Abstract
Dimerization of the prototypic family B G protein-coupled secretin receptor is determined by the lipid-exposed face of transmembrane segment four (TM4), and has substantial functional importance, facilitating G protein coupling. Recently, we demonstrated that the human secretin receptor elicits an inter-receptor bioluminescence resonance energy transfer (BRET) signal with most other human family B peptide receptors, except for the calcitonin receptor. In this study we have explored the occurrence and importance of calcitonin receptor oligomerization. Static and saturation receptor BRET were utilized to demonstrate that, unlike the human calcitonin receptor that does not yield a significant homomeric BRET signal, the rabbit calcitonin receptor exhibits strong resonance energy transfer. Within the lipid-exposed face of TM4, rabbit and human calcitonin receptors differ by a single amino acid (Arg236 in human; His in rabbit), while Thr253 that occurs in human and rabbit calcitonin receptors is unique across family B receptors. Mutating Arg236 or Thr253 of the human calcitonin receptor to residues found in the rabbit calcitonin receptor or the human secretin receptor (R236H, R236Y and T253A) resulted in generation of significant BRET signals. Similarly, mutation of Val250 of the human calcitonin receptor to another key lipid-facing residue found in the secretin receptor (V250I) also increased the receptor BRET signal. These data support the consistent theme of lipid-exposed residues of TM4 being important for the dimerization of the calcitonin receptor. However, rabbit and human calcitonin receptor constructs bound calcitonin and stimulated cAMP similarly, suggesting that differences in BRET could reflect differences in orientation or in the stability of homo-dimeric receptor complexes, which were nevertheless similarly effective in eliciting the functions attributed to that complex. The likelihood of human calcitonin receptor dimerization, even in the absence of a significant BRET signal, was further supported by data demonstrating that the peptide representing TM4 of this receptor that disrupts the rabbit receptor BRET signal, produced a right shift in the cAMP concentration-response curves for both rabbit and human receptors.
1. INTRODUCTION
Dimerization is an important structural and functional theme for family B guanine nucleotide-binding protein (G protein)-coupled receptors (GPCRs) [1–3]. The secretin receptor, as prototypic of this family, associates with itself in a constitutive manner that is established during biosynthesis, and is not subsequently affected by agonist binding [4, 5]. The homo-oligomeric complexes of secretin receptors are homo-dimers, rather than higher order complexes, with the dimers formed along the lipid-exposed face of transmembrane segment four (TM4) of this receptor [6–8]. The G protein-coupled secretin receptor homo-dimeric complex is associated with the high affinity state of that receptor that also leads to the most potent cAMP responses to natural agonist occupation [6]. Consistent with this interpretation, non-dimerizing secretin receptor mutants have been shown to bind secretin with lower affinity and to support less potent cAMP responses to secretin stimulation [6].
Homo-oligomerization has also been reported for many other members of family B GPCRs [4, 5, 7–13], and hetero-oligomerization has been reported for several of these receptors [4, 14]. Of particular interest, the human secretin receptor was reported to form hetero-oligomeric complexes with eight other members of this family based on bioluminescence resonance energy transfer (BRET) signals, but in that series co-expression of that receptor with the human calcitonin receptor did not yield a significant BRET signal [14]. In another report, the rabbit calcitonin receptor has been shown to form homo-oligomeric complexes [13]. It was, therefore, not clear whether the human calcitonin receptor lacks key residues for specific interaction with the secretin receptor, or whether it might have differences in orientation within a oligomeric complex or be less efficient in oligomerization. It was also unclear what the functional implications of this might be.
In the current work, we have carefully assessed the capacity for human and rabbit calcitonin receptors to oligomerize, using both static and saturation BRET methodologies, and attempting to disrupt homo-dimeric receptor complexes with TM4 peptide. We also utilized receptor site-directed mutagenesis to examine the contribution to oligomerization of lipid-facing residues within TM4, focusing particularly on those residues that are distinct in the two species of calcitonin receptors and those that are unique among the members of this receptor subfamily. These studies supported the importance of this interface for forming receptor homo-dimers, and also that variation in the stability or orientation of such complexes occurs among members of this family. Once the propensity for forming oligomeric complexes was clear, we also studied the function of those complexes, including their ligand binding characteristics and the ability of calcitonin to elicit cAMP.
2. MATERIALS AND METHODS
2.1. Materials
Molecular biology reagents were from New England BioLabs (Ipswich, MA). Human calcitonin was from Bachem (Torrance, CA, USA). Coelenterazine h was from Biotium (Hayward, CA). cAMP assay kits that utilize LANCE technology and Optiplates were from PerkinElmer (Wellesley, MA). Dulbecco’s modified Eagle’s medium (DMEM) was from Invitrogen (Carlsbad, CA) and Fetal Clone II supplement was from (Hyclone laboratories, Logan, UT). Other reagents were analytical grade.
2.2. Receptor constructs
Receptor constructs tagged with Renilla luciferase (Rlu) at the carboxyl terminus were inserted into the pcDNA3.0 eukaryotic expression vector. Human calcitonin receptor (hCTR) constructs with yellow fluorescent protein (YFP) at the carboxyl terminus were prepared using Gateway technology (Invitrogen, Carlsbad, CA), as described previously [4]. The untagged rabbit calcitonin receptor (rCTR) construct was a generous gift from Dr. William Horne (Harvard Medical School, Boston, MA). Analogous Rlu- and YFP-tagged rCTR receptor constructs were prepared by inserting tags before the stop codon. hCTR mutant constructs were prepared using the QuikChange mutagenesis kit (Stratagene, La Jolla, CA). All sequences were confirmed by direct DNA sequencing.
2.3. Transmembrane segment 4 peptides
The peptides corresponding to TM4 of the calcitonin receptor (LRWYYLLGWGFPLVPTTI) and the secretin receptor (LQGFVAFGWGSPAIFVALWAIARHFLE) were synthesized on Pal resin (Sigma, St Louis, MO) using manual techniques with Fmoc (N-(9-fluorenyl)methoxycarbonyl)-protected amino acids, as we have reported [30]. The deprotected peptides were purified to homogeneity using semi-preparative reversed-phase HPLC with a solvent system of acetonitrile/0.1% trifluoroacetic acid. The peptides were dissolved in 20 % acetonitrile/water mixture, and were diluted in Krebs-Ringers-HEPES (KRH) medium (25 mM HEPES, pH 7.4, 104 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM KH2PO4, 1.2 mM MgSO4) to yield an acetonitrile concentration of 0.3% at the time of use in receptor assays.
