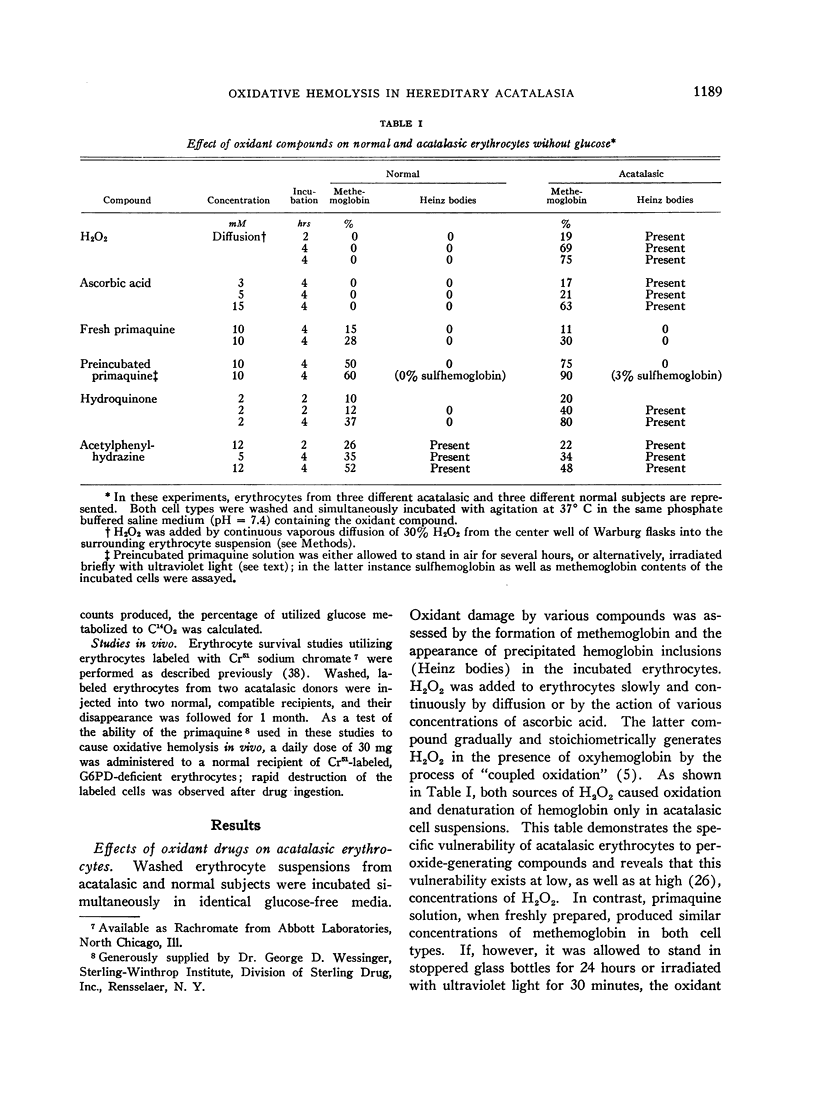
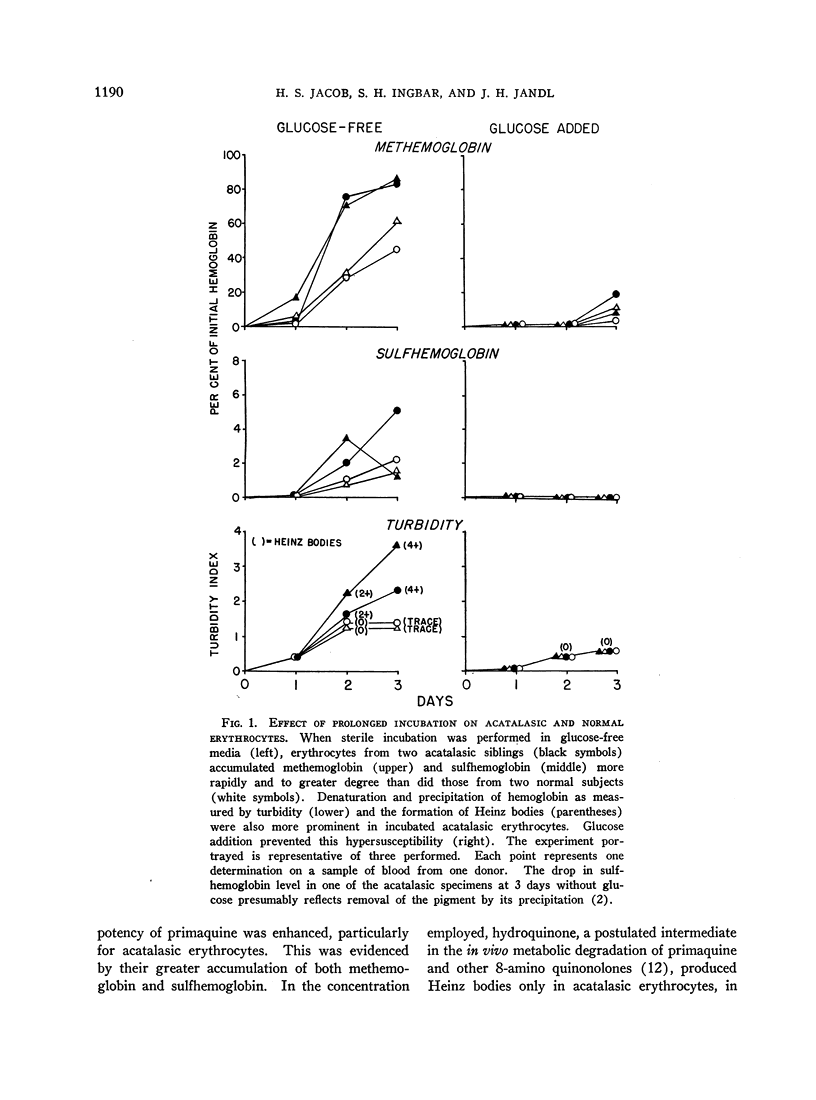
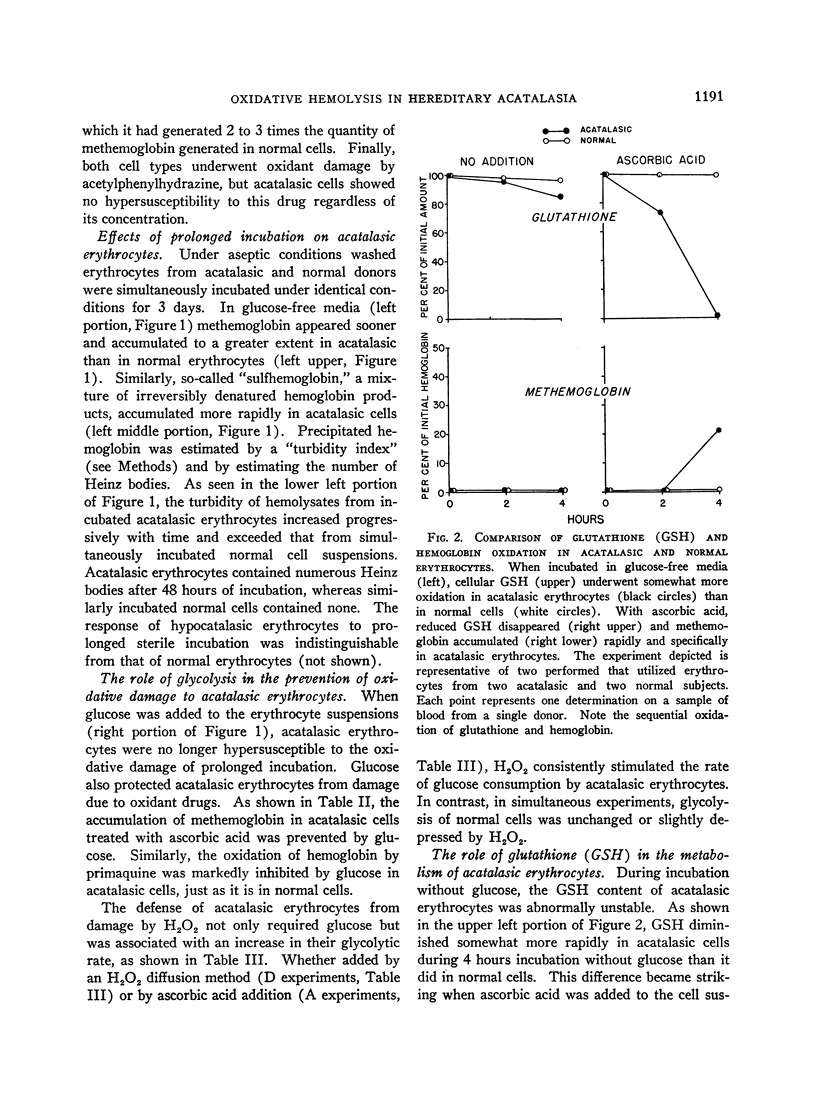
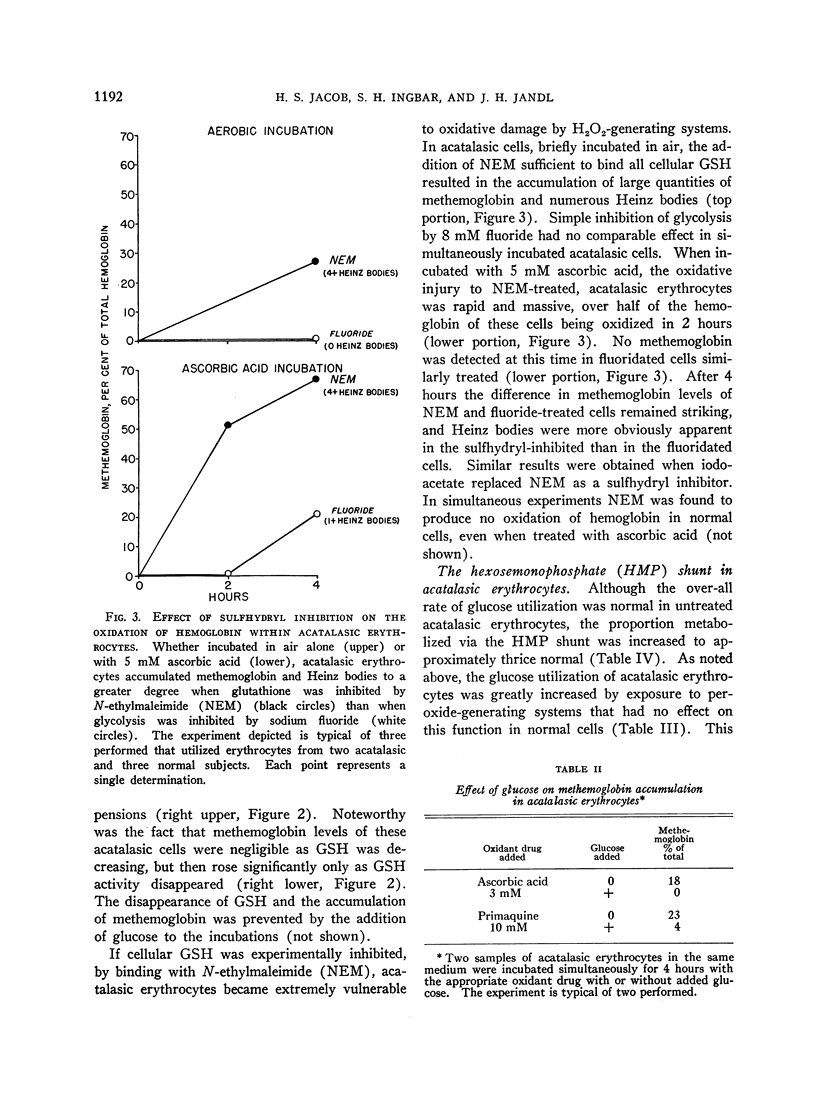
Full text
PDF

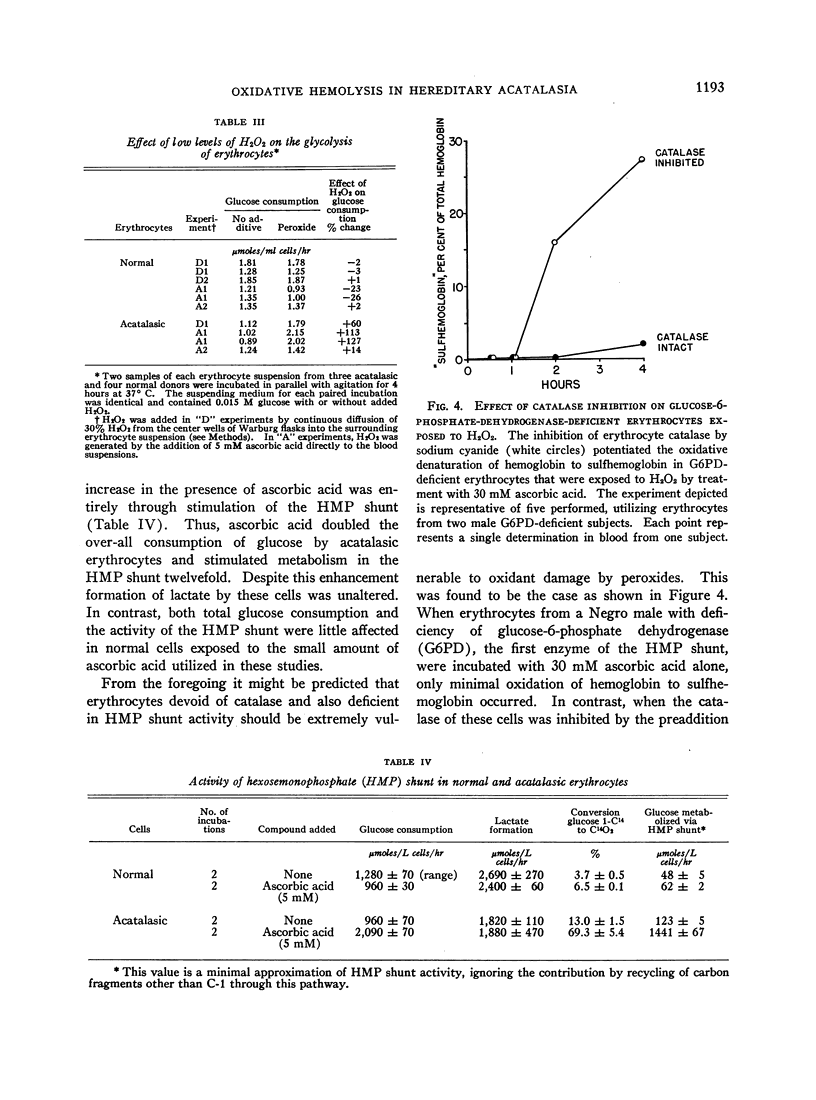
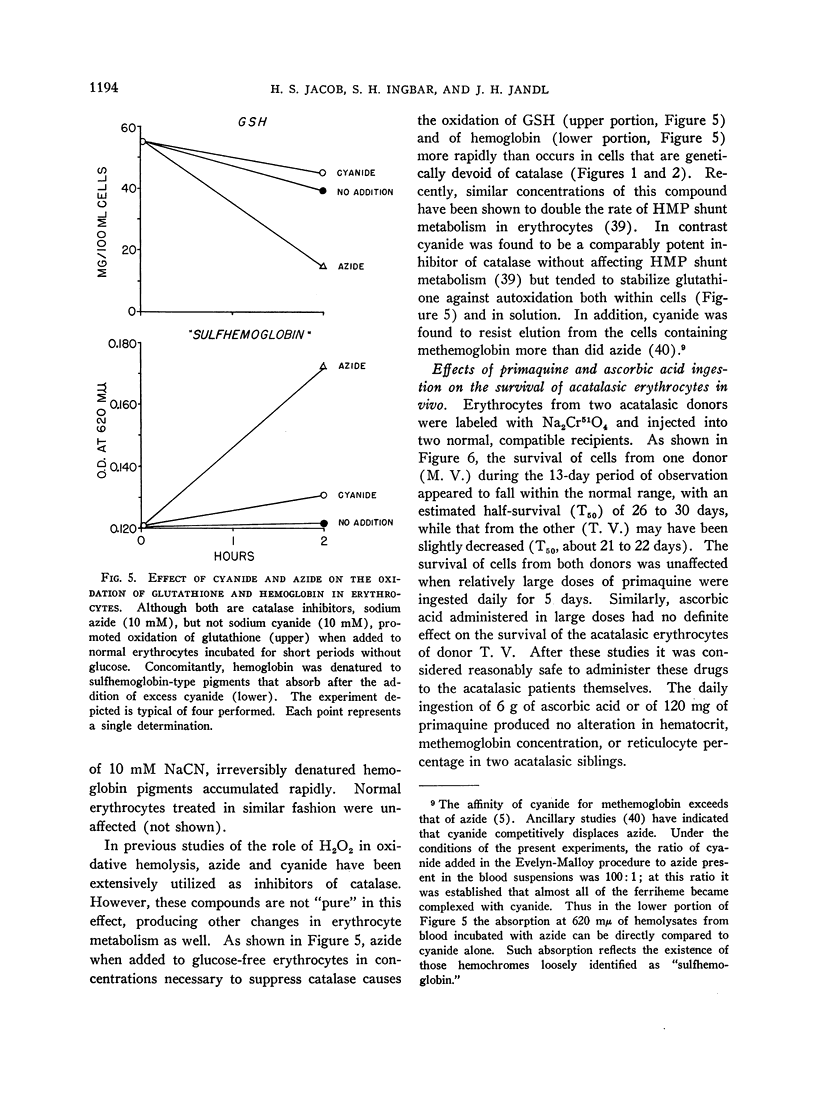
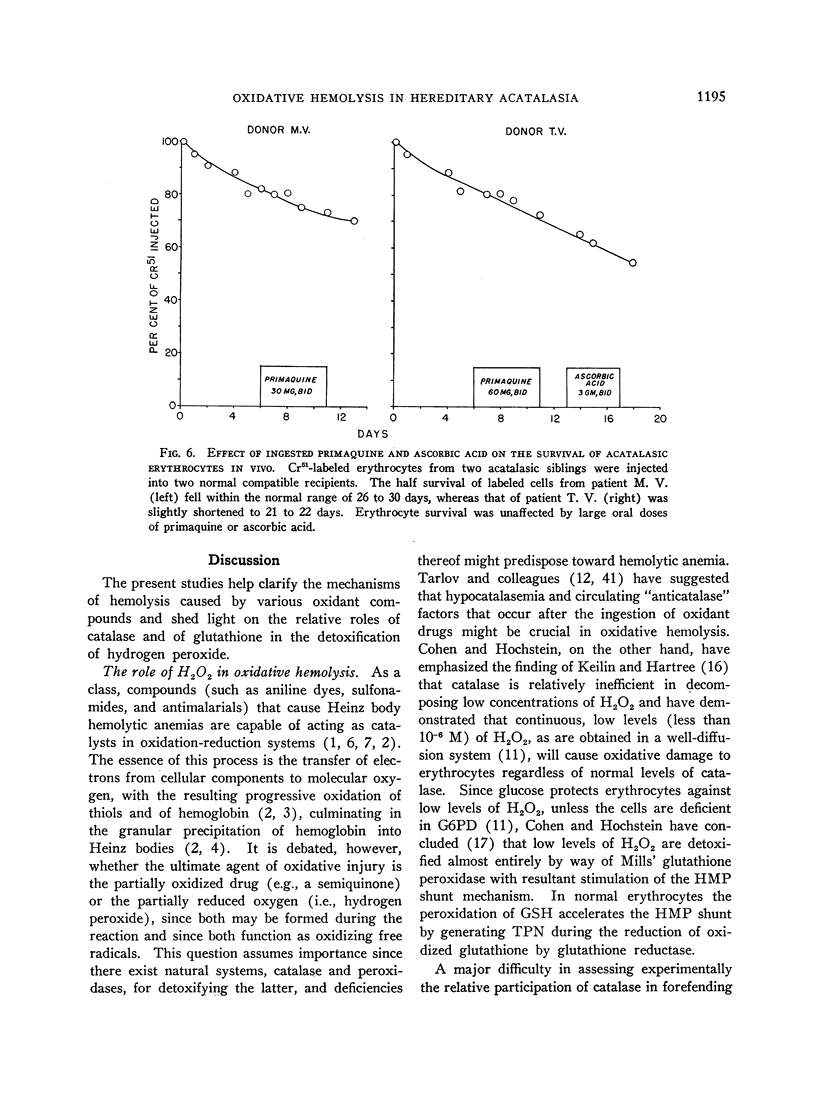




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AEBI H., HEINIGER J. P., BUETLER R., HAESSIG A. Two cases of acatalasia in Switzerland. Experientia. 1961 Oct 15;17:466–466. doi: 10.1007/BF02158293. [DOI] [PubMed] [Google Scholar]
- AEBI H., JEUNET F., RICHTERICH R., SUTER H., BUTLER R., FREI J., MARTI H. R. Observations in two Swiss families with acatalasia. Enzymol Biol Clin (Basel) 1962;2:1–22. doi: 10.1159/000458069. [DOI] [PubMed] [Google Scholar]
