Abstract
Introduction
Niaspan, An Extended-Release Formulation Of Niacin (Vitamin B3), Has Been Widely Used To Increase High Density Lipoprotein (HDL) Cholesterol And To Prevent Cardiovascular Diseases And Stroke. In This Study, We Tested Whether Niaspan Administered Acutely After Stroke Is Neuroprotective.
Methods
Adult Male Rats (N=8/Group) Were Subjected To 2hs Of Middle Cerebral Artery Occlusion (Mcao) And Treated With Or Without Different Doses Of Niaspan (20mg/Kg, 40mg/Kg Or 80mg/Kg) At 2 Hours And 24 Hours After Mcao. A Battery Of Functional Outcome Tests Was Performed, And Serum HDL And Triglycerides Were Measured. Rats Were Sacrificed At 7 Days After Mcao And Lesion Volumes Were Measured. The Optimal Dose Of Niaspan Treatment Of Stroke Was Chosen For Immunostaining: Deoxynucleotidyl Transferase–Mediated Dutp Nick-End Labeling (TUNEL), Cleaved Caspase-3, Tumor Necrosis Factor Alpha (TNF-Alpha), Vascular Endothelial Growth Factor (VEGF), Phosphorylated Phosphatidylinositol 3-Kinase (P-PI3K). Another Set Of Rats (N=4/Group) Were Killed At 7 Days After Mcao For Western Blot Assay.
Results
Niaspan Dose-Dependently Reduced Infarct Volume And Improved Functional Outcome After Stroke. No Significant Difference In HDL And Triglyceride Levels Was Detected Between Niaspan Treatments And Mcao Control Groups. Niaspan Treatment Significantly Decreased The Number Of TUNEL-Positive Cells (105±17) And Cleaved Caspase-3 Expression (381±33) In The Ischemic Brain Compared To Mcao Control (165±18; 650±61, Respectively; P<0.05). Niaspan Treatment Significantly Reduced The Expression Of TNF-Alpha (9.7±1.1% Vs. 16±2.2%; P<0.05) And Negative Correlations Were Observed Between The Functional Tests And The Expression Of TNF-Alpha (R=−0.71, P<0.05). Niaspan Treatment Also Significantly Increased The Expression Of VEGF (5.2±0.9%) And PI3K/Akt (0.381±0.04%) In The Ischemic Brain Compared With Non-Treated Mcao Control (2.6±0.4%; 0.24±0.03, Respectively; P<0.05). The Functional Outcome Was Positively Correlated With P-PI3K (R=0.7, P<0.05).
Conclusions
Treatment Of Stroke With Niaspan At 2 Hours After Mcao Reduces Infarct Volume And Improves Neurological Outcome And Provides Neuroprotection. The Neuroprotective Effects Of Niaspan Were Associated With Reduction Of Apoptosis And Attenuation Of TNF-Alpha Expression. VEGF And The PI3K/Akt Pathway May Contribute To The Niaspan-Induced Neuroprotection After Stroke.
Keywords: Niaspan, Neuroprotection, Apoptosis, Cleaved Caspase-3, TNF-Alpha, VEGF, PI3K/Akt
Introduction
Niacin Is A Lipid Altering Agent And Has Been Widely Used To Prevent Cardiovascular Diseases, Stroke And Atherosclerosis (Meyers Et Al., 2004). Niacin Is The Most Effective Medication In Current Clinical Use For Increasing High Density Lipoprotein (HDL) Cholesterol (Elam Et Al., 2000). Niaspan, An Extended-Release Formulation Of Niacin (Vitamin B3), Reduces The Niacin-Induced Major Side Effects Of Flush And Hepatotoxicity (Carlson, 2004). We Have Previously Demonstrated That Niaspan Treatment Of Experimental Stroke In Rats Starting 24 Hours After Stroke, Significantly Increases HDL Cholesterol, Enhances Local Cerebral Blood Flow, Promotes Angiogenesis And Arteriogenesis, And Improves Functional Outcome Without Affecting Infarct Volume (Chen Et Al., 2009; Chen Et Al., 2007). Extended-Release Niacin Decreases The Level Of C-Reactive Protein In Patients With Stable Coronary Artery Disease (Kuvin Et Al., 2006) And Inhibits Vascular Inflammation (Ganji Et Al., 2009; Wu Et Al.). The Inflammatory Response Is Characterized By The Local Expression Of Various Inflammatory Cytokines In The Brain (Degraba, 1998). Tumor Necrosis Factor Alpha (TNF-Alpha) Which Is Detected As Early As 1 Hour After The Onset Of Ischemia, Contributes To The Progression Of Neuronal Injury After Stroke (Morales Et Al., 2008; Sehara Et Al., 2007; Suzuki Et Al., 2009). Several Different Strategies To Inhibit TNF-Alpha Effects In Acute Stroke Have Been Reported To Reduce The Degree Of Ischemic Injury In Animal Models (Shohami Et Al., 1999). Niacin Is Metabolized In The Liver To Nicotinamide. Administration Of Nicotinamide Up To 2 Hours After Cerebral Ischemia Protects Against Both Necrosis And Apoptosis, And Reduces Infarct Volume (Mokudai Et Al., 2000; Yang Et Al., 2002a). In This Study We Tested The Hypothesis That Early Treatment Of Stroke With Niaspan Decreases Inflammation After Stroke And Induces Neuroprotective Effects.