2.4. Cell culture and transfections
COS-1 cells were used for transient expression of receptor constructs. Cells were plated at a density of 0.5×106 cells/dish in sterile 10 cm tissue culture dishes in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5 % Fetal Clone II. After 24 hrs, cells were transfected with approximately 3 µg of DNA per dish using the diethylaminoethyl (DEAE)-dextran method [8].
2.5. Fluorescence microscopy
Receptor expression at the cell surface was established using fluorescence microscopy for YFP localization. Receptor-bearing COS-1 cells grown on 25-mm cover slips were washed with phosphate-buffered saline (PBS) and fixed in 2 % (w/v) paraformaldehyde in PBS for 30 min. Cover slips were mounted on microscopic slides using Vectashield (Vector Laboratories, Burlingame, CA). Imaging was accomplished with a Zeiss LSM 510 confocal microscope (Thornwood, NY) using the YFP setting (excitation, 488 nm argon laser; emission, LP505 filter; pinhole diameter 2.6 airy units, Plan-Apochromat 63X/1.4NA oil). All constructs underwent normal biosynthesis and trafficking, with clear cell surface fluorescence observed (data not shown).
2.6. BRET studies
Suspensions of approximately 25,000 receptor-bearing COS-1 cells/well were used for bioluminescence and fluorescence measurements in 96-well white Optiplates, as described previously [7]. Cells were studied 48–72 h after transfection. BRET assays were initiated by mixing 5 µM coelenterazine h (Renilla luciferase-specific substrate) with the cell suspension. Signals were collected immediately using a 2103 Envision fluorescence plate reader configured with the <700 nm mirror and with dual emission filter sets for luminescence (460 nm, bandwidth 25 nm) and fluorescence (535 nm, bandwidth 25 nm). Fluorescence of YFP was acquired by exciting the samples at 485 nm. The BRET ratio was calculated based on the ratio of YFP and Rlu emission, as described previously [7]
Saturation BRET experiments were also performed as described previously [7]. COS-1 cells were transfected with a fixed amount of Rlu-tagged receptor constructs as donors (1.0 µg DNA/dish) and with increasing amounts of YFP-tagged constructs as acceptors (0.3 µg to 6 µg DNA/dish). BRET assays were performed 48–72 h later. The BRET ratios were plotted against the ratios of Rlu/YFP and curves were fit and evaluated based on R2 values using Prism 3.0. (GraphPad, San Diego, CA).
2.7. Receptor-binding assays
Membranes were isolated from COS-1 cells transiently expressing each of the receptor constructs. Cells were harvested mechanically using a cell scraper 48–72 h after transfection, and the membrane fraction was isolated from a sucrose gradient after centrifugation, as we have previously described [15]. Membranes were resuspended in Krebs-Ringers-HEPES (KRH) medium containing 25 mM HEPES, pH 7.4, 104 mM NaCl, 5 mM KCl, 2 mM CaCl2,1 mM KH2PO4, 1.2 mM MgSO4, 0.01% soybean trypsin inhibitor, and 1 mM phenylmethylsulfonyl fluoride, and were stored at −80 °C until use.
Radioligand binding studies were performed using these cell membranes, as described previously [16]. Membranes were mixed with with 1–2 pM 125I-human calcitonin (prepared and purified in our laboratory to yield approximate specific radioactivity of 2,000 Ci/mmol) in the absence or presence of increasing concentrations (1 pM to 1 µM) of unlabelled human calcitonin for 1 h at room temperature in KRH medium, pH 7.4, containing 0.01 % soybean trypsin inhibitor and 0.2 % bovine serum albumin. Receptor-bound was separated from free radioligand using centrifugation and repeated washing with ice-cold KRH medium. Receptor-bound radioactivity was quantified using a γ-spectrometer. Non-saturable binding was determined in the presence of 1 µM calcitonin, and was less than 18 percent of total binding. Saturable binding data were analyzed using the LIGAND program [17] and plotted using the non-linear least-squares curve-fitting routine in Prism (GraphPad, San Diego, CA).
2.8. cAMP assays
Calcitonin-stimulated cAMP accumulation in receptor-bearing COS-1 cells was quantified using the LANCE assay from PerkinElmer (Wellesley, MA) in the 384-well white Optiplate format using a 2103 Envision plate reader (PerkinElmer), as described previously [8]. Receptor-bearing COS-1 cells were studied 48–72 h after transfection. Cells were stimulated with increasing concentrations of calcitonin (1pM to 1µM) in KRH medium (pH 7.4) containing 0.2 % bovine serum albumin, 0.01 % soybean trypsin inhibitor, 0.1 % bacitracin, and 1 mM 3-isobutyl-1-methylxanthine for 30 min at 37 °C.
2.9. Statistical analysis
Differences between calcitonin binding and signaling parameters at distinct receptor constructs were evaluated for statistical significance using one-way analysis of variance (ANOVA) and Dunnett’s test in Prism (GraphPad, San Diego, CA). Values less than 0.05 were considered to be statistically significant.
