Abstract
The free-energy landscape of glycerol permeation through the aquaglyceroporin GlpF has been estimated in the literature by the nonequilibrium method of steered molecular dynamics (SMD) simulations and by the equilibrium method of adaptive biasing force (ABF) simulations. However, the ABF results qualitatively disagree with the SMD results that were based on the Jarzynski equality (JE) relating the equilibrium free-energy difference to the nonequilibrium work of the irreversible pulling experiments. In this paper, I present a new SMD study of the glycerol permeation through GlpF to explore the free-energy profile of glycerol along the permeation channel. Instead of the JE in terms of thermodynamic work, I use the fluctuation-dissipation theorem (FDT) of Brownian dynamics (BD), in terms of mechanical work, for extracting the free-energy difference from the nonequilibrium work of irreversible pulling experiments. The results of this new SMD-BD-FDT study are in agreement with the experimental data and with the ABF results.
The glycerol uptake facilitator1–3 (GlpF), found in Escherichia coli, is responsible for passive transport of water and small hydrophilic species, such as linear polyalcohols. In recent years, considerable efforts have been invested on the glycerol permeation through GlpF4–8. To study the slow diffusion of glycerol in GlpF4 with all-atom molecular dynamics (MD), steered MD (SMD) technique9 has been employed to sample permeation paths of glycerol whose center of mass is pulled through the porins of GlpF5. Work by the pulling force is recorded for each path sampled. The equilibrium free-energy profile across the conduction pathway has been estimated with the Jarzynski equality (JE)10 relating the desired free-energy difference to the measured work of irreversible pulling. More recently, the adaptive biasing force11, 12 (ABF), an equilibrium approach, has been employed to explore the free-energy landscape of glycerol through the GlpF porins7. The results of the equilibrium ABF method qualitatively disagree with the nonequilibrium SMD-JE study5. Ref. 8 gives a different equilibrium study of the transport of glycerol through GlpF. Using the umbrella sampling simulations, Ref. 8 predicts a free-energy profile with a barrier height of 3.2 kcal/mol (13.5 kJ/mol) in contrast with the prediction of 8.7 kcal/mol in Ref. 7. In light of these existing studies, in this paper, I present a new SMD study of glycerol permeation, using the fluctuation-dissipation theorem of the Brownian dynamics (BD-FDT)13 instead of the JE for the extraction of equilibrium free energy from the nonequilibrium work of irreversible pulling experiments. The results of this nonequilibrium SMD-BD-FDT study agree well with the equilibrium ABF study7 and with the experimental data4. Note that the difference between this study and the SMD-JE study of Ref. 5 is not running longer simulations but using BD-FDT instead of JE.
GlpF possesses a homotetrameric structure3: Each monomer forms an independently functional pore (channel). The core of this protein consists of two half-membrane spanning repeats related by an essentially two-fold symmetry. The N–termini of the α-helical repeats convene approximately at the center of the channel, where the Asn–Pro–Ala (NPA) motifs are located. The selectivity filter (SF), the narrowest section of the pore, is about 8 Å from the center of the channel, namely, from the central plane of the lipid bilayer. In this work, the z–axis of the Cartesian coordinates is chosen to be normal to the water-membrane interface, pointing from periplasm to cytoplasm. The origin of the coordinates is set so that z = 2.7 Å at the mid point between the N-termini of Asn 68 and Asn 203.
This work uses the all-atom model of the GlpF-membrane system studied in Refs. 5–7, consisting of GlpF homotetramer embedded in a fully hydrated palmitoyloleyl-phosphatidyl-ethanolamine (POPE) bilayer. Four glycerol molecules are placed in the vicinity of the four pores of GlpF. The glycerol-protein-lipid-bilayer complex is explicitly solvated with TIP3 waters. Soldium and Chlorine ions are added and the entire system is neutralized. The whole system consists of 106,245 atoms. All simulations of this work were performed using NAMD 2.614. The periodic boundary conditions were employed. The pressure and the temperature were maintained at 1 bar and 300 K respectively. The Langevin damping coefficient was chosen to be 5/ps. The particle-mesh Ewald method was utilized to compute electrostatic interactions. The time step of 1 fs was used for short-range interactions and 4 fs for long-range forces. Covalent bonds of hydrogens were fixed to their equilibrium length. The all-atom CHARMM27 force field15 was adopted.