The Pathophysiological Events After Cerebral Ischemia, Leading To Neuronal Death And Functional Disabilities Are Complex And Interconnected. The Acute Neuronal Injury Is Followed By A Second Round Of Neuronal Death In The Neighboring Area Of The Ischemic Core (Kirino, 2000). Apoptosis And Inflammation Are Among The Key Factors That Contribute To The Delayed Injury In The Penumbra (Becker, 1998; Graham And Chen, 2001). Caspases, A Superfamily Of Cysteinyl-Aspartate Protease Are Essential Players In Cell Death (Schulz Et Al., 1999). Administration Of Broad Spectrum Caspase Inhibitors To Ischemic Rodents Induced Neuroprotection (Rabuffetti Et Al., 2000).
Vascular Endothelial Growth Factor (VEGF) And The Phosphatidylinositol 3-Kimase (PI3K)/Akt Signaling Pathway Promote Neuroprotection Post Stroke. VEGF Is An Angiogenic And Neuroprotective Factor That Can Increase Axonal Outgrowth, Block Neuronal Apoptosis, And Promote Neurogenesis (Jin Et Al., 2000). The PI3-K/Akt Pathway Has Been Implicated In The Ability Of VEGF To Enhance Neuroprotective Effect (Kilic Et Al., 2006). The PI3K/Akt Signaling Pathway Plays A Key Role In Cell Survival And Proliferation (Osaki Et Al., 2004). The Neuroprotective Effect Of PI3-K/Akt Has Also Been Attributed To Its Anti-Apoptotic Action And Suppression Of Inflammatory Response (Yin Et Al., 2007).
In The Present Study, Using A Rat Model Of Middle Cerebral Artery Occlusion (Mcao), We Tested Whether Niaspan Treatment Would Provide Neuroprotection In Stroke As Measured By Neurological Behavioral Tests And Infarct Volume. We Also Investigated Whether This Neuroprotection Is Associated With Reduced Apoptosis And TNF-Alpha Expression. In Addition, We Examined The Effect Of Niaspan Treatment On VEGF And PI3K/Akt Activity In The Ischemic Brain.
Material And Methods
Animal Middle Cerebral Artery Occlusion (Mcao) Model And Experimental Groups
Adult Male Wistar Rats Weighing 270–300g Were Employed In All Experiments. Transient Right Middle Cerebral Artery Occlusion (Mcao) Was Induced For 2 Hours By Advancing A 4-0 Surgical Nylon Suture (18.5–19.5 Mm) Determined By The Animal Weight, With Its Tip Rounded By Heating Near A Flame, To Block The Origin Of The MCA, Using A Method Of Intraluminal Vascular Occlusion Modified In Our Laboratory (Chen Et Al., 2006). Rectal Temperature Was Maintained At 37°c Throughout The Surgical Procedure Using A Feedback Regulated Water Heating System. Experimental Groups Consist Of Rats Subjected To Transient Mcao (N=8/Group) And Randomly Treated With Different Doses Of Niaspan (Dissolved In Water; Kos Pharmaceuticals, Canburry, NJ):
Control: Water Was Gavaged At 2 Hours And 24 Hours After Mcao.
Niaspan 20 Mg/Kg Was Gavaged At 2 Hours And 24 Hours After Mcao.
Niaspan 40 Mg/Kg Was Gavaged At 2 Hours And 24 Hours After Mcao.
Niaspan 80 Mg/Kg Was Gavaged At 2 Hours And 24 Hours After Mcao.
All Rats Were Sacrificed At 7 Days After Mcao.
Functional Tests
A Modified Neurological Severity Score (Mnss) And Foot-Fault Test Were Performed Before Mcao, And At 1, 3 And 7 Days After Mcao By An Investigator Who Was Blinded To The Experimental Groups.
Modified Neurological Severity Score (Mnss)
Neurological Function Was Graded On A Scale Of 0 To 18 (Normal Score, 0; Maximal Deficit Score, 18). Mnss Is A Composite Of Motor, Sensory, Reflex, And Balance Tests. In The Severity Scores Of Injury, 1 Score Point Was Awarded For The Inability To Perform The Test Or For The Lack Of A Tested Reflex; Thus, The Higher Score, The More Severe Is The Injury (Chen Et Al., 2001b).