3. RESULTS
3.1. Transmembrane segment four (TM4) sequences
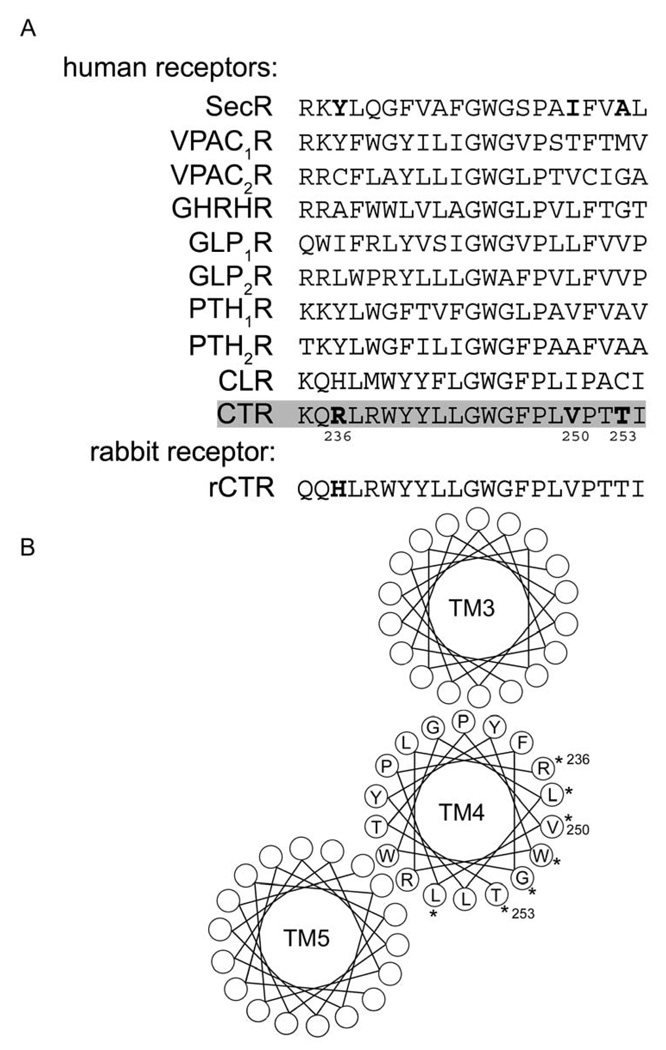
The major determinant for human secretin receptor dimerization has been reported to be the lipid-exposed face of TM4 [6]. That prototypic family B GPCR also formed hetero-oligomers with eight other members of this family, with the human calcitonin receptor the only other human receptor in this series to not elicit a significant BRET signal when co-expressed with the human secretin receptor [14]. Evaluation of the sequence of TM4 of these receptors provided interesting insights (alignment shown in Fig 1, along with helical wheel diagram of the proposed orientation of the TM4 segment of the human calcitonin receptor). The human calcitonin receptor sequence was notable for having two residues (Arg236 and Thr253), both of which are predicted to face the lipid, that are distinct from analogous residues in TM4 of all the other human family B receptor members. These two residues have, therefore, been the major focus of the current investigation. We also examined Val250, even though a valine is present in this position in the VPAC2 and PTH1 receptors, since the analogous lipid-exposed residue in the secretin receptor (Ile) plays a key role for its dimerization [8].
Figure 1. Alignment of TM4 sequences of family B GPCRs and predicted helical wheel orientation of residues.
Shown above (A) is the alignment of amino acid sequences of proposed TM4 segments of human family B GPCRs, and of the rabbit calcitonin receptor (rCTR). Highlighted are hCTR residues, Arg236, Val250, and Thr253, which are the sites of mutagenesis in the current study. Shown below (B) is the predicted helical wheel organization of TM3, TM4, and TM5 of hCTR receptor (based on the GLP1 receptor model by Donnelly [35]). Asterisks depict the TM4 residues that are predicted to be exposed to the surrounding lipid in the membrane bilayer.
There may be species differences in the tendency for GPCRs to oligomerize and for the stability of oligomeric complexes. There is a report in the literature supporting the presence of homo-oligomerization of the rabbit calcitonin receptor (rCTR) [13], while no investigations of the possible homo-oligomerization of the human calcitonin receptor (hCTR) have been reported. The sequence of TM4 of the rabbit receptor differs from the human receptor in one of the two residues noted above, with human residue Arg236 corresponding to a histidine residue in the rabbit; an analogous histidine residue is found in the human calcitonin receptor-like receptor (CLR), which also forms receptor dimers [7]. We prepared hCTR mutants in which Arg236 was converted to His (R236H) as present in rCTR and CLR, or to Tyr (R236Y) as present in this position in the secretin receptor. Similarly, Val250 was converted to Ile (V250I), and Thr253 was converted to Ala (T253A), as present in the secretin receptor at analogous positions (Fig. 1A).
3.2. Calcitonin receptor BRET studies
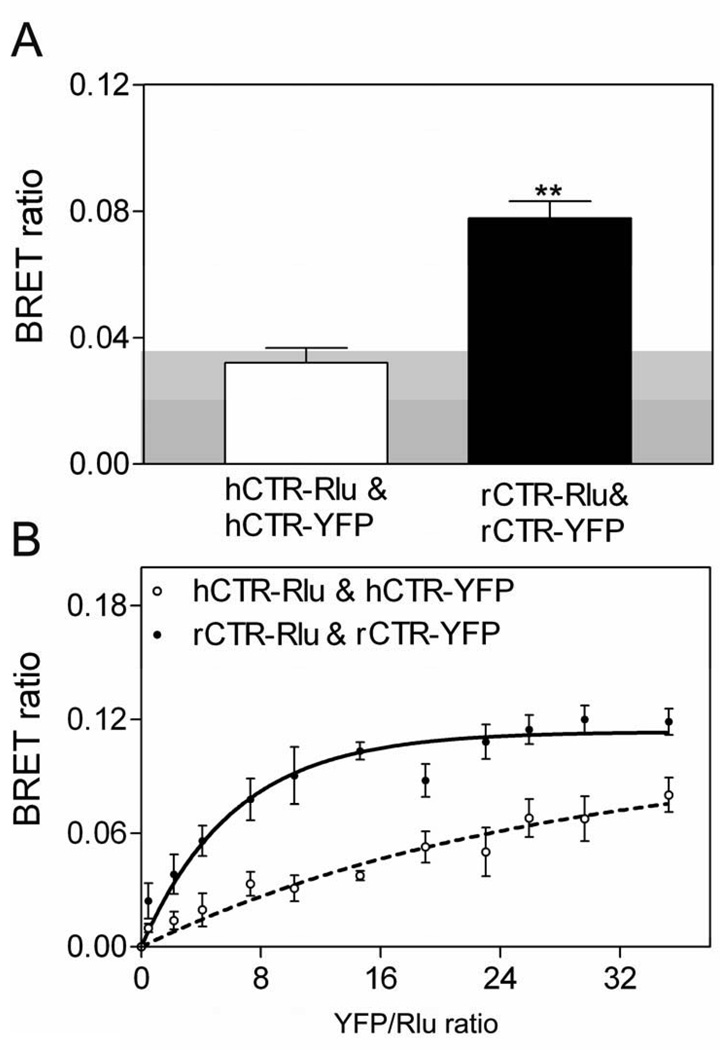
BRET studies were performed to evaluate possible homo-oligomerization of calcitonin receptor constructs. The human receptor (hCTR) yielded no significant BRET signal above background, while the rabbit receptor (rCTR) produced a significant static BRET signal (Fig 2A). These results were confirmed using saturation BRET analysis, differentiating a possible bystander signal, from random interactions between donor and acceptor, from what is thought to represent a significant specific interaction. In saturation BRET studies (Fig 2B), the BRET ratio for rCTR increased and reached an asymptote, representing a positive signal, while that for hCTR was not different from a linear fit, consistent with non-specific or transient interactions. It is important to recognize that these BRET signals represent average signals collected from a population of cells. While a positive static BRET signal that is confirmed by saturation BRET studies likely provides strong evidence for the presence of oligomeric complexes, a negative signal does not rule out such complexes. Since energy transfer is dependent on the orientation and distance between donor and acceptor, an absent BRET signal could reflect excessive distance or non-optimal orientation of tags on receptors that are part of a complex. Additionally, the kinetics of association could affect the intensity of the BRET signal, with transient or relatively unstable complexes producing a reduced signal. While recent reports have established the transient nature of some receptor interactions [18, 19], even some documented short-lived receptor oligomers can yield a positive BRET signal.
