Abstract
Dynamic combinatorial libraries are mixtures of compounds that exist in a dynamic equilibrium and can be driven to compositional self adaptation via selective binding of a specific assembly of certain components to a molecular target. We present here an extension of this initial concept to dynamic libraries that consists of two levels, the first formed by the coordination of terpyridine-based ligands to the transition metal template, and the second, by the imine formation with the aldehyde substituents on the terpyridine moieties. Dialdehyde 7 has been synthesized, converted into a variety of ligands, oxime ethers L11–L33 and acyl hydrazones L44–L77, and subsequently into corresponding cobalt complexes. A typical complex, Co(L22)22+ is shown to engage in rapid exchange with a competing ligand L11 and with another complex, Co(L22)22+ in 30% acetonitrile/water at pH 7.0 and 25°C. The exchange in the corresponding Co(III) complexes is shown to be much slower. Imine exchange in the acyl hydrazone complexes (L44–L77) is strongly controlled by pH and temperature. The two types of exchange, ligand and imine, can thus be used as independent equilibrium processes controlled by different types of external intervention, i.e., via oxidation/reduction of the metal template and/or change in the pH/temperature of the medium. The resulting double-level dynamic libraries are therefore named orthogonal, in similarity with the orthogonal protecting groups in organic synthesis. Sample libraries of this type have been synthesized and showed the complete expected set of components in electrospray ionization MS.
Rapid development of combinatorial chemistry and high-throughput experimentation in basic and applied research (1–4) generates increasing demand in new approaches that simplify and expedite synthesis and screening of diverse arrays of compounds and materials. Dynamic combinatorial chemistry (5–11) has attracted increasing interest over recent years as a new approach that combines in one system the library generation and screening processes. The dynamic libraries are designed as mixtures of components that can reversibly interconvert in a dynamic equilibrium that is driven by molecular recognition of a specific molecular target toward that assembly or a subset of components that form the library constituent best bound to the target.
One of the key issues in designing dynamic libraries is the choice of the reversible chemical reaction that can interconvert the library components. A number of such reactions have been tested, which include transacylation (12–15), imine exchange (16–20), isomerization (21–23), thiol-disulfide exchange (24, 25), and ligand exchange in coordination complexes (17, 18, 26).
A logical development of dynamic combinatorial chemistry would aim toward the combination of two or more reversible processes within one library. To extend the comparison with “static” chemical libraries, such multilevel organization in the dynamic library would be similar to advancing from a static library of components having just one type of chemical bond between the functional units (e.g., peptide and nucleotide libraries) to more diverse arrays of compounds in which building blocks are held together by a variety of linkages (e.g., libraries of synthetic organic molecules). Combinations of two reactions in dynamic libraries have been reported previously (17, 27); however, the highly labile bonds hampered isolation and characterization of library components and restricted control over library composition.
One may distinguish two types of such multilevel dynamic libraries: orthogonal, when the reversible processes operate independently (e.g., imine formation and metal ion coordination or imine formation and intramolecular rearrangement) and nonorthogonal, when the processes may interfere/cross over (e.g., imine formation and Michael addition).
In this paper, we report an example of dynamic libraries on the basis of two types of reactions, metal coordination and imine exchange, which can be controlled independently of each other by varying the set of conditions. These libraries are referred to below as orthogonal, because the two processes, in principle, do not interfere, and analogously to the orthogonal protecting groups in organic chemistry.
Materials and Methods
Instrumental Techniques.
NMR spectra were recorded on Varian Unity 300, 400, and 500 MHz instruments. MS analysis was performed by using a model API-3,000 triple quadrupole mass spectrometer (Applied Biosystems/Sciex) coupled to liquid chromatography and equipped with a turbo ion-spray interface. The mass spectrometer was operated at unit resolution. Samples were introduced into the ionization source at a flow rate of 5 μl/min with a syringe pump. The ion-spray needle was operated at +5,500 V, and the orifice voltage was set at 35–40 eV. Full-scan MS spectra were acquired over the mass range m/z 50–3,000 by scanning the first quadrupole (Q1) with 0.1 step size at a scan rate of 3 seconds. The product–ion spectra of each analyte were obtained with collision energy ranging 5 to 60 eV. Solutions were prepared in 30% acetonitrile/70% 0.014 M ammonium acetate (pH 7.0) with sample concentration ranging from 5 to 30 μM. Some MS spectra were acquired by using an autosampler and LC-MS in a column-by-pass mode. Typically, a chromatogram was recorded at a flow rate of 200 μl/min with the same mobile phase that was used for the continuous infusion. The ion source temperature was set at 300°C.
Synthesis.
All commercial reagents were purchased from Aldrich. The synthesis of ligand 7 is published as supplemental data on the PNAS web site, www.pnas.org.
Oxime Ethers 8a.