Foot-Fault Test
Animals Were Placed On An Elevated Grid Floor (45 Cm × 30 Cm), 2.5 Cm Higher Than A Solid Base Floor, With 2.5 Cm × 2.5 Cm Diameter Openings. Animals Tend To Move On The Grid With Their Paws Placed On The Wire Frame. When Animals Inaccurately Place A Limb, The Limb Falls Through One Of The Openings In The Grid. When The Limb Falls Through And Pulls Back Quickly Without Touching The Solid Base, The Behavior Is Called A Half Fault And Counted As 1 Foot-Fault Score. If The Limb Falls Through And Touches The Base For Support, This Situation Is Called A Full Fault And Counted As 2 Foot-Fault Scores. Both Foot-Fault Scores And The Number Of Total Steps For Both Forelimbs In A Trial With At Least 40 Movements Were Measured. The Asymmetry Score Were Given By Subtracting The Percent Of Ipsilateral Forelimb Faults From The Percent Of Contralateral Forelimb Faults (Barth Et Al., 1990; Schallert Et Al., 2000).
Histological And Immunohistochemical Assessment
At Seven Days After Mcao, Animals (N=8/Group) Were Sacrificed And Brains Were Fixed By Transcardial Perfusion With Saline, Followed By Perfusion And Immersion In 4% Paraformaldehyde Before Being Embedded In Paraffin. Seven Coronal Sections Of Tissue Were Processed And Stained With Hematoxylin And Eosin (H&E) For Calculation Of Volume Of Cerebral Infarction (Swanson Et Al., 1990). The Indirect Lesion Area, In Which The Intact Area Of The Ipsilateral Hemisphere Was Subtracted From The Area Of The Contralateral Hemisphere, Was Calculated Using The Global Lab Image Analysis System (Data Translation, Malboro, MA) (Swanson Et Al., 1990). Lesion Volume Is Presented As A Volume Percentage Of The Lesion Compared With The Contralateral Hemisphere.
Immunohistochemical Staining
The Optimal Niaspan Dose Which Reduces Infarct Volume And Improves Functional Outcome, Was Selected For Immunostaining. A Standard Paraffin Block Was Obtained From The Center Of The Lesion (Bregma −1 Mm To +1 Mm). A Series Of 6 μM Thick Sections Were Cut From The Block. Every 10th Coronal Section For A Total 5 Sections Was Used For Immunohistochemical Staining. Antibody Against Cleaved Caspase-3 (Rabbit Polyclonal Igg; Dilution 1:200, Cell Signaling Technology, Danvers, Massachusetts), TNF-Alpha (Rabbit Polyclonal Igg; Dilution 1:200; Abcam PLC, Cambridge, Massachusetts), VEGF (Goat Polyclonal Igg; Dilution 1:200; Cruz Biotech Inc, Santa Cruz, California), And Phosphorylated Phosphoinositide-3 Kinase (P-PI3K; Goat Polyclonal Igg; Dilution 1:200; Cruz Biotech Inc, Santa Cruz, California) Immunostaining Were Performed. Control Experiments Consisted Of Staining Brain Coronal Tissue Sections As Outlined Above, But The Primary Antibodies Were Omitted, As Previously Described (Li Et Al., 1998). Terminal Deoxynucleotidyl Transferase–Mediated Dutp Nick-End Labeling (TUNEL) For Measuring Apoptosis Was Performed Using A Commercial Kit (Apoptag Kit, Chemicon, S7100).
Quantitation
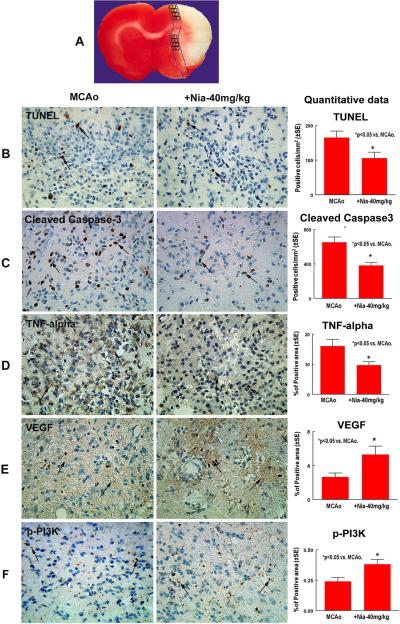
For Measurement Of Apoptotic Markers, Each Cleaved Caspase-3 And TUNEL Immunostained Coronal Section Was Digitized Using A 20× Objective, Via The MCID Computer Imaging Analysis System. Five Sections From The Standard Reference Coronal Section And 8 Brain Field Within Each Section Were Acquired And The Total Number Of Cleaved Caspase-3 And TUNEL Positive Cells In The 8 Fields Of The Ischemic Border Area (IBZ, See Figure 2A) Which Is Adjacent To The Ischemic Core Were Counted Using The MCID Computer Imaging Analysis System (Imaging Research, St. Catharines, Canada). The Total Number Of Positive Cells Per Mm2 Area Is Presented.
Fig. 2. Niaspan Treatment Of Stroke Reduces Apoptosis, Attenuates TNF-Alpha Expression And Increases VEGF And PI3K Activity.
