Abstract
The presence and activity of dendritic cells (DC) in retina is controversial, as these cells are difficult to identify in retina due to limited markers and sparse numbers. Transgenic mice that express green fluorescent protein (GFP) on the CD11c promoter to label DC allowed the visualization and quantification of retinal DC. Two retina injury models, the optic nerve crush (ONC) and light injury, were used to study their injury response. Many GFP+ DC were tightly associated with retinal ganglion cell nerve fibers following ONC, while very few microglia (GFP−CD11b+ cells) were found in close contact. The GFP+ cells were greatly elevated in the outer plexiform layer following photic injury. All of the GFP+ DC were CD11b+, suggesting a myeloid origin. In addition, the GFP+ DC upregulated expression of MHC class II after injury, while the GFP−CD11b+ microglia did not. This study shows that DC were found in the retina and that they rapidly responded to neural injuries. We propose that they are a previously overlooked population, distinct from microglia, and may be important in the injury response.
Keywords: retina, retinal ganglion cell, microglia, mice, neuronal death, injury
Introduction
Several types of bone marrow (BM)-derived CD45+ cells participate in immunity and inflammation in CNS, including perivascular cells (PVC), microglia (MG), and dendritic cells (DC) (Dick, 1999; Gregerson et al., 2004; Hickey and Kimura, 1988; Xu et al., 2007a). MG have been the focus of studies to understand the local response to neural injury. In addition to their well-known ability to scavenge dead or dying neurons (Streit et al., 2004), they have been reported to promote survival of injured neurons (Sanders and Jones, 2006).
There is significant controversy as to whether DC exist in quiescent retina and function as antigen presenting cells (APC). Part of the difficulty is that normal retina has very few DC, and there are few markers for elucidation of their phenotype, function and origin. Evidence for DC in the inflamed retina has been found using the experimental autoimmune uveoretinitis (EAU) model for retinal autoimmune disease in which CD11c+ cells were recruited to retina by the inflammation during pathogenesis of the disease (Jiang et al., 1999). Of several markers associated with murine DC, CD11c is most frequently used. However, antibodies to murine CD11c are difficult to use in immunohistochemistry. Using flow cytometry and antibodies 33D1 (Brasel et al., 2000) and CD205, a small number of MHC class II+ putative DC were identified in retina (Gregerson and Yang, 2003; Xu et al., 2007a). The relationship between MG and DC is uncertain, and it is not entirely clear whether retinal DC or other cells with APC ability are derived from MG, recruited from the circulation (Gregerson and Kawashima, 2004), or derived from local progenitor cells.
To visualize and study the responses of retinal DC, we took advantage of a transgenic mouse line (CD11c-DTR) where CD11c+ DC express both the diphtheria toxin receptor (DTR) and green fluorescent protein (GFP) under the CD11c promoter (Jung et al., 2002). This mouse model allows identification of DC by their expression of GFP and provides a method for depletion of the DC by treating the mice with diphtheria toxin (DTx). The response of retinal DC to neural injury was examined by analysis for changes in the number and localization of DC following two distinct retinal injuries. First, DC changes occurring after a unilateral optic nerve crush (ONC) were examined bilaterally in the retina. The axonal damage induced by ONC leads to retinal ganglion cell (RGC) death, and triggers a retinal MG response (Bodeutsch et al., 1999; Panagis et al., 2005; Sautter and Sabel, 1993; Yoles and Schwartz, 1998). Second, the effect of light-induced retinal damage on DC was examined. Constant light is a well-known model of injury to the retinal photoreceptor cells (LaVail et al., 1987). Both injury models resulted in increased numbers of retinal GFP+ DC in areas of the retina associated with the injured cells. Examination of the retinas whose RGC were injured by the ONC revealed that far more GFP+ DC than GFP− MG were tightly associated with the damaged axons. A smaller, but significant GFP+ DC response was also found in the retina of the unmanipulated contralateral eye.
Materials and Methods
Mice
All mice were on the B6 background. CD11c-DTR transgenic mice express a chimeric protein comprised of GFP and the DTR using the CD11c promoter (Jung et al., 2002). Mice were handled in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and University of Minnesota IACUC guidelines.
Immunostaining of retinal whole mounts
Mice were euthanized by CO2 inhalation, and perfused with 12 mL of 2 U/mL heparin in Dulbecco’s Phosphate-Buffered Saline (DPBS) to remove circulating CD45+ cells in the vasculature. The retinas were removed as described by Winkler (Winkler, 1981), and fixed in 4% paraformaldehyde for 12 min. Tissues were washed 3 times with DPBS for 5 min each. After washing, the retinas were blocked in 10% normal donkey serum (Jackson Immuno Research) with 0.1% Triton X-100 for 1 h at RT. Tissues were incubated overnight at 4 °C with primary antibody (CD45, BD Pharmingen; CD11b, M1/70, BD Pharmingen; rabbit anti-GFP, Invitrogen; β3-tubulin, TU-20, Abcam; MHC-II, M5/114, eBioscience). Retinas were washed again 3 times with DPBS for 5 min each. Secondary antibody (biotinylated anti-rabbit IgG, Vector; biotinylated anti-rat IgG, Vector; AF 488/Streptavidin, Invitrogen; AF 594 anti-rat IgG, Invitrogen; AF 405/Streptavidin, Invitrogen; AF 488 anti-mouse IgG, Invitrogen) was added and the tissues were incubated for 3 h at room temperature. In some experiments, fluorescent-conjugated isolectin B4 (Invitrogen) was included with the secondary antibodies to stain for vascular endothelial cells. Once more, the tissues were washed 3 times with DPBS and flat mounted, vitreous side up, onto slides. All immunostained retinal flatmounts were examined using a confocal scanning laser microscope (Olympus FluoView 1000, IX81 Inverted Confocal, Japan). Z-stacks of confocal images were reconstructed and analyzed using ImageJ software (National Institutes of Health, USA).
ONC injury
An ONC was done following anesthesia with ketamine/xylazine (87 mg/Kg : 13 mg/Kg) given IM. 0.5% proparacaine drops were applied topically. A lateral canthotomy was made to access the posterior pole. The conjunctiva was incised laterally and peeled back to the posterior segment. The optic nerve was exposed by gentle blunt-dissection of surrounding muscle bundles with watchmaker forceps. The nerve was partially clamped for 4 sec, 2 mm from the posterior pole of the globe with self-closing forceps (Li et al., 1999) to obtain a consistent injury. Sham-operated controls were done similarly, except that the forceps were not allowed to close on the nerve.
Light injury
The mice were exposed to constant light (3.6 × 103 lux) for four days. Some shade was available under their food and water supply, or when the mice were huddled together. The pupils were not dilated and the lids were not retracted. Light intensity was measured using a Sekonic Auto-Lumi L-158 light meter. After light exposure, the mice recovered in normal cyclic light for 3 days. The retinas were harvested, and stained for GFP+ cells and blood vessels.
