcia-Cardena G, Frangos JA, Mennone A, Groszmann RJ, Sessa WC. Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. J Clin Invest. 1997;100:2923–2930. doi: 10.1172/JCI119842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S, Tarbell JM. Effect of fluid flow on smooth muscle cells in a 3-dimensional collagen gel model. Arterioscler Thromb Vasc Biol. 2000;20:2220–2225. doi: 10.1161/01.atv.20.10.2220. [DOI] [PubMed] [Google Scholar]
- 33.McAllister TN, Du T, Frangos JA. Fluid shear stress stimulates prostaglandin and nitric oxide release in bone marrow-derived preosteoclast-like cells. Biochem Biophys Res Commun. 2000;270:643–648. doi: 10.1006/bbrc.2000.2467. [DOI] [PubMed] [Google Scholar]
- 34.Klein-Nulend J, Semiens CM, Ajubi NE, Nijweide PJ, Burger EH. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not by periosteal fibroblasts—correlation with prostaglandin upregulation. Biochem Biophys Res Commun. 1995;217:640–648. doi: 10.1006/bbrc.1995.2822. [DOI] [PubMed] [Google Scholar]
- 35.Smalt R, Mitchell FT, Howard RL, Chambers TJ. Induction of NO and prostaglandin E2 in osteoblasts by wall-shear stress but not mechanical strain. Am J Physiol. 1997;273:E751–E758. doi: 10.1152/ajpendo.1997.273.4.E751. [DOI] [PubMed] [Google Scholar]
- 36.Higgins GM, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 37.Carnovale CE, Scapini C, Alvarez ML, Favre C, Monti J, Carrillo MC. Nitric oxide release and enhancement of lipid peroxidation in regeneration rat liver. J Hepatol. 2000;32:798–804. doi: 10.1016/s0168-8278(00)80249-4. [DOI] [PubMed] [Google Scholar]
- 38.Rudnick DA, Perlmutter DH, Muglia LJ. Prostaglandins are required for CREB activation and cellular proliferation during liver regeneration. Proc Natl Acad Sci USA. 2001;98:8885–8890. doi: 10.1073/pnas.151217998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelm M, Feelisch M, Seussen A, Strauer BE, Schrader J. Release of endothelium derived nitric oxide in relation to pressure and flow. Cardiovasc Res. 1991;25:831–836. doi: 10.1093/cvr/25.10.831. [DOI] [PubMed] [Google Scholar]
- 40.Gallis B, Corthals GL, Goodlett DR, Ueba H, Kim F, Presnell SR, Figeys D, Harrison DG, Berk BC, Aebersold R, Corson MA. Identification of flow-dependent endothelial nitric-oxide synthase phosphorylation sites by mass spectrometry and regulation of phosphorylation and nitric oxide production by the phosphatidylinositol 3-kinase inhibitor LY294002. J Biol Chem. 1999;274:30101–30108. doi: 10.1074/jbc.274.42.30101. [DOI] [PubMed] [Google Scholar]
- 41.Uberall F, Werner-Felmayer G, Schubert C, Grunicke HH, Wachter H, Fuchs D. Neopterin derivatives together with cyclic guanosine monophosphate induce c-fos gene expression. FEBS Lett. 1994;352:11–14. doi: 10.1016/0014-5793(94)00899-x. [DOI] [PubMed] [Google Scholar]
- 42.Zhuang D, Kawajiri H, Takahashi Y, Yoshimoto T. Suppression of Prostaglandin E2-mediated c-fos mRNA induction by interleukin-4 in murine macrophages. J Biochem. 2000;127:451–456. doi: 10.1093/oxfordjournals.jbchem.a022627. [DOI] [PubMed] [Google Scholar]
- 43.Fitzgerald J, Hughes-Fulford M. Mechanically induced c-fos expression is mediated by cAMP in MC3T3 osteoblasts. FASEB J. 1999;13:553–557. doi: 10.1096/fasebj.13.3.553. [DOI] [PubMed] [Google Scholar]
- 44.Sievi E, Lahteenmaki TA, Alanko J, Vuorinen P, Vapaatalo H. Nitric oxide as a regulator of prostacyclin synthesis in cultured rat heart endothelial cells. Arzneimittelforschung. 1997;47:1093–1098. [PubMed] [Google Scholar]
- 45.Osanai T, Fujita N, Fujiwara N, Nakano T, Takahashi K, Guan W, Okumura WK. Cross talk of shear-induced production of prostacyclin and nitric oxide in endothelial cells. Am J Physiol. 2000;278:H233–H238. doi: 10.1152/ajpheart.2000.278.1.H233. [DOI] [PubMed] [Google Scholar]
- 46.Osanai T, Akutsu N, Fujita N, Nakano T, Takahashi K, Guan W, Okumura K. Cross talk between prostacyclin and nitric oxide under shear in smooth muscle cell: role in monocyte adhesion. Am J Physiol. 2001;281:H177–H182. doi: 10.1152/ajpheart.2001.281.1.H177. [DOI] [PubMed] [Google Scholar]
- 47.Hendrickson RJ, Cappadona C, Yankah EN, Sitzmann JV, Cahill PA, Redmond EM. Sustained pulsatile flow regulates endothelial nitric oxide synthase and cyclooxygenase expression in co-cultured vascular endothelial and smooth muscle cells. J Mol Cell Cardiol. 1999;31:619–629. doi: 10.1006/jmcc.1998.0898. [DOI] [PubMed] [Google Scholar]
- 48.Fischer H, Becker JC, Boknik P, Huber V, Luss H, Neumann J, Schmitz W, Domschke W, Konturek JW. Expression of endothelial cell-derived nitric oxide synthase (eNOS) is increased during gastric adaptation to chronic aspirin intake in humans. Aliment Pharmacol Ther. 1999;13:507–514. doi: 10.1046/j.1365-2036.1999.00489.x. [DOI] [PubMed] [Google Scholar]