Figure 2. Calcitonin receptor BRET.
Shown are the data for static (A) and saturation (B) BRET obtained from COS-1 cells transiently expressing Rlu- and YFP-tagged hCTR and rCTR constructs. The shaded area represents the background BRET signal (0.038) generated when Rlu-tagged CTR was co-expressed with soluble YFP. rCTR exhibited a significant BRET ratio above background (p<0.001), as well as a saturation BRET curve that increased to reach an asymptote. hCTR did not yield a significant BRET signal, and its saturation BRET curve was not different from a linear fit. The data are presented as means ± S.E.M. of 4–5 independent experiments.
3.3. hCTR mutant receptor BRET studies
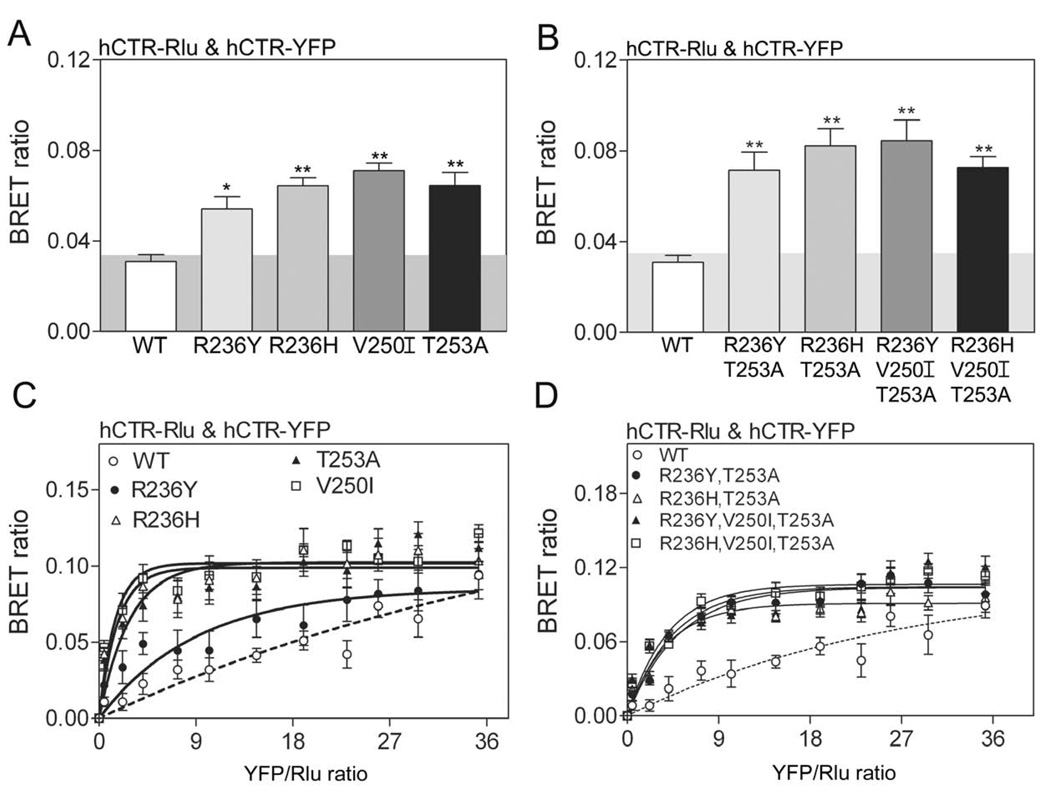
BRET was also utilized to evaluate the ability of hCTR mutants to form homo-oligomers when transiently expressed in COS-1 cells. We studied a series of mutations of single or multiple putative lipid-exposed residues within TM4 of hCTR. BRET studies were performed in COS-1 cells expressing both Rlu- and YFP-tagged constructs, either as single residue mutations (R236Y; R236H; V250I; T253A), two residue mutations (R236Y, T253A; R236H, T253A), or three residue mutations (R236Y, V250I, T253A; R236H, V250I, T253A). Static and saturation BRET data from constructs with single and multiple mutations are shown in Figure 3. Mutation of each of the selected lipid-exposed residues in TM4 of hCTR to analogous residues found in the relevant position in rCTR, CLR or the secretin receptor resulted in a stronger and statistically significant BRET signal. Saturation BRET studies confirmed the significance of these signals. Of interest, the R236H human calcitonin receptor construct generated not only a significant BRET signal with the wild type human calcitonin receptor (BRET ratio 0.06 ± 0.01), but also with the wild type rabbit calcitonin receptor (BRET ratio 0.08 ± 0.01).
Figure 3. BRET of human calcitonin receptor mutants.
Shown are the data for static (A,B) and saturation (C,D) BRET generated when COS-1 cells transiently expressed single or multiple mutations within TM4 of hCTR. Each of the mutants generated a significant BRET signal above background (p<0.001), while wild type hCTR did not. Saturation BRET analysis confirmed the significance of the static BRET signals. The data are presented as means ± S.E.M. of data from 4–5 independent experiments.