Flow cytometry
Mice were euthanized, perfused, and retinas removed as described above. The retinas were dissociated using Liberase/Blendzyme3 (Roche) and DNAse. The dissociated retinas were washed with DPBS and incubated for 10 min at room temperature in 100 µL FACS buffer (DPBS with 2% FBS and 0.02% sodium azide) containing 0.5 µg anti-mouse CD16/CD32 (Fc block). 0.5 to 4 µL of the appropriate fluorescent-labeled antibodies (eBioscience and BD) were added and incubated for 20 min on ice. Cells were washed, resuspended in FACS buffer and collected using a FACSCalibur and CellQuest software (BD Biosciences) and analyzed using CellQuest or FlowJo (TreeStar, Ashland, OR) software.
Intraocular DTx treatment
Anterior chamber inoculation was done by a transcorneal deposition into the anterior chamber (AC) of the eye. Mice were anesthetized with ketamine (87 mg/kg) and xylazine (13 mg/kg) given IM, followed by 0.5% proparacaine eye drops. The pupil was dilated with 1 % tropicamide eye drops. The tip of a 30 gauge needle was used at a shallow angle to put a slit into the cornea near the limbal-scleral junction. Approximately 1 µL of aqueous humor was allowed to escape. A 33 gauge blunt canula attached to a 10 µL Hamilton syringe was used to slowly pass 1 – 1.5 µL of saline or DTx (5 ng) into the AC.
Results
Morphology and distribution of GFP+ cells in retina
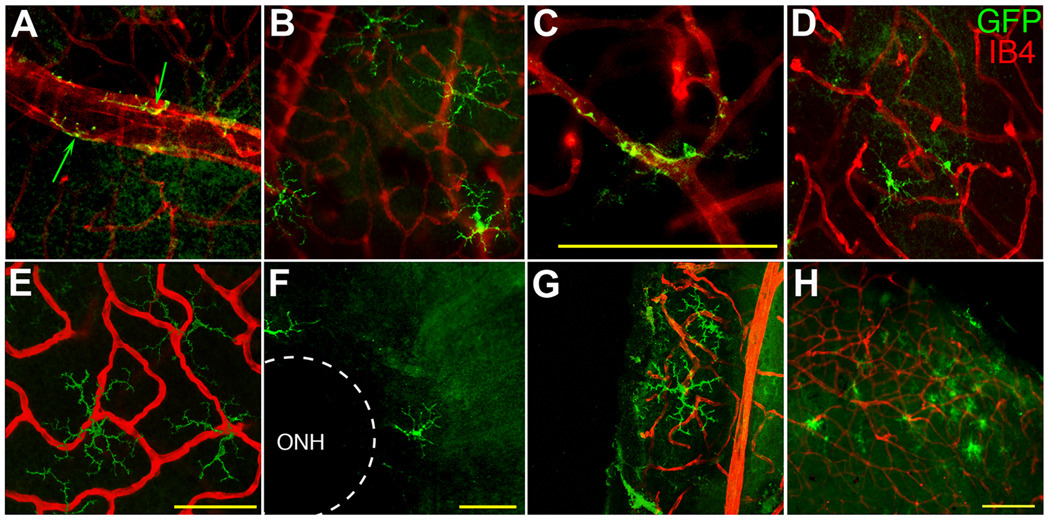
Since a small number of putative DC have been reported in quiescent retina by immunofluorescence (IF) (Xu et al., 2007b) and by flow cytometry (Gregerson and Yang, 2003), we examined the retina by IF for these cells in the CD11c-DTR mouse, in which the DC express GFP. Although the GFP does not give robust autofluorescence for microscopy, anti-GFP antibody staining detected the cells in retinal whole mounts with high sensitivity. To facilitate examination of the distribution, morphology and number of the GFP+ cells, retinal wholemounts were double stained with anti-GFP and isolectin B4, which labels blood vessels, and observed by confocal microscopy (Fig. 1). The GFP+ cells had two distinct morphologies. Perivascular GFP+ cells were found in the ganglion cell/nerve fiber layer (GCL/NFL), where they frequently exhibited a close association with large blood vessels (Fig. 1A). Highly ramified GFP+ cells, without evidence of vascular association, were found among the extensively branched smaller blood vessels of the GCL (Fig. 1B). A few GFP+ cells were associated with branch points in intermediate-sized vessels (Fig. 1C). Few GFP+ cells were found in the inner plexiform layer (IPL) (Fig. 1D). None were found in the inner nuclear layer (INL). Together, the inner layers of the retina contained 38 ± 17 GFP+ cells/retina. A similar number (57 ± 25) of ramified cells was found in the outer plexiform layer (OPL) (Fig. 1E), yielding a total of 95 ± 42 GFP+ cells/quiescent retina (N = 14). A side view of Fig. 1E revealed that the ramified GFP+ cells in the OPL were remarkably two-dimensional (supplemental Fig. S1). The lateral distribution was highly variable; GFP+ cells were found in the peripappillary region (Fig. 1F), peripheral retina (Fig. 1B), and the far periphery (Fig. 1G). Small clusters were occasionally found in the periphery (Fig. 1H). This distribution differs from that of MG, which are well-known to be concentrated in the GCL/IPL, with a much smaller number in the OPL (Garcia-Valenzuela et al., 2005; Kezic et al., 2008; Santos et al., 2010). These results were replicated in the CD11c-DTR mice following staining for CD11b, a widely used marker for MG (supplemental Fig. S2). The lateral distribution of the CD11b+ cells was uniform. The number of GFP+ cells was a small fraction (approximately 2 %) of the number of MG per retina (4291 ± 545 CD11b+ cells; N = 8) found in normal, quiescent retinas.
Fig. 1.
Distribution of GFP+ cells in quiescent retina from CD11c-DTR mice. GFP+ cells (green) were detected with AF488-labeled anti-GFP; blood vessels (red) were stained with AF594-labeled isolectin B4 (IB4). GFP+ cells in the GCL were found to exhibit either a highly elongated PVC morphology with large blood vessels (A, arrows), or a highly ramified morphology (B). A few GFP+ cells in the GCL were associated with branch points in smaller blood vessels (C). GFP+ cells in the IPL (D) and OPL (E) were also highly ramified. Examination of the lateral distribution of the GFP+ cells showed cells that were close to the optic nerve head (ONH) (F) or in the far periphery (G). Occasional clusters of GFP+ cells were found in the same focal plane as the extensively branched network of blood vessels near the surface of the retina (H). Scale bars, 50 µm (A – G), or 100 µm (H).