Dialdehyde 7 (0.05 g, 0.17 mmol) was dissolved in 10 ml of pyridine. O-methylhydroxylamine hydrochloride (0.042 g, 0.51 mmol) was added, and the reaction mixture was stirred overnight at room temperature. The solvent was evaporated, and the solid residue dissolved in 15 ml of CHCl3 and washed with 0.1 M K2HPO4 at pH 8.0 (4 × 15 ml). Organic phase was dried over MgSO4. Evaporation of solvent gave 0.043 g (73%) of the product. L11: 1H NMR (500 MHz, CDCl3): δ = 4.03 (s, 6H, CH3), 7.97 (t, JH-4′,H-3′ = 7.93 Hz, 1H; H-4′), 8.14 (dd, JH-4,H-3 = 8.54 Hz, JH-4,H-6 = 1.83 Hz 2H; H-4), 8.15 (s, 1H; HC = ), 8.49 (d, JH-3′,H-4′ = 7.93 Hz, 2H; H-3′), 8.63 (d, JH-3,H-4 = 8.54 Hz, 2H; H-3), 8.8 (d, JH-6,H-4 = 1.83 Hz, 2H; H-6). Electrospray ionization (ESI) MS: m/z, M + 1 = 348.2 L22: 1H NMR (500 MHz, CDCl3): δ = 1.36 (t, J = 7.14 Hz, 6H; CH3) 4.29 (q, J = 7.14, 4H; CH2), 7.96 (t, JH-4′,H-3′ = 7.69 Hz, 1H; H-4′), 8.12 (dd, JH-4,H-3 = 8.24 Hz, JH-4,H-6 = 2.19 Hz 2H; H-4), 8.15 (s, 1H; HC = ), 8.48 (d, JH-3′,H-4′ = 7.69 Hz, 2H; H-3′), 8.61 (d, JH-3,H-4 = 8.24 Hz, 2H; H-3), 8.8 (d, JH-6,H-4 = 2.19 Hz, 2H; H-6). ESI MS: m/z, M + 1 = 376.3
Acyl Hydrazones 8a.
The following procedure is given for L44 and was identical for L55, L66, and L77. Acetylhydrazide (0.038 g, 0.51 mmol) and 7 (0.05 g, 0.17 mmol) were dissolved in 15 ml of anhydrous EtOH. The solution was refluxed overnight and then allowed to stand at room temperature. A white precipitate formed was washed several times by cold EtOH. Yield 0.052 g, 76%. L44: 1H NMR (500 MHz, DMSO): δ = 1.98 (s, 2.2H; CH3) 2.25 (s, 3.7H; CH3), 8.1 (s, 1H), 8.13 (t, 1H), 8.27 (s, 1H), 8.28 (dd, 1H), 8.3 (dd, 1H), 8.48 (m, 2H), 8.68 (m, 2H), 8.94 (s, 2H), 11.49 (s, 1.2H), 11.63 (s, 0.8H). According to peaks of protons that correspond to the CH3 and NH groups, the ratio of anti/syn isomers in the mixture is 60/30. ESI MS: m/z, M + 1 = 402.6. L55: 1H NMR (500 MHz, DMSO): δ = 7.56 (t, J = 7.33 Hz, 4H; CH) 7.62 (t, J = 7.33 Hz, 2H; CH), 7.95 (d, J = 7.33 Hz, 4H; CH), 8.16 (t, JH-4′,H-3′ = 7.324 Hz, 2H; H-4′), 8.38 (d, JH-4,H-3 = 7.94 Hz, 2H; H-4), 8.52 (d, JH-3′,H-4′ = 7.324 Hz, 2H; H-3′), 8.61 (s, 1H; HC⩵), 8.75 (d, JH-3,H-4 = 7.94 Hz, 2H; H-3), 9.01 (s, 2H; H-6), 12.12 (s, 2H; NH). ESI MS: m/z, M + 1 = 526.2 L66: 1H NMR (500 MHz, DMSO): δ = 7.9 (dd, J = 8.9 Hz, J = 5.4 Hz 2H; CH), 8.17 (t, JH-4′,H-3′ = 7.3 Hz, 2H; H-4′), 8.36 (d, JH-4,H-3 = 7.75 Hz, 2H; H-4), 8.49 (d, JH-3′,H-4′ = 7.3 Hz, 2H; H-3′), 8.63 (s, 1H; HC⩵), 8.73 (d, JH-3,H-4 = 7.75Hz, 2H; H-3), 8.82 (d, J = 8.9 Hz, 2H; CH), 8.97 (d, J = 5.4 Hz, 2H; CH), 9.15 (s, 2H; H-6), 9.7 (s, 2H; CH), 11.9 (s, 2H; NH). ESI MS: m/z, M + 1 = 528.18. L77: 1H NMR (500 MHz, DMSO): δ = 3.86 (s, 1H), 7.73 (d, J = 8.7 Hz, 4H; CH), 8.05 (d, J = 8.7 Hz, 4H), 8.17 (t, JH-4′,H-3′ = 7.23 Hz, 2H; H-4′), 8.36 (d, JH-4,H-3 = 7.75 Hz, 2H; H-4), 8.49 (d, JH-3′,H-4′ = 7.23 Hz, 2H; H-3′), 8.63 (s, 1H; HC⩵), 8.73 (d, JH-3,H-4 = 7.75Hz, 2H; H-3), 9.15 (s, 2H; H-6), 12.2 (s, 2H; NH). ESI MS: m/z, M + 1 = 558.18.
General Procedure for Co(8)22+ Complexes.