- ALLEN D. W., JANDL J. H. Oxidative hemolysis and precipitation of hemoglobin. II. Role of thiols in oxidant drug action. J Clin Invest. 1961 Mar;40:454–475. doi: 10.1172/JCI104273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAVEN G. H., WHITE J. C. Oxidation of phenylhydrazines in the presence of oxyhaemoglobin and the origin of Heinz bodies in erythrocytes. Nature. 1954 Feb 27;173(4400):389–391. doi: 10.1038/173389b0. [DOI] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- BEUTLER E. The glutathione instability of drug-sensitive red cells; a new method for the in vitro detection of drug sensitivity. J Lab Clin Med. 1957 Jan;49(1):84–95. [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GLUTATHIONE PEROXIDASE: THE PRIMARY AGENT FOR THE ELIMINATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES. Biochemistry. 1963 Nov-Dec;2:1420–1428. doi: 10.1021/bi00906a038. [DOI] [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. Glucose-6-phosphate dehydrogenase and detoxification of hydrogen peroxide in human erythrocytes. Science. 1961 Dec 1;134(3492):1756–1757. doi: 10.1126/science.134.3492.1756. [DOI] [PubMed] [Google Scholar]
- GRUNERT R. R., PHILLIPS P. H. A modification of the nitroprusside method of analysis for glutathione. Arch Biochem. 1951 Feb;30(2):217–225. [PubMed] [Google Scholar]
- HARLEY J. D., MAUER A. M. Studies on the formation of Heinz bodies. II. The nature and significance of Heinz bodies. Blood. 1961 Apr;17:418–433. [PubMed] [Google Scholar]
- HILL A. S., Jr, HAUT A., CARTWRIGHT G. E., WINTROBE M. M. THE ROLE OF NONHEMOGLOBIN PROTEINS AND REDUCED GLUTATHIONE IN THE PROTECTION OF HEMOGLOBIN FROM OXIDATION IN VITRO. J Clin Invest. 1964 Jan;43:17–26. doi: 10.1172/JCI104889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. I. Mechanism of hemolysis. J Clin Invest. 