Characterization of retinal GFP+ cells
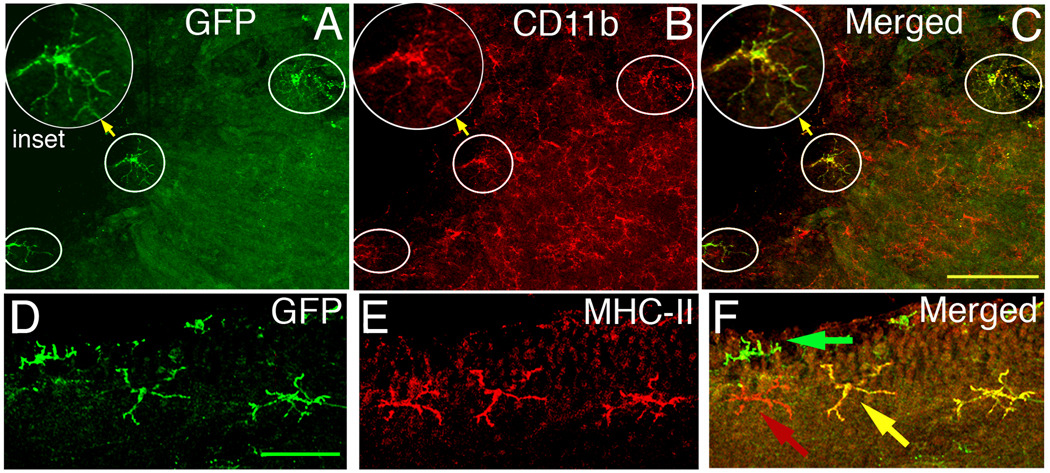
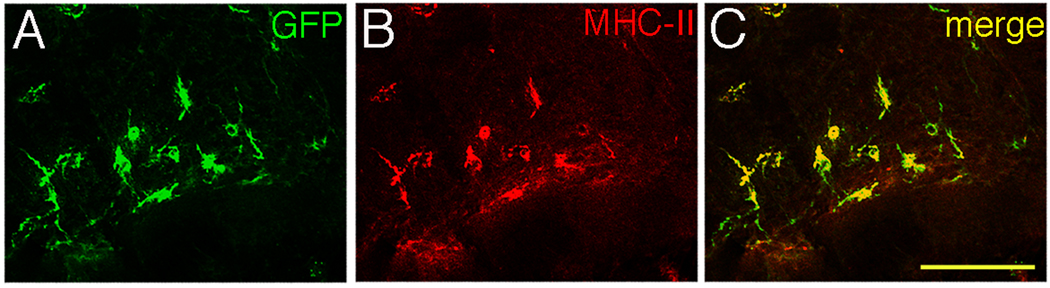
Dual staining of retinal wholemounts for CD11b and GFP revealed that all GFP+ cells were also CD11b+ (Fig. 2A–C, circled cells), raising the possibility that this subset of DC has been previously misidentified as CD45+CD11b+ MG. Immunofluorescent staining of retinal wholemounts showed a small number of clearly MHC class II+GFP+ cells in a ramified morphology (Fig. 2D–F). The GFP+ cells had a much higher frequency of class II expression than did the GFP− MG (Table 1). The results in Fig. 2D–F are not representative of the proportions of cells that stain for MHC class II and GFP, but demonstrate the reliability of the IF protocol, and its capacity for detecting double positive cells.
Fig. 2.
Retinal GFP+ cells also express CD11b+ and MHC class II. (Top) Area of CD11c-DTR retina near the ONH stained with anti-GFP antibody (A) and CD11b (B). Circled cells are the GFP+ cells from panel A. (C) Merge of A and B showing that all GFP+ cells expressed CD11b, but most CD11b+ cells were GFP− (Bottom) Double staining for GFP+ and MHC class II+ cells in the naïve CD11c-DTR retina (D–F). Scale bars, 100 µm (A–C), or 50 µm (D–F).
Table 1.
Upregulation of MHC Class II on the GFP+ DC by an ONC.
| Dendritic Cells | Microglia | ||
|---|---|---|---|
| Retina | GFP+MHC-II+ | GFP+MHC-II− | GFP−MHC-II+ |
| Naïve | 4.4% | 95.5% | 0.2% |
| ONC injureda | 49.6% | 50.4% | <0.1% |
Seven days post-ONC.
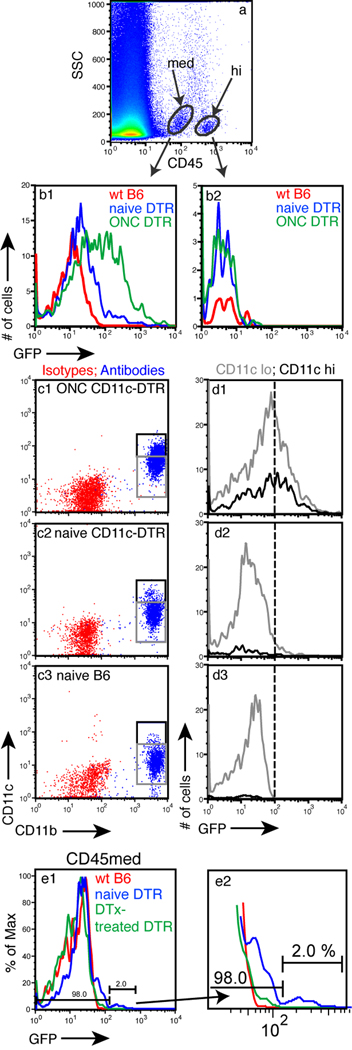
Further characterization of GFP+ cells in the CD11c-DTR retina was done by FACS. The difficulty of staining even lightly-fixed tissue with the available antibodies for CD11c or CD45 were resolved in flow cytometry. Unlike lymphoid tissue, retinal tissue contained two distinct CD45+ populations, one with high expression of CD45 (CD45hi) and one with medium expression (CD45med) (Fig 3A). The GFP+ cells were concentrated in the CD45med population; the CD45hi population was GFP− (Fig. 3B1 vs 3B2). Analysis of the CD11c+ cells in the CD45med population showed that all CD11c+ cells in normal CD11c-DTR retina were also CD11b+ (Fig. 3C2), confirming our previous IF results (Fig. 2). When CD11c+CD11b+ cells were analyzed for GFP expression, the GFP+ cells were found to be a subpopulation of the CD11c+CD11b+ cells compared to wt B6 mice controls (Fig. 3D2 vs 3D3). Although GFP expression was dependent on CD11c, the level of GFP expression in the retinal cells did not correlate with the level of CD11c expression (Fig. 3D). This result prompted analysis to confirm the fidelity of the GFP expression on other populations of CD11c+ cells. CD11c-DTR mouse lymphoid tissue was examined by flow cytometry and showed that GFP expression was accurately represented in the DC populations, and that the expression levels of CD11c and GFP were well-correlated (supplemental Fig. S3).
Fig. 3.
The retinal CD45med population contains the GFP+CD11c+ cells. (A) FACS analysis of retinal cell suspensions showing distinct CD45+ populations. (B1 and B2) GFP expression in the CD45med and CD45hi populations from retinas of CD11c-DTR mice (blue), control B6 mice (red), and CD11c-DTR mice 7 d following an ONC (green). (C) FACS analysis of the CD45med population for CD11b and CD11c from the indicated mice. (D) Analysis of GFP levels in the CD11b+CD11clo (gray box) and CD11b+CD11chi (black box) populations. (E1) Injection of DTx into the eye depletes the GFP+ population. (E2) Enlargement of profile showing detail of the DTx-depleted and control populations.
To further confirm that the GFP+ cells seen by flow cytometry were also DTx sensitive, and represented the GFP+ cells seen by IF, retinas from CD11c-DTR mice were collected 36 h after intraocular injection of 5 ng DTx. Flow cytometry showed that the GFP+ cells, which were approximately 2 % of the total CD45+ cells, were lost after DTx treatment (Fig. 3E).