Cobalt(II) chloride hexahydrate (1 eq) was added to water solution containing 2 eq of the ligand. Reaction mixture was stirred at 50°C until complete dissolution of the ligand. The complex was then precipitated with excess of saturated solution of NH4PF6 at 0°C. The precipitate was washed with water at 0°C and dried in vacuo. The product was purified by recrystallization from acetonitrile by means of ether diffusion in solution. [CoL11L11](PF6)2: 1H NMR (500 MHz, CD3CN): δ = 4.12 (s, 12H, CH3), 9.1 s, 9.58 s, 21.96 s, 44.56 s, 53.51 s, 98.4 s. ESI MS: M/2 = 376.7. MS/MS: M/2 = 347.8, 319.4, 290.5, 262.2. [CoL22L22](PF6)2: 1H NMR (500 MHz, CD3CN): δ = 1.28 (t,, J = 7.14 Hz, 12H, CH3), 4.25 (q, J = 7.14 Hz, 8H; CH2), 9.07 s, 9.61 s, 22.69 s, 48.9 s, 56.85 s, 103.01 s. ESI MS: M/2 = 405.1. MS/MS: M/2 = 382 (-O-CH2CH3), 367.7 (-CH = N-), 354.8 (-CH2CH3), 319.5 (-CH = n = OCH2CH3), 283.4 (-CH = n = OCH2CH3), 262.2 (-CH = N-O-). [CoL44L44](PF6)2: 1H NMR (500 MHz, D2O): δ = 1.77 s, 8.85 s, 9.29 s, 12.74 s, 21.84 s, 40.94 s, 51.83 s, 97.23 s. ESI MS: M/2 = 431.3. MS/MS: M/2 = 401.7, 371.8, 342.3, 312.8. [CoL55L55](PF6)2: 1H NMR (500 MHz, CD3CN): δ = 7.46 (t, J = 6.72 Hz, 8H), 7.56 (t, J = 6.72 Hz, 4H), 7.68 (t, J = 6.72 Hz, 8H), 8.53 s, 9.34 s, 12.0 s, 22.61 s, 46.15 s, 54.74 s, 101.26 s. ESI MS: M/2 = 555.1. MS/MS: M/2 = 494.3, 438.9, 373.3, 312.7 [each time, NHC(O)-C6H5 groups fall out]. [CoL66L66](PF6)2: ESI MS: M/2 = 557.6. MS/MS: M/2 = 496.3, 435.1, 373.9, 312.0 [each time, NHC(O)-NC5H4 groups fall out]. [CoL77L77](PF6)2: ESI MS: M/2 = 587.6. MS/MS: M/2 = 518.6, 449.3, 380.2, 312.3 [each time, NHC(O)-C6H4-OH groups fall out].
General Procedure for Co(8)23+ Complexes.
To the water solution of [Co(8)2]Cl2, 10-fold molar excess of H2O2 30% and 2 eq of 10% HCl were added. The reaction mixture was stirred overnight at room temperature. Co3+ complexes were precipitated by saturated solution of NH4PF6 at 0°C. Precipitate was washed with H2O at 0°C and dried in vacuo. The complex was purified by recrystallization from acetonitrile by means of ether diffusion in solution. [CoL11L11](PF6)3: 1H NMR (500 MHz, CD3CN): δ = 3.9 (s, 12H, CH3), 7.13 (d, JH-6,H-4 = 1.52 Hz, 4H; H-6), 7.89 (s, 4H; n = CH), 8.43 (dd, JH-4,H-6 = 1.52 Hz, JH-4,H-3 = 8.24 Hz 4H; H-4), 8.58 (d, JH-3,H-4′ = 8.24 Hz, 4H; H-3), 9.06 (m, 6H, H-4′, H-3′). [CoL22L22](PF6)3: 1H NMR (500 MHz, CD3CN): δ = 1.21 (t, J = 7.01 Hz, 12H, CH3), 4.15 (q, J = 7.01 Hz, 8H; CH2), 7.14 (d, JH-6,H-4 = 1.52 Hz, 4H; H-6), 7.88 (s, 4H; n = CH), 8.43 (dd, JH-4,H-6 = 1.52 Hz, JH-4,H-3 = 8.24 Hz 4H; H-4), 8.57 (d, JH-3,H-4′ = 8.24 Hz, 4H; H-3), 9.06 (m, 6H, H-4′, H-3′).
General Procedure for the Hydrazone Library Formation.
The single-level library was formed by mixing individual complexes Co(L44)22+, Co(L66)22+, Co(L77)22+ in equimolar amounts in 30% acetonitrile in 0.01 M aqueous NH4OAc, pH 7.0 (see sample details in Fig. 5). The double-level library was formed by the equilibration of the Co(L44)2+ (3 × 10−4 M) with two acyl hydrazine substituents, nicotinic hydrazide (1.2 × 10−3 M), and 4-hydroxybenzoylhydrazide (1.2 × 10−3 M). The reaction mixture was incubated for 14 h at 60°C, pH 3.0, 0.01 M aqueous NH4OAc in 30% MeCN.
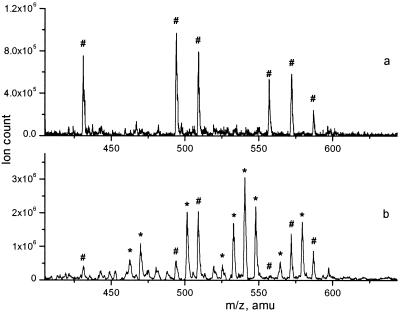
Figure 5.