Panel A Shows Quantification Field Of View In The Ischemic Border Zone. Panels B–D Show That Niaspan 40mg/Kg Treatment Significantly Decreased The Number Of TUNEL-Positive (B), Cleaved Caspase-3 (C), And TNF-Alpha (D) Expression In The Ischemic Brain Compared To Mcao Control Rats (P<0.05). Panels E–F Show That Niaspan 40mg/Kg Treatment Significantly Increased The Expression Of VEGF (E) And P-PI3K (F) In The Ischemic Border Compared With Non-Treatment Mcao Control Rats (P<0.05). SE = Standard Error. *P< 0.05 Vs Mcao (N=8/Group).
For Quantitative Measurements Of TNF-Alpha, VEGF And P-PI3K, Five Slides From Each Brain, With Each Slide Containing 8 Fields From The IBZ Were Digitized Under A 40× Objective (Olympus BX40) Using A 3-CCD Color Video Camera (Sony DXC-970MD) Interfaced With An MCID Image Analysis System (Imaging Research, St. Catharines, Canada) (Calza Et Al., 2001; Chen Et Al., 2003a; Chen Et Al., 2003b). Data Were Analyzed In A Blinded Manner And Presented As Percentage Of Positive Area For, TNF-Alpha, VEGF And P-PI3K, Respectively.
Western Blot
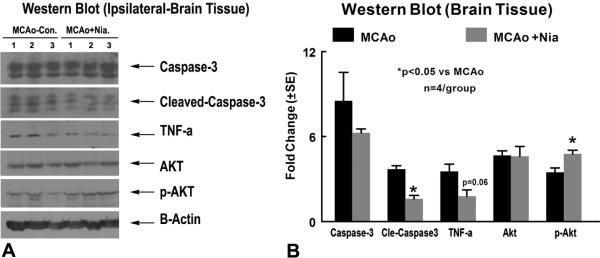
Another Set Of Rats (N=4/Group) Were Killed At 7 Days After Mcao And Brain Tissue Were Extracted From The Ipsilateral Ischemic Area. Equal Amounts Of Cell Lysate Were Subjected To Western Blot Analysis, As Previously Described (Chen Et Al., 2003b). Specific Proteins Were Visualized Using A Supersignal West Pico Chemiluminescence Kit (Pierce). The Following Primary Antibodies Were Used: Anti-B-Actin (Dilution 1:2000; Santa Cruz Biotechnology, Santa Cruz, CA), Anti-Cleaved Caspase-3 (Dilution 1:1000; Cell Signaling Technology), Anti-TNF-Alpha (Dilution 1:500; Abcam PLC.), Anti-Phospho-Akt (Ser473; Dilution 1:1000, Cell Signaling Technology).
HDL Cholesterol And Triglycerides Measurements
Whole Blood HDL And Triglycerides Were Measured Before Treatment And 7 Days After Niaspan Treatment Using Cardiochek P•A Analyzer And HDL And Triglycerides Check Strips (Polymer Technology System, Inc. Indianapolis, IN), According To The Manufacturer's Instructions. 15μl Of Blood Was Collected From The Tail Vein. The Data Are Presented As Mg/Dl Values.
Statistical Analysis
Animals Subjected To Mcao Were Randomized Into One Of Four Groups Including Control Group, Niaspan 20 Mg/Kg, Niaspan 40 Mg/Kg, And Niaspan 80 Mg/Kg. Behavior Tests (Mnss And Foot-Fault Test) Were Performed Before Mcao At 1, 3 And 7 Days After Mcao.
The Global Test Using Generalized Estimating Equations (GEE) (Lu Et Al., 2003) Was Used To Test The Group Difference On Functional Recovery Measured From Multiple Behavior Tests. The Pair-Wise Treatment Comparison Was Conducted If The Overall Treatment Effect Was Detected At 0.05 Level. If The Global Test Was Significant At The 0.05 Level, Tests For Group Differences Were Conducted On Each Functional Test At The 0.05 Level. Otherwise, The Pair-Wise Treatment Comparison Or The Treatment Comparisons On Each Functional Outcome Were Considered As Exploratory. The Global Test On Multiple Outcomes Is More Efficient Than A Single Outcome, When The Group Effects Are Consistent On All The Outcomes (E.G., The Positive Correlation). The Correlation Among Two Outcomes Was Estimated Using GEE. The Most Effective Dose Of Niaspan Treatment Of Stroke Was Identified From The GEE Analysis.
Immunostaining Measures Including Cleaved Caspase-3, TUNEL, TNF-Alpha, VEGF And P-PI3k, Were Compared Between Rats Treated With Most Effective Dose Of Niaspan And Control Using Two-Sample T-Tests For Normally Distributed Variables And Wilcoxon Rank-Sum Tests For Non-Normal Variables. Spearman Correlation Coefficients Were Calculated For Immunostaining Measurements And The 7 Day Functional Recovery Measures Were Stratified By The Treatment Groups.
