Abstract
BACKGROUND
Aging results in decreased neuromuscular function, which is likely associated with neurologic alterations. At present little is known regarding age-related changes in intracortical properties.
METHODS
In this study we used transcranial magnetic stimulation (TMS) to measure intracortical facilitation (ICF), short- and long-interval intracortical inhibition (SICI and LICI), motor evoked potential amplitude, and silent period duration in young and older adults (21.4±0.8 yrs and 70.9±1.8 yrs). These variables were assessed from the flexor carpi radialis muscle of the non-dominant arm under resting conditions, and during a submaximal contraction (intensity 15% maximum strength).
RESULTS
Older adults exhibited increased SICI and LICI in comparison to young adults (SICI: 29.0±9.2% vs. 46.2±4.8% of unconditioned pulse; LICI: 6.5±1.7% vs. 15.8±3.3% of unconditioned pulse; p=0.04), and less ICF under resting conditions (74.6±8.7% vs. 104.9±6.9% of unconditioned pulse; p=0.02). These age-related differences disappeared during contraction, although the older adults did exhibit a longer silent period during contraction (112.5±6.5 vs. 84.0±3.9 msec; p<0.01).
CONCLUSIONS
Collectively, these findings suggest increased GABA mediated intracortical inhibition with age.
Keywords: Transcranial magnetic stimulation, muscle, motor evoked potential, aging, sarcopenia, dynapenia, electromyography
INTRODUCTION
In 2006, there were ~ 37 million older adults in the United States (CDC 2008), and projections forecast that by 2030 this age cohort will increase to approximately 71.5 million people representing nearly 20 percent of the total U.S. population (CDC 2008). Aging is associated with a myriad of physiological and functional changes, with one dramatic change being a reduction in neuromuscular function (e.g., decreased muscle strength and motor performance) (Frontera et al. 1991; Clark and Manini 2008). Over the past decade numerous studies have been conducted to identify age-related changes in excitability and behavioral properties of the spinal motorneurons (Laidlaw et al. 2000; Semmler et al. 2000; Scaglioni et al. 2003; Chalmers and Knutzen 2004; Kido et al. 2004; Kornatz et al. 2005; Christie and Kamen 2006). These studies have demonstrated that aging results in altered motorneuron properties, such as decreased spinal excitability (Kido et al. 2004), increased variability in motor unit discharge rate (Laidlaw et al. 2000) and a lower incidence of doublet discharges (Christie and Kamen 2006). However, at present relatively little scientific investigation has focused on determining the age-related changes in intracortical properties.
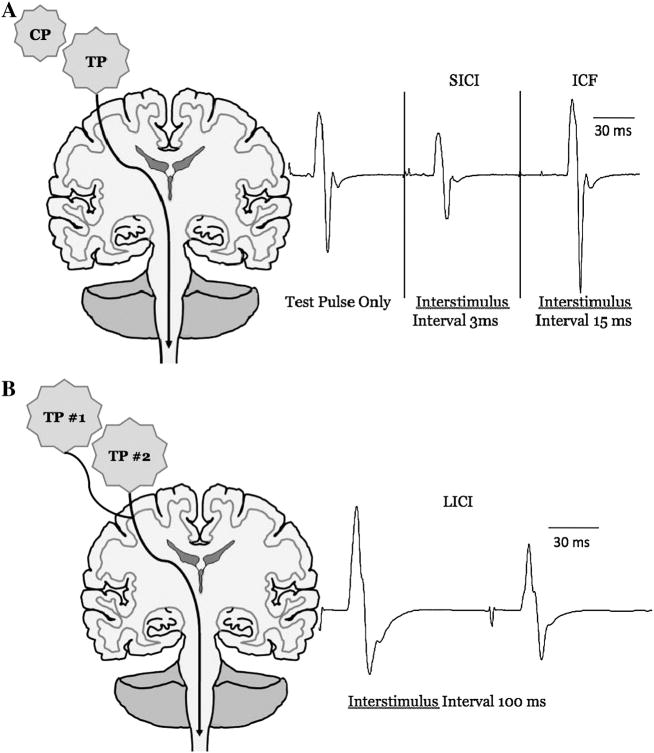
Over the past decade transcranial magnetic stimulation (TMS) has gained increasing popularity as a tool to elucidate information about human motor cortex excitability (Kobayashi and Pascual-Leone 2003). Single pulse TMS can be used to assess corticospinal excitability by eliciting motor evoked potentials (MEPs) and silent periods (SP). Unfortunately, because these responses are mediated at both the cortical and spinal levels it is difficult to determine where differences or changes in these parameters are being spatially mediated (Kobayashi and Pascual-Leone 2003). Cortico-cortical excitability can be more directly examined by using paired-pulse TMS, which combines a conditioning stimulus with a test stimulus at different interstimulus intervals (Figure 1) (Ziemann et al. 1995; Ziemann et al. 1996; Ziemann 2003; Ziemann 2004). For example, when a subthreshold conditioning pulse (i.e. 5% less than motor threshold) precedes a suprathreshold test pulse (i.e. 30% above MT) by 15 ms the MEP amplitude associated with the test pulse is greater than that of a single unconditioned pulse of the same intensity (intracortical facilitation [ICF]). Conversely, when the interstimulus interval is 3 ms the test motor response is reduced by the conditioning pulse (short-interval intracortical inhibition [SICI]), and the test motor response is also inhibited when two suprathreshold pulses are separated by 100 ms (long-interval intracortical inhibition [LICI]). It is generally thought that SICI is mediated by gamma-aminobutyric acid (GABA) type A receptors (GABAA) (Ziemann 2003; Florian et al. 2008), LICI is mediated by GABA type B receptors (GABAB) (McDonnell et al. 2006; Florian et al. 2008), and ICF is mediated by excitatory glutamatergic interneurons and N-methyl-D-aspartate (NMDA) receptors (Ziemann et al. 1995; Ziemann 2003; Ziemann 2004; Reis et al. 2008). In general, SICI and ICF are considered to be mediated locally within the primary motor cortex (M1). LICI is also commonly suggested to be mediated within M1 (Nakamura et al. 1997; Reis et al. 2008), although recent evidence suggests that it can also be influenced by spinal mechanisms (McNeil et al. 2009).