(a) ESI MS spectrum of the single-level library formed by mixing individual complexes Co(L44)22+, Co(L66)22+, and Co(L77)22+ (3 × 10−4 M each) in 30% acetonitrile/0.01 M aqueous NH4OAc, at pH 7.0, 25°C. The spectrum is taken 10 min after mixing. (b) ESI MS spectrum of the double-level library, formed by the equilibration of the Co(L44)2+ (3 × 10−4 M) with nicotinic hydrazide (1.2 × 10−3 M) and 4-hydroxybenzoylhydrazide (1.2 × 10−3 M) after incubation for 14 h at 60°C, pH 3.0. Signals of complexes containing Lxx are marked only with #; those containing Lxy are marked with *.
Results and Discussion
Library Design and Building-Block Synthesis.
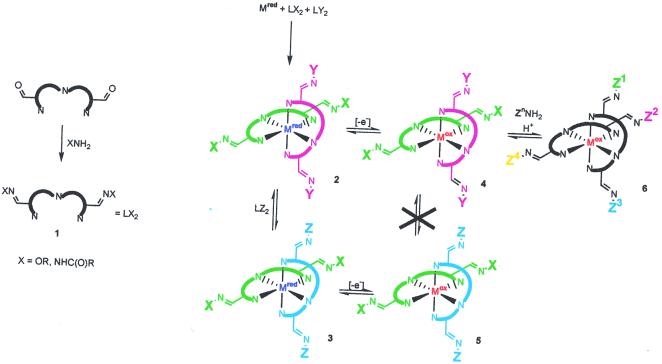
As the first level of dynamic library, we chose to use two tridentate ligands assembling on a transition metal of octahedral coordination geometry (Scheme S1). The diversity of compounds (Scheme S1, 2) in this case can be generated by using ligands with varying substituents, similar to the static libraries reported by the Hamilton group (28). If excess of the ligands is present in the system, their exchange in the metal coordination sphere will result in the interconversion of all possible combinations, thereby forming a dynamic library (3, Scheme S1). Notably, the rate of such an exchange depends on the oxidation state of the central metal ion. Generally, the ligand exchange rate constants in transition metals decrease by many orders of magnitude on increasing the oxidation number (29). It should therefore be possible to form the libraries and perform the exchange with the reduced form of the metal and then “freeze” the components by oxidation (Scheme S2, 4–5) for the analysis of the resulting library composition.
Scheme 1.
Scheme 2.
To form the second level of the dynamic library, the ligands were appended with the aldehyde groups, onto which one could attach various substituents through imination reactions, e.g., with hydroxylamines or hydrazines (Scheme S1, 1). The resulting imines can then engage in reversible reactions with the excess of the amines.
Because combinatorial libraries are routinely tested for interactions with biological targets, the important consideration for the library design is its compatibility with aqueous solutions. Neither ligand exchange nor certain types of imine exchange (ref. 19; S. Lohmann & J.-M.L., unpublished results), interfere with water, which makes this type of dynamic library sufficiently robust to be subjected to the interaction with biopolymer targets under physiological conditions.
The two above reactions, ligand and imine exchange, are expected to act as independent processes, which result in different degrees of library diversification and are controlled by different modifications of conditions. Ligand exchange leads to the scrambling of substituents in a pairwise mode and is controlled by the reduction/oxidation of the central metal. Imine exchange results in complete scrambling of substituents in the library components (Scheme S1, 6). Imine exchange is subject to acidic catalysis (30, 31); it proceeds much faster at low pH and is markedly slowed down in neutral and basic aqueous solutions. Therefore, it becomes possible to switch both or any of the exchange equilibria on and off either by adding an oxidizing/reducing agent or by changing the pH of the solution. This is important for the application of the dynamic libraries in an evolutionary selection/variation (22) or in the preequilibrated mode (25).
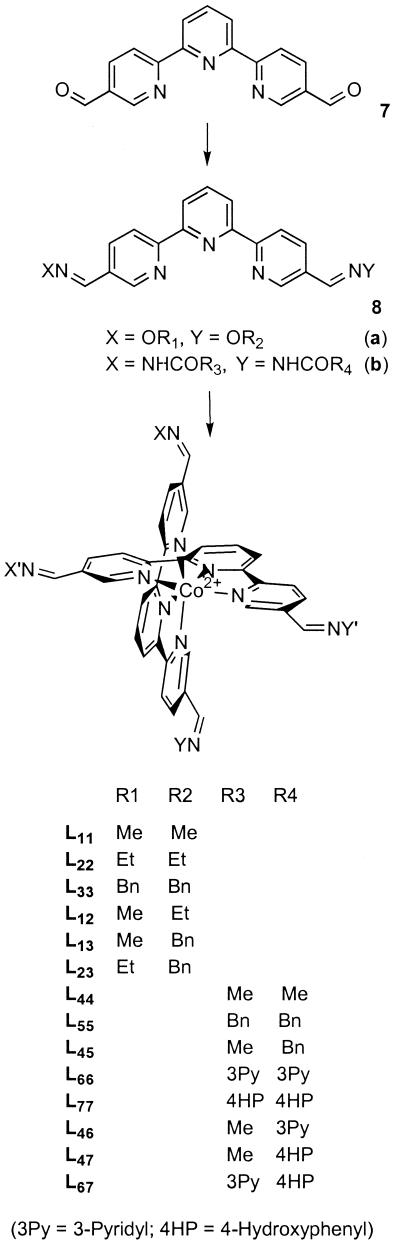
The terpyridine unit was chosen as the tridentate ligand because of its well-known ability to form strong complexes with octahedral transition metals (32). Dialdehyde 7 was synthesized (see supplementary material published on the PNAS web site) as the ligand that meets the criteria outlined in Scheme S1. The reaction of 7 with Co(II) chloride in protic solvents did not result in a single product because of the formation of a mixture of acetals. However, on conversion of the ligand to the imine form via reactions with alkoxyamines or acylhydrazides (Scheme S2), the resulting imines of general structure 8 readily formed corresponding complexes of Co(II), which could be further oxidized to Co(III). Both types of complexes were stable in aqueous solutions and were further studied as possible components of the dynamic libraries.