Results
Neurological Functional Outcome (Figure 1A And 1B)
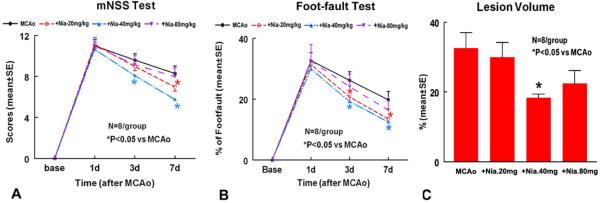
Fig. 1. Neurological Outcome And Lesion Volume After Stroke.
Panels A–B Show Mnss (A) And Foot-Fault (B) Tests After Stroke In The 4 Experimental Groups (Mcao Control, Niaspan 20mg/Kg, Niaspan 40mg/Kg And Niaspan 80mg/Kg Treatment Groups). Panel C Shows The Lesion Volume In The 4 Experimental Groups. SE = Standard Error. *P< 0.05 Vs Mcao (N=8/Group).
To Test Whether Early Niaspan Treatment Of Stroke Rats Regulates Functional Outcome, A Battery Of Functional Tests Was Performed. On Day 3, The Global Test Showed That Rats Treated With Niaspan 40 Mg/Kg Had Significantly Improved Functional Recovery Compared To Mcao Control Group (P=0.029), And Marginally Improved Functional Outcome After Stroke Was Detected In The Niaspan 20 Mg/Kg Treatment Group Compared To The Mcao Control Group (P=0.06). Pair-Wise Comparisons Indicated That Rats In The 40 Mg/Kg And 20 Mg/Kg Treatment Groups Had Significantly Lower Foot-Fault Scores Compared To Control (P=0.012 And P=0.043, Respectively). The Only Significant Pair-Wise Comparison For Mnss Was Between Rats Treated With 40 Mg/Kg And Mcao Control Rats (P=0.008). On Day 7, The Global Test Showed That Niaspan 20mg/Kg (P<0.05) And Niaspan 40mg/Kg (P<0.05) Treatment Of Stroke Significantly Improved Functional Recovery Compared To Mcao Control, Respectively. Global Test Showed That The Most Effective Dose Is Niaspan 40mg/Kg. Pair-Wise Comparisons Indicated That Rats In The 40 Mg/Kg And 20 Mg/Kg Treatment Groups Had Significantly Lower Foot-Fault Scores (P=0.009 And P=0.005, Respectively) And Mnss Test (P<.0001 And P=0.018) Compared To Controls.
Lesion Volume (Figure 1C)
A One-Way ANOVA Model Was Used To Test The Difference In Ischemic Lesion Volume Between Treatment Groups. Pairwise Comparisons Indicated That The Only Significant Decrease In Infarct Volume Was Detected Between Niaspan 40 Mg/Kg (18.30 ± 1.09%) And Control (32.57±4.37%, P=0.011) Groups. The Data Suggest That The Most Effective Niaspan Dose In This Experimental Protocol Was 40mg/Kg.
HDL And Triglycerides Levels
To Test Whether The Two Dose Niaspan 40mg/Kg Treatment Regulates HDL And Triglyceride Level, We Measured HDL And Triglycerides Before Treatment And At 7 Days After Niaspan Treatment. The Data Show That No Significant Difference (P>0.1) In HDL And Triglyceride Levels Was Detected Before Treatment Between Niaspan 40mg/Kg (33±2.29 And 91.33±9.11, Respectively; N=6) And Mcao Control Group (31.33±2.04 And 98.33±12.6, Respectively; N=6). The Levels Of HDL And Triglycerides Did Not Differ Significantly (P>0.1) At 7 Days In The Niaspan Treated Group (30.08± 2.28 And 77.4±3.14, Respectively; N=6) Compared With Mcao Control Group (29.83±3.1 And 70.16 ±3.02, Respectively; N=6).
Niaspan Treatment Decreases Expression Of TUNEL And Cleaved Capase-3
To Test Whether Niaspan 40mg/Kg Treatment Regulates Apoptosis, Two Apoptotic Markers, TUNEL And Cleaved Caspase-3, Were Employed. Figure 2B–C Show That Early Niaspan Treatment Significantly Decreased The Number Of TUNEL-Positive Cells In The Ischemic Brain, As Well As The Number Of Cleaved Caspase-3 Positive Cells Compared To Mcao Control Rats (P<0.05). Cleaved Caspase-3 Expression In Niaspan Treatment Group Significantly (P<0.05) Decreased In The Ischemic Brain Compared To Mcao Control As Measured By Western Blot (Figure 3). These Data Indicate That Niaspan Has An Antiapoptotic Role In The Treatment Of Stroke.
Fig. 3. Western Blot Assay: Niaspan Treatment Of Stroke Reduces TNF-Alpha And Cleaved Caspase-3 Expression And Increases P-Akt Activity In The Ischemic Brain.
Panel A Shows Western Blot Assay. Panel B Shows Western Blot Quantification Data (N=4/Group).
