Abstract
Bone marrow transplantation (BMT) is a potentially curative treatment for patients with leukemia and lymphoma. Tumor eradication is promoted by the anti-tumor activity of donor T cells contained in the transplant; however, donor T cells also mediate the serious side effect of graft-versus-host disease (GVHD). Separation of GVHD from graft anti-tumor activity is an important goal of research in improving transplant outcome. One approach is to take advantage of the immunomodulatory activity of regulatory NKT cells and CD4+ CD25+ Treg of host and/or donor origin. Both host and donor NKT cells and donor Treg are able to prevent GVHD in murine models. In this review, we summarize the mechanisms of NKT cell- and Treg-mediated protection against GVHD in mice while maintaining graft anti-tumor activity. In addition, we also examine the interactions between NKT cells and Treg in the context of BMT, and integrate the data from murine experimental models with the observations made in humans.
Keywords: Bone marrow transplantation, Natural killer T cells, Regulatory T cells
Introduction
Currently, bone marrow transplantation (BMT) in humans is used predominantly for the treatment of cancers of the hematopoietic and immune systems including leukemia, lymphoma, and myeloma [1–5]. BMT's major advantage over other treatment modalities is that it can be curative; however, these benefits are complicated by serious and potentially lethal side effects, such as immunodeficiency and graft-versus-host disease (GVHD). All patients require “conditioning” to prevent the host immune cells from rejecting the donor-cell transplant, and to facilitate engraftment of donor hematopoietic progenitor cells in the host BM. Previously, conditioning of patients prior to transplant involved intensive myeloablative irradiation and/or chemotherapy to kill tumor cells. In the past decade, nonmyeloablative conditioning has been increasingly used as the conditioning regimen of choice for allogeneic transplantation, especially in older individuals (>55 years old), who are the most frequently diagnosed group with leukemia and lymphoma [6–8]. In the case of nonmyeloablative conditioning, tumor killing depends on the anti-tumor activity of the immune cells in the allogeneic transplant rather than on the tumor killing capacity of the conditioning regimen [9, 10]. The reduced intensity of nonmyeloablative conditioning reduces transplant-related morbidity and mortality especially in elderly patients. In allogeneic transplantation, T cells within the graft make the predominant contribution to graft-versus-tumor (GVT) activity, especially in the large majority of patients who receive T-cell-replete grafts from HLA-matched donors [3]. In contrast, in patients given HLA-mismatched transplants (matched at only one HLA haplotype between donor and recipient), it appears that both graft T cells and NK cells play important roles in anti-tumor activity [11].
A major toxicity associated with BMT is GVHD, an attack by the allogeneic nonregulatory effector (also termed “conventional”) CD4+ and CD8+ T cells in the graft against recipient target organs including the skin, intestines, and liver [12]. Aggressive depletion of T cells in the allogeneic graft can markedly reduce the incidence of acute GVHD; however, this is not without costs. Depleting T cells from the graft has several drawbacks, including delayed immune reconstitution [13], poorer donor-cell engraftment [14], as well as reducing the GVT effect of allo-BMT [14]. Accordingly, the goals of many preclinical and clinical studies of BMT have been to keep donor T cells in the graft to maintain anti-tumor and anti-microbial activity, and facilitate stable engraftment of the donor cells, while attempting to reduce donor T-cell-mediated acute and chronic GVHD [15, 16]. After BMT, the host immune and hematopoietic cells are replaced by donor cells that have engrafted resulting in the establishment of chimerism that is either a mixture of host and donor cells (mixed chimerism) or only the donor cells (complete chimerism). Complete chimerism, i.e. eradication of all host hematolymphoid cells including tumor cells, via graft anti-tumor activity and complete donor-cell replacement of the hematopoietic and immune systems can result in a cure. Increasing the number of donor T cells in the transplant facilitates complete chimerism, and decreasing the number can result in graft rejection [14, 15]. One approach to the separation of GVHD and anti-tumor activity is to harness the regulatory properties of T cells, including that exerted by NKT cells and CD4+CD25+ Treg, to reduce GVHD while preserving graft anti-tumor activity, anti-infection protection, and engraftment facilitation [17–19]. This review summarizes the way in which both host and donor NKT cells and CD4+CD25+ Treg contribute to the separation of GVHD and GVT activity in both preclinical and clinical models of allogeneic BMT.
Donor NKT cells can prevent GVHD
NKT cells are a small subset of innate immune cells that recognize the antigen presenting molecule CD1d instead of MHC class I or II [20, 21]. The majority of NKT cells express a TCR with an invariant TCR α-chain (Vα14 in mice and Vα24 in humans) that mediates NKT-cell recognition of specific glycolipid ligands within the context of CD1d [20–23]. Both endogenous and exogenous glycolipids are recognized [21]. Type I NKT cells express the invariant TCR, and the smaller subset of type II NKT cells express a noninvariant TCR that also recognizes CD1d [22, 23]. After activation, NKT cells can produce both Th1 cytokines such as IFN-γ and Th2 cytokines such as IL-4 [21]; the balance between Th1 and Th2 cytokine secretion by NKT cells is dependent on NKT cell interactions with APC, the nature of the activating ligands, the cytokine milieu (pro-inflammatory promoting Th1 polarization; and anti-inflammatory promoting Th2 polarization). NKT-cell polarization can also be altered by a variety of immunomodulatory treatments including in vivo or ex vivo irradiation [21, 24]. Due to NKT cells’ unique capacity for rapid and early production of cytokines, polarization of NKT cells can in turn alter polarization of the cytokine profile of conventional T cells exposed to their cognate ligands [20–23], thus serving to rapidly shift a coordinated immune cascade in a pro-inflammatory or anti-inflammatory direction. NKT cells are particularly resistant to apoptosis, including irradiation-induced apoptosis via the p53/Bcl-2 pathway, due to their high levels of expression of anti-apoptotic proteins including Bcl-2 [25]. The mouse liver and the BM are the tissues that have the highest fraction of NKT cells among total T cells [20–23, 26]. NKT cells in all peripheral lymphoid tissues contain both CD4+ and CD4–CD8– subsets, and the BM contains an additional CD8+ NKT-cell subset [20–23, 26, 27].