Figure 1. The change of motor evoked potential (MEP) sizes obtained with paired pulse TMS.
A: Measurement of short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF). The intensity of the conditioning pulse (CP) was set 5% below active motor threshold, and the test pulse (TP) was set to evoke MEP’s between 0.5–1 mV. At short interstimulus intervals (e.g., 3-msec) the CP inhibits the MEP in comparison to the TP only (SICI), whereas at longer interstimulus intervals (e.g., 15-msec) it facilitates the MEP (ICF). B: Measurement of long-interval intracortical inhibition (LICI). To quantify LICI two TP were delivered at an interstimulus interval of 100-msec. This results in the second MEP being inhibited in comparison to the first MEP.
To our knowledge only a handful of studies have examined age-related changes in intracortical facilitatory and/or inhibitory properties using paired-pulse TMS, and these studies have reported discrepant findings (Peinemann et al. 2001; Kossev et al. 2002; Oliviero et al. 2006; Smith et al. 2009). For example, one study observed an age-related decrease in SICI (Peinemann et al. 2001), one observed no age-related effects (Oliviero et al. 2006; Smith et al. 2009), and two observed an age-related increase in SICI (Kossev et al. 2002). The majority of the aforementioned studies examined middle-aged individuals, as opposed to older/elderly adults (≥65 yrs) (Peinemann et al. 2001; Kossev et al. 2002; Smith et al. 2009). Additionally, no studies have performed a comprehensive analysis of age-related changes in ICF, SICI and LICI. Accordingly, the purpose of this study was to compare ICF, SICI and LICI between young and older adults. Because we have previously observed state-dependent adaptations (e.g., resting conditions versus during voluntary contraction) in response to studies investigating the effects of disuse atrophy on neurologic properties (Clark et al. 2008b; Clark et al. In Press), we measured ICF, SICI, and LICI at rest and during a submaximal, isometric wrist flexion contraction.
MATERIALS AND METHODS
General Overview of the Study Design
Twenty-one young adults (21.4±0.8 yrs) and nine older adults (70.9±1.8 yrs) participated in the experiment. These subjects underwent an orientation and familiarization session followed by a testing session involving the assessment of neuromuscular function of the non-dominant arm. In addition to measuring wrist flexion muscle strength, we utilized electrical stimulation to measure the amplitude of the compound muscle fiber action potential (Mmax), and single- and paired-pulse TMS to measure resting and active motor threshold, MEP amplitude, SP duration and ICF, SICI, and LICI. Because many neurophysiologic properties are activity state dependent (Ridding et al. 1995b; Ridding and Rothwell 1999; Aagaard et al. 2002; Clark et al. 2008a) we obtained our respective TMS-based outcomes under resting conditions and during a submaximal isometric wrist flexion muscle contraction at an intensity of 15% of maximal voluntary contraction (MVC) strength. Specific details on these procedures are provided below.
Subjects
Twenty-one young subjects (11 women and 10 men, 173.5±2.55 cm, 70.1±2.9 kg, 23.1±0.5 kg/m2) and 9 older subjects (4 women and 5 men, 170.1±3.6 cm, weight 78.4±4.0 kg, 26.9±1.3 kg/m2) completed the study. Subjects were excluded if they were taking any of the following medications (many of which are known to affect physiological properties of TMS measures of cortical excitability (Ziemann 2004)): alpha blockers, antibiotics, antipsychotics, benzodiazepines, beta-blockers, calcium channel blockers, systemic corticosteroids, direct thrombin inhibitors, heparins, muscle relaxants, Parkinson’s disease drugs, neuromuscular blocking agents, sedatives, and psycostimulants. Subjects were also excluded if they had any of the following conditions: Alzheimer’s, Amyotrophic lateral sclerosis (ALS), anxiety disorders, blindness, cancer, chronic obstructive pulmonary disease (COPD), congestive heart failure, depression, diabetes, drug or alcohol abuse in the last six months, emphysema, epilepsy, frequent faintness/dizziness, frequent pain in heart/chest, migraine headaches, smoked in the last six months, heart attack, HIV, kidney disease, liver disease, macular degeneration, multiple sclerosis, Parkinson’s disease, rheumatoid arthritis, seizures, severe anemia, sickle cell anemia, spasticity, stroke, other neurological disorders, and other psychiatric disorders. In addition, subjects were excluded if they had any known orthopedic limitations that would affect their ability to complete the testing protocol, or reported nicotine related products. Lastly, subjects were excluded if they reported any contraindications or cautionary signs for being exposed to a strong magnetic field as defined by the Institute for Magnetic Resonance Safety, Education, and Research (http://www.IMRSER.org/). There were some prescription medications that were allowed in the older adult subjects, which were (the number of subjects on these medications are noted in parentheses): diuretic (3), statins (3), ACE inhibitor (3), proton pump inhibitor (2), NSAID (2), 5-alpha-reductase inhibitors (1), fibrates (1), biguanide (1), bisphosphonates (1). Subjects were asked to not consume alcohol (abstain for 24-hrs) or caffeine (abstain for 4-hrs) prior to the testing sessions. The Ohio University Institutional Review Board approved the study.
Electrical & Mechanical Recordings
Electrical signals were recorded from the non-dominant flexor carpi radialis (FCR) muscle using bipolar surface electrodes as we have previously reported (band-pass filtered: 10–500 Hz, sampling rate: 5 kHz (Clark et al. 2008b). To quantify wrist flexion forces subjects were seated with the elbow at 90°, the hand pronated and the forearm supported (Biodex System 4). The wrist joint was aligned with the axis of rotation of a torque motor to which a lever arm was attached (Figure 2), and the lever arm adapter was held at a fixed length for all subjects so that the absolute torque measurement was held constant across individuals. The signal was scaled to maximize its resolution (208.7 mV/N-m; Biodex Researchers Tool Kit Software), smoothed over a 10-point running average, and sampled at 625 hertz (MP150 Biopac Systems). Subjects received visual feedback of all exerted forces on a 53-cm computer monitor located 1 m directly in front of them.