Niaspan Treatment Attenuates TNF-Alpha Expression In The Ischemic Brain
To Further Investigate The Effect Of Niaspan 40mg/Kg Treatment On The Inflammatory Reaction In The Ischemic Brain, We Measured The Expression Of TNF-Alpha In The Ipsilateral Ischemic Border Area. Figure 2D Shows That Niaspan Treatment Significantly Reduced The Expression Of TNF-Alpha In The Ischemic Border Compared With Mcao Control Rats (P<0.05). Figure 3 Shows That TNF-Alpha Expression Decreased (P=0.06) In The Niaspan Treatment Group Compared To Mcao Control. Significant Negative Correlations Were Observed Between The Functional Tests At Day 7 And The Expression Of TNF-Alpha (R= −0.71, P<0.05).
Niaspan Treatment Increases VEGF, PI3K/Akt Activity In The Ischemic Brain
In Order To Investigate Mechanisms Underlying The Niaspan-Induced Neuroprotection After Stroke, VEGF And P-PI3K Activity Was Measured In The Ischemic Border. Figure 2E–F Show That Niaspan 40mg/Kg Treatment Significantly Increased The Expression Of VEGF (E) And P-PI3K (F) In The Ischemic Border Compared With Non-Treatment Mcao Control Rats (P<0.05). The Western Blot Data (Figure 3) Show That P-Akt Activity Significantly (P<0.05) Increased In The Treatment Group Compared To Mcao Control Group. The Functional Outcome At Day 7 Was Positively Correlated With P-PI3K (R=0.7, P<0.05).
Discussion
The Results Of The Current Study Indicate That Niaspan Treatment Of Stroke Starting At 2 Hours After Mcao Is Neuroprotective. The Neuroprotective Effect, Observed In The 40mg/Kg Niaspan Treatment Group Rather Than In 20mg/Kg And 80mg/Kg, Was Demonstrated By Significant Infarct Volume Reduction And Functional Improvement After Mcao In Rats. The Neuroprotective Effects Of Niaspan 40mg/Kg Were Associated With Reduction Of Apoptosis And Attenuation Of TNF-Alpha Expression. Niaspan Treatment Also Significantly Increased VEGF And PI3K/Akt Activity In The Ischemic Brain.
Niaspan Treatment Of Stroke Decreases Expression Of TUNEL And Cleaved Caspase-3
Apoptotic Cell Death Is A Highly Regulated Process That In Many Cases Requires Activation Of Caspases (Manabat Et Al., 2003). Caspase-3 Is The Most Abundant Caspase Family Member In The Adult And Neonatal Nervous System (Le Et Al., 2002). During Ischemia, Caspase-3 Is Cleaved And Activated Where Upon It Degrades Multiple Substrates In Cytoplasm And Nucleus Leading To Cell Death (Le Et Al., 2002; Namura Et Al., 1998). Genetic Deletion Of Caspase-3 Rendered Neurons Resistant To Ischemic Injury Both In Vivo And In Vitro (Le Et Al., 2002). Moreover, Inhibition Of Caspase-3 Like Activity In The Brain Prevented DNA Fragmentation And Other Apoptotic Nuclear Changes Resulting From Ischemic Injury (Luo Et Al., 2002). Niacin And Nicotinamide Are Water-Soluble B Group Vitamins. Nicotinamide, The Amide Form Of Niacin, Is The Precursor Of The Coenzyme Beta-Nicotinamide Adenine Dinucleotide (NAD+) And Plays A Significant Role In Cell Survival And Metabolism. Nicotinamide Is Formed Through The Conversion Of Niacin In The Liver Or Through Hydrolysis Of NAD+ (Li Et Al., 2006). Nicotinamide Prevents Neuronal Degeneration Against Trauma, Axonal Degeneration, Oxidative Stress, Cerebral Ischemia, And Spinal Cord Injury (Li Et Al., 2006). Nicotinamide May Foster Cellular Protection Through Modulation Of Caspase Activity (Maiese And Chong, 2003) During Cerebral Vascular Endothelial Injury (Chong Et Al., 2002) And Prevent Nitric Oxide-Induced Neuronal Injury (Lin Et Al., 2000). Our Data Demonstrate That Niaspan 40mg/Kg Treatment Inhibits Cleaved Caspase-3 Expression And Decreases DNA Fragmentation In The Brain After Transient Focal Cerebral Ischemia. Therefore, Niaspan Has An Anti-Apototic Role In The Treatment Of Stroke After Focal Ischemia In Rats.