Initially, it was thought that all T-cell subsets in the BMT contributed to GVHD; however, examination of purified subsets showed that the CD4–CD8– T cells inhibited GVHD [28]. Given that almost all CD4–CD8– T cells in the BM were NKT cells, the marrow NKT cells were studied for their ability to inhibit GVHD after allotransplantation in murine models [18]. These studies first compared the ability of sorted CD4+ and CD8+ T cells obtained from the peripheral blood versus those from BM of donor C57BL/6 (H-2b) mice to induce acute GVHD in lethally irradiated MHC-mismatched BALB/c (H-2d) hosts co-injected with stringently T-cell-depleted C57BL/6 marrow cells [18]. The peripheral blood T cells were at least 30-fold more potent than the marrow T cells on a per cell basis as judged by mortality of the hosts during a 100-day observation period. In fact, no significant mortality was induced by the sorted donor marrow T cells in the dose range tested; however, depletion of NKT cells from the sorted marrow T cells or use of sorted marrow T cells from IL-4–/– donors resulted in lethal GVHD [18]. Sorted donor IL-4–/– NKT cells that secreted high levels of IFN-γ without IL-4 (“unopposed IFN-γ”) exacerbated histopathological changes of GVHD and overall GVHD mortality when added back to donor marrow T cells depleted of NKT cells. The add-back of WT NKT cells ameliorated GVHD [18]. The experimental results showed that NKT cells potently suppress acute lethal GVHD in an IL-4-dependent manner and that the GVHD-inhibitory CD4–CD8– T-cells (“natural suppressor” cells) are NKT cells [17, 26, 29, 30]. The results also showed that CD4+ or CD8+ T cells that secrete a Th1-polarized cytokine profile (e.g. IFN-γ and IL-2, but not IL-4) induce GVHD. In contrast, conventional donor T cells that secrete a Th2-type profile (e.g. IL-4 but not IFN-γ or IL-2) are weak inducers of GVHD [31–33].
Donor Treg can prevent GVHD
Human Treg isolated from peripheral blood have been shown to suppress alloresponses in the MLR [34, 35], and several previous reports have indicated a role for murine Treg in tolerance induction to alloantigens [36]. It is therefore not surprising that the role of Treg in protection against GVHD has been studied extensively. Taylor et al. [37] showed in an allogeneic BMT model that depletion of CD25+ cells from the donor CD4+ T-cell population completely abrogated ex vivo tolerization to host alloantigens and resulted in loss of protection against GVHD. The same model was used to investigate the capacity of naturally occurring, unmanipulated, CD4+CD25+ T cells to suppress or prevent GVHD after allogeneic transplantation [38]. The C57BL/6→BALB/c transplantation model demonstrated that un-manipulated donor-type Treg induced significant protection from GVHD lethality when co-transferred with CD4+CD25– conventional effector T cells, which otherwise induce uniform lethal GVHD [38]. Over 90% of BALB/c recipients given a high dose of 4.5 × 105 sorted C57BL/6-derived CD4+CD25– T cells were rescued from lethal GVHD by co-injecting the animals with an equal number of freshly isolated CD4+CD25+ T cells from the C57BL/6 donors. Given that as few as 0.25 × 105 CD4+CD25– T cells, transplanted together with 1.5 × 106 T-cell-depleted BM cells, caused severe GVHD and death of half of the animals within 70 days, the data demonstrate the potent GVHD regulatory capacity of donor Treg after myeloablative allotransplantation [38].
GVHD after allogeneic transplantation is characterized by donor leukocyte infiltrates in the skin, gut, and liver [38, 39]. Recruitment, activation, and expansion of mature donor T cells at these sites is regarded as one of the key processes during the initiation phase of GVHD, which leads to severe tissue damage, multi-organ failure and potentially death of the transplant recipient [39]. The presence of both CD4+CD25- T cells and CD4+CD25+ donor T cells in GVHD target tissues (gut and liver) 5 days after their co-transfer into lethally irradiated hosts was observed in mice “rescued” from GVHD [38]. This indicates that CD4+CD25+ T cells co-migrate with CD4+CD25– T cells to target organs of GVHD and then restrict the local expansion and/or tissue damaging proinflammatory activity of the CD4+CD25– T cells at these sites [38]. CD4+CD25+ T cells have been shown to regulate the expansion of CD4+CD25– T cells after co-transfer into lymphocyte-deficient mice, Treg can proliferate at sites of their alloregulation without loss of their suppressive function [40, 41].
Although Treg maintain their allosuppressor function against CD4+CD25– T cells in vitro in the presence of anti-IL-10 Ab [34], in vivo studies using transfer of Treg from IL-10–/– mice together with WT effector CD4+CD25– T cells into lethally irradiated allogeneic recipients resulted in significantly reduced protection from GVHD as compared with that found after co-transfer of WT Treg [38]. This and other data [40, 41] support both a likely cell-contact-dependent mechanism of allo-suppressor activity of Treg in vitro, and the noncontact-dependent mechanisms such as the secretion of the suppressive cytokine, IL-10, in vivo.