Figure 2.
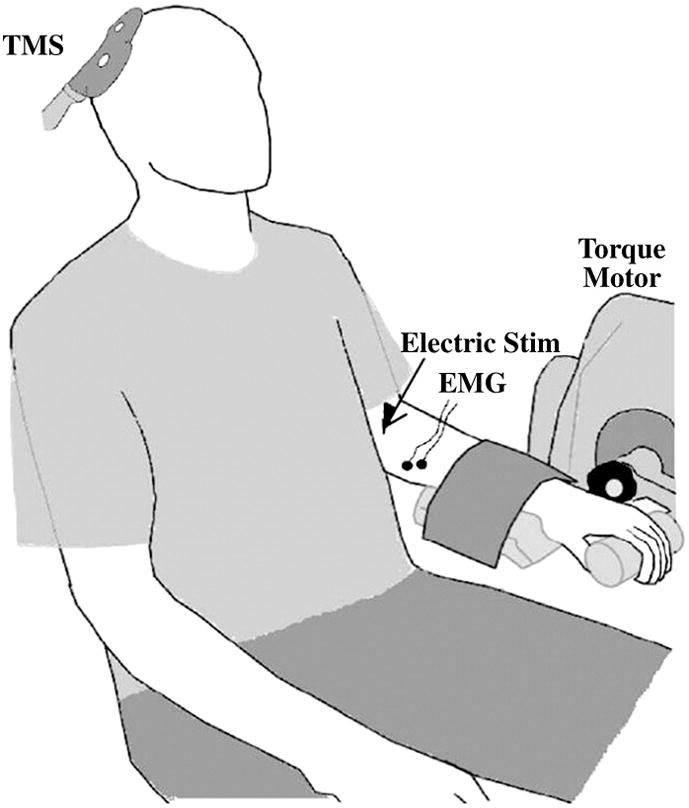
The experimental setup.
Voluntary Wrist Flexion Strength
To assess maximal wrist flexion strength, subjects performed a minimum of 3 MVCs with a 1–2 minute rest period between each contraction. If subjects continually produced more force with increasing trials, or if the two highest trials were not within 5% of each other, additional trials were performed until a plateau was reached. During testing verbal encouragement was provided.
Electrical Stimulation and Mmax
We measured maximal compound muscle fiber action potential (Mmax) via supramaximal electrically stimulation of the median nerve (Clark et al. 2008a; Cowley et al. 2008). Electrical stimulation intensity was delivered as single pulses (500-μs pulse duration) using a constant current stimulator (model DS7A, Digitimer, Hertfordshire, UK). Intensity was increasedincrementally until an increase in stimulation intensity produced no increasein evoked muscle action potential amplitude. The maximum peak-to-peak (p-p) amplitude was considered the Mmax.
Transcranial Magnetic Stimulation
TMS pulses were delivered using two connected Magstim 2002 stimulators (The Magstim Co. Ltd) through one 70-mm figure-of-eight focal coil. The TMS coil was positioned tangentially to the scalp and laterally at 45 degrees from the midline (Brasil-Neto et al. 1992) so that the induced current flowed in a lateral-posterior to medial-anterior direction in the brain that predominantly activates corticospinal neurons transsynaptically (Werhahn et al. 1994). The stimulation location that elicited the largest p-p amplitude of the FCR MEP was identified and marked on a lycra cap for coil placement.
Next, motor thresholds (MT) were determined while subjects were seated in the dynamometer by delivering single pulses at gradually increasing stimulation intensities as we have previously described (Clark, Issac et al. 2008; Damron, Hoffman et al. 2008). Both resting and active MT were determined and expressed as a percent of the maximal stimulator output. MTs were determined by using a TMS intensity well below MT and gradually increasing the intensity in 2% increments until the TMS stimulus intensity that induced suprathreshold MEPs was observed. Resting MT was defined as the stimulation intensity eliciting MEPs with a p-p amplitude of ≥ 50 microV in at least 3 of 6 trials. During this assessment the muscle was completely relaxed as monitored by the EMG signal. During the active MT assessment subjects performed a wrist flexion contraction at an intensity of 15% MVC (a force matching task displayed on a computer monitor). Before the active MT determination began, subjects performed a minimum of 6 trials during which the interference/background EMG associated with the submaximal contraction was recorded. The maximum p-p amplitude associated with the voluntary contraction trials were averaged and considered the maximum EMG activity during a 15% of maximum contraction. The active MT was subsequently defined as the lowest TMS intensity required to evoke MEPs with a p-p amplitude ≥ two times that present in at least 3 of 6 of these voluntary trials. We chose to quantify threshold in this manner rather than using an absolute amplitude (e.g., 300 μV) in an attempt to minimize the influence of numerous factors which are known to influence the amplitude of the voluntary signal and are likely to differ between younger and older adults (e.g., thickness of subcutaneous tissue layers, skin impedance, etc) (Farina et al. 2004).
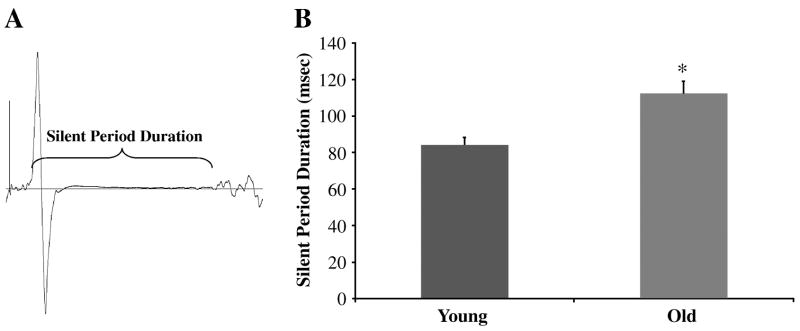
MEP amplitude and silent period (SP) duration were evaluated using single pulse TMS. To assess changes in MEP amplitude at rest single pulses were delivered at 130% of resting MT. The p-p amplitude of the MEPs was calculated and averaged over 6 trials and expressed as a percentage of Mmax. In addition, changes in MEP amplitude during the 15% MVC contraction were assessed by applying a suprathreshold stimulus (130% AMT) over six trials, while the subject contracted at 15% MVC. From these trials MEP amplitude and SP duration were determined and averaged. A single, blinded investigator visually defined the MEP onset and return of the interference EMG signal, and the duration between these two events was calculated to represent the SP (Figure 3). We have previously reported that this quantification method displays high inter-rater reliability (r=0.97), and a week-to-week coefficient of variation among healthy subject of ~12% (Damron et al. 2008).
