Abstract
WT1 is a transcription factor that is aberrantly overexpressed in acute and chronic leukemias. Overexpression of WT1 in pediatric acute myeloid leukemia has been reported, but the prognostic significance is unclear because sample sizes in these studies have been relatively small. WT1 expression was measured by quantitative RT-PCR in samples obtained at diagnosis from 155 pediatric AML patients treated on a cooperative group protocol. Neither overall survival nor event-free survival was correlated with WT1 expression.
Keywords: Wilms tumor 1, acute myeloid leukemia, prognosis, prognostic factor
Introduction
WT1, originally identified as the Wilms’ tumor suppressor gene, is expressed primarily in genitourinary and hematopoietic progenitors. Hematopoietic expression of WT1 is inversely correlated with the degree of differentiation. WT1 is overexpressed in hematologic malignancies, with higher levels observed in less differentiated leukemias. WT1 expression in normal bone marrow, peripheral blood, and lymph nodes is minimally detectable.[1] Significantly worse overall survival (OS) was reported in adult patients with de novo acute myeloid leukemia (AML) expressing high levels of WT1.[2] Disease-free survival (DFS), as well as OS, of AML patients with high WT1 expression was significantly decreased in a recent study, although the effect was more evident in patients younger than 60 years of age.[3] Other studies have not found an association between WT1 expression and survival, but these may have been limited by decreased sensitivity of detection of their RT-PCR assays.[4,5]
Elevated WT1 expression is seen in approximately 80% of children with AML, but the clinical significance of this finding is unclear. Poorer outcome was reported in children with acute leukemia and elevated WT1 at diagnosis.[6] Trka et al. corroborated these findings in children with AML. High WT1 copy after induction predicted elevated risk of relapse and death in children with AML, but this may simply reflect the presence of minimal residual disease.[7] Other researchers demonstrated elevated WT1 expression in pediatric patients with favorable cytogenetics; high WT1 copy was thus associated with increased OS.[8] These studies have been limited by small sample size. We present data from a large multi-institutional pediatric AML study suggesting that WT1 expression does not impact OS or event-free survival (EFS).
Materials and methods
Patients were enrolled on Pediatric Oncology Group (POG) study 9421 from 1995 to 1999. Details of the trial were described previously.[9,10] RNA was isolated from bone marrow aspirates obtained at diagnosis using the Qiagen RNEasy Kit (Valencia, CA). Poly (A)+ RNA was amplified using the MessageAmp aRNA Kit from Ambion (Austin, TX). One microgram of RNA from available samples was reverse transcribed using random hexamer primers. The cDNA was amplified by quantitative real-time polymerase chain reaction (RT-PCR) to evaluate total WT1 relative expression. Primers spanning the 6 Kb intron 1, previously validated by Siehl et al[11] and our lab, were used (sense strand: 5′-CGCTATTCGCAATCAGGGTTAC; antisense strand: 5′-ATGGGATCCTCATGCTTGAATG). Reactions (25μL each) were prepared using 12.5 μL iQ SYBR Green Supermix (Bio-Rad, Hercules, CA), 0.5 μL of cDNA, and 0.5 μL of 10μM primer mix. The reaction protocol proceeded as follows: 95° C for 3 minutes, 95° C for 15 seconds, 60° C for 1 minute, steps 2 and 3 were repeated for 40 cycles, followed by melt curve analysis. Individual melt curves were analyzed to assure PCR product purity. GAPDH threshold cycles (Ct) ranged from 10.1 to 33.9. Samples with GAPDH Ct ≥ 30, indicative of poor RNA integrity, were excluded from analysis. Of 193 samples with acceptable GAPDH Ct, one patient was ineligible. Another was missing an identification number. They were excluded from the analysis. Of the remaining samples, 34 were relapse specimens, leaving 157. These samples represented eligible patients without duplicates. Two patients with Down syndrome were excluded, restricting analysis to those with de novo AML. Relative expression was calculated using the 2−δδCt method[12] using GAPDH as an internal control and normalized to WT1 expression in the human leukemia cell line K562, assigned a value of 100%. The relationship between WT1 expression and clinical endpoints for the 155 evaluable patients, including OS and EFS, was determined using Kaplan-Meier survival analysis. OS was defined as time from study entry to death. EFS was defined as time from study entry to induction failure, relapse or death. The influence of WT1 expression on clinical outcome was analyzed within subgroups comprising patients with normal karyotype, t(8;21), inv16, FLT3-ITD, or 11q23 translocations.