Donor Treg can separate acute GVHD and the GVT effect
As mentioned in the Introduction, a key requirement of successful BMT is maintaining anti-tumor effects while protecting against GVHD (i.e. separating GVHD from GVT). Complete protection from lethal GVHD has been observed after transplantation of C57BL/6 Treg and CD4+CD25– effector T cells at a 1:1 ratio into lethally irradiated BALB/c animals, while lower ratios of CD4+CD25+ Treg to CD4+CD25– conventional T cells did not protect from GVHD [38]. This 1:1 ratio was also then evaluated for the impact of Treg on effector T cell-associated GVT effect while maintaining protection from GVHD. Donor CD4+CD25+ Treg induced a profound suppression of the expansion of effector T cells in secondary lymphoid organs and GVHD target tissues at this ratio, which was associated with lower serum levels of key GVHD-inducing inflammatory cytokines, such as IFN-γ and TNF-α [19]. Taken together, these findings verify that two major predictors of lethal GVHD are suppressed after co-transplantation of Treg, namely excessive donor T-cell proliferation and the development of a pro-inflammatory cytokine milieu.
In two independent BALB/c-derived tumor models (the leukemia line A20, which infiltrates the BM; and BCL1, which primarily invades liver and spleen), it has been demonstrated that donor Treg do not interfere with the GVT activity of conventional effector T cells after allotransplantation. Treg permitted the elimination of tumors resulting in long-term survival of tumor-bearing recipients [19]. In addition, Treg did not abrogate the ability of allo-stimulated CD8+ T cells to kill tumor cells in vitro [19]. While the mechanisms by which Treg allow for GVT activity and also protect against acute GVHD are not entirely clear, the experimental results indicate that Treg do not inhibit the direct cytolytic function of CD8+ T cells that mediate tumor killing [19]. In contrast, prevention of GVHD by Treg is likely to be mediated by blockade of CD8+ T-cell expansion; expansion coupled-with proinflammatory cytokine secretion appears to be more important in the development of GVHD than direct cytolysis.
In contrast, Treg block expansion of cytolytic CD8+ T cells after BMT, which is important in preventing GVHD, as expansion coupled with pro-inflammatory cytokine secretion appears to be more important in the development of GVHD than direct cytolysis.
Conditioning with TLI and anti-T-cell Ab can prevent GVHD: Host NKT-cell dependence
Although regulatory donor T cells including NKT cells and Treg can inhibit GVHD, it is also possible that host Treg that persist after conditioning for BMT can inhibit GVHD. This is more likely to be the case after nonmyeloablative conditioning regimens that do not thoroughly deplete host immune and hematopoietic cells. Given that the nonmyeloablative regimen of total lymphoid irradiation (TLI) had been reported to protect hosts from GVHD as compared with total body irradiation (TBI), several studies were performed to search for residual host T cells with regulatory properties after TLI [42].
The TLI regimen of multiple fractionated doses of irradiation targeted to the lymph nodes, spleen, and thymus was used initially in humans as a successful treatment for Hodgkin's disease [43]. Inbred mice and rats, and outbred dogs conditioned with TLI before allogeneic BMT were protected from acute GVHD even with high doses of infused donor T cells [44, 45]. BALB/c (host strain) spleen cells after TLI treatment were reported to adoptively transfer resistance to GVHD in TBI-conditioned BALB/c hosts injected with C57BL/6 donor splenic T cells [46, 47]. Furthermore, spleen cells assayed immediately after TLI were found to secrete high levels of IL-4 and low levels of IFN-γ after in vitro activation with anti-CD3 mAb compared with normal spleen cells [48, 49]. Thus, a change in the composition of T cells and their cytokine profile after TLI was theorized to contribute to protection against GVHD [47–49].
Given the potent ability of NKT cells to bias the immune response and NKT cells’ relative resistance to irradiation-induced apoptosis, the role of NKT cells in GVHD protection after TLI was investigated. Immunofluorescent staining and multicolor analysis of the spleen cells in C57BL/6 and BALB/c mice showed a progressive increase in the percentage of NKT cells as the number of TLI doses increased [47, 49]. After 17 treatments, the proportion of NKT cells rose from ~1% in unirradiated controls to ~60–70% among the residual T cells. Addition of anti-thymocyte serum (ATS) or anti-thymocyte globulin (ATG) to the irradiation regimen resulted in a further increase in the percentage of NKT cells to > 90% of all TCRαβ+ cells [47].
The marked change in the T-cell subset composition was explained by the profound depletion of non-NKT cells as compared with NKT cells due to the relative radio-resistance of NKT cells [24]. The sorted NKT cells in the spleen after TLI secreted considerably higher levels of IL-4 after in vitro activation compared with the sorted NKT cells from untreated mice. Given that the protection against GVHD was associated with the increase in the percentage of NKT cells and increased IL-4 secretion in BALB/c hosts, the severity of GVHD was determined in WT, NKT cell-deficient CD1d–/–, and IL-4–/– hosts conditioned with TLI/ATS and given allotransplants of BM and PBMC [47, 49, 50]. The survival of the WT hosts was significantly improved compared with that of CD1d–/– or IL-4–/– hosts, indicating that host CD1d-reactive NKT cells that secrete IL-4 contribute to protection against GVHD as compared with WT T cells [47, 49]. Sorted T cells from IL-4–/– mice given TLI failed to inhibit GVHD and worsened the survival of BALB/c hosts [47, 49]. Cumulatively, these experiments demonstrate the IL-4-dependent GVHD-suppressive capacity of both host and donor-type regulatory NKT cells on donor GVHD-effector T cells after allotransplantation.