1962 Apr;41:779–792. doi: 10.1172/JCI104536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. II. Studies in vivo. J Clin Invest. 1962 Jul;41:1514–1523. doi: 10.1172/JCI104607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. INCREASED CELL MEMBRANE PERMEABILITY IN THE PATHOGENESIS OF HEREDITARY SPHEROCYTOSIS. J Clin Invest. 1964 Aug;43:1704–1720. doi: 10.1172/JCI105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., ENGLE L. K., ALLEN D. W. Oxidative hemolysis and precipitation of hemoglobin. I. Heinz body anemias as an acceleration of red cell aging. J Clin Invest. 1960 Dec;39:1818–1836. doi: 10.1172/JCI104206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., GREENBERG M. S., YONEMOTO R. H., CASTLE W. B. Clinical determination of the sites of red cell sequestration in hemolytic anemias. J Clin Invest. 1956 Aug;35(8):842–867. doi: 10.1172/JCI103338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Properties of catalase. Catalysis of coupled oxidation of alcohols. Biochem J. 1945;39(4):293–301. [PMC free article] [PubMed] [Google Scholar]
- MILLS G. C. Hemoglobin catabolism. I. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative breakdown. J Biol Chem. 1957 Nov;229(1):189–197. [PubMed] [Google Scholar]
- MILLS G. C., RANDALL H. P. Hemoglobin catabolism. II. The protection of hemoglobin from oxidative breakdown in the intact erythrocyte. J Biol Chem. 1958 Jun;232(2):589–598. [PubMed] [Google Scholar]
- SZEINBERG A., MARKS P. A. Substances stimulating glucose catabolism by the oxidative reactions of the pentose phosphate pathway in human erythrocytes. J Clin Invest. 1961 Jun;40:914–924. doi: 10.1172/JCI104330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHARA S. Progressive oral gangrene due to acatalasemia (colored motion picture). Laryngoscope. 1954 Aug;64(8):685–688. doi: 10.1288/00005537-195408000-00004. [DOI] [PubMed] [Google Scholar]
- TAKAHARA S. Progressive oral gangrene probably due to lack of catalase in the blood (acatalasaemia); report of nine cases. Lancet. 1952 Dec 6;2(6745):1101–1104. doi: 10.1016/s0140-6736(52)90939-2. [DOI] [PubMed] [Google Scholar]
- TARLOV A. R., BREWER G. J., CARSON P. E., ALVING A. S. Primaquine sensitivity. Glucose-6-phosphate dehydrogenase deficiency: an inborn error of metabolism of medical and biological significance. Arch Intern Med. 1962 Feb;109:209–234. doi: 10.1001/archinte.1962.03620140081013. [DOI] [PubMed] [Google Scholar]
- TARLOV A. R., KELLERMEYER R. W. The hemolytic effect of primaquine. XI. Decreased catalase activity in primaquine-sensitive erythrocytes. J Lab Clin Med. 1961 Aug;58:204–216. [PubMed] [Google Scholar]