Niaspan Treatment Attenuates TNF-Alpha Expression In The Ischemic Brain
TNF-Alpha Is One Of The Key Immunomodulatory And Pro-Inflammatory Cytokines Up-Regulated After Focal Stroke And Plays A Detrimental Role In Neuronal Survival (Hallenbeck, 2002; Wang Et Al., 2004). Administration Of TNF-Alpha During An Ischemic Brain Insult Has Been Shown To Augment The Injury, As Evidenced By Increased Tissue Damage And Neurological Deficits (Barone Et Al., 1997). TNF-Alpha Is Neurotoxic And Capable To Induce Apoptosis Through The Activation Of Caspase Cascade (Hsu Et Al., 1996; Rabuffetti Et Al., 2000). Inhibition Of The Effect Of TNF-Alpha In Acute Stroke, Such As Using An Anti-TNF Neutralizing Antibody (Hosomi Et Al., 2005) And Inhibition Of Soluble TNF-Alpha Receptor Type1 (Nawashiro Et Al., 1997) Reduce Ischemic Damage And Improves Functional Outcome After Stroke. Nicotinamide Is A Potent Inhibitor Of Proinflammatory Cytokines Such As TNF-Alpha In Vitro And In Vivo (Chen Et Al., 2001a; Fukuzawa Et Al., 1997; Ungerstedt Et Al., 2003). In Addition, Niacin Also Has Anti-Inflammatory Properties And Inhibits Vascular Inflammation By Processes Independent Of Changes In Plasma Lipid And HDL Levels (Ganji Et Al., 2009; Wu Et Al.). Our Results Indicate That Niaspan 40mg/Kg Treatment Significantly Reduced The Expression Of TNF-Alpha In The Ischemic Border Compared With Mcao Control Rats (P<0.05), And TNF-Alpha Expression Significantly And Negatively Correlated With Functional Outcome After Stroke. This Anti-Inflammatory Effect Of Niaspan Was Not Associated With Changes In HDL Or Triglycerides. Therefore, The Reduction Of TNF-Alpha Expression May Contribute To Niaspan Treatment Induced Neuroprotection Effects Which Appear Independent Of Increasing HDL.
Niaspan Treatment Increases VEGF And PI3K/Akt Activity
VEGF Was First Found In Vascular Endothelial Cells And Was Characterized As An Angiogenic And Vessel Permeability Factor (Keck Et Al., 1989; Leung Et Al., 1989). VEGF Also Has Neuroprotective Effects In Vitro (Jin Et Al., 2000), And Protects Degenerating Motoneurons In Rodent Models Of Amyotrophic Lateral Sclerosis (Storkebaum Et Al., 2005). VEGF Antisense Treatment Increased Ischemia-Induced Neuronal Damage, Including Increased Numbers Of TUNEL-Positive Cells, Larger Infarct Volume (Yang Et Al., 2002b). Furthermore, Exogenous Administration (Sun Et Al., 2003) Or Overexpression Of VEGF Reduces Hypoxic Ischemic Brain Infarct And Decreases Hypoxic Neuronal Death (Sun And Guo, 2005) And Reduces Neurological Deficit (Zhang Et Al., 2000). Two PI3K Inhibitors, Wortmanin And LY294002, Reversed The Neuroprotective Effect Of VEGF In Immortalized Hippocampal Neuronal Cell Line (HN33). Inhibition Of PI3K/Akt Pathway, Completely Prevented VEGF Neuroprotection In In Vivo And In Vitro Models Of ALS (Lunn Et Al., 2009). PI3K/Akt Promotes Niaspan-Induced Neurogenesis (Chen Et Al., 2009; Chen Et Al., 2007). Activation Of Protein Kinase B (Akt1) Is A Necessary Requirement For Nicotinamide Cytoprotection And The Inhibition Of PI3K Phosphorylation Of Akt1 Eliminated The Cytoprotective Effect Of Nicotinamide Of Hippocampal Neurons (Chong Et Al., 2004). Our Data Show That Niaspan 40mg/Kg Treatment Significantly Increased The Expression Of VEGF, P-PI3K, And P-Akt In The Ischemic Brain Compared With Non-Treatment Mcao Control Rats And The Increased P-PI3K Expression Correlated With Functional Outcome After Stroke (R=0.7, P<0.05). Therefore, The PI3K/Akt Pathway May Be Involved In Niaspan-Induced Neuroprotective Effect.
In Summary, We Demonstrated That Niaspan Dose-Dependently Provides Neuroprotection In A Rat Stroke Model. Treatment Of Stroke With Niaspan 40mg/Kg At 2hs After Mcao Reduces Infarct Volume And Improves Neurological Outcome After Stroke. The Neuroprotective Effects Of Niaspan (40mg/Kg) Were Associated With Reduction Of Apoptosis And Attenuation Of TNF-Alpha Expression. VEGF And PI3K/Akt Pathway May Contribute To The Niaspan-Induced Neuroprotection After Stroke.
Acknowledgements
The Authors Wish To Thank Qinge Lu And Supata Santra For Technical Assistance.
Sources Of Funding This Work Was Supported By National Institute On Aging RO1 AG031811 (JC), National Institute Of Neurological Disorders And Stroke PO1 NS23393 (MC) And 1R41NS064708 (JC), And American Heart Association Grant 09GRNT2300151 (JC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures None.