Conditioning with TLI and anti-T-cell Ab can separate GVHD and the GVT effect
Further studies demonstrated that BALB/c mice given nonmyeloablative conditioning with TLI/ATS followed by BM and splenocyte transplants from C57BL/6 donors are protected not only against acute GVHD, but also against the progressive growth of the BCL1 lymphoma [51]. High doses of splenocytes (60 × 106) and BM cells (50 × 106) were infused in order to maximize donor chimerism and GVT activity. Using this regimen, almost all WT BALB/c hosts conditioned with TLI/ATS became more than 80% donor-type chimeras in all cell lineages tested (T cells, B cells, monocytes, and granulocytes) in the peripheral blood when assayed at days 28 and 100 after transplantation [51].
TLI/ATS conditioning protected BALB/c hosts from acute lethal GVHD even with a very high dose of 60 × 106 spleen cells (a source of donor effector T cells for GVHD induction) as compared with conditioning with a single dose of TBI or TBI/ATS [51]. Protection was dependent on residual host invariant NKT (iNKT) cells expressing the Vα14Jα18 invariant TCR [52], as demonstrated by Ja18–/– BALB/c hosts that lack the invariant Vα14Jα18 TCRα-chain expressing iNKT cells succumbing to acute GVHD after TLI/ATS and allogeneic BMT [51]. Graft anti-tumor activity was clearly demonstrated by the survival of hosts given TLI/ATS followed by allogeneic BMT, compared with the uniform tumor progression in WT hosts given the TLI/ATS conditioning regimen and BCL1 lymphoma without transplants. Progressive tumor growth was also seen after the transplantation of BM and spleen cells from CD8–/– or perforin–/– donors, indicating that donor peripheral CD8+ T cells expressing the perforin cytolytic molecule were required for killing and clearance of the BCL1 tumor cells [51]. These studies further demonstrated in vivo that a transplant-conditioning regimen (TLI/ATS), which enriches for host iNKT cells, does not inhibit the GVT effect of donor allo-reactive T cells against a potent hematolymphoid malignancy. Furthermore, the results indicate that iNKT cells do not suppress GVT activtiy.
Interactions between host NKT cells and donor Treg protect from GVHD
Donor CD4+CD25+ Treg are required for protection against GVHD after TLI/ATS conditioning, because all WT hosts given CD4+CD25+ T cells or total CD4+ T-cell-depleted grafts developed lethal GVHD after transplantation [53]. Addition of purified Treg to CD4–/– donor BMT into TLI/ATS-conditioned hosts restored protection against GVHD colon injury at day 6 [53]. An important influence of host NKT cells and host IL-4 on the accumulation of donor Treg in the TLI/ATS-conditioned host spleen 6 days after transplantation was observed. Donor Treg division and accumulation were markedly reduced in IL-4–/– or NKT cell-deficient Jα18–/– hosts compared with WT hosts [53]. Injection of WT BALB/c NKT cells markedly increased donor Treg division and accumulation in both Jα18–/– and IL-4–/– hosts. The ability of NKT cells to restore Treg division was dependent on their production of IL-4, because IL-4–/– NKT cells failed to significantly increase Treg expansion [53]. NKT cells from irradiated mice develop a Th2 bias via unknown direct or indirect mechanisms, with increased IL-4 secretion after activation [47–49]. In control experiments, two other key cytokines secreted by host NKT cells, IFN-γ and IL-10, were not required for either donor Treg expansion or protection against early GVHD colitis after TLI/ATS and allogeneic BMT [53]. It is of interest that expanded donor Treg in GVHD-protected transplanted WT hosts secreted significantly higher levels of IL-10 than Treg from untreated donor mice [53].
Notably, injection of BALB/c NKT cells into iNKT-deficient Jα18–/– hosts reduced the donor CD8+ T-cell accumulation in the colon only if CD4+CD25+ T cells were present in the donor cell inoculum [53]. In addition, donor Treg were able to significantly reduce the accumulation of donor CD8+ T cells in the host colon at day 6, even when conventional donor effector CD4+CD25– T cells were absent from the donor graft. Treg reduced accumulation of both CD4+ and CD8+ T cells in the colon, although the reduction was more dramatic with donor CD8+ T cells. Reduction of CD4+ and CD8+ T-cell accumulation was associated with reduced colon injury. This effect could potentially be explained by the potent IL-10-secreting capacity of the in vivo expanded Treg. The results in the GVHD model are consistent with those in the autoimmune colitis model in which Treg inhibit inflammation in an IL-10-dependent manner [54]. Cumulatively, these studies [55, 56], as well as others not detailed here due to space limitations, demonstrate that iNKT cells can induce expansion of Treg through either IL-4- or IL-2-dependent mechanism.
Clinical studies of prevention of GVHD after conditioning with TLI and anti-T-cell Ab
In the clinical realm, acute GVHD remains a major clinical problem after both myeloablative and nonmyeloablative BMT, with 20–65% of patients developing severe (≥grade II) acute GVHD in multi-center trials [57, 58]. Mortality due to severe acute GVHD accounts for approximately 50% of nonrelapse deaths.
A novel alternative approach to prevent acute GVHD in allogeneic BMT is to manipulate the immune system to take advantage of T cells with regulatory properties. As discussed, given that both iNKT cells and CD4+CD25+ Treg have been reported to inhibit acute GVHD in rodents [18, 19, 38, 47], the conditioning regimen of TLI and anti-T-cell Ab, which alters the host/donor T-cell function and balance, was adapted for the treatment of humans with hematolymphoid malignancies [8, 59].