Injury response of the retinal DC
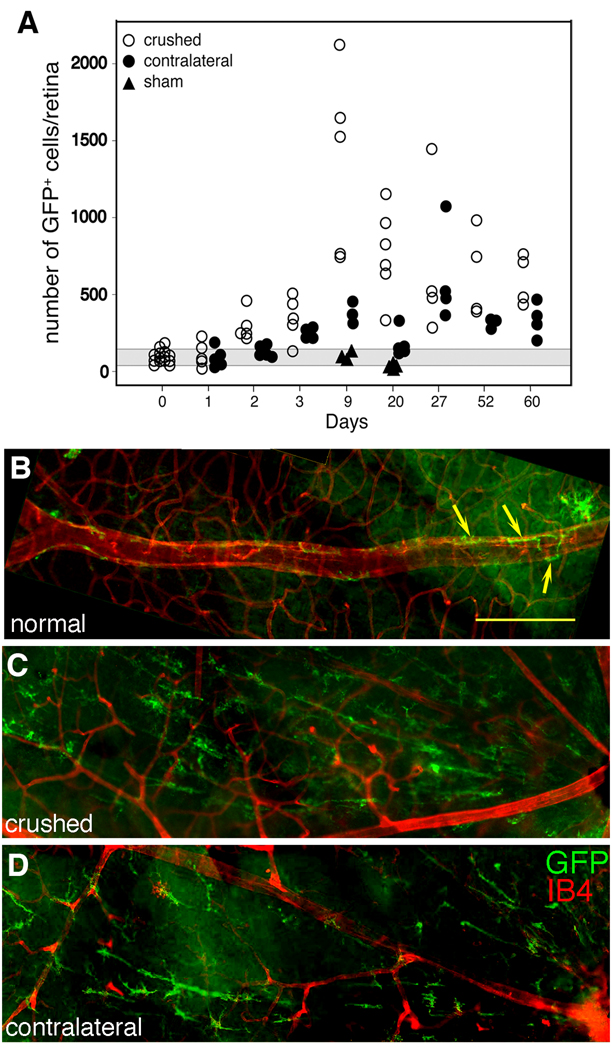
To determine if the retinal GFP+ DC population responded to neural injury, an ONC was performed on CD11c-DTR mice to induce apoptosis in RGC. Following ONC, GFP levels increased in the CD11c+CD11b+ cells from the CD45med population (Fig. 3B1, D). IF analysis showed a rapid and significant increase in GFP+ cell numbers (Fig. 4A), with a 10-fold increase at day 9 in ipsilateral retinas of ONC-injured mice. Significantly increased numbers of DC (p < 0.001) were seen as early as day 2 post-ONC in the ipsilateral retina, and elevated numbers persisted at least 60 days. The number of GFP+ cells in the contralateral retina increased with a lag of 1 – 2 days compared with the ONC-injured retina (Fig. 4A). Although not reaching the numbers found in the injured retina, the increase in the contralateral retina was still significantly above the numbers in control retina (p < 0.001, day 3). Sham-operated controls were normal in number, distribution, and morphology of CD11c+ cells on days 9 and 20 post-ONC (Fig. 4A).
Fig. 4.
Redistribution and morphological changes in retinal CD11c+ cells after an ONC of CD11c-DTR mice. (A) Numbers of GFP+ cells in retinas in the crushed (ipsilateral) and uncrushed (contralateral) retinas of ONC mice and in sham-operated control mice. The gray area represents the mean ± 1 SD of the number of GFP+ cells in unmanipulated control mice. (B) Normal retina. Arrows point to GFP+ cells tightly aligned on the large blood vessel (IB4, red). A single ramified GFP+ cell is visible (upper right). (C) Ipsilateral retina 24 d post-ONC showing elongated, radial orientation of the GFP+ cells. (D) The contralateral retina showing a similar radial arrangement of GFP+ cells, but to a lesser degree. The optic nerve head is on the right in all panels. Scale bar, 100 µm.
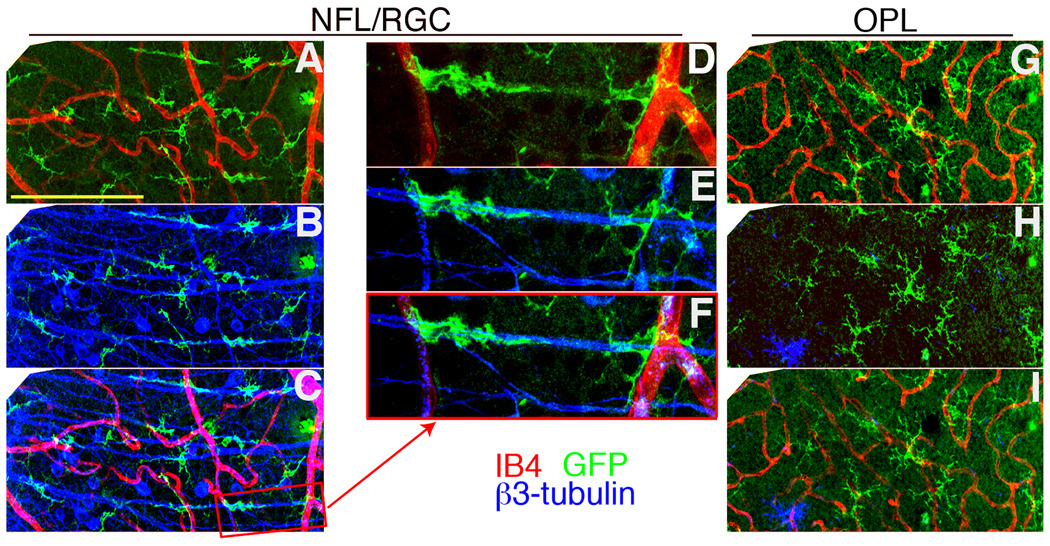
Changes in morphology of GFP+ cells were also found post-ONC. In the normal GCL, ramified and perivascular GFP+ cells were seen (Fig. 4B, yellow arrows). A large increase in GFP+ cells with an elongated morphology and a distinct radial orientation was found at 7 d post-ONC (not shown), and at 24 d post-ONC (Fig. 4C). Examination of unmanipulated contralateral eyes also revealed the presence of elongated GFP+ cells, but to a lesser degree (Fig. 4D). The source of the radial pattern of GFP+ staining was analyzed and was found to result from the association of GFP+ cells with the β3-tubulin+ axons of the RGC (Fig. 5), which have a radial pattern converging at the optic nerve head. Examination of optical sections from different layers of a retina collected 10 d post-ONC revealed that the DC were closely associated with the nerve fibers of the RGC in the injured retina (Fig. 5A–C). This relationship was illustrated by examination of an enlargement of panel C, where DC can be seen wrapped around a nerve fiber (Fig. 5D–F). There was an increase in GFP+ cell numbers in the OPL (Fig. 5G–I) but there were no changes in DC morphology, probably because RGC axons are not found there.
Fig. 5.