3.4. Functional characterization of calcitonin receptor mutants
Homo-dimerization of the secretin receptor has been reported to be important for attaining the high affinity state of that receptor [6] and for improving the efficiency of G protein coupling (with Gs) and cAMP responses [6]. Similar studies were performed with the CTR constructs prepared for the current studies. All the constructs bound calcitonin saturably. Data are shown in Table 1. No statistically significant differences in binding affinity or receptor density were observed.
Table 1.
Binding and biological activity parameters for calcitonin receptor constructs
| Constructs | Ki (nM) | Bmax (pmol/mg protein) | EC50 (nM) |
|---|---|---|---|
| WT hCTR | 0.28±0.1 | 7.9±2.4 | 0.21±0.04 |
| WT rCTR | 0.61±0.27 | 5.2±0.7 | 0.13±0.09 |
| (R236Y)hCTR | 0.19± 0.03 | 5.1±0.7 | 0.06±0.02* |
| (R236H)hCTR | 0.14±0.02 | 6.3±2.7 | 0.21±0.06 |
| (V250I)hCTR | 0.11±0.02 | 6.3±0.8 | 0.10±0.02 |
| (T253A)hCTR | 0.21±0.05 | 8.2±2.0 | 0.12±0.01 |
| (R236Y,T253A)hCTR | 0.56±0.18 | 7.4±0.9 | 0.08±0.03 |
| (R236H,T253A)hCTR | 0.45±0.03 | 5.5±1.2 | 0.10±0.05 |
| (R236Y,V250I,T253A)hCTR | 1.05±0.33 | 5.4±0.9 | 0.33±0.04 |
| (R236H,V250I,T253A)hCTR | 0.92±0.29 | 5.3±1.6 | 0.03±0.01* |
Data are expressed as means±S.E.M. of values from 4–6 independent experiments. Receptor binding parameters (Ki and Bmax) for calcitonin receptor constructs were derived from homologous competition-binding studies with 125I-human calcitonin and unlabelled human calcitonin. Biological activity data (EC50 values) for concentrations of calcitonin needed to stimulate half-maximal cAMP responses are also listed.
represent differences from the analogous values for wild type human calcitonin receptor that are significant using ANOVA analysis at the level of p<0.05.
Table 1 also summarizes the cAMP responses to calcitonin in the various constructs. Both wild type hCTR and rCTR constructs responded to calcitonin with simlar cAMP responses. This was also true for most of the site mutants that generated significant BRET signals. Only R236Y and R236H,V250I,T253A human calcitonin receptor constructs had increased potency of calcitonin-stimulated cAMP that reached statistical significance. However, there were no clear correlations between the intensity of BRET signals and the calcitonin binding or calcitonin-stimulated cAMP responses in this series.
3.5. Use of transmembrane segment four peptides to disrupt calcitonin receptor oligomerization
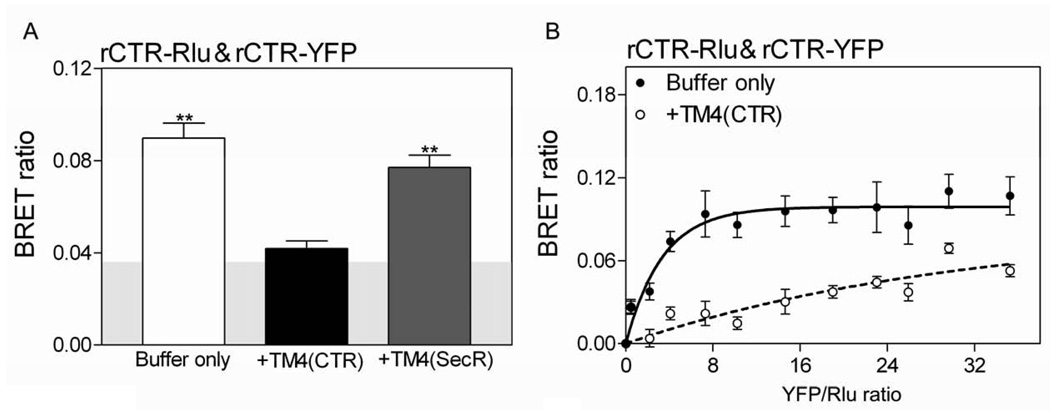
Competition with TM4 peptides has previously been utilized to disrupt dimeric secretin receptor complexes and to examine the functional significance of such complexes [8]. In the current work, we have, therefore, utilized TM4 peptide competition to further explore the possible presence and the potential functional significance of calcitonin receptor oligomerization. We studied TM4 peptides from both the calcitonin receptor and the secretin receptor. Since only the rabbit calcitonin receptor and not the human calcitonin receptor produce a significant receptor BRET signal (data shown above), the rabbit receptor system was utilized to explore the ability of these TM4 peptides to disrupt the complex. Indeed, the calcitonin receptor TM4 peptide was effective in reducing the rabbit calcitonin receptor BRET signal to background levels (Fig 4A), and this was confirmed with saturation BRET studies (Fig 4B). Of interest, there was clearly structural specificity for this disruption, with the TM4 peptide from the secretin receptor much less effective in reducing calcitonin receptor BRET.
Figure 4. Effects of TM4 peptides on calcitonin receptor BRET.
Shown are the static BRET (A) and saturation BRET ratios (B) for COS cells expressing tagged rCTR constructs in the presence or absence of TM4 peptides from the calcitonin receptor and the secretin receptor. The static BRET signal was significantly reduced with the calcitonin receptor TM4 peptide to a level not different from background. This indicates disruption of rCTR oligomers. Saturation BRET curves (plotted as ratios of YFP fluorescence to Rlu luminescence) in the absence or presence of TM4 peptide (10−5M) were obtained with a fixed amount of donor and increasing amounts of acceptor. The curve generated in the presence of the calcitonin receptor TM4 peptide was not significantly different from a linear fit, consistent with disruption of the complex. The data are presented as means ± S.E.M. of data from four independent experiments performed in triplicate.