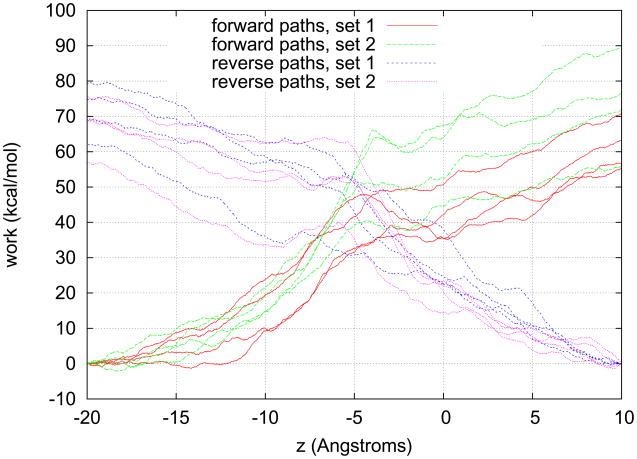
Starting from the fully equilibrated structure of Ref. 7, equilibrium Langevin dynamics was run for 4 ns while the center of mass of each glycerol was fixed. Then constant-velocity SMD simulations9 were performed to pull the four glycerols through the four channels. Two sets of pulling paths were sampled: Each set has four forward paths (from periplasm to cytoplasm) and four reverse paths (from cytoplasm to periplasm). The work along these pulling paths are plotted in Fig. 1.
Fig. 1.
Work along the paths of irreversible pulling. Four glycerol molecules are pulled through the four permeation channels: The horizontal axis is the z-coordinate of the center of mass of one given glycerol molecule being pulled. For forward paths, the vertical axis is WA→z, work done to the glycerol molecule by the pulling force when its center of mass is pulled from zA = −20Å to z. For reverse paths, the vertical axis is WB→z, work done to the glycerol molecule by the pulling force when its center of mass is pulled from zB = 10Å to z. The pulling speed is 0.05Å/ps. The spring constant k = ∞. Each curve is the average work among the four glycerols simultaneously pulled through the four channels.
The widely used JE10 relates the irreversible work to the equilibrium free energy as follows
| (1) |
counting only the forward pulling paths. The BD-FDT of Ref. 13 counts both the forward and reverse pulling paths,
| (2) |
Here, in Eqs. (1) and (2), WA→z is the work done to pull a glycerol from State A to a state in which the center of mass of the pulled glycerol is located at z. Wz→A = WB→A − WB→z is the work along the reverse pulling back to State A. In State A, the center of mass of the pulled glycerol zA = 20Å and in State B, zB= 10Å. GA is the Gibbs free energy in State A. G(z) is the free energy when the center of mass of the pulled glycerol is equal to z. The brackets stand for statistical averages over the forward or reverse paths respectively. It is noted that Eq. (2) agrees with the FR formula of Ref. 16 in the Gaussian approximation.
Note that the JE is in terms of the thermodynamic work Wt while the FDT is in terms of the mechanical work Wm. The two definitions of work are as follows:
| (3) |
| (4) |
where λ(t) is the control parameter as a function of time t. dz is the infinitesimal displacement of the pulled molecule and F is the pulling force. In the constant velocity SMD, the center of mass of the pulled molecule is attached to a spring with spring constant k.9 The pulling force
| (5) |
The control parameter
| (6) |
where z0 is the initial center-of-mass coordinate of glycerol and v is the pulling speed. The potential energy
| (7) |
Evidently, the mechanical work [Eq. (4)] is not equal to the thermodynamic work [Eq. (3)] except in the limit of infinitely stiff spring. The stiff-spring approximation was given by Park and Schulten in Ref. 9 as an expansion in powers of 1/k. In this report, the pulling experiments were performed in the k → ∞ limit by fixing the z – coordinate of the center of mass of glycerol at each time step to λ(t). In the infinitely stiff spring limit, k → ∞, the stiff-spring approximation becomes exact. The pulling force F is equal to the z – component of the total force on the pulled glycerol exerted by all other molecules of the system. The center of mass of the pulled molecule simply follows the control parameter, dz = λ̇dt and the work in Eq. (3) is equal to the work in Eq. (4). Shown in Fig. 1 are work curves along eight forward paths and eight reverse paths. The pulling speed was set to v = 0.05Å/ps. As can be seen from Fig. 1, pulling at such a speed is clearly irreversible, WA→z+Wz→A ≠ 0.