The overall incidence of severe acute GVHD in patients given TLI/ATG was markedly lower than that reported in studies of nonmyeloablative conditioning with TBI, chemotherapeutic agents, or both [7, 57, 58, 60–66]. This reduction is especially noteworthy because 45% of patients were at high risk for severe GVHD because they received transplants from unrelated donors. In the previous studies, the incidence of acute GVHD grades II-IV was more than 50% in recipients of unrelated donor transplants [7, 58, 64–66], whereas the TLI/ATG regimen demonstrated overall incidence of acute GVHD this grades II-IV of under 5%; however, about a third of patients given TLI/ATG developed chronic GVHD, a form of GVHD that is associated with the GVT effect [67]. The majority of patients with lymphoid malignant diseases who entered the TLI/ATG study in partial remission developed complete remission after transplantation including patients who had clearing of the tumor outside the TLI radiation field [8, 59].
Post-BMT, a marked increase in the production of IL-4 by donor CD4+ T cells in the transplant recipients was observed, as compared with IL-4 production by CD4+ T cells in the normal control subjects [59]. These donor CD4+ T cells also showed a marked reduction in their proliferative response to third-party alloantigenic stimulation in the MLR [59]. Donor CD4+ T cells from patients who underwent conditioning with TBI, rather than TLI/ATG showed neither an increased production of IL-4 nor a reduced proliferative response in the MLR. TLI/ATG resulted in an alteration of host T-cell subsets such that the ratio of host iNKT to conventional T cells significantly increased at the completion of TLI/ATG administration. In particular, the reduction of non-NKT cells was 200-fold, and the reduction of iNKT cells was ninefold. TLI/ATG conditioning was well tolerated and associated with low rates of nonrelapse mortality, irrespective of whether the graft was from a related or unrelated donor [8, 59]. Immunosuppressive medications were withdrawn from the majority of patients one year after transplantation, as no such medication was required for the control of chronic GVHD.
Concluding remarks
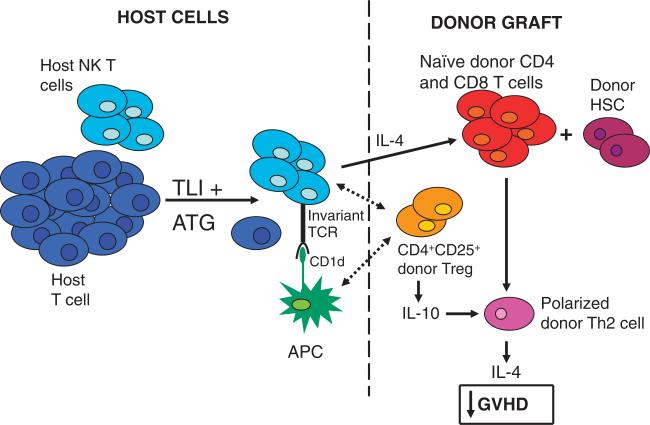
Figure 1 shows the interactions between the Treg, NKT, and conventional T cells that promote the separation of the undesirable development of GVHD from the desirable immune functions of donor T cells after conditioning of laboratory animals and humans with TLI and anti-T-cell Ab for transplantation. The conditioning regimen changes the balance of host T-cell subsets to favor NKT cells over the host naïve conventional T cells [25]; the latter cells being the major mediators of graft rejection [25]. The altered balance immediately after conditioning is mainly due to differences in resistance to apoptosis induced by irradiation, which is mediated by the p53/Bcl-2 apoptotic pathway [24, 25]. The increased expression of anti-apoptotic proteins by the regulatory as compared with the conventional naïve T-cell subsets accounts for the differences [24, 25]. The changed balance allows for the host NKT cells to prevent GVHD by interacting with the donor Treg and donor conventional T cells (Fig. 1). The irradiated host NKT cells develop a Th2 bias that results in the increased expression of IL-4, which causes the donor conventional T cells to develop a Th2 bias that has been shown to ameliorate GVHD [32, 33]. In addition, the host NKT-cell production of IL-4 promotes the expansion of donor Treg (Fig. 1) [53]. Donor Treg secrete IL-10 and suppress the GVHD capacity of conventional donor T cells by reducing the conventional T cells’ capacity to expand and accumulate in the target tissues of GVHD, including the large and small intestines, and by reducing their capacity to produce proinflammatory Th1 cytokines [53]. Treg do not prevent the donor CD8+ T cells from differentiating into cytolytic cells, which can kill tumor cells via direct lysis and maintain GVT activity [19]. The differential effects of Treg on the different functions of the conventional donor T cells result in the suppression of GVHD and the preservation of graft anti-tumor activity.
Figure 1.
Immune and hematopoietic cell interactions after BMT in mice conditioned with TLI and ATG. Conventional host CD4+ and CD8+ T cells are more profoundly depleted than host NKT cells by the conditioning regimen. The balance of T-cell subsets is changed to favor the NKT cells, which develop a Th2 bias after conditioning. After transplantation of donor hematopoietic and immune cells, the host NKT cells can be activated by interactions with CD1d on APC of host or donor origin. IL-4 produced by the host NKT cells polarizes donor conventional T cells to a Th2 pattern that ameliorates GVHD. In addition, host NKT cell production of IL-4 promotes expansion of donor Treg and polarizes them toward production of IL-10. The latter cells further suppress the ability of the donor conventional T cells to induce GVHD. Although the expansion and production of inflammatory cytokines by donor conventional T cells is suppressed, their ability to kill host tumor cells via direct cytolysis, and to promote engraftment of donor hematopoietic stem cells is maintained. Dotted lines distinguish host and donor cell contributions to these interactions.