Results and discussion
We performed quantitative RT-PCR to measure relative WT1 levels in 155 samples obtained from eligible de novo patients on POG 9421. WT1 expression was compared to expression in K562 cells, a standard adopted by several other groups [13–15]. Defining K562 expression as 100%, expression in the AML samples ranged from 0% to 166.2% and was normally distributed if expression was plotted logarithmically (Figure 1A). Twenty-one percent of samples had low expression levels (≤ 0.1%). High WT1 expression, defined as > 10% compared to K562, was seen in 20 samples (13%). No significant correlation of outcome with WT1 expression level was found when patient samples were divided into either 2, 3, or 4 groups based on relative WT1 expression (Figure 1B). To assess potential interactions between WT1 and established prognostic factors in pediatric AML, we performed subgroup analysis to determine the effect of WT1 expression on survival of patients with normal karyotype (n=54), t(8;21) (n=17), inv16 (n=19), FLT3-ITD (n=34), or translocations involving 11q23 (n=31). WT1 expression did not influence OS or EFS in any of these subgroups (data not shown).
Figure 1.
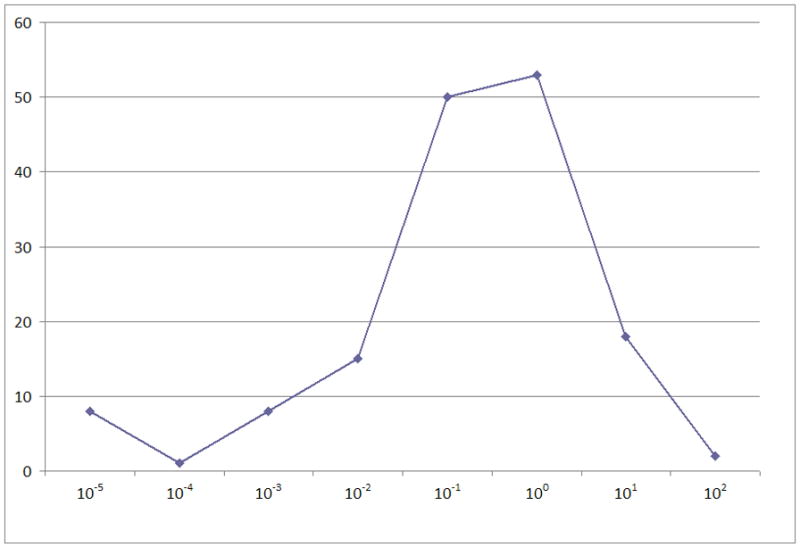
To exclude selection bias conferred by the availability of WT1 data, a group of 405 study subjects for whom there was insufficient RNA to determine WT1 expression level was compared to the current group. The proportion of patients with inv 16 was significantly higher in patients with WT1 expression available (12.3%) vs. patients for whom WT1 was unavailable (6.5%), p=0.040. The proportion of patients with FLT3-ITD was significantly higher in patients with WT1 available (16.2%) vs. patients for whom WT1 was unavailable (7.6%), p=0.039. The median WBC count (×103/μL) was also significantly higher in patients with WT1 available (median WBC=42.05) vs. patients without WT1 expression data (median WBC=19.95), p<0.001. Despite these differences, there was no significant difference between the two groups for the primary endpoints of this analysis, – OS and EFS (data not shown). These two groups of patients were also compared for OS and EFS from study entry for normal karyotype, inv16, t(8;21), 11q23 and FLT3-ITD subgroups, and no significant differences were found.