Figure 3. Older adults exhibited a longer silent period than young adults.
A: Example EMG recording demonstrating the silent period of a subject following single pulse TMS. B: The older adults silent period was ~ 28 msec longer than the young adults (*P<0.01).
SICI, ICF and LICI were evaluated using paired magnetic pulses under resting conditions and during contraction. To quantify SICI, ICF and LICI at rest the second (test) stimulus was set at an intensity that, when it was given alone, evoked an MEP of ~ 0.5 to 1.0 mV p-p amplitude (mean stimulus intensity for young adults was 75.6±3.1% of stimulator output and for older adults it was 78.0±4.7% of stimulator output (p=0.56)). During contraction this stimulus intensity was set to evoke an MEP of ~ 2.0 mV p-p amplitude (mean stimulus intensity for young adults was 58.6±3.0% of stimulator output and for older adults it was 67.7±5.5% of stimulator output (p=0.08)). It should be noted that the stimulus intensities for the resting condition were on average 60±0.06% above resting MT, and for the active contraction 28±0.05% above resting MT. This detail is important based on the observation that LICI amplitude ratios decrease with increasing conditioning stimulus intensity, and that they reach an asymptote for peak inhibition at levels around 10% above resting MT (Hammond and Garvey 2006). Accordingly, our level of conditioning stimulus was relatively similar between groups and well above the asymptote threshold for peak inhibition making it unlikely that subtle differences in this parameter influence our between group comparisons. For SICI and ICF quantification the intensity of the first (conditioning) stimulus was set to 95% of active MT at rest and during contraction, and for LICI quantification the first (conditioning) stimulus was set at the intensity used for the aforementioned test pulses. The interstimulus intervals for assessing SICI, ICF, and LICI were 3, 15, and 100 ms, respectively. A total of 8 trials of each of these 4 conditions (test pulse given alone, ICF, SICI and LICI trials) were randomly performed in blocks and averaged. The resting trials were performed first followed by the contraction trials. During the 15% MVC contractions subjects were provided with a target line and asked to exert the respective target level of force for ~ 5 s with TMS pulses being delivered between the 3rd–5th second. We chose a contraction intensity of 15% MVC as our pilot experiments indicated that during higher intensity contractions the ability to observe intracortical inhibition was diminished. SICI, ICF and LICI are operationally defined by expressing the mean p-p amplitude of the conditioned MEP, at each interstimulus interval, as a percentage of the mean p-p amplitude of the unconditioned test pulse.
Statistics
Univariate analysis of variance procedures were used to determine group differences (young vs. old) on the respective dependent variables. For all analyses, significance was set at a P-value ≤0.05. Data are presented as means ± SEM. Additionally, to further aid in interpretation we also report the effect size (ES; partial η2), which represents the proportion of total variation attributable to a given factor when partialing out other factors from the total non-error variation. The SPSS statistical package (version 14.0, Chicago, IL) was used for data analysis.
RESULTS
Muscle Strength
No differences were observed in absolute levels of voluntary muscle strength between young and older adults (19.6±1.8 vs. 19.2±2.2 N•m; P=0.92, ES=0.00). The older adults were ~ 15% weaker than the young adults when muscle strength was expressed relative to BMI (0.85±0.08 vs. 0.72±0.08), however this difference was not significantly different (P=0.48; ES=0.02).
Motor Thresholds
Older adults exhibited a higher resting motor threshold than young adults (53.3±5.5 vs. 44.1±1.7% of maximum stimulator output; p=0.04, ES=0.14). No age group differences were observed for active motor threshold (Young Adults: 39.9±1.8% of maximum stimulator output, Old Adults: 45.6±4.8% of maximum stimulator output; P=0.18; ES=0.06).
Mmax, MEP Amplitude and Silent Period
Resting MEP amplitude expressed in absolute terms was not significantly different between young and older adults (0.45±0.06 vs. 0.31±0.04 mV; p=0.16), although a large effect size was observed for young adults to demonstrate larger absolute resting MEPs (ES=0.65). Similarly, active MEP amplitude expressed in absolute terms was significantly greater in young adults when compared to older adults (2.36±0.28 vs. 1.34±0.19; p=0.02; ES=0.96). Because age-related differences in Mmax could influence the absolute MEP values independent of differences in corticospinal excitability we also expressed the MEPs relative to Mmax. While Mmax did not differ between the younger and older adults (6.5±0.6 vs. 4.8±1.1 mV; p=0.15), we did observe a moderate to large effect size for young adults to exhibit larger Mmax values (ES=0.48). Interestingly, when the resting and active MEP amplitudes were normalized to Mmax no differences existed between the younger and older adults (Resting Condition: 8.5±1.9 vs. 7.1±1.0% of Mmax; P=0.46, ES=0.02; Active Condition: 36.3±6.7 vs. 37.3±3.5% of Mmax; P=0.89; ES=0.01). Older adults exhibited a longer silent period in comparison to the young adults (Figure 3) (P<0.01; ES=0.37).
ICF, SICI, and LICI
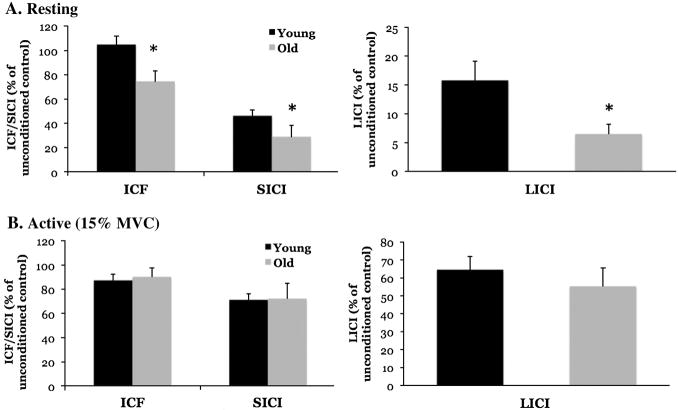
Older adults exhibited less ICF and more SICI and LICI when compared to young adults under resting conditions (Figure 4A) (ICF P=0.02; ES=0.19; SICI P=0.04; ES=0.14; LICI P=0.04; ES=0.15). No differences were observed in ICF, SICI or LICI during an active contraction (Figure 4B) (ICF P=0.69, ES=0.01; SICI P=0.97; ES=0.00; LICI P=0.42; ES=0.02).