In conclusion, both NKT cells and CD4+CD25+ Treg of host and donor origin can regulate allo-immunity after BMT. The cells interact with each other in complex ways to prevent GVHD and graft rejection that require further elucidation. Treg can thus be harnessed to suppress undesirable allo-immune responses while maintaining desirable allo-responses. The application of Treg manipulation in the context of allogeneic BMT for the treatment of human hematolymphoid cancer is an important new direction in the field. Potency of Treg after human BMT is likely to be dependent on the maintenance of Treg's ability to migrate to appropriate sites via receptors such as CD62L and CCR5, and on their ability to expand via homeostatic proliferation and alloactivation as shown in preclinical studies [68–71].
Acknowledgements
Work in the Strober laboratory was supported by NIH grants P01 CA49605 and R01 AI1037683 and by the Leukemia and Lymphoma Foundation.
Abbreviations
- ATG
anti-thymocyte globulin
- ATS
anti-thymocyte serum
- BMT
BM transplantation
- GVHD
graft-versus-host disease
- GVT
graft-versus-tumor
- iNKT
invariant NKT
- TBI
total body irradiation
- TLI
total lymphoid irradiation
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Hart DP, Peggs KS. Current status of allogeneic stem cell transplantation for treatment of hematologic malignancies. Clin. Pharmacol. Ther. 2007;82:325–329. doi: 10.1038/sj.clpt.6100283. [DOI] [PubMed] [Google Scholar]
- 2.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, Wadleigh M, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. J. Am. Med. Assoc. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, Burnett AK, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111:1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 4.Alyea E, Weller E, Schlossman R, Canning C, Mauch P, Ng A, Fisher D, et al. Outcome after autologous and allogeneic stem cell transplantation for patients with multiple myeloma: impact of graft-versus-myeloma effect. Bone Marrow Transplant. 2003;32:1145–1151. doi: 10.1038/sj.bmt.1704289. [DOI] [PubMed] [Google Scholar]
- 5.Zuckerman T, Rowe JM. Hematopoietic stem cell transplantation for adults with acute lymphoblastic leukemia. Curr. Opin. Hematol. 2009;16:453–459. doi: 10.1097/MOH.0b013e3283309a40. [DOI] [PubMed] [Google Scholar]
- 6.Maris MB, Niederwieser D, Sandmaier BM, Storer B, Stuart M, Maloney D, Petersdorf E, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 7.Mielcarek M, Martin PJ, Leisenring W, Flowers ME, Maloney DG, Sandmaier BM, Maris MB, Storb R. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 8.Kohrt HE, Turnbull BB, Heydari K, Shizuru JA, Laport GG, Miklos DB, Johnston LJ, et al. TLI and ATG conditioning with low risk of graft-versus-host disease retains antitumor reactions after allogeneic hematopoietic cell transplantation from related and unrelated donors. Blood. 2009;114:1099–1109. doi: 10.1182/blood-2009-03-211441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marks DI, Lush R, Cavenagh J, Milligan DW, Schey S, Parker A, Clark FJ, et al. The toxicity and efficacy of donor lymphocyte infusions given after reduced-intensity conditioning allogeneic stem cell transplantation. Blood. 2002;100:3108–3114. doi: 10.1182/blood-2002-02-0506. [DOI] [PubMed] [Google Scholar]
- 10.Martino R, Caballero MD, Perez-Simon JA, Canals C, Solano C, Urbano-Ispizua A, Bargay J, et al. Evidence for a graft-versus-leukemia effect after allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning in acute myelogenous leukemia and myelodysplastic syndromes. Blood. 2002;100:2243–2245. doi: 10.1182/blood-2002-02-0400. [DOI] [PubMed] [Google Scholar]
- 11.Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, Stern M, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433–440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi SW, Levine JE, Ferrara JL. Pathogenesis and management of graft-versus-host disease. Immunol. Allergy Clin. North Am. 2010;30:75–101. doi: 10.1016/j.iac.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boeckh M, Erard V, Zerr D, Englund J. Emerging viral infections after hematopoietic cell transplantation. Pediatr. Transplant. 2005;9:48–54. doi: 10.1111/j.1399-3046.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 14.Bunjes D. The current status of T-cell depleted allogeneic stem-cell transplants in adult patients with AML. Cytotherapy. 2001;3:175–188. doi: 10.1080/146532401753174007. [DOI] [PubMed] [Google Scholar]
- 15.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 16.Sprangers B, Fevery S, Van Wijmeersch B, De Somer L, Waer M, Billiau AD. Can graft-versus-leukemia reactivity be dissociated from graft-versus-host disease? Front. Biosci. 2007;12:4568–4594. doi: 10.2741/2411. [DOI] [PubMed] [Google Scholar]
- 17.Palathumpat V, Dejbakhsh-Jones S, Holm B, Wang H, Liang O, Strober S. Studies of CD4- CD8- alpha beta bone marrow T cells with suppressor activity. J. Immunol. 1992;148:373–380. [PubMed] [Google Scholar]