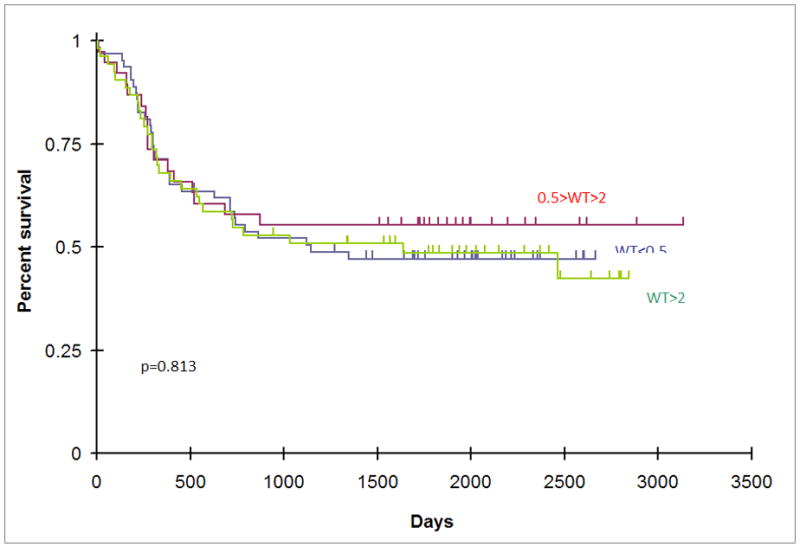
Trka et al. reported superior survival of pediatric AML patients with WT1 expression less than 0.01% of the level of K562 cells. Spanaki et al. reported a worse outcome for patients with WT1 expression greater than 10% of the level of K562 cells. In our patient cohort, there was no difference in OS or EFS of patients with WT1 expression ≤ 0.01% compared with the rest of the cohort, nor was there a difference in event free or overall survival of patients with WT1 expression ≥ 10% (Figure 2A, Figure 2B). To ensure that the lack of prognostic significance in our dataset was not a result of an arbitrarily selected cut point, multiple univariate Cox regression analyses were performed testing each WT1 cut point in the 10th to 90th percentile range of WT1 expression (0.0066176 – 15.7490131). In these 113 individual comparisons, there were no p-values for differences in OS or EFS <0.05 at any dichotomization between the 10–90 percentile, nor was there a discernible trend to significance at either extreme of WT1 expression, further suggesting that WT1 expression was not correlated with outcome in these patients. The findings of Trka et al. and Spanaki et al. are therefore likely due to their small sample sizes (47 and 14 patients respectively).
Figure 2.


The ubiquitous expression of WT1 in AML may explain why its expression level has no effect on OS, just as PML-RARα expression level in acute promyelocytic myeloid leukemia has no prognostic significance. While expression level may not affect prognosis in AML, this does not preclude an important role for WT1 in AML biology. For example, WT1 expression after treatment may be used as a marker for minimal residual disease (MRD), as suggested by the data from Lapillone et al.[7] It may also serve as a therapeutic target for small molecule inhibition or immunotherapy.
While expression level of wild-type WT1 may not play a significant prognostic role in AML, WT1 mutations have been associated with poor prognosis in normal-karyotype adult AML. A mutation frequency of 10% has been reported in large cohorts, predominantly clustering in exons 7 and 9. Patients exhibiting mutations have significantly reduced disease-free and overall survival.[16,17] Similar findings were recently reported in childhood AML.[18] Sample availability in our study precluded WT1 mutational analysis.
Another relevant question is whether WT1 isoform expression influences outcome. Preclinical evidence supports differential roles among WT1 isoforms in hematopoiesis and leukemogenesis. Transfection of 32D cl3 cells, a non-transformed murine myeloblast cell line, with isoforms excluding KTS and exon 5 potentiated granulocytic differentiation in response to G-CSF.[19] By contrast, transfection with isoforms including KTS and exon 5 produced 32D cl3 cells which were resistant to G-CSF induced differentiation.[20] Siehl and associates found there was a relative excess of exon 5-containing variants in AML patient samples. They did not find significant variation in WT1 expression among samples representing different myeloid leukemia FAB subtypes.[11] In contrast, other groups found M5 AML to be associated with significantly lower WT1 expression, as measured by RT-PCR, in comparison to other FAB subtypes. [2,7,15] It is not known whether WT1 isoforms are similarly differentially expressed in pediatric AML. Evaluation of isoform expression may elucidate the role of WT1 in pediatric leukemia.
Acknowledgments
We thank Mr. Greg McCarty for technical assistance. This research was funded by the Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant (T32), #5T32CA060441-15 (SAN and JEF), the Children’s Cancer Foundation, and NIH grant CA120535 (RJA). Dr. Arceci is the King Fahd Endowed Chair in Pediatric Oncology at Johns Hopkins.