GFP+ cells were found tightly associated with nerve fibers after an ONC-injury to CD11c-DTR mice. (A–C) A three micron thick retinal section of the NFL/GCL 10 d post-ONC stained for (A) GFP+ cells (green) and blood vessels (IB4, red), (B) GFP+ cells and nerve fibers (β3-tubulin; blue). (C) Merged image of A and B. (D–F) A higher magnification of the boxed area in image C is shown. (G–I) Confocal stack of the OPL from the same field shown in A–C. Scale bars, 100 µm (A–C, G–I), or 50 µm (D–F).
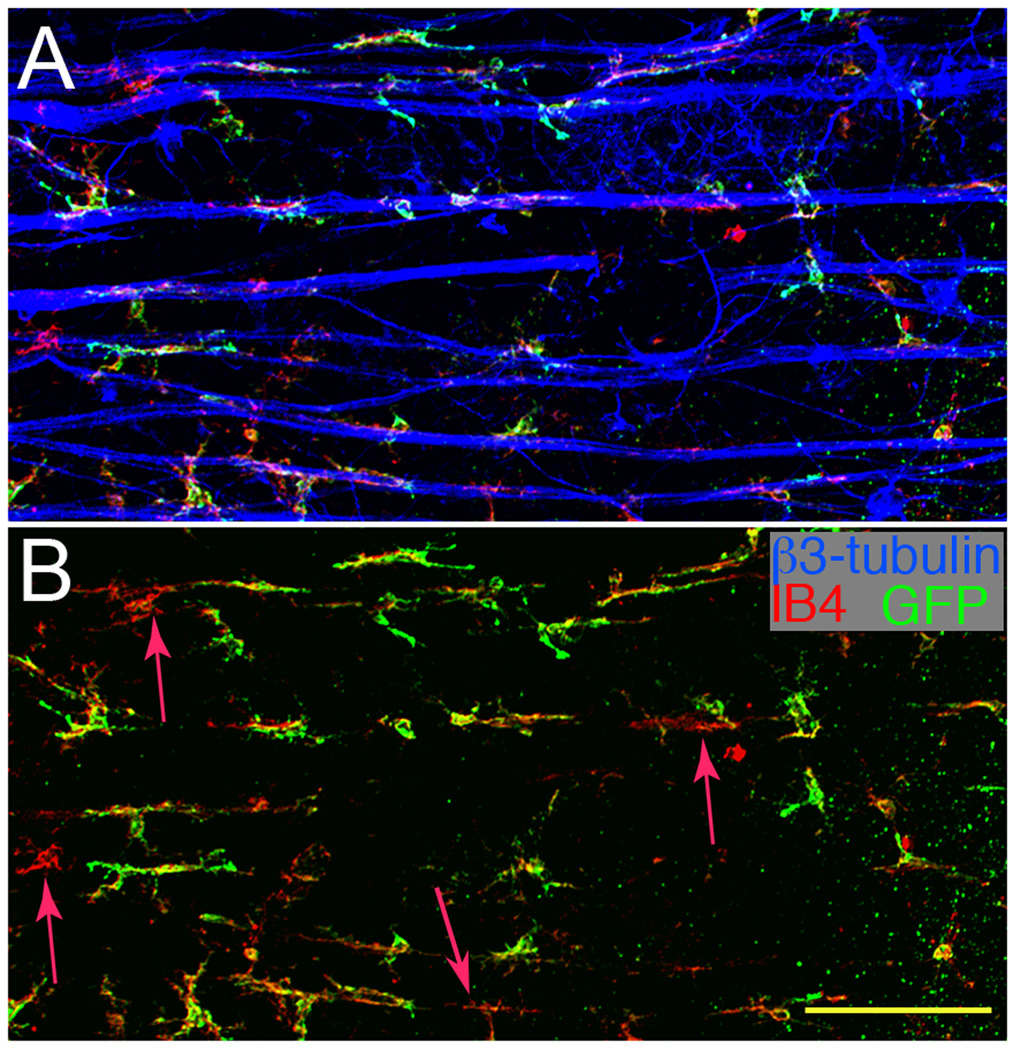
Since MG greatly outnumber the DC in retina, it was important to ask if they were also found closely associated with the RGC axons. Staining retinal wholemounts post-ONC for CD11b, GFP, and β3-tubulin (nerve fibers) showed that the majority of cells closely associated with nerve fibers were GFP+CD11b+ DC (Fig. 6A). Few CD11b+GFP−MG were found; only 4 were present in the field shown (Fig. 6B, red arrows). Overall, 92.6% of the cells in close contact with the nerve fibers (150 of 162 cells in representative fields) were GFP+ DC. The elevated number of GFP+ cells could represent the influx of a discreet lineage of cells, or result from activation of the CD11c promoter in MG.
Fig. 6.
The majority of the cells associated with nerve fibers are GFP+CD11b+. (A) A three micron thick optical section of the NFL/RGC from a CD11c-DTR mouse 7 d post-ONC stained for GFP+ cells (green), CD11b+ cells (red), and nerve fibers (blue). (B) Same field as A analyzed for GFP+CD11b+ cells showing very few GFP−CD11b+ cells. Red arrows mark the four cells near nerve fibers that were CD11b+ but GFP−. Scale bar, 100 µm.
To further confirm that the GFP+ retinal cells differed from the MG, their sensitivity to DTx was examined. A single intraocular injection of 5 ng of DTx given 7 d post-ONC rapidly killed the GFP+ cells responding in the RGC/NFL layers in the retina (supplemental Fig. S4C, D), but the underlying retina was normally populated with MG. The susceptibility of MG to multiple DTx treatments was evaluated in CD11c-DTR mice. Three injections at 2 day intervals gave a sustained reduction in the number of the GFP+ cells in the uncrushed retina, from 95 ± 42 to 10 ± 15. DTx treatment post-ONC also depleted the GFP+ cells, but did not affect the number of CD11b+ MG. The mice were given an ONC to one eye and 13 d later were depleted of their GFP+CD11c+ cells by injecting 5 ng DTx into the AC of the injured eye. At 2 day intervals, the mice received two more injections of DTx. The retinas were harvested 2 d later for counting (Table 2). These results further demonstrated that the two populations were distinct.
Table 2.
DTx treatment reduces retinal CD11c+, but not CD11b+, cell numbers.
| Naïve | ONC and DTx treated | P valuea | |
|---|---|---|---|
| CD11b+ cells | 4291 ± 545 (n = 8) | 3971 ± 627 (n = 7) | = 0.58 |
| CD11c+ cells | 95 ± 42 (n = 14) | 14 ± 14 (n = 4)b | < 0.005 |
T-test comparing cells counts of naïve vs. ONC/DTx-treated retinas.
Without DTx treatment, 597 ± 163 GFP+ cells were found.