Figure 4. Older adults exhibit differences in intracortical properties.
A: Older adults exhibit less intracortical facilitation (ICF) than younger adults, and more short-interval intracortical inhibition (SICI) and long-interval intracortical inhibition (LICI) under resting conditions (*P<0.05). B: No age-related differences were observed for ICF, SICI or LICI during an active contraction.
Control Experiment
While we did not observe a significant difference in active MT between younger and older adults, there as a modest mean difference. Accordingly, the absolute conditioning stimulus intensity (95% of active MT) for the ICF and SICI contractions was slightly lower for the younger adults. To examine whether the difference in absolute CS could explain the between group differences in ICF and SICI at rest we conducted a follow-up control experiment. Here, we retrospectively examined our older adult data and identified three older adults with a very similar active MT (42–44% of stimulator output)- who correspondingly received conditioning stimuli of ~ 41 (range: 40–42%). Subsequently, we recruited four additional young adults and measured resting ICF and SICI with an absolute conditioning stimulus equal to this subset of older adults (41%) as well as relative to active MT (95% of active MT). The data from this control experiment indicated that the subset of older adults exhibited ICF and ICF similar to that observed in the complete older cohort (74.9 and 11.8% of unconditioned pulse). Similarly, when the younger adults were also assessed at a 95% of active MT they also exhibited ICF and SICI similar to that observed in the complete younger cohort (106.7 and 51.2% of unconditioned pulse). When the younger adults conditioning stimulus was set to the same absolute value as the subset of older adults (41% of stimulator output), ICF was nominally altered (104.3% of unconditioned pulse), and SICI was increased slightly (44.1% of unconditioned pulse)- albeit to a considerably lesser extent than observed between young and old. Thus, these findings indicate that while some of the age related differences in resting ICF and SICI may be related to subtle differences in active MT (and the absolute stimulus intensity)-it does not appear that this is able to fully explain our observed differences.
DISCUSSION
This study used transcranial magnetic stimulation to examine differences in intracortical properties between young and older adults. The most novel findings of this work are that older adults exhibited less intracortical facilitation and more intracortical inhibition (SICI and LICI) under resting conditions, which disappeared during a submaximal muscle contraction. We also observed that older adults had a higher resting motor threshold and a prolonged silent period. Below we will first discuss our findings with respect to the current literature and then discuss their physiological interpretation and functional implications.
Influence of Age on Cortical Excitability
Previous investigations on age-related changes in ICF and SICI have reported discrepant findings (Peinemann et al. 2001; Kossev et al. 2002; Oliviero et al. 2006; Smith et al. 2009). Peinemann and colleagues reported that middle-aged individuals (mean age 51 yrs) exhibited less SICI in the first dorsal interosseus than young adults, and no differences in ICF (Peinemann et al. 2001). This study utilized biphasic pulses, which have been suggested to activate both facilitatory and inhibitory neurons (Arai et al. 2005). Consequently, it is difficult to compare their findings to studies using monophasic pulses. Among studies using monophasic pulses, Kossev and colleagues observed that middle-aged adults (mean age 56 yrs) exhibited more SICI and less ICF than young adults under resting conditions (Kossev et al. 2002), whereas Oliviero et al. and Smith et al. observed that older adults (mean ages of 71 and 63 years, respectively) exhibited a comparable amount of SICI as younger adults (Oliviero et al. 2006). Directly interpreting and comparing these aforementioned studies to the present is difficult based on i) large between study differences in the ages of the older adults (e.g., mid-50’s in one study versus early-70’s in the present), and ii) critical methodological differences between the studies. Regarding key methodological differences, it has recently been reported that the degree of SICI correlates with the conditioning stimulus intensity when expressed relative to an individuals threshold for SICI induction, and that this SICI threshold is more closely related to active motor threshold in comparison to resting motor threshold (Orth et al. 2003). Consequently, it is difficult to directly compare the findings of Kossev et al. to ours because in their study the conditioning stimulus intensity was set relative to resting motor threshold whereas in the present study our conditioning stimulus intensity was relative to active motor threshold. Similarly, the studies by Oliviero et al. and Smith et al. differed from the present study in that the previous studies conditioning stimulus for measuring SICI were set at an absolute 5% of stimulator output below each subject’s active motor threshold, whereas in the present study we set the conditioning stimulus intensity relative to active motor threshold (95% of active motor threshold). Interestingly, Smith et al. observed age-differences in active motor threshold, and because their conditioning stimulus intensity was set at an absolute level below active motor threshold the older adults received a slightly higher relative intensity stimulus. Further they reported that the higher conditioning stimulus intensity explained ~ 15% of the between subject variability in SICI (Smith et al. 2009). Because we did not observe significant age-related differences in active motor threshold and because our conditioning stimulus intensity was set relative to active motor threshold it is likely that this confound less influences our results. However, we should note that we also observed that the conditioning stimulus intensity explained ~ 16% of the between subject variability in SICI (R2= 0.16, p=0.02), which is consistent with Smith and colleagues. Accordingly, our findings of increased SICI and decreased ICF in older adults help to clarify the question of whether there are age-related changes in these parameters, and when these data are collectively considered it appears that advancing age does increase SICI and decrease ICF under resting conditions. In addition to demonstrating increases in SICI, the older adults also exhibited increased levels of LICI, which to our knowledge has never been reported with regards to age-related changes.