- 18.Zeng D, Lewis D, Dejbakhsh-Jones S, Lan F, Garcia-Ojeda M, Sibley R, Strober S. Bone marrow NK1.1(−) and NK1.1(+) T cells reciprocally regulate acute graft versus host disease. J. Exp. Med. 1999;189:1073–1081. doi: 10.1084/jem.189.7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat. Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 20.Kronenberg M, Kinjo Y. Innate-like recognition of microbes by invariant natural killer T cells. Curr. Opin. Immunol. 2009;21:391–396. doi: 10.1016/j.coi.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kronenberg M, Engel I. On the road: progress in finding the unique pathway of invariant NKT cell differentiation. Curr. Opin. Immunol. 2007;19:186–193. doi: 10.1016/j.coi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 23.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat. Rev. Immunol. 2002;2:557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 24.Yao Z, Liu Y, Jones J, Strober S. Differences in Bcl-2 expression by T-cell subsets alter their balance after in vivo irradiation to favor CD4+ Bcl-2hi NKT cells. Eur. J. Immunol. 2009;39:763–775. doi: 10.1002/eji.200838657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nador RG, Hongo D, Yao Z, Strober S. The changed balance of regulatory and naive T cells promotes tolerance after TLI and anti-T-cell antibody conditioning. Am. J. Transplant. 2009 doi: 10.1111/j.1600-6143.2009.02942.x. e-publication 20041865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng D, Hoffmann P, Lan F, Huie P, Higgins J, Strober S. Unique patterns of surface receptors, cytokine secretion, and immune functions distinguish T cells in the bone marrow from those in the periphery: impact on allogeneic bone marrow transplantation. Blood. 2002;99:1449–1457. doi: 10.1182/blood.v99.4.1449. [DOI] [PubMed] [Google Scholar]
- 27.Zeng D, Gazit G, Dejbakhsh-Jones S, Balk SP, Snapper S, Taniguchi M, Strober S. Heterogeneity of NK1.1+ T cells in the bone marrow: divergence from the thymus. J. Immunol. 1999;163:5338–5345. [PubMed] [Google Scholar]
- 28.Palathumpat V, Dejbakhsh-Jones S, Holm B, Strober S. Different subsets of T cells in the adult mouse bone marrow and spleen induce or suppress acute graft-versus-host disease. J. Immunol. 1992;149:808–817. [PubMed] [Google Scholar]
- 29.Sykes M, Hoyles KA, Romick ML, Sachs DH. In vitro and in vivo analysis of bone marrow-derived CD3+, CD4−, CD8−, NK1.1+ cell lines. Cell. Immunol. 1990;129:478–493. doi: 10.1016/0008-8749(90)90222-d. [DOI] [PubMed] [Google Scholar]
- 30.Strober S, Cheng L, Zeng D, Palathumpat R, Dejbakhsh-Jones S, Huie P, Sibley R. Double negative (CD4-CD8- alpha beta+) T cells which promote tolerance induction and regulate autoimmunity. Immunol. Rev. 1996;149:217–230. doi: 10.1111/j.1600-065x.1996.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 31.Fowler DH, Kurasawa K, Husebekk A, Cohen PA, Gress RE. Cells of Th2 cytokine phenotype prevent LPS-induced lethality during murine graft-versus-host reaction. Regulation of cytokines and CD8+ lymphoid engraftment. J. Immunol. 1994;152:1004–1013. [PubMed] [Google Scholar]
- 32.Krenger W, Snyder KM, Byon JC, Falzarano G, Ferrara JL. Polarized type 2 alloreactive CD4+ and CD8+ donor T cells fail to induce experimental acute graft-versus-host disease. J. Immunol. 1995;155:585–593. [PubMed] [Google Scholar]
- 33.Fowler DH, Breglio J, Nagel G, Eckhaus MA, Gress RE. Allospecific CD8+ Tc1 and Tc2 populations in graft-versus-leukemia effect and graft-versus-host disease. J. Immunol. 1996;157:4811–4821. [PubMed] [Google Scholar]
- 34.Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 2001;193:1295–1302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang SM, Tang Q, Bluestone JA. CD4+CD25+ regulatory T cells in transplantation: progress, challenges and prospects. Am. J. Transplant. 2007;7:1457–1463. doi: 10.1111/j.1600-6143.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 37.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J. Exp. Med. 2001;193:1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Exp. Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snider D, Liang H. Early intestinal Th1 inflammation and mucosal T cell recruitment during acute graft-versus-host reaction. J. Immunol. 2001;166:5991–5999. doi: 10.4049/jimmunol.166.10.5991. [DOI] [PubMed] [Google Scholar]
- 40.Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A, Bandeira A. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J. Immunol. 2001;166:3008–3018. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 41.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4+CD25+ suppressor T cells in vivo. Nat. Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 42.Slavin S, Strober S. Induction of allograft tolerance after total lymphoid irradiation (TLI): development of suppressor cells of the mixed leukocyte reaction (MLR). J. Immunol. 1979;123:942–946. [PubMed] [Google Scholar]
- 43.Kaplan HS, Rosenberg SA. Extended-field radical radiotherapy in advanced Hodgkin's disease: short-term results of 2 randomized clinical trials. Cancer Res. 1966;26:1268–1276. [PubMed] [Google Scholar]
- 44.Slavin S, Fuks Z, Kaplan HS, Strober S. Transplantation of allogeneic bone marrow without graft-versus-host disease using total lymphoid irradiation. J. Exp. Med. 1978;147:963–972. doi: 10.1084/jem.147.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gottlieb M, Strober S, Hoppe RT, Grumet FC, Kaplan HS. Engraftment of allogeneic bone marrow without graft-versus-host disease in mongrel dogs using total lymphoid irradiation. Transplantation. 1980;29:487–491. [PubMed] [Google Scholar]
- 46.King DP, Strober S, Kaplan HS. Suppression of the mixed leukocyte response and of graft-vs-host disease by spleen cells following total lymphoid irradiation (TLI). J. Immunol. 1981;126:1140–1145. [PubMed] [Google Scholar]
- 47.Lan F, Zeng D, Higuchi M, Huie P, Higgins JP, Strober S. Predominance of NK1.1+TCR alpha beta+ or DX5+TCR alpha beta+ T cells in mice conditioned with fractionated lymphoid irradiation protects against graft-versus-host disease: “natural suppressor” cells. J. Immunol. 2001;167:2087–2096. doi: 10.4049/jimmunol.167.4.2087. [DOI] [PubMed] [Google Scholar]