Exchange Processes in the Co(8)22+ and Co(8)23+ Complexes.
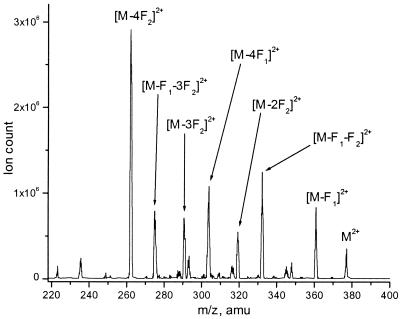
The exchange processes in the Co(8)2 complexes were monitored in by 1H NMR and ESI MS. These techniques provide extensive structural information and are complementary to each other. The paramagnetic Co(II) complexes yield proton NMR spectra with relatively broad signals that span over a range of 110 ppm. The ESI MS spectra of the Co(II) complexes showed strong doubly charged molecular peaks at MW/2 with very little or no fragmentation. The MS/MS of the molecular peaks showed patterns consistent with the structure, the most prominent fragments originating from the cleavage of the N—O and the Ar—CN bonds (Fig. 1). The core Co-terpyridine unit was stable to gas-phase fragmentation. Not only were fragments of the terpyridine unit absent in the MS/MS, but also the ligand dissociation from the metal was not observed, and all fragments originating from the substituent cleavage always retained the initial 2+ charge. This fact demonstrates that despite their kinetic lability in the ligand exchange discussed below, the Co(II) complexes are thermodynamically very stable species. In most experiments, the ESI MS was used as the method of choice for the studies of Co(II) complexes.
Figure 1.
MS/MS of the Co(L11)22+ molecular peak. The labeled fragment peaks correspond to the losses of OCH3 (F1) and CH = NOCH3 (F2).
The corresponding diamagnetic Co(III) complexes showed the expected high-resolution NMR spectra. Unlike with the reduced form, the molecular peaks (M/3) did not dominate in the ESI MS spectra of the Co(III) complexes. It came as a surprise that these spectra contained sizeable peaks of the corresponding Co(II) complexes whose intensity was up to three times that of Co(III) peaks. Proton NMR showed that the injected solution did not contain any impurities of Co(II). Therefore, the Co(II) peaks are likely to originate because of partial reduction in the mass spectrometer source, either in the droplet or in the gas phase. Variations in the injected solvent had little effect on the spectral pattern. For these reasons, the exchange in Co(III) complexes was primarily monitored by the proton NMR.
A–Ligand Exchange.
The ligand exchange in the Co(II) complexes was studied in two reactions of a symmetrical complex Co(L22)22+ (see Scheme S2): one with an excess of the competing terpyridyl ligand L11 (Fig. 2), another in the reaction with a similar complex Co(L11)22+ (Fig. 3). In both reactions, the signals of the symmetrical complexes, as well as that of the crossproduct CoL11L222+, were monitored by the ESI MS.
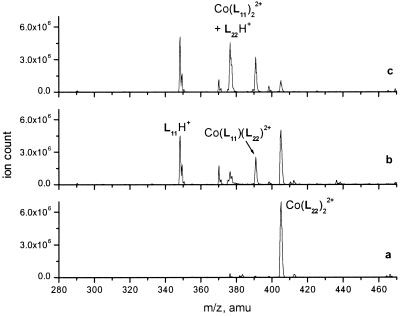
Figure 2.
ESI MS spectra of 5 × 10−6 M Co(L22)22+ in 30% acetonitrile in 0.01 M aqueous NH4OAc, pH 7.0 (a) and its mixture with 5 × 10−5 L11 taken in 2 min (b) and 45 min (c) after mixing at 25°C.
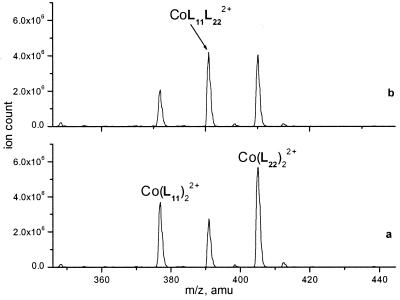
Figure 3.
ESI MS spectra of the mixture of Co(L11)22+ and Co(L22)22+ (5 × 10−6 M each) in 30% acetonitrile in 0.01 M aqueous NH4OAc, pH 7.0 taken in 2 min (a) and 14 min (b) after mixing at 25°C.
The reaction with the 10-fold excess of the external ligand in 30% aqueous acetonitrile resulted in a fairly rapid exchange in the dilute solutions at room temperature (Fig. 2). As expected, the starting complex was converted into an equilibrated mixture of three complexes with the dominating fraction of Co(L11)22+. Although detailed kinetic studies of the process were not performed, the second-order reaction rate constant of the complex–ligand reaction was estimated from the single run as 15 M−1s−1.