References
- Barone FC, et al. Tumor Necrosis Factor-Alpha. A Mediator Of Focal Ischemic Brain Injury. Stroke. 1997;28:1233–44. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- Barth TM, et al. Effects Of MK-801 On Recovery From Sensorimotor Cortex Lesions. Stroke. 1990;21:III153–7. [PubMed] [Google Scholar]
- Becker KJ. Inflammation And Acute Stroke. Curr Opin Neurol. 1998;11:45–9. doi: 10.1097/00019052-199802000-00008. [DOI] [PubMed] [Google Scholar]
- Calza L, et al. Nerve Growth Factor Control Of Neuronal Expression Of Angiogenetic And Vasoactive Factors. Proc Natl Acad Sci U S A. 2001;98:4160–5. doi: 10.1073/pnas.051626998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson LA. Niaspan, The Prolonged Release Preparation Of Nicotinic Acid (Niacin), The Broad-Spectrum Lipid Drug. Int J Clin Pract. 2004;58:706–13. doi: 10.1111/j.1368-5031.2004.00233.x. [DOI] [PubMed] [Google Scholar]
- Chen CF, et al. The Protective Effect Of Niacinamide On Ischemia-Reperfusion-Induced Liver Injury. J Biomed Sci. 2001a;8:446–52. doi: 10.1007/BF02256606. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Niaspan Treatment Increases Tumor Necrosis Factor-Alpha-Converting Enzyme And Promotes Arteriogenesis After Stroke. J Cereb Blood Flow Metab. 2009;29:911–20. doi: 10.1038/jcbfm.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, et al. Niaspan Increases Angiogenesis And Improves Functional Recovery After Stroke. Ann Neurol. 2007;62:49–58. doi: 10.1002/ana.21160. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Therapeutic Benefit Of Intracerebral Transplantation Of Bone Marrow Stromal Cells After Cerebral Ischemia In Rats. J Neurol Sci. 2001b;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Vascular Endothelial Growth Factor Mediates Atorvastatin-Induced Mammalian Achaete-Scute Homologue-1 Gene Expression And Neuronal Differentiation After Stroke In Retired Breeder Rats. Neuroscience. 2006;141:737–44. doi: 10.1016/j.neuroscience.2006.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, et al. Intravenous Administration Of Human Bone Marrow Stromal Cells Induces Angiogenesis In The Ischemic Boundary Zone After Stroke In Rats. Circ Res. 2003a;92:692–9. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Statins Induce Angiogenesis, Neurogenesis, And Synaptogenesis After Stroke. Ann Neurol. 2003b;53:743–51. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, et al. Nicotinamide Modulates Mitochondrial Membrane Potential And Cysteine Protease Activity During Cerebral Vascular Endothelial Cell Injury. J Vasc Res. 2002;39:131–47. doi: 10.1159/000057762. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, et al. The NAD+ Precursor Nicotinamide Governs Neuronal Survival During Oxidative Stress Through Protein Kinase B Coupled To FOXO3a And Mitochondrial Membrane Potential. J Cereb Blood Flow Metab. 2004;24:728–43. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- Degraba TJ. The Role Of Inflammation After Acute Stroke: Utility Of Pursuing Anti-Adhesion Molecule Therapy. Neurology. 1998;51:S62–8. doi: 10.1212/wnl.51.3_suppl_3.s62. [DOI] [PubMed] [Google Scholar]
- Elam MB, et al. Effect Of Niacin On Lipid And Lipoprotein Levels And Glycemic Control In Patients With Diabetes And Peripheral Arterial Disease: The ADMIT Study: A Randomized Trial. Arterial Disease Multiple Intervention Trial. JAMA. 2000;284:1263–70. doi: 10.1001/jama.284.10.1263. [DOI] [PubMed] [Google Scholar]
- Fukuzawa M, et al. Inhibitory Effect Of Nicotinamide On In Vitro And In Vivo Production Of Tumor Necrosis Factor-Alpha. Immunol Lett. 1997;59:7–11. doi: 10.1016/s0165-2478(97)00088-6. [DOI] [PubMed] [Google Scholar]
- Ganji SH, et al. Niacin Inhibits Vascular Oxidative Stress, Redox-Sensitive Genes, And Monocyte Adhesion To Human Aortic Endothelial Cells. Atherosclerosis. 2009;202:68–75. doi: 10.1016/j.atherosclerosis.2008.04.044. [DOI] [PubMed] [Google Scholar]
- Graham SH, Chen J. Programmed Cell Death In Cerebral Ischemia. J Cereb Blood Flow Metab. 2001;21:99–109. doi: 10.1097/00004647-200102000-00001. [DOI] [PubMed] [Google Scholar]
- Hallenbeck JM. The Many Faces Of Tumor Necrosis Factor In Stroke. Nat Med. 2002;8:1363–8. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- Hosomi N, et al. Tumor Necrosis Factor-Alpha Neutralization Reduced Cerebral Edema Through Inhibition Of Matrix Metalloproteinase Production After Transient Focal Cerebral Ischemia. J Cereb Blood Flow Metab. 2005;25:959–67. doi: 10.1038/sj.jcbfm.9600086. [DOI] [PubMed] [Google Scholar]
- Hsu H, et al. TRADD-TRAF2 And TRADD-FADD Interactions Define Two Distinct TNF Receptor 1 Signal Transduction Pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- Jin KL, et al. Vascular Endothelial