With respect to our single-pulse TMS data we observed a longer silent period in older adults, and no differences in MEP amplitude. In contrast, several studies have reported that older adults have reduced MEP amplitudes in comparison to younger adults (Rossini et al. 1992; Sale and Semmler 2005; Oliviero et al. 2006; Talelli et al. 2008; Fujiyama et al. 2009). However, most of these studies did not normalize their MEP amplitudes to Mmax and simply reported absolute differences in mV values, which are highly influenced by non-physiologic factors that are likely to differ between young and old (Rossini et al. 1992; Oliviero et al. 2006; Fujiyama et al. 2009). To illustrate this, our resting and active MEP amplitudes were virtually identical when expressed relative to the Mmax; however, when the absolute mV values were examined the older adults tended to exhibit MEPs and Mmax values ~30–40% smaller than younger adults. Among the studies that have normalized the MEP amplitude, divergent findings exist with reports of reduced MEP (Sale and Semmler 2005; Talelli et al. 2008), and no age differences (Hunter et al. 2008). Thus, our findings of no age-differences in normalized MEP amplitude in a forearm muscle is in agreement with Hunter and colleagues (2008) who assessed an upper arm muscle, and in disagreement with the work of Sale and Semmler (2005) and Talelli and colleagues (2008) who assessed intrinsic hand muscles. It is possible that these discrepant findings may be due to differences in segmental inhibition between muscle groups, as there is evidence suggesting that arm muscles have more segmental inhibition than intrinsic hand muscles (Triggs et al. 1993). Similarly, studies examining the silent period duration of arm muscles have observed no differences with age (Hunter et al. 2008; Fujiyama et al. 2009), whereas those examining the intrinsic hand muscles have observed that older adults exhibit a shorter duration (Sale and Semmler 2005; Oliviero et al. 2006). Interestingly, our finding of a longer silent period in the forearm muscles in older adults is in disagreement with all of these aforementioned studies. It is possible that these discrepant findings are related to between-study methodological disparities such as the subject populations or TMS parameters.
Age Related Changes in Cortical Properties Are State-Dependent
The concept and investigation of state-dependency is important as the neural impact of a TMS external stimulus represents an interaction with the ongoing brain activity at the time of stimulation- making the effects not only determined by the properties of that stimulus, but also by the activation state of the brain (Silvanto and Pascual-Leone 2008). Neurophysiologic properties and adaptations are well known to be activity state dependent (Ridding et al. 1995b; Ridding and Rothwell 1999; Aagaard et al. 2002; Clark et al. 2008b), and task specific differences in corticospinal excitability have been observed between younger and older adults (Sale and Semmler 2005). Thus, we expected to find state-dependent differences in intracortical properties. Voluntary contraction is well known to increase both efferent and afferent neuronal activity and reduce the excitability of inhibitory circuits in cortical areas that project to the active muscle (Vallbo 1971; Ridding et al. 1995b). As previously stated, we observed reduced ICF and increased SICI and LICI at rest in older adults, but these differences disappeared during contraction. These findings are consistent with the observation that age-related differences in SICI disappear when vibration is applied to a muscle to selectively activate the group Ia muscle spindle afferent fibers (Burke and Schiller 1976; Kossev et al. 2002). Accordingly, it is possible that the increased afferent activity associated with contraction mechanistically contributed to the elimination of our observed age-related differences at rest. This assertion is supported by the findings that intracortical inhibitory circuits are particularly sensitive to afferent activity (Rosenkranz et al. 2003; Rosenkranz and Rothwell 2003). However, because LICI, SICI and the silent period duration are reduced with the magnitude of voluntary contraction (Hammond and Vallence 2007), it is possible that any increase of intracortical inhibition caused by increasing sensory feedback from the contracting muscle is overridden by a reduction in inhibition caused by increasing voluntary drive.
Physiology and Functional Implications of Altered Cortical Properties
Research over the last 10–15 years has resulted in a better understanding of the physiological underpinning of TMS-based outcome measures, and in general ICF, SICI, and LICI are thought to be cortical in origin, particularly under resting conditions. Based on pharmacological studies, ICF appears to be highly influenced by glutamatergicfacilitation tempered by persisting GABAergic inhibition, and SICI and LICI are mediated by GABAergic inhibition at the intracortical level (Ziemann et al. 1995; Ziemann 2003; McDonnell et al. 2006; Florian et al. 2008). Specifically, it has been suggested that SICI is mediated by GABA type A receptors (GABAA) (Ziemann 2003; Florian et al. 2008), whereas LICI is mediated by GABA type B receptors (McDonnell et al. 2006; Florian et al. 2008). Our observation of increased SICI and LICI in older adults indicates increased GABAergic inhibition with age under resting conditions, and our observation of decreased ICF is either a result of the increased inhibition or a separate decrease in glutamatergic facilitation. In addition to increased SICI and LICI, we observed a longer silent period in older adults. The SP has been proposed to be mediated by GABAB receptors (Ziemann 2003); thus suggesting a mechanistic link to LICI. We observed a longer SP in older adults during contraction without a difference in LICI during contraction. Divergent differences in the SP and LICI have previously been reported as evidenced by fatiguing exercise reducing LICI and prolonging the SP (Benwell et al. 2007). The SP is an indicator of inhibition during contraction with changes in its duration being mediated at both the cortical and spinal levels (Chen et al. 1999); thus, determining the spatial localization of these differences is difficult. Overall our results of increased SICI, LICI, and silent period duration suggest increased GABA mediated inhibition with age.
From a functional perspective intracortical inhibition is fundamental in modulating motor cortex output during fine movements (Liepert et al. 1998; Stinear and Byblow 2004), and excessive intracortical inhibition following stroke is believed to limit recovery by reducing dexterity (Butefisch et al. 2003; Butefisch et al. 2006). Additionally, an abnormal balance between inhibitory and excitatory mechanisms has been implicated in neurodegenerative diseases that manifest with disordered movement (Ridding et al. 1995a; Hanajima et al. 1996; Hanajima and Ugawa 2000). Accordingly, it is probable that the age-associated changes in cortical excitability results in functional consequences, but further work is needed to better understand the specific implications of these the neurophysiologic changes.