The second process expected to occur in the dynamic library, the ligand exchange between two different complexes, was studied in an equimolar mixture of two symmetrical complexes with different ligands (Fig. 3). Unexpectedly, this reaction turned out to be visually much faster than the complex–ligand exchange. The mixing of the two complexes at micromolar concentrations produced a sizeable peak of the crossproduct in the first injection into the mass spectrometer, and the equilibrium between the components was established within a few minutes. The second-order reaction rate constant was estimated as 1,300 M−1 s−1, nearly two orders of magnitude higher than that for the exchange with the free ligand! We are unaware of any documented precedents of such behavior. Although detailed mechanistic studies are in order, it seems probable that the ligand exchange between the two complexes occurs without complete dissociation of the terpyridyl moiety from the metal coordination sphere, and perhaps a cooperative transfer of two ligands is involved.
The exchange in the Co(III) complexes was studied under similar conditions, albeit at a higher concentration necessary for NMR (3 × 10−4 M [CoL22]Cl3, 3 × 10−3 M L11). The exchange in the oxidized form, as expected, was much slower than in the reduced one. The second-order rate constant determined by fitting the curve of the starting complex consumption was 5.0 × 10−3 M−1 s−1 at 25°C. As results from this value, the half-reaction time of the oxidized complex under the conditions of Fig. 2 would be 3 × 106 s, i.e., no meaningful exchange would occur for several days. These results are in line with our library design (Scheme S1), e.g., it appears possible to freeze the rapid exchange of the components by oxidizing the metal ion core.
The above studies were performed with the oxime ether derivatives of the terpyridyl ligands (X = NOR, Scheme S2). Because no major mechanistic differences would be expected for the acylhydrazone substituents (X = NNHCOR, Scheme S2), these ligands were not studied in detail, but the corresponding exchange products were always observed in the MS of their Co(II) complex mixtures.
B–Imine Exchange.
To explore the second level of exchange in the prospective dynamic libraries of the Co-terpyridine complexes, sample symmetrical complexes Co(Lx)22+ were mixed with an excess of amino components, which would compete for the imine formation with the terpyridyl side-chains and were incubated in a variety of conditions. The imine exchange was expected to produce a larger variety of products than the ligand exchange because of the appearance of nonsymmetrically substituted ligands.
Previous studies of the imine exchange between oxime ethers and alkoxyamines have shown that this reaction is subject to acidic catalysis, exhibits high activation enthalpy, and occurs through different mechanisms depending on the reactant structural features (19). We tested the complexes Co(L22)22+ and Co(L22)23+ in the reaction with O-methylhydroxylamine. In a typical run, 3 × 10−4 M Co(L22)22+ or Co(L22)23+ reacted with 6 × 10−3 M L11, monitoring by ESI MS or 1H NMR, respectively. We failed to detect any exchange products for either Co(II) or the Co(III) complexes within 15 h in the pH range between 3.0 and 8.0 and between 25 and 70°C.
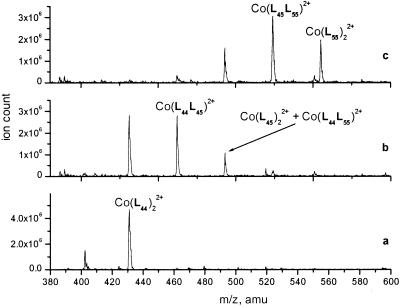
Such an exceptional stability of the oxime ether derivatives prompted us to test a similar exchange with the acylhydrazones, which are more labile to the imine exchange and have previously been used as components of dynamic libraries (ref. 20; S. Lohmann & J.-M.L., unpublished results). We studied the exchange reaction between the symmetrical complex Co(L44)22+ and benzoyl hydrazine. The series of reversible exchange processes was expected to yield a six-component mixture of Co(L44)22+, Co(L44L45)2+, Co(L45)22+, Co(L44L55)2+, Co(L45L55)2+, and Co(L55)22+, with two components, Co(L45)22+ and Co(L44L55)2+, being isobaric. Virtually no exchange was observed at pH 7.0 and 5.0 on overnight incubation at ambient temperature (Fig. 4a). However, the reaction at pH 3.0 showed partial conversion of the starting complex to that with ligands containing the new imine (Fig. 4b). The same reaction performed at 60°C resulted in nearly statistical distribution of the components substituted by the two imines (Fig. 4c). The kinetic studies (see details in supplementary material) based on consumption of the starting material monitored by mass spectrometry yielded the second-order reaction rate constant (9.3 ± 0.3) × 10−3 M−1 s−1 for the reaction between Co(L44)22+ and benzoyl hydrazine at pH 3.0 and 60°C.
Figure 4.
ESI MS spectra of the mixture of Co(L44)22+ (3 × 10−4 M) and benzoylhydrazide (6 × 10−3 M) after incubation for 14 h: (a) at 25°C, pH 7.0; (b) 25°C, pH 3.0; (c) 60°C, pH 3.0.
Formation of Sample Libraries of Substituted Co(II) Terpyridyl Complexes.
The double-level library design offers flexibility in control over the library composition and equilibration. As follows from Scheme S1, the libraries can be created by using n symmetrically substituted ligands (2, 3) forming the mixture of complexes that rapidly equilibrate and form all possible pairwise combinations, the total number of which is n × (n + 1)/2 (single-level library). This relatively limited level of diversity may nevertheless be important if the high symmetry of the resulting library components is justified by the structure of the biological target. Alternatively, one can perform complete scrambling of the substituents (6) via the imine exchange and thus increase the diversity proportionally to n4, albeit reduced by the symmetry considerations (double-level library).