Elaborate multistage application of the JE has been performed in Ref. 5. Each channel was divided into 12 sections of varying width. The JE was applied to each section for the free-energy profile over that section. It should be noted the pulling directions were opposite for the neighboring sections. Presumably, equilibrations were done for each section when the four paths were sampled. And the JE estimates of the free energy profiles must have been dependent upon the pulling directions. In this work, the JE in Eq. (1) and the FDT in Eq. (2) are applied in the single-stage manner. Namely, a glycerol is pulled from State A to State B without intermediate equilibration, sampling one forward path. Then system is equilibrated at State B (with the glycerol’s center of mass fixed at zB) for 1 ns. From there the glycerol is pulled back to State A, sampling a reverse path. To achieve better statistics, the work was averaged among the four glycerol molecules as they are simultaneously pulled through the four channels. Fig. 2 shows the results of the free-energy estimates using the JE in Eq. (1) and the FDT in Eq. (2). Eight forward paths were used for the ensemble average in Eq. (1). Eight forward and eight reverse paths were used for the ensemble average in Eq. (2). The single-stage application of the FDT produces the free energy profile (Fig. 2(a)) in agreement with the equilibrium ABF results7: A single barrier of 9.5 kcal/mol located in the SF region, which is also in agreement with the experimental measurements4. However, the single-stage JE results (Fig. 2(b)) are far from the equilibrium ABF results and far from the experimental data.
Fig. 2.
Free-energy of glycerol permeation along the permeation channel of GlpF. (a) The FDT results. (b) The JE results: the exponential form (red) and the second moment expansion of the JE (green). State A is chosen as the reference point:GA = 0. The results of Henin et al’s ABF study was approximated from the curve in Fig. 1 of Ref. 7.
In summary, the free-energy landscape of glycerol permeation through GlpF has been determined in a new nonequilibrium SMD approach employing the Brownian dynamics FDT instead of the JE. The resultant free-energy profile agrees well with the equilibrium ABF study of Ref. 7 and with the experimental data of Ref. 4. It is reasonable to expect that the Brownian dynamics FDT of Ref. 13 is also suitable for exploring free-energy landscapes in many other nonequilibrium pulling experiments.
Acknowledgments
The author thanks R. Renthal for sharing his biological insights and C. Chipot for providing the coordinate and structure files of GlpF. He acknowledges support from an NIH SC3 grant (GM084834), the UTSA CBI, and the Texas Advanced Computing Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heller KB, Lin EC, Wilson TH. J Bacteriol. 1980;144:274. doi: 10.1128/jb.144.1.274-278.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stroud RM, Nollert P, Miercke L. Adv Protein Chem. 2003;63:291. doi: 10.1016/s0065-3233(03)63011-1. [DOI] [PubMed] [Google Scholar]
- 3.Stroud RM, Miercke LJW, O’Connell J, Khademi S, Lee JK, Remis J, Harries W, Robles Y, Akhavan D. Curr Opin Struct Biol. 2003;13:424. doi: 10.1016/s0959-440x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 4.Borgnia MJ, Agre P. Proc Natl Acad Sci. 2001;98:2888. doi: 10.1073/pnas.051628098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen MO, Park S, Tajkhorshid E, Schulten K. Proc Natl Acad Sci. 2002;99:6731. doi: 10.1073/pnas.102649299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen MO, Tajkhorshid E, Schulten K. Structure. 2001;9:1083. doi: 10.1016/s0969-2126(01)00668-2. [DOI] [PubMed] [Google Scholar]
- 7.Henin J, Tajkhorshid E, Schulten K, Chipot C. Biophysical J. 2008;94:832. doi: 10.1529/biophysj.107.115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hub JS, De Groot BL. Proc Natl Acad Sci USA. 2008;105:1198. doi: 10.1073/pnas.0707662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isralewitz B, Baudry J, Gullingsrud J, Kosztin D, Schulten K. J Mol Graphics. 2001;19:13. doi: 10.1016/s1093-3263(00)00133-9. [DOI] [PubMed] [Google Scholar]; Park S, Schulten K. J Chem Phys. 2004;120:5946. doi: 10.1063/1.1651473. [DOI] [PubMed] [Google Scholar]
- 10.Jarzynski C. Phys Rev Lett. 1997;78:2690. [Google Scholar]
- 11.Darve E, Pohorille A. J Chem Phys. 2001;115:9169. [Google Scholar]
- 12.Henin J, Chipot C. J Chem Phys. 2004;121:2904. doi: 10.1063/1.1773132. [DOI] [PubMed] [Google Scholar]
- 13.Chen LY. J Chem Phys. 2008;129:144113. doi: 10.1063/1.2992153. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chen LY, Bastien DA, Hugo HE. Phys Chem Chem Phys. 2010 doi: 10.1039/B926889H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel L, Kale RD, Schulten K. J Comput Chem. 2005;26:1781. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacKerell AD, Jr, Bashford D, Bellott M, Dunbrack RL, Jr, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Reiher WE, III, Roux B, Schlenkrich M, Smith JC, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M. J Phys Chem B. 1998;102:3586. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]; Feller SE, MacKerell AD., Jr J Phys Chem B. 2000;104:7510. [Google Scholar]
- 16.Kosztin I, Barz B, Janosi L. J Chem Phys. 2006;124:064106. doi: 10.1063/1.2166379. [DOI] [PubMed] [Google Scholar]