Study Limitations
There are several limitations of the present study that should be noted. For example, we did not further sub-categorize our older adults into sub-groups (e.g., pre-frail or frail) making it difficult to know whether our observed changes are similar across all older adults or whether they differ by functional status. We also did not conduct neuropsychological tests or neuroimaging assessments in order to exclude the effects of specific organic factors. This is particularly important to consider in light of the recent modeling work by Wagner et al. indicating that cortical atrophy (which is well-known to occur with advancing age (Salat et al. 2004)) decreases the magnitude of current densities induced on the cortex with TMS (Wagner et al. 2008). Thus, it is possible that cortical atrophy may have influenced our findings irrespective of age-related changes in cortical excitability. We also did not obtain measures of manual dexterity or assess the effects of handedness-making it hard to draw inferences about the functional effects.
Conclusion
In this study we examined age-related differences in cortical excitability, and observed that older adults exhibited several differences most notably under resting conditions. Specifically, we observed that older adults demonstrate increases in SICI and LICI, along with a longer silent period duration. ICF also differed with age, as older adults demonstrated reduced levels. Collectively, these findings indicate increased GABA mediated inhibition with age. Interestingly, the age-related differences in SICI, LICI and ICF were not evident during muscle contraction suggesting state-dependent adaptations in association with aging. Further work is required to better understand the functional relevance of changes in intracortical properties on muscle performance.
Acknowledgments
This work was supported in part by a Research and Scholarly Advancement Fellowship (RSAF) to Marisa McGinley through the Ohio University College of Osteopathic Medicine. The project described was supported by Award Number R15HD065552 from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development to BC Clark. The content is solelythe responsibility of the authors and does not necessarilyrepresent the official views of the Eunice Kennedy Shriver National Institute Of Child Health & Human Development or the National Institutes of Health.
Footnotes
Disclosure statement: The authors have no actual or potential conflicts of interest associated with this work.
Human subjects approval: The Ohio University Institutional Review Board approved the study, and subjects provided written informed consent prior to study participation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aagaard P, Simonsen EB, et al. Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol. 2002;92(6):2309–18. doi: 10.1152/japplphysiol.01185.2001. [DOI] [PubMed] [Google Scholar]
- Arai N, Okabe S, et al. Comparison between short train, monophasic and biphasic repetitive transcranial magnetic stimulation (rTMS) of the human motor cortex. Clin Neurophysiol. 2005;116(3):605–13. doi: 10.1016/j.clinph.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Benwell NM, Mastaglia FL, et al. Differential changes in long-interval intracortical inhibition and silent period duration during fatiguing hand exercise. Exp Brain Res. 2007;179(2):255–62. doi: 10.1007/s00221-006-0790-2. [DOI] [PubMed] [Google Scholar]
- Burke D, Schiller HH. Discharge pattern of single motor units in the tonic vibration reflex of human triceps surae. J Neurol Neurosurg Psychiatry. 1976;39(8):729–41. doi: 10.1136/jnnp.39.8.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butefisch CM, Kleiser R, et al. Post-lesional cerebral reorganisation: evidence from functional neuroimaging and transcranial magnetic stimulation. J Physiol Paris. 2006;99(4–6):437–54. doi: 10.1016/j.jphysparis.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Netz J, et al. Remote changes in cortical excitability after stroke. Brain. 2003;126(Pt 2):470–81. doi: 10.1093/brain/awg044. [DOI] [PubMed] [Google Scholar]
- CDC. F. I. F. o. A.-R. Statistics. U.S. Government Printing Office; Washington, DC: 2008. Older Americans 2008: Key Indicators of Well-Being. [Google Scholar]
- Chalmers GR, Knutzen KM. Recurrent inhibition in the soleus motor pool of elderly and young adults. Electromyogr Clin Neurophysiol. 2004;44(7):413–21. [PubMed] [Google Scholar]
- Chen R, Lozano AM, et al. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res. 1999;128(4):539–42. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Christie A, Kamen G. Doublet discharges in motoneurons of young and older adults. J Neurophysiol. 2006;95(5):2787–95. doi: 10.1152/jn.00685.2005. [DOI] [PubMed] [Google Scholar]
- Clark B, Issac LC, et al. Neuromuscular plasticity during and following 3-weeks of human forearm cast immobilization. J Appl Physiol. 2008a;105:868–878. doi: 10.1152/japplphysiol.90530.2008. [DOI] [PubMed] [Google Scholar]
- Clark BC, Issac LC, et al. Neuromuscular plasticity during and following 3 wk of human forearm cast immobilization. J Appl Physiol. 2008b;105(3):868–78. doi: 10.1152/japplphysiol.90530.2008. [DOI] [PubMed] [Google Scholar]
- Clark BC, Manini TM. Sarcopenia ≠ dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63(8):829–34. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- Clark BC, Taylor JL, et al. Cast immobilization increases long-interval intracortical inhibition. Muscle and Nerve. doi: 10.1002/mus.21694. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley PM, Clark BC, et al. Kinesthetic motor imagery and spinal excitability: the effect of contraction intensity and spatial localization. Clin Neurophysiol. 2008;119(8):1849–56. doi: 10.1016/j.clinph.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Damron LA, Dearth DJ, et al. Quantification of the corticospinal silent period evoked via transcranial magnetic stimulation. J Neurosci Methods. 2008;173(1):121–8. doi: 10.1016/j.jneumeth.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Farina D, Merletti R, et al. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96(4):1486–95. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- Florian J, Muller-Dahlhaus M, et al. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. J Physiol. 2008;586(2):495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera WR, V, Hughes A, et al. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol. 1991;71(2):644–50. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Garry MI, et al. Age-related differences in inhibitory processes during interlimb coordination. Brain Res. 2009;1262:38–47. doi: 10.1016/j.brainres.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Hammond G, Vallence AM. Modulation of long-interval intracortical inhibition and the silent period by voluntary contraction. Brain Res. 2007;1158:63–70. doi: 10.1016/j.brainres.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Hammond GR, Garvey CA. Asymmetries of long-latency intracortical inhibition in motor cortex and handedness. Exp Brain Res. 2006;172(4):449–53. doi: 10.1007/s00221-006-0349-2. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y. Intracortical inhibition of the motor cortex in movement disorders. Brain Dev. 2000;22(Suppl 1):S132–5. doi: 10.1016/s0387-7604(00)00140-6. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, et al. Ipsilateral cortico-cortical inhibition of the motor cortex in various neurological disorders. J Neurol Sci. 1996;140(1–2):109–16. doi: 10.1016/0022-510x(96)00100-1. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Todd G, et al. Recovery from supraspinal fatigue is slowed in old adults after fatiguing maximal isometric contractions. J Appl Physiol. 2008;105(4):1199–209. doi: 10.1152/japplphysiol.01246.2007. [DOI] [PubMed] [Google Scholar]