Class II expression of DC following ONC
Since significant changes in morphology and numbers of GFP+ cells were seen after ONC, MHC class II expression was examined as a marker of DC activation. At ten days post-ONC, retinal wholemounts were immunostained for GFP and MHC class II. Comparison of retinas from untreated and ONC-treated mice showed that class II expression was dramatically upregulated on GFP+ cells, but not on the MG, by an ONC (Fig. 7; Table 1). The frequency of MHC class II+ cells in normal retina was quite low; 8 of 180 GFP+ cells in quiescent retina were class II+ (4.4%). Overall, approximately 15 cells/retina were MHC class II+. In the same retinas, only 9 MG (GFP−CD11b+) were found to stain for MHC class II, approximately 0.2% of the MG. After an ONC, 63 of 127 GFP+ cells (49.6%) were class II+, but only a very low number of MHC class II+ MG (only 1 field contained a single positive cell) was found in two retinas. Overall, an ONC injury led to the selective upregulation of MHC class II in the GFP+ DC population.
Fig. 7.
Double staining for GFP+ and MHC class II+ cells in the ONC-injured CD11c-DTR retina. (A) GFP+ cells, (B) MHC class II+ cells, (C) A and B merged. In contrast to naïve retina (Fig. 2I–K), the MHC class II+ cells are also GFP+ after an ONC. Scale bar, 100 µm.
Light injury and the retinal GFP+ DC
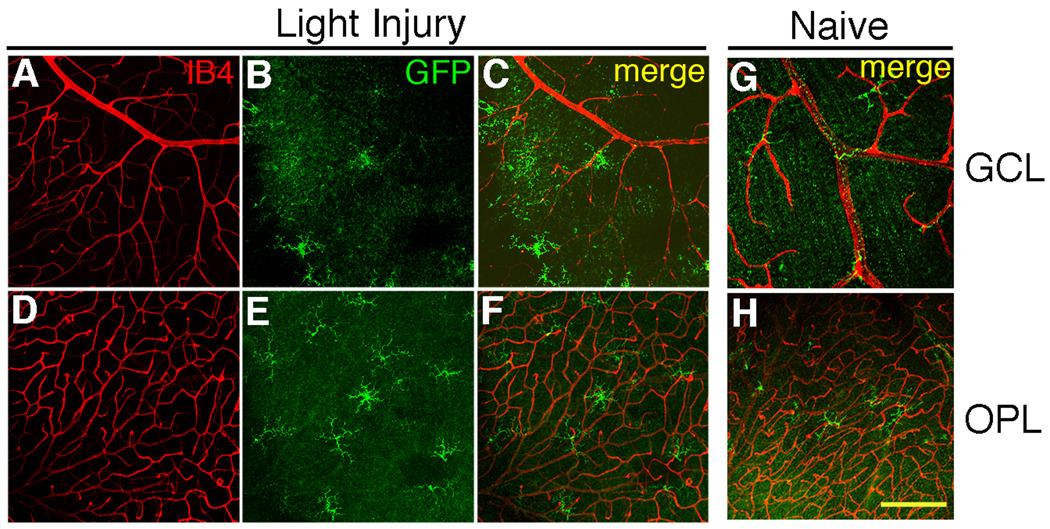
To determine if the DC response to retinal injury was broadly representative of DC activity in the CNS, light-induced retinal damage was performed. Light-induced retinal injury is a well-known model that is often used to study neural remodeling and mechanisms of resistance to light damage (LaVail et al., 1987; Mittag et al., 1999; O'Driscoll et al., 2008; Oishi et al., 2008). Unlike the ONC, which injures the axons of the RGC, light damage injures the photoreceptor cells. To determine if the GFP+ DC responded to a subtle photoreceptor cell injury, CD11c-DTR mice were exposed in their cages to 3.6 × 103 lux (overhead) continuously for 4 days followed by 3 days of normal light/dark housing conditions. This light exposure did not lead to detectable morphological damage to the retina when sampled 3 or 14 days post-light exposure (data not shown), consistent with the resistant B6 phenotype (LaVail et al., 1987). However, the GFP+ DC substantially increased in number due to the light exposure (Table 3). They were concentrated in the OPL proximal to the photoreceptors, and exhibited a ramified morphology (Fig. 8). The DC injury response appeared to reflect the location of the injured neurons.
Table 3.
Upregulation of GFP+ cells in the OPL by exposure to bright light.
| Treatment | |||
|---|---|---|---|
| GFP+ cell counts | Controla | Light-treatedb | P valuec |
| Total / retina | 95 ± 42 | 614 ± 90 | < 0.001 |
| Inner retina (GCL, IPL, INL) | 38 ± 17 | 94 ± 14 | < 0.001 |
| OPL | 57 ± 25 | 519 ± 75 | < 0.001 |
| Number of retinas | 14 | 7 | |
Conventional light/dark housing.
Four days continuous light followed by 3 days conventional light/dark cycle, then harvest.
Control vs. light-treated retinas.
Fig. 8.
Light injury causes a change in the distribution and number of GFP+ cells. (A–C) Optical sections from the GCL. (D–F) Same image field as in A–C, but the optical sections were taken from the OPL. (A, D) Blood vessels stained with IB4 (red). (B, E) GFP+ cells (green). (C, F) Merged. (G) GFP+ cells in the GCL of naive retinas. (H) OPL from the same field as G. Scale bar, 50 µm. Cell counts are shown in Table 3.
Discussion
Identifying and assigning functions to the various myeloid cell types in retina can be difficult due to similarities in cell surface phenotype and morphology, as well as difficulty in isolation and purification. As a result, the cells that perform innate immune functions in retina have been frequently described as MG. We propose that an important population of cells of the innate immune system in the retina has been overlooked. Our evidence shows that a sub-population of CD11b+ cells are DC, identified as GFP+ myeloid cells that upregulate MHC class II when stimulated by a retinal injury to CD11c-DTR mice. The GFP−CD11b+ MG did not upregulate MHC class II. These cells responded rapidly to two distinct retinal injuries: a crush injury to the axons of retinal ganglion cells, and light-induced injury to the photoreceptor cells. If conventional MG markers (CD11b, Iba-1, F4/80) had been used without attention to DC markers, these reactive cells would have been identified as MG. Consequently, it is uncertain what functions that have been ascribed to MG were instead due to the activity of this DC sub-population.
Since MG are widely regarded to be the injury-responsive population of myeloid cells in the retina and CNS, we sought strategies that would reveal the presence and response of retinal DC to injury. The ONC procedure, which produces a neural injury in which RGC degenerate via apoptosis, is thought to be a potent inducer of MG activation, and is widely used to study the MG response (Bodeutsch et al., 1999; Bodeutsch and Thanos, 2000; Macharadze et al., 2009; Panagis et al., 2005). An ONC in the CD11c-DTR mice induced a GFP+CD11b+ DC response in the NFL, where the DC were found to form intimate contacts with the axons of the injured RGC. The increase of GFP+CD11b+ cells at the area of injury was detectable by 2 days, reached a peak at 9 days post crush, and was still elevated at 60 days post crush. Interestingly, we found few cells that were GFP−CD11b+ in the NFL following the ONC. This observation suggests that this population of DC, rather than MG, contains the responders to neuronal injury. A similar population of GFP+ cells was found in the CD45med population from brain (data not shown).