- 48.Field EH, Rouse TM. Alloantigen priming after total lymphoid irradiation alters alloimmune cytokine responses. Transplantation. 1995;60:695–702. doi: 10.1097/00007890-199510150-00014. [DOI] [PubMed] [Google Scholar]
- 49.Lan F, Zeng D, Higuchi M, Higgins JP, Strober S. Host conditioning with total lymphoid irradiation and antithymocyte globulin prevents graft-versus-host disease: The role of CD1-reactive natural killer T cells. Biol. Blood Marrow Transplant. 2003;9:355–363. doi: 10.1016/s1083-8791(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 50.Liu YP, Li Z, Nador RG, Strober S. Simultaneous protection against allograft rejection and graft-versus-host disease after total lymphoid irradiation: role of natural killer T cells. Transplantation. 2008;85:607–614. doi: 10.1097/TP.0b013e31816361ce. [DOI] [PubMed] [Google Scholar]
- 51.Pillai AB, George TI, Dutt S, Teo P, Strober S. Host NKT cells can prevent graft-versus-host disease and permit graft antitumor activity after bone marrow transplantation. J. Immunol. 2007;178:6242–6251. doi: 10.4049/jimmunol.178.10.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seino K, Taniguchi M. Functionally distinct NKT cell subsets and subtypes. J. Exp. Med. 2005;202:1623–1626. doi: 10.1084/jem.20051600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pillai AB, George TI, Dutt S, Strober S. Host natural killer T cells induce an interleukin-4-dependent expansion of donor CD4+CD25+ Foxp3+ T regulatory cells that protects against graft-versus-host disease. Blood. 2009;113:4458–4467. doi: 10.1182/blood-2008-06-165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J. Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu R, La Cava A, Bai XF, Jee Y, Price M, Campagnolo DI, Christadoss P, et al. Cooperation of invariant NKT cells and CD4+CD25+ T regulatory cells in the prevention of autoimmune myasthenia. J. Immunol. 2005;175:7898–7904. doi: 10.4049/jimmunol.175.12.7898. [DOI] [PubMed] [Google Scholar]
- 56.Jiang S, Game DS, Davies D, Lombardi G, Lechler RI. Activated CD1d-restricted natural killer T cells secrete IL-2: innate help for CD4+ CD25+ regulatory T cells? Eur. J. Immunol. 2005;35:1193–1200. doi: 10.1002/eji.200425899. [DOI] [PubMed] [Google Scholar]
- 57.Maris MB, Sandmaier BM, Storer BE, Chauncey T, Stuart MJ, Maziarz RT, Agura E, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104:3535–3542. doi: 10.1182/blood-2004-06-2275. [DOI] [PubMed] [Google Scholar]
- 58.Georges GE, Maris M, Sandmaier BM, Malone DG, Feinstein L, Niederweiser D, Shizuru JA, et al. Related and unrelated nonmyeloablative hematopoietic stem cell transplantation for malignant diseases. Int. J. Hematol. 2002;76:184–189. doi: 10.1007/BF03165242. [DOI] [PubMed] [Google Scholar]
- 59.Lowsky R, Takahashi T, Liu YP, Dejbakhsh-Jones S, Grumet FC, Shizuru JA, Laport GG, et al. Protective conditioning for acute graft-versus-host disease. N. Engl. J. Med. 2005;353:1321–1331. doi: 10.1056/NEJMoa050642. [DOI] [PubMed] [Google Scholar]
- 60.Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, Varadi G, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 61.Childs R, Clave E, Contentin N, Jayasekera D, Hensel N, Leitman S, Read EJ, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999;94:3234–3241. [PubMed] [Google Scholar]
- 62.Giralt S, Thall PF, Khouri I, Wang X, Braunschweig I, Ippolitti C, Claxton D, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97:631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 63.McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, Chauncey TR, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 64.Niederwieser D, Maris M, Shizuru JA, Petersdorf E, Hegenbart U, Sandmaier BM, Maloney DG, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–1629. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 65.Schetelig J, Kroger N, Held TK, Thiede C, Krusch A, Zabelina T, Dubiel M, et al. Allogeneic transplantation after reduced conditioning in high risk patients is complicated by a high incidence of acute and chronic graft-versus-host disease. Haematologica. 2002;87:299–305. [PubMed] [Google Scholar]
- 66.Spitzer TR, McAfee S, Sackstein R, Colby C, Toh HC, Multani P, Saidman S, et al. Intentional induction of mixed chimerism and achievement of antitumor responses after nonmyeloablative conditioning therapy and HLA-matched donor bone marrow transplantation for refractory hematologic malignancies. Biol. Blood Marrow Transplant. 2000;6:309–320. doi: 10.1016/s1083-8791(00)70056-5. [DOI] [PubMed] [Google Scholar]
- 67.Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, Hochberg EP, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nguyen VH, Zeiser R, Dasilva DL, Chang DS, Beilhack A, Contag CH, Negrin RS. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007;109:2649–2656. doi: 10.1182/blood-2006-08-044529. [DOI] [PubMed] [Google Scholar]
- 69.Ermann J, Hoffmann P, Edinger M, Dutt S, Blankenberg FG, Higgins JP, Negrin RS, et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105:2220–2226. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 70.Taylor PA, Panoskaltsis-Mortari A, Swedin JM, Lucas PJ, Gress RE, Levine BL, June CH, et al. L-selectin(hi) but not the L-selectin(lo) CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood. 2004;104:3804–3812. doi: 10.1182/blood-2004-05-1850. [DOI] [PubMed] [Google Scholar]
- 71.Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, Taylor PA, McKinnon KP, Su L, Blazar BR, Serody JS. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood. 2005;106:3300–3307. doi: 10.1182/blood-2005-04-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]