- Kido A, Tanaka N, et al. Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol. 2004;82(4):238–48. doi: 10.1139/y04-017. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2(3):145–56. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Kornatz KW, Christou EA, et al. Practice reduces motor unit discharge variability in a hand muscle and improves manual dexterity in old adults. J Appl Physiol. 2005;98(6):2072–80. doi: 10.1152/japplphysiol.01149.2004. [DOI] [PubMed] [Google Scholar]
- Kossev AR, Schrader C, et al. Increased intracortical inhibition in middle-aged humans; a study using paired-pulse transcranial magnetic stimulation. Neurosci Lett. 2002;333(2):83–6. doi: 10.1016/s0304-3940(02)00986-2. [DOI] [PubMed] [Google Scholar]
- Laidlaw DH, Bilodeau M, et al. Steadiness is reduced and motor unit discharge is more variable in old adults. Muscle Nerve. 2000;23(4):600–12. doi: 10.1002/(sici)1097-4598(200004)23:4<600::aid-mus20>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Liepert J, Classen J, et al. Task-dependent changes of intracortical inhibition. Exp Brain Res. 1998;118(3):421–6. doi: 10.1007/s002210050296. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, et al. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173(1):86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Martin PG, et al. The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. J Physiol. 2009 doi: 10.1113/jphysiol.2009.180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, et al. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498(Pt 3):817–23. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliviero A, Profice P, et al. Effects of aging on motor cortex excitability. Neurosci Res. 2006;55(1):74–7. doi: 10.1016/j.neures.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Orth M, Snijders AH, et al. The variability of intracortical inhibition and facilitation. Clin Neurophysiol. 2003;114(12):2362–9. doi: 10.1016/s1388-2457(03)00243-8. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, et al. Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci Lett. 2001;313(1–2):33–6. doi: 10.1016/s0304-3940(01)02239-x. [DOI] [PubMed] [Google Scholar]
- Reis J, Swayne OB, et al. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 2008;586(2):325–51. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Inzelberg R, et al. Changes in excitability of motor cortical circuitry in patients with Parkinson’s disease. Ann Neurol. 1995a;37(2):181–8. doi: 10.1002/ana.410370208. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Afferent input and cortical organisation: a study with magnetic stimulation. Exp Brain Res. 1999;126(4):536–44. doi: 10.1007/s002210050762. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, et al. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995b;487(Pt 2):541–8. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Pesenti A, et al. Focal reduction of intracortical inhibition in the motor cortex by selective proprioceptive stimulation. Exp Brain Res. 2003;149(1):9–16. doi: 10.1007/s00221-002-1330-3. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Rothwell JC. Differential effect of muscle vibration on intracortical inhibitory circuits in humans. J Physiol. 2003;551(Pt 2):649–60. doi: 10.1113/jphysiol.2003.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Desiato MT, et al. Age-related changes of motor evoked potentials in healthy humans: non-invasive evaluation of central and peripheral motor tracts excitability and conductivity. Brain Res. 1992;593(1):14–9. doi: 10.1016/0006-8993(92)91256-e. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14(7):721–30. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Sale MV, Semmler JG. Age-related differences in corticospinal control during functional isometric contractions in left and right hands. J Appl Physiol. 2005;99(4):1483–93. doi: 10.1152/japplphysiol.00371.2005. [DOI] [PubMed] [Google Scholar]
- Scaglioni G, Narici MV, et al. Effect of ageing on the electrical and mechanical properties of human soleus motor units activated by the H reflex and M wave. J Physiol. 2003;548(Pt 2):649–61. doi: 10.1113/jphysiol.2002.032763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmler JG, Steege JW, et al. Motor-unit synchronization is not responsible for larger motor-unit forces in old adults. J Neurophysiol. 2000;84(1):358–66. doi: 10.1152/jn.2000.84.1.358. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21(1):1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AE, Ridding MC, et al. Age-related changes in short-latency motor cortex inhibition. Exp Brain Res. 2009 doi: 10.1007/s00221-009-1945-8. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Impaired modulation of intracortical inhibition in focal hand dystonia. Cereb Cortex. 2004;14(5):555–61. doi: 10.1093/cercor/bhh017. [DOI] [PubMed] [Google Scholar]
- Talelli P, Waddingham W, et al. The effect of age on task-related modulation of interhemispheric balance. Exp Brain Res. 2008;186(1):59–66. doi: 10.1007/s00221-007-1205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggs WJ, Cros D, et al. Cortical and spinal motor excitability during the transcranial magnetic stimulation silent period in humans. Brain Res. 1993;628(1–2):39–48. doi: 10.1016/0006-8993(93)90935-g. [DOI] [PubMed] [Google Scholar]
- Vallbo AB. Muscle spindle response at the onset of isometric voluntary contractions in man. Time difference between fusimotor and skeletomotor effects. J Physiol. 1971;218(2):405–31. doi: 10.1113/jphysiol.1971.sp009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T, Eden U, et al. Transcranial magnetic stimulation and brain atrophy: a computer-based human brain model study. Exp Brain Res. 2008;186(4):539–50. doi: 10.1007/s00221-007-1258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U. Pharmacology of TMS. Suppl Clin Neurophysiol. 2003;56:226–31. [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115(8):1717–29. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, et al. Inhibition of human motor cortex by ethanol. A transcranial magnetic stimulation study. Brain. 1995;118(Pt 6):1437–46. doi: 10.1093/brain/118.6.1437. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, et al. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996;109(1):127–35. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]