Light damage of the retina is another often-used model for activation of a MG response (Harada et al., 2002; Ni et al., 2008; Santos et al., 2010; Zhang et al., 2004; Zhang et al., 2005). The results obtained from the light damage experiments in the CD11c-DTR mice confirm in a different model that GFP+CD11b+ DC respond to neuronal injury. Continuous exposure of the mice to bright light led to increased numbers of GFP+CD11b+ cells in the OPL, near the photoreceptor cells that were injured by the light treatment. Retinal MG have been reported to respond rapidly to retinal laser injury by a mechanism that is enhanced by CX3CR1, although the MG population of the quiescent retina was not affected by CX3CR1-deficiency (Lee et al., 2008; Liang et al., 2009). Using the CX3CR1+GFP+ transgenic mice, the GFP+ cells were shown to have a highly dynamic morphology, and to migrate rapidly toward the site of injury (Lee et al., 2008). Whether or not these cells include the CD11c-DTR+GFP+ subset of retinal cells we have identified is uncertain at this time. Myeloid DC also express GFP in the CX3CR1-GFP mice, and their expression of GFP by macrophages/MG and DC has not been reported to differ in lymphoid tissue, the gut, or the retina (del Rio et al., 2008; Kezic et al., 2008; Niess and Adler, 2010).
Mice expressing another marker of macrophages and DC, the MacGreen mice (csf1R-promoter driven GFP expression), have been used to study myeloid cells in tissues. These mice produce myeloid cells in which virtually all CD11b+, or F4/80+ cells, and myeloid DC, are GFP+ (MacDonald et al., 2005), including the DC of the cornea (Chinnery et al., 2008). Since CD11b and F4/80 label MG, as well as the GFP+ cells we have found in the CD11c-DTR mice, it is unlikely that the retinal DC would be discriminated from MG by GFP expression in the MacGreen mice. In preliminary studies, we have tried to induce expression of GFP in cultures of microglia purified from CD11c-DTR retina, using GM-CSF and/or IFNγ, but no induction of GFP was found. Parallel positive controls from CD11c-depleted bone marrow from the same animals responded to the same treatment by strongly upregulating GFP expression. This result is consistent with retinal GFP+ cells having a lineage distinct from the MG.
We found that the GFP+ DC response to a unilateral ONC was expressed bilaterally, although the response in the contralateral eye was substantially less robust. Compared to the injured eye, the number of GFP+CD11b+ cells was lower in the contralateral NFL, but still significantly higher than normal retina. Most of these cells maintained their ramified morphology. A sympathetic glial response in the contralateral retina to an injury has been previously observed in rodents, most often by documenting a response by MG (CD11b+ cells) after an optic nerve axotomy or crush (Bodeutsch et al., 1999; Macharadze et al., 2009; Panagis et al., 2005)
(Bodeutsch et al., 1999; Macharadze et al., 2009; Panagis et al., 2005). Although there is evidence for retino-retinal projections in newborn/young rats (Muller and Hollander, 1988), the number of these projections maintained in adult rats is extremely small (Macharadze et al., 2009), and unlikely to account for the response in the contralateral eye. Instead, the uncrossed retinal projections reaching the ipsilateral superior colliculus appear to account for the stimulus in the contralateral retina (Macharadze et al., 2009). If the retino-retinal projections are, in fact, too few to provide the contralateral injury stimulus, the stimulus for the GFP+ DC is likely to result from signals sent via uninjured neurons through the superior colliculus to the contralateral retina, and received by the GFP+ DC. The sympathetic nature of the DC response; i.e., an injury to one retina elicits a DC response in the contralateral retina, suggests that the initial signals that lead to recruitment of DC can be transmitted to remote sites by neurons, and cautions against customary use of the opposite eye as a control in studies of retinal injury and physiology.
The misidentification of DC in the retinal injury response may contribute to confusion in studies of MG turnover. For example, in a recent report, Kaneko found very little repopulation of retinal MG by 12 months post-grafting in quiescent retina (Kaneko et al., 2008), consistent with previous literature. It was found that potent injuries transiently increased the number of donor-derived cells in the retina, which subsequently declined to low levels during convalescence. Despite their acquisition of MG-like properties, the cells recruited by the injury appeared to maintain a distinct lineage. While there was an injury-promoted influx of MG-like cells of BM graft origin, their numbers decreased relative to host MG as the injury waned. Using a model similarly based on tracking progeny of labeled BM grafts and quantitation, Xu et al found a rapid turnover of MG that was substantial by 12 – 14 weeks, and nearly complete by 6 months post-BM grafting in normal retina (Xu et al., 2007a). These retinas exhibited a MG response to optic nerve transection in the ipsilateral retina; the contralateral retina was not reported. There was evidence for proliferation, but identification of the cells was unclear. Thus, these recent studies of the cells of innate immunity in the retina have reached widely disparate conclusions about their origins, turnover, and relationships with circulating precursors, whether in quiescent or injured retina.
The GFP+ cells of retina and brain differed from those in peripheral lymphoid tissue, which showed a direct relationship between the level of CD11c and GFP expression. Further, the GFP+ cells in lymphoid tissue were CD45hi, unlike those from retina and brain, which were CD45med. While future studies are needed to dissect out the specific roles of the DC identified in the retina by GFP expression, these studies demonstrate that MG may not be the primary responders of the innate immune response to retinal injury. This is supported by the presence of activated DC around the nerve fibers after ONC, and their presence in the OPL after light-induced injury.