References
- 1.Inoue K, Sugiyama H, Ogawa H, et al. WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood. 1994;84(9):3071–3079. [PubMed] [Google Scholar]
- 2.Bergmann L, Miething C, Maurer U, et al. High levels of Wilms’ tumor gene (wt1) mRNA in acute myeloid leukemias are associated with a worse long-term outcome. Blood. 1997;90(3):1217–1225. [PubMed] [Google Scholar]
- 3.Barragan E, Cervera J, Bolufer P, et al. Prognostic implications of Wilms’ tumor gene (WT1) expression in patients with de novo acute myeloid leukemia. Haematologica. 2004;89(8):926–933. [PubMed] [Google Scholar]
- 4.Gaiger A, Schmid D, Heinze G, et al. Detection of the WT1 transcript by RT-PCR in complete remission has no prognostic relevance in de novo acute myeloid leukemia. Leukemia. 1998;12(12):1886–1894. doi: 10.1038/sj.leu.2401213. [DOI] [PubMed] [Google Scholar]
- 5.Schmid D, Heinze G, Linnerth B, et al. Prognostic significance of WT1 gene expression at diagnosis in adult de novo acute myeloid leukemia. Leukemia. 1997;11(5):639–643. doi: 10.1038/sj.leu.2400620. [DOI] [PubMed] [Google Scholar]
- 6.Spanaki A, Linardakis E, Perdikogianni C, et al. Quantitative assessment of WT1 expression in diagnosis of childhood acute leukemia. Leuk Res. 2007;31(4):570–572. doi: 10.1016/j.leukres.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Lapillonne H, Renneville A, Auvrignon A, et al. High WT1 expression after induction therapy predicts high risk of relapse and death in pediatric acute myeloid leukemia. J Clin Oncol. 2006;24(10):1507–1515. doi: 10.1200/JCO.2005.03.5303. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues PC, Oliveira SN, Viana MB, et al. Prognostic significance of WT1 gene expression in pediatric acute myeloid leukemia. Pediatr Blood Cancer. 2006 doi: 10.1002/pbc.20953. [DOI] [PubMed] [Google Scholar]
- 9.Ravindranath Y, Chang M, Steuber CP, et al. Pediatric Oncology Group (POG) studies of acute myeloid leukemia (AML): a review of four consecutive childhood AML trials conducted between 1981 and 2000. Leukemia. 2005;19(12):2101–2116. doi: 10.1038/sj.leu.2403927. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien MM, Taub JW, Chang MN, et al. Cardiomyopathy in children with Down syndrome treated for acute myeloid leukemia: a report from the Children’s Oncology Group Study POG 9421. J Clin Oncol. 2008;26(3):414–420. doi: 10.1200/JCO.2007.13.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siehl JM, Reinwald M, Heufelder K, et al. Expression of Wilms’ tumor gene 1 at different stages of acute myeloid leukemia and analysis of its major splice variants. Ann Hematol. 2004;83(12):745–750. doi: 10.1007/s00277-004-0941-0. [DOI] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Inoue K, Ogawa H, Sonoda Y, et al. Aberrant overexpression of the Wilms tumor gene (WT1) in human leukemia. Blood. 1997;89(4):1405–1412. [PubMed] [Google Scholar]
- 14.Boublikova L, Kalinova M, Ryan J, et al. Wilms’ tumor gene 1 (WT1) expression in childhood acute lymphoblastic leukemia: a wide range of WT1 expression levels, its impact on prognosis and minimal residual disease monitoring. Leukemia. 2006;20(2):254–263. doi: 10.1038/sj.leu.2404047. [DOI] [PubMed] [Google Scholar]
- 15.Trka J, Kalinova M, Hrusak O, et al. Real-time quantitative PCR detection of WT1 gene expression in children with AML: prognostic significance, correlation with disease status and residual disease detection by flow cytometry. Leukemia. 2002;16(7):1381–1389. doi: 10.1038/sj.leu.2402512. [DOI] [PubMed] [Google Scholar]
- 16.Virappane P, Gale RE, Hills R, et al. Mutation of the Wilms’ tumor 1 gene is a poor prognostic factor associated with chemoresistance in normal karyotype acute myeloid leukemia. Blood (ASH Annual Meeting Abstracts) 2007;110 Abstract 361. [Google Scholar]
- 17.Paschka P, Marcucci G, Ruppert AS, et al. Wilms tumor 1 (WT1) gene mutations predict poor outcome in adults with cytogenetically normal (CN) acute myeloid leukemia (AML): a Cancer and Leukemia Group B (CALGB) study. Blood (ASH Annual Meeting Abstracts) 2007;110 doi: 10.1200/JCO.2007.15.2058. Abstract 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollink IH, van den Heuvel-Eibrink MM, Zimmermann M, et al. Clinical relevance of Wilms’ tumor 1 gene mutations in childhood acute myeloid leukemia. Blood. 2009 doi: 10.1182/blood-2008-09-177949. [DOI] [PubMed] [Google Scholar]
- 19.Loeb DM, Summers JL, Burwell EA, et al. An isoform of the Wilms’ tumor suppressor gene potentiates granulocytic differentiation. Leukemia. 2003;17(5):965–971. doi: 10.1038/sj.leu.2402906. [DOI] [PubMed] [Google Scholar]
- 20.Inoue K, Tamaki H, Ogawa H, et al. Wilms’ tumor gene (WT1) competes with differentiation-inducing signal in hematopoietic progenitor cells. Blood. 1998;91(8):2969–2976. [PubMed] [Google Scholar]