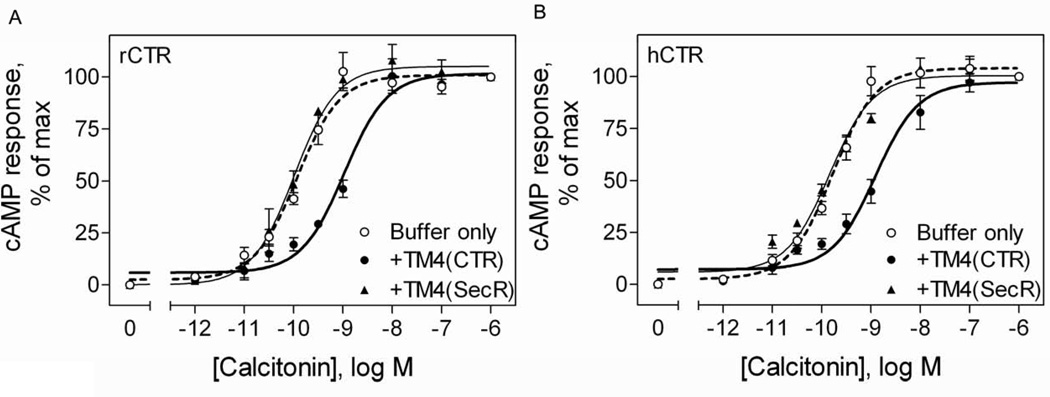
The functional impact of competitive disruption of the calcitonin receptor oligomeric complexes was studied by determining the impact on the calcitonin-stimulated cAMP responses. Indeed, like the secretin receptor previously studied [8], disruption of oligomeric complexes of the rabbit calcitonin receptor resulted in a rightward shift of the cAMP concentration-response curve (Fig 5A) No such shift was observed for calcitonin-stimulated cAMP responses from calcitonin receptors when the secretin receptor TM4 peptide was utilized (Fig 5A). Remarkably, an analogous rightward shift in cAMP responses was observed when the calcitonin receptor TM4 peptide was utilized in analogous assays with the human calcitonin receptor (Fig 5B). This was true despite the absence of a significant BRET signal from the human calcitonin receptors.
Figure 5. Effects of disruption of calcitonin receptor dimerization by TM4 peptide on the biological activity.
Shown are the calcitonin-stimulated cAMP responses in COS cells expressing rCTR (A) and hCTR (B) in the absence or presence of TM4 peptides from calcitonin and secretin receptors. COS cells transiently expressing either rCTR or hCTR were incubated with 10−5M TM4 peptides. Only the TM4 peptide from the calcitonin receptor significantly reduced the potency of calcitonin-stimulated cAMP responses in intact cells. Data represent means ± S.E.M. of data from 4–5 independent experiments performed in duplicate.
4. DISCUSSION
While the oligomerization of GPCRs is now well accepted [3], there are considerable differences in consequences for receptor function among different receptors. The oligomerization of some GPCRs is differentially affected by ligand binding, with this reported as augmenting, reducing, and having no effect on different members of this superfamily [20–22]. The oligomeric state of some GPCRs can affect ligand binding affinity and even specificity, efficiency and selectivity of G protein coupling, signaling pathways activated, and receptor regulation [6, 22–29].
It is quite interesting that the themes for GPCR oligomerization seem to be distinct for the different families of GPCRs [8, 23, 30]. The clearest and most compelling theme is that for family C GPCRs, where dimerization seems to be the rule, with some dimeric receptor complexes even stabilized by covalent disulfide bonding [31]. Here, heterodimerization has been shown to change the ligand specificity and signaling of these complexes [30, 31]. The most extensively studied group of GPCRs are family A receptors, where there has been no consistent theme linking oligomerization and functional impact.
While family B GPCRs have been less extensively studied than family A GPCRs, for this group the theme of structurally-specific dimerization is well established [6, 32, 33]. Here, the secretin receptor has been the prototype revealing that the dimerization is established through the lipid-facing surface of TM4, with the dimeric complex structurally symmetrical [8]. In this family, the hetero-oligomerization of the secretin receptor with eight other members of the family in BRET studies [14], suggested that a common face of the receptor was used and that there was overlap in the region of the receptor giving rise to the interaction interface. Equally notable in that series [14] was the absence of a significant BRET signal when the human secretin receptor was co-expressed with the human calcitonin receptor.
In the current work, we have carefully studied the capacity for human and rabbit calcitonin receptors to oligomerize. While we were able to utilize receptor BRET studies to confirm the homo-oligomerization of the rabbit receptor that was reported previously [13], we also found that an analogous assay with the human calcitonin receptor gave no significant BRET signal above background. These observations were further confirmed using saturation BRET conditions. It should be emphasized that the all donors and acceptors used in these resonance transfer studies were at the carboxyl terminus of the relevant receptors.
We initially focused our attention on the lipid-facing residues within TM4, previously shown to contribute to the homo-dimerization interface for the prototypic secretin receptor (TM4 sequences are illustrated in Figure 1) [6, 8], exploring the possibility that differences in this region between the human and rabbit calcitonin receptors could explain the observed differences in receptor BRET signals. The human calcitonin receptor is indeed different from the rabbit calcitonin receptor for one of the TM4 lipid-facing residues (Arg236 in the human receptor is a His in the rabbit). Additionally, two other key residues in the dimerization interface of the secretin receptor are distinct in both the human and rabbit calcitonin receptors (Val250 and Thr253 in the human calcitonin receptor are Ile and Ala, respectively, in the human secretin receptor). Mutant receptors in which we changed each of these residues in the human calcitonin receptor to those in the rabbit calcitonin receptor or the human secretin receptor were prepared. These were studied for their effects on receptor oligomerization utilizing BRET-based interaction assays. Indeed, these mutations resulted in a greater tendency for the human calcitonin receptor to form oligomeric complexes as supported by the generation of a significant BRET signal.
These data are consistent with a generalized role for lipid-exposed residues within TM4 of family B GPCRs to contribute to the propensity to form or to the stability of receptor oligomers. Some receptors in this family may have a greater tendency than others to form such structures, both as homomeric and heteromeric receptor complexes. It is critical to emphasize that the resonance transfer techniques, such as BRET and FRET, reflect average signals in the cuvette. These can be influenced by the strength of the signal from a small number or percentage of receptors in the analysis, such as by short distances between donor and acceptor or by very stable complexes, or by a large number or percentage of more distant interactions or less stable complexes. The dynamics of receptor oligomerization has recently been investigated using the techniques of fluorescence recovery after photobleaching and internal reflectance microscopy with particle tracking [18, 19, 34], supporting the transient nature of some receptor complexes. Substantial variation in the stability and/or orientation of oligomeric complexes of GPCRs may exist. Family C GPCRs seem to form highly stable complexes, while different family A GPCRs are reported to have transient interactions and stable interactions. To date, no family B GPCRs have been directly studied.