To test both levels of library formation, we used three acyl hydrazide substituents that form the set of ligands L44, L46, L47, L66, L67, L77, Scheme S2. The pairwise combinations of three symmetrical ligands, L44, L66, and L77 are expected to form a total of 6 complexes, all of different molecular masses (Table 1). The complete scrambling of the substituents should result in the double-level library with the total of 21 components that yield 15 unique molecular masses (Table 1). The larger number of substituents was not used to ensure reliable detection of all components in the double-level library.§
Table 1.
Compositions of single- and double-level dynamic libraries consisting of complexes 8b with three different substituents (R = Me, 3Py, 4HP; see Scheme S2)
| No. | Complex | MW/2 | Single-level library* | Double-level library* |
|---|---|---|---|---|
| 1 | Co(L44)22+ | 430.63 | + | + |
| 2 | Co(L44)(L46)2+ | 462.13 | + | |
| 3 | Co(L44)(L47)2+ | 469.60 | + | |
| 4 | Co(L44)(L66)2+ | 493.64 | + | +† |
| 5 | Co(L46)(L46)2+ | 493.64 | +† | |
| 6 | Co(L44)(L67)2+ | 501.14 | +† | |
| 7 | Co(L46)(L47)2+ | 501.14 | +† | |
| 8 | Co(L44)(L77)2+ | 508.64 | + | +† |
| 9 | Co(L47)(L47)2+ | 508.64 | +† | |
| 10 | Co(L46)(L66)2+ | 525.14 | + | |
| 11 | Co(L66)(L47)2+ | 532.65 | +† | |
| 12 | Co(L46)(L67)2+ | 532.65 | +† | |
| 13 | Co(L46)(L77)2+ | 540.15 | +† | |
| 14 | Co(L47)(L67)2+ | 540.15 | +† | |
| 15 | Co(L47)(L77)2+ | 547.65 | + | |
| 16 | Co(L66)22+ | 556.65 | + | + |
| 17 | Co(L66)(L67)2+ | 564.15 | + | |
| 18 | Co(L66)(L77)2+ | 571.65 | + | +† |
| 19 | Co(L67)(L67)2+ | 571.65 | +† | |
| 20 | Co(L67)(L77)2+ | 579.15 | + | |
| 21 | Co(L77)22+ | 586.65 | + | + |
Components observed in the MS (Fig. 5) are marked with +;
Detection of the peak indicates the presence of at least one of the isobaric components.
The single-level library was formed by mixing three individual complexes Co(L44)2+, Co(L66)2+, and Co(L77)2+. After the rapid equilibration, the mass spectrum showed the formation of the three expected crossproducts, along with the starting components (Fig. 5a, Table 1). The double-level library, formed by the equilibration of the Co(L44)2+ with two remaining acyl hydrazine substituents also yielded the masses of all expected components (Fig. 5b, Table 1). Although one of the components, CoL44L772+, was observed only in minor amounts, the overall spectrum was remarkably clean, with all major peaks corresponding to the library components. The acyl hydrazone exchange reaction thus appears to be a robust technique for the library equilibration in aqueous solutions with few, if any, side reactions involved and all products easily detectable.
Although we have shown that the oxime exchange reaction is not a viable option for dynamic equilibration in the current system, the oximation of 7 followed by the complex formation offers a rapid way to the diverse libraries that can be equilibrated only via the ligand exchange reaction. A spectrum of such a library that yields complete representation of all theoretically expected masses is given in the supplementary material.
Conclusions
We have shown that two types of reversible reactions can be combined to create a double-level orthogonal dynamic combinatorial library. Several features of such a library design distinguish it from the previously known static and dynamic libraries. First, the orthogonality of the reversible processes, i.e., the ability to perform the ligand and imine exchange independently on each other, offers a higher degree of control over the library composition and its organization in the interaction with a prospective target. Second, the different nature of the building-block linkages at the two levels simplifies deconvolution and analysis of potentially very complex libraries of the kind. For example, dissociation of the individual complexes isolated from the library followed by ligand analysis will allow one to easily discriminate between isobaric components, such as complexes Co(L45)22+ and Co(L44L55)2+ (see Fig. 4). This straightforward deconvolution approach paves the way not only to higher numbers of components that can be screened simultaneously, but also to the addition of extra levels of complexity, e.g., new reversible linkages, to the library components. Finally, the important factor is compatibility of the reported libraries with the aqueous solutions and their suitability for interaction with biopolymers. The exchange reactions used in our system are benign to biological targets. In the general structure, all components display substituents on a large, rigid, and positively charged scaffold. These features render them potential ligands for nucleic acids, particularly RNA (33), which is the subject of our ongoing studies.
We are grateful to the National Institutes of Health for financial support of this work (Grant no. 6035201 from National Institute of General Medical Sciences). The mass spectrometer was obtained with Shared Instrumentation Grant no. S10RR14572 from the National Center for Research Resources, National Institutes of Health. We thank the Pharmaceutical Sciences instrumentation facility at the University at Buffalo for data acquisition.
Supplementary Material
Abbreviation
- ESI
electrospray ionization
Footnotes
The use of four substituents would yield the double-level library of 65 components, 46 molecular masses. Such a spectrum would be overcrowded for the precise direct analysis; however, much larger levels of diversity are conceivable for the screening of dynamic libraries, where the signal-to-noise ratio in the spectrum is selectively increased on equilibration with the target.