Supplementary Material
Acknowledgements
The authors thank Thien Sam and Katie Pierson for technical assistance and the Biomedical Image Processing Laboratory at the University of Minnesota for their help with confocal microscopy. We thank Drs. Walter Low, Phil Peterson, and Maxim Cheeran for critiques of the manuscript. This work was supported by the US National Institutes of Health (R01-EY011542, R01-EY016376 to D.S.G., T32-EY07133-17 to U.L., and P30-EY011374), Research to Prevent Blindness, Inc, and the Minnesota Lions Clubs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bodeutsch N, et al. Unilateral injury to the adult rat optic nerve causes multiple cellular responses in the contralateral site. J Neurobiol. 1999;38:116–128. doi: 10.1002/(sici)1097-4695(199901)38:1<116::aid-neu9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Bodeutsch N, Thanos S. Migration of phagocytotic cells and development of the murine intraretinal microglial network: an in vivo study using fluorescent dyes. Glia. 2000;32:91–101. doi: 10.1002/1098-1136(200010)32:1<91::aid-glia90>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Brasel K, et al. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 2000;96:3029–3039. [PubMed] [Google Scholar]
- Chinnery HR, et al. Cutting edge: Membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol. 2008;180:5779–5783. doi: 10.4049/jimmunol.180.9.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio ML, et al. CX3CR1+ c-kit+ bone marrow cells give rise to CD103+ and CD103-dendritic cells with distinct functional properties. J Immunol. 2008;181:6178–6188. doi: 10.4049/jimmunol.181.9.6178. [DOI] [PubMed] [Google Scholar]
- Dick AD. Immune regulation of uveoretinal inflammation. Dev Ophthalmol. 1999;30:187–202. doi: 10.1159/000060744. [DOI] [PubMed] [Google Scholar]
- Garcia-Valenzuela E, et al. Multilayered retinal microglial response to optic nerve transection in rats. Mol Vis. 2005;11:225–231. [PubMed] [Google Scholar]
- Gregerson DS, Kawashima H. APC derived from donor splenocytes support retinal autoimmune disease in allogeneic recipients. J. Leukoc. Biol. 2004;76:383–387. doi: 10.1189/jlb.0404249. [DOI] [PubMed] [Google Scholar]
- Gregerson DS, et al. The antigen-presenting activity of fresh, adult parenchymal microglia and perivascular cells from retina. J Immunol. 2004;172:6587–6597. doi: 10.4049/jimmunol.172.11.6587. [DOI] [PubMed] [Google Scholar]
- Gregerson DS, Yang J. CD45-positive cells of the retina and their responsiveness to in vivo and in vitro treatment with IFN-gamma or anti-CD40. Invest Ophthalmol Vis Sci. 2003;44:3083–3093. doi: 10.1167/iovs.02-1014. [DOI] [PubMed] [Google Scholar]
- Harada T, et al. Microglia-Muller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J Neurosci. 2002;22:9228–9236. doi: 10.1523/JNEUROSCI.22-21-09228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey W, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Jiang HR, et al. Macrophages and dendritic cells in IRBP-induced experimental autoimmune uveoretinitis in B10RIII mice. Invest. Ophthalmol. Vis. Sci. 1999;40:3177–3185. [PubMed] [Google Scholar]
- Jung S, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H, et al. Characteristics of Bone Marrow-Derived Microglia in the Normal and Injured Retina. Invest Ophthalmol Vis Sci. 2008;49:4162–4168. doi: 10.1167/iovs.08-1738. [DOI] [PubMed] [Google Scholar]
- Kezic J, et al. Retinal microglia and uveal tract dendritic cells and macrophages are not CX3CR1 dependent in their recruitment and distribution in the young mouse eye. Invest Ophthalmol Vis Sci. 2008;49:1599–1608. doi: 10.1167/iovs.07-0953. [DOI] [PubMed] [Google Scholar]
- LaVail MM, et al. Genetic regulation of light damage to photoreceptors. Invest Ophthalmol Vis Sci. 1987;28:1043–1048. [PubMed] [Google Scholar]
- Lee JE, et al. Ex vivo dynamic imaging of retinal microglia using time-lapse confocal microscopy. Invest Ophthalmol Vis Sci. 2008;49:4169–4176. doi: 10.1167/iovs.08-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. Experimental induction of retinal ganglion cell death in adult mice. Invest. Ophthalmol. Vis. Sci. 1999;40:1004–1008. [PubMed] [Google Scholar]
- Liang KJ, et al. Regulation of dynamic behavior of retinal microglia by CX3CR1 signaling. Invest Ophthalmol Vis Sci. 2009;50:4444–4451. doi: 10.1167/iovs.08-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald KP, et al. The colony-stimulating factor 1 receptor is expressed on dendritic cells during differentiation and regulates their expansion. J Immunol. 2005;175:1399–1405. doi: 10.4049/jimmunol.175.3.1399. [DOI] [PubMed] [Google Scholar]
- Macharadze T, et al. Interretinal transduction of injury signals after unilateral optic nerve crush. Neuroreport. 2009;20:301–305. doi: 10.1097/WNR.0b013e32832027e6. [DOI] [PubMed] [Google Scholar]
- Mittag TW, et al. Light-induced retinal damage in mice carrying a mutated SOD I gene. Exp Eye Res. 1999;69:677–683. doi: 10.1006/exer.1999.0748. [DOI] [PubMed] [Google Scholar]
- Muller M, Hollander H. A small population of retinal ganglion cells projecting to the retina of the other eye. An experimental study in the rat and the rabbit. Exp Brain Res. 1988;71:611–617. doi: 10.1007/BF00248754. [DOI] [PubMed] [Google Scholar]
- Ni YQ, et al. Neuroprotective effects of naloxone against light-induced photoreceptor degeneration through inhibiting retinal microglial activation. Invest Ophthalmol Vis Sci. 2008;49:2589–2598. doi: 10.1167/iovs.07-1173. [DOI] [PubMed] [Google Scholar]
- Niess JH, Adler G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J Immunol. 2010;184:2026–2037. doi: 10.4049/jimmunol.0901936. [DOI] [PubMed] [Google Scholar]
- O'Driscoll C, et al. Basic fibroblast growth factor-induced protection from light damage in the mouse retina in vivo. J Neurochem. 2008;105:524–536. doi: 10.1111/j.1471-4159.2007.05189.x. [DOI] [PubMed] [Google Scholar]
- Oishi A, et al. Granulocyte colony-stimulating factor protects retinal photoreceptor cells against light-induced damage. Invest Ophthalmol Vis Sci. 2008;49:5629–5635. doi: 10.1167/iovs.08-1711. [DOI] [PubMed] [Google Scholar]
- Panagis L, et al. Unilateral optic nerve crush induces bilateral retinal glial cell proliferation. Eur. J. Neurosci. 2005;21:2305–2309. doi: 10.1111/j.1460-9568.2005.04046.x. [DOI] [PubMed] [Google Scholar]
- Sanders VM, Jones KJ. Role of immunity in recovery from a peripheral nerve injury. J Neuroimmune Pharmacol. 2006;1:11–19. doi: 10.1007/s11481-005-9004-0. [DOI] [PubMed] [Google Scholar]
- Santos AM, et al. Microglial response to light-induced photoreceptor degeneration in the mouse retina. J Comp Neurol. 2010;518:477–492. doi: 10.1002/cne.22227. [DOI] [PubMed] [Google Scholar]
- Sautter J, Sabel BA. Recovery of brightness discrimination in adult rats despite progressive loss of retrogradely labelled retinal ganglion cells after controlled optic nerve crush. Eur J Neurosci. 1993;5:680–690. doi: 10.1111/j.1460-9568.1993.tb00533.x. [DOI] [PubMed] [Google Scholar]
- Streit WJ, et al. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation. 2004;1:14. doi: 10.1186/1742-2094-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler BS. Glycolytic and oxidative metabolism in relation to retinal function. J Gen Physiol. 1981;77:667–692. doi: 10.1085/jgp.77.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, et al. Turnover of resident retinal microglia in the normal adult mouse. Glia. 2007a;55:1189–1198. doi: 10.1002/glia.20535. [DOI] [PubMed] [Google Scholar]
- Xu H, et al. Identification of novel dendritic cell populations in normal mouse retina. Invest Ophthalmol Vis Sci. 2007b;48:1701–1710. doi: 10.1167/iovs.06-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoles E, Schwartz M. Elevation of intraocular glutamate levels in rats with partial lesion of the optic nerve. Arch Ophthalmol. 1998;116:906–910. doi: 10.1001/archopht.116.7.906. [DOI] [PubMed] [Google Scholar]
- Zhang C, et al. Neuroprotection of photoreceptors by minocycline in light-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2004;45:2753–2759. doi: 10.1167/iovs.03-1344. [DOI] [PubMed] [Google Scholar]
- Zhang C, et al. Activation of microglia and chemokines in light-induced retinal degeneration. Mol Vis. 2005;11:887–895. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.