It is particularly notable that the functional analysis of the human and rabbit calcitonin receptors revealed no significant differences in cAMP assay, despite the previous demonstration that the Gs protein-coupled homo-dimeric form of the secretin receptor is the high affinity form that is most effective in stimulating cAMP [6]. Either this could suggest that calcitonin binding and signaling are not affected by dimerization the same way as the secretin receptor, or that the human calcitonin receptor without a significant average BRET signal could reflect a transient homo-dimeric complex that is not stable enough to increase the signal in this assay, or that the complex exists in an alternate orientation, but that in either case is adequate to facilitate G protein coupling and signaling.
Indeed, the observation that all of the human calcitonin receptor mutants that produced significant BRET signals bound calcitonin with similar affinities to that of wild type human calcitonin receptor further supports the concept that the wild type human receptor interacts adequately, although potentially in a transient manner, to stabilize its G protein interaction and to yield high affinity binding. These data are also consistent with the recent reports of the kinetics and efficiencies of oligomerization varying among receptors, while retaining full function that is attributed to the oligomeric states [19, 34]. The only differences that were observed for cAMP responses among the human calcitonin receptor mutants relative to the wild type receptor were for R236Y and R236H,V250I,T253A human calcitonin receptor constructs that displayed increased potency; both constructs yielding significantly elevated BRET signals. While the trends for some of the other mutants with high BRET values were in a similar direction, they did not reach statistical significance. It might be that the kinetics of oligomerization and G protein coupling at the human calcitonin receptor are quite close to that necessary for optimal signaling, and small improvements in the structure of this interface produces greater cAMP responses.
We attempted to provide additional experimental evidence for the possibility that the human calcitonin receptor was, indeed, capable of homo-dimerization, with the resulting effect of facilitating G protein coupling and more potent stimulation of cAMP production, despite the absence of a significant receptor BRET signal. This was accomplished using TM4 peptides that, for the secretin receptor, competitively disrupt receptor homo-dimers to reveal functional differences between receptor monomers and dimer [8]. Because the rabbit calcitonin receptor generated a strong receptor BRET signal, we utilized that experimental system to demonstrate that the TM4 peptide from the calcitonin receptor disrupted rabbit calcitonin receptor homo-dimers and thereby generated a rightward shift in the calcitonin-stimulated cAMP concentration-response curve. There was clear structural specificity to this effect, with the TM4 peptide from another family B GPCR, the secretin receptor, unable to disrupt rabbit calcitonin receptor BRET or to produce a similar rightward shift in the cAMP response. When these TM4 peptides were co-expressed with the human calcitonin receptor, the calcitonin receptor TM4 peptide elicited the same rightward shift in cAMP responsiveness. Indeed, this provides still more presumptive evidence that the human calcitonin receptor forms homo-dimers, despite not producing a significant receptor BRET signal and this is likely to be a conserved component of family B GPCR function.
ACKNOWLEDGEMENTS
The authors thank M.-L.Augustine and D. I. Pinon for their technical assistance.
This work was supported by grants to LJM from the National Institutes of Health (DK46577) and from the Fiterman Foundation. PMS is a Principal Research Fellow of the National Health and Medical Research Council of Australia.
Abbreviations
- BRET
bioluminescence resonance energy transfer
- hCTR
human calcitonin receptor
- rCTR
rabbit calcitonin receptor
- DMEM
Dulbecco’s modified Eagle’s medium
- GPCR
G protein-coupled receptor
- KRH
Krebs-Ringers-HEPES
- PBS
phosphate-buffered saline
- Rlu
Renilla luciferase
- YFP
yellow fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gurevich VV, Gurevich EV. How and why do GPCRs dimerize? Trends Pharmacol Sci. 2008;29:234–240. doi: 10.1016/j.tips.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohse MJ. Dimerization in GPCR mobility and signaling. Curr Opin Pharmacol. 2009 doi: 10.1016/j.coph.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Milligan G. G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br J Pharmacol. 2009;158:5–14. doi: 10.1111/j.1476-5381.2009.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harikumar KG, Morfis MM, Lisenbee CS, Sexton PM, Miller LJ. Constitutive formation of oligomeric complexes between family B G protein-coupled vasoactive intestinal polypeptide and secretin receptors. Mol Pharmacol. 2006;69:363–373. doi: 10.1124/mol.105.015776. [DOI] [PubMed] [Google Scholar]
- 5.Lisenbee CS, Miller LJ. Secretin receptor oligomers form intracellularly during maturation through receptor core domains. Biochemistry. 2006;45:8216–8226. doi: 10.1021/bi060494y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao F, Harikumar KG, Dong M, Lam PC, Sexton PM, Christopoulos A, Bordner A, Abagyan R, Miller LJ. Functional importance of a structurally distinct homodimeric complex of the family B G protein-coupled secretin receptor. Mol Pharmacol. 2009;76:264–274. doi: 10.1124/mol.109.055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harikumar KG, Happs RM, Miller LJ. Dimerization in the absence of higher-order oligomerization of the G protein-coupled secretin receptor. Biochim Biophys Acta. 2008;1778:2555–2563. doi: 10.1016/j.bbamem.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harikumar KG, Pinon DI, Miller LJ. Transmembrane segment IV contributes a functionally important interface for oligomerization of the Class II G protein-coupled secretin receptor. J Biol Chem. 2007;282:30363–30372. doi: 10.1074/jbc.M702325200. [DOI] [PubMed] [Google Scholar]
- 9.Heroux M, Hogue M, Lemieux S, Bouvier M. Functional calcitonin gene-related peptide receptors are formed by the asymmetric assembly of a calcitonin receptor-like receptor homo-oligomer and a monomer of receptor activity-modifying protein-1. J Biol Chem. 2007;282:31610–31620. doi: 10.1074/jbc.M701790200. [DOI] [PubMed] [Google Scholar]