References
- 1.Lowe G. Chem Soc Rev. 1995;24:309. [Google Scholar]
- 2.Balkenhohl F, Von dem Bussche-Hunnefeld C, Lansky A, Zechel C. Angew Chem Int Ed Engl. 1996;35:2289–2337. [Google Scholar]
- 3.Williard X, Pop I, Bourel L, Horvath D, Baudelle R, Melnyk P, Deprez B, Tartar A. Eur J Med Chem. 1996;31:87–98. [Google Scholar]
- 4.Maehr H. Bioorg Med Chem. 1997;5:473–491. doi: 10.1016/s0968-0896(96)00277-5. [DOI] [PubMed] [Google Scholar]
- 5.Ganesan A. Angew Chem Int Ed Engl. 1998;37:2828–2831. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2828::AID-ANIE2828>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 6.Eliseev A V. Curr Opin Drug Discov Dev. 1998;1:106–115. [PubMed] [Google Scholar]
- 7.Eliseev A V, Lehn J M. Combinatorial Chemistry in Biology. 1999;243:159–172. doi: 10.1007/978-3-642-60142-2_9. [DOI] [PubMed] [Google Scholar]
- 8.Lehn J M. Chem Eur J. 1999;5:2455–2463. [Google Scholar]
- 9.Klekota B, Miller B L. Trends Biotechnol. 1999;17:205–209. doi: 10.1016/s0167-7799(99)01305-0. [DOI] [PubMed] [Google Scholar]
- 10.Cousins G R L, Poulsen S A, Sanders J K M. Curr Opin Chem Biol. 2000;4:270–279. doi: 10.1016/s1367-5931(00)00088-0. [DOI] [PubMed] [Google Scholar]
- 11.Karan C, Miller B L. Drug Discov Today. 2000;5:67–75. doi: 10.1016/s1359-6446(99)01431-2. [DOI] [PubMed] [Google Scholar]
- 12.Swann P G, Casanova R A, Desai A, Frauenhoff M M, Urbancic M, Slomczynska U, Hopfinger A J, Lebreton G C, Venton D L. Biopolymers. 1996;40:617–625. doi: 10.1002/(sici)1097-0282(1996)40:6<617::aid-bip3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Brady P A, Sanders J K M. J Chem Soc Perkin Trans. 1997;1:3237–3253. [Google Scholar]
- 14.Rowan S J, Sanders J K M. Chem. Commun. 1997. 1407–1408. [Google Scholar]
- 15.Rowan S J, Lukeman P S, Reynolds D J, Sanders J K M. N J Chem. 1998;22:1015–1018. [Google Scholar]
- 16.Huc I, Lehn J M. Proc Natl Acad Sci USA. 1997;94:2106–2110. doi: 10.1073/pnas.94.6.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klekota B, Hammond M H, Miller B L. Tetrahedron Lett. 1997;38:8639–8642. [Google Scholar]
- 18.Klekota B, Miller B L. Tetrahedron. 1999;55:11687–11697. [Google Scholar]
- 19.Polyakov V A, Nelen M I, Nazarpack-Kandlousy N, Ryabov A D, Eliseev A V. J Phys Org Chem. 1999;12:357–363. [Google Scholar]
- 20.Cousins G R L, Poulsen S A, Sanders J K M. Chem. Commun. 1999. 1575–1576. [Google Scholar]
- 21.Eliseev A V, Nelen M I. J Am Chem Soc. 1997;119:1147–1148. [Google Scholar]
- 22.Eliseev A V, Nelen M I. Chem Eur J. 1998;4:825–834. [Google Scholar]
- 23.Berl V, Huc I, Lehn J M, DeCian A, Fischer J. Eur. J. Org. Chem. 1999. 3089–3094. [Google Scholar]
- 24.Hioki H, Still W C. J Org Chem. 1998;63:904–905. [Google Scholar]
- 25.Ramström O, Lehn J M. ChemBioChem. 2000;1:41–48. doi: 10.1002/1439-7633(20000703)1:1<41::AID-CBIC41>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 26.Hasenknopf B, Lehn J M, Boumediene N, Dupontgervais A, Van Dorsselaer A, Kneisel B, Fenske D. J Am Chem Soc. 1997;119:10956–10962. [Google Scholar]
- 27.Epstein, D. M., Choudhary, S., Churchill, M. R., Keil, K. M., Eliseev, A. V. & Morrow, J. R. (2001) Inorg. Chem., in press. [DOI] [PubMed]
- 28.Goodman M S, Jubian V, Linton B, Hamilton A D. J Am Chem Soc. 1995;117:11610–11611. [Google Scholar]
- 29.Helm L, Merbach A E. Coord Chem Rev. 1999;187:151–181. [Google Scholar]
- 30.Jencks W P. Catalysis in Chemistry and Enzymology. New York: Dover; 1987. [Google Scholar]
- 31.Patai S. The Chemistry of the Carbon-Nitrogen Double Bond. London: Interscience; 1970. [Google Scholar]
- 32.Swiegers G F, Malefetse T J. Chem Rev. 2000;100:3483–3537. doi: 10.1021/cr000023w. [DOI] [PubMed] [Google Scholar]
- 33.Hermann T, Westhof E. Curr Opin Biotechnol. 1998;9:66–73. doi: 10.1016/s0958-1669(98)80086-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.