- 10.Kraetke O, Wiesner B, Eichhorst J, Furkert J, Bienert M, Beyermann M. Dimerization of corticotropin-releasing factor receptor type 1 is not coupled to ligand binding. J Recept Signal Transduct Res. 2005;25:251–276. doi: 10.1080/10799890500468838. [DOI] [PubMed] [Google Scholar]
- 11.Langer I, Gaspard N, Robberecht P. Pharmacological properties of Chinese hamster ovary cells coexpressing two vasoactive intestinal peptide receptors (hVPAC1 and hVPAC2) Br J Pharmacol. 2006;148:1051–1059. doi: 10.1038/sj.bjp.0706816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pioszak AA, Harikumar KG, Parker NR, Miller LJ, Xu HE. Dimeric arrangement of the parathyroid hormone receptor and a structural mechanism for ligand-induced dissociation. J Biol Chem. 2010;285:12435–12444. doi: 10.1074/jbc.M109.093138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seck T, Baron R, Horne WC. The alternatively spliced deltae13 transcript of the rabbit calcitonin receptor dimerizes with the C1a isoform and inhibits its surface expression. J Biol Chem. 2003;278:23085–23093. doi: 10.1074/jbc.M211280200. [DOI] [PubMed] [Google Scholar]
- 14.Harikumar KG, Morfis MM, Sexton PM, Miller LJ. Pattern of intra-family heterooligomerization involving the G-protein-coupled secretin receptor. J Mol Neurosci. 2008;36:279–285. doi: 10.1007/s12031-008-9060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadac EM, Ghanekar DV, Holicky EL, Pinon DI, Dougherty RW, Miller LJ. Relationship between native and recombinant cholecystokinin receptors: role of differential glycosylation. Pancreas. 1996;13:130–139. doi: 10.1097/00006676-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Harikumar KG, Hosohata K, Pinon DI, Miller LJ. Use of probes with fluorescence indicator distributed throughout the pharmacophore to examine the peptide agonist-binding environment of the family B G protein-coupled secretin receptor. J Biol Chem. 2006;281:2543–2550. doi: 10.1074/jbc.M509197200. [DOI] [PubMed] [Google Scholar]
- 17.Munson PJ, Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 18.Fonseca JM, Lambert NA. Instability of a class a G protein-coupled receptor oligomer interface. Mol Pharmacol. 2009;75:1296–1299. doi: 10.1124/mol.108.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hern JA, Baig AH, Mashanov GI, Birdsall B, Corrie JE, Lazareno S, Molloy JE, Birdsall NJ. Formation and dissociation of M1 muscarinic receptor dimers seen by total internal reflection fluorescence imaging of single molecules. Proc Natl Acad Sci U S A. 2010;107:2693–2698. doi: 10.1073/pnas.0907915107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng ZJ, Miller LJ. Agonist-dependent dissociation of oligomeric complexes of G protein-coupled cholecystokinin receptors demonstrated in living cells using bioluminescence resonance energy transfer. J Biol Chem. 2001;276:48040–48047. doi: 10.1074/jbc.M105668200. [DOI] [PubMed] [Google Scholar]
- 21.Ding WQ, Cheng ZJ, McElhiney J, Kuntz SM, Miller LJ. Silencing of secretin receptor function by dimerization with a misspliced variant secretin receptor in ductal pancreatic adenocarcinoma. Cancer Res. 2002;62:5223–5229. [PubMed] [Google Scholar]
- 22.Patel RC, Kumar U, Lamb DC, Eid JS, Rocheville M, Grant M, Rani A, Hazlett T, Patel SC, Gratton E, Patel YC. Ligand binding to somatostatin receptors induces receptor-specific oligomer formation in live cells. Proc Natl Acad Sci U S A. 2002;99:3294–3299. doi: 10.1073/pnas.042705099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breit A, Lagace M, Bouvier M. Hetero-oligomerization between beta2- and beta3-adrenergic receptors generates a beta-adrenergic signaling unit with distinct functional properties. J Biol Chem. 2004;279:28756–28765. doi: 10.1074/jbc.M313310200. [DOI] [PubMed] [Google Scholar]
- 24.Canals M, Lopez-Gimenez JF, Milligan G. Cell surface delivery and structural re-organization by pharmacological chaperones of an oligomerization-defective alpha(1b)-adrenoceptor mutant demonstrates membrane targeting of GPCR oligomers. Biochem J. 2009;417:161–172. doi: 10.1042/BJ20081227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O'Dowd BF. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- 26.Han Y, Moreira IS, Urizar E, Weinstein H, Javitch JA. Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat Chem Biol. 2009;5:688–695. doi: 10.1038/nchembio.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salahpour A, Angers S, Mercier JF, Lagace M, Marullo S, Bouvier M. Homodimerization of the beta2-adrenergic receptor as a prerequisite for cell surface targeting. J Biol Chem. 2004;279:33390–33397. doi: 10.1074/jbc.M403363200. [DOI] [PubMed] [Google Scholar]
- 28.Terrillon S, Barberis C, Bouvier M. Heterodimerization of V1a and V2 vasopressin receptors determines the interaction with beta-arrestin and their trafficking patterns. Proc Natl Acad Sci U S A. 2004;101:1548–1553. doi: 10.1073/pnas.0305322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uberti MA, Hall RA, Minneman KP. Subtype-specific dimerization of alpha 1-adrenoceptors: effects on receptor expression and pharmacological properties. Mol Pharmacol. 2003;64:1379–1390. doi: 10.1124/mol.64.6.1379. [DOI] [PubMed] [Google Scholar]
- 30.White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 31.Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 32.Guo W, Shi L, Filizola M, Weinstein H, Javitch JA. Crosstalk in G protein-coupled receptors: changes at the transmembrane homodimer interface determine activation. Proc Natl Acad Sci U S A. 2005;102:17495–17500. doi: 10.1073/pnas.0508950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harikumar KG, Dong M, Cheng Z, Pinon DI, Lybrand TP, Miller LJ. Transmembrane segment peptides can disrupt cholecystokinin receptor oligomerization without affecting receptor function. Biochemistry. 2006;45:14706–14716. doi: 10.1021/bi061107n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dorsch S, Klotz KN, Engelhardt S, Lohse MJ, Bunemann M. Analysis of receptor oligomerization by FRAP microscopy. Nat Methods. 2009;6:225–230. doi: 10.1038/nmeth.1304. [DOI] [PubMed] [Google Scholar]
- 35.Donnelly D. The arrangement of the transmembrane helices in the secretin receptor family of G-protein-coupled receptors. FEBS Lett. 1997;409:431–436. doi: 10.1016/s0014-5793(97)00546-2. [DOI] [PubMed] [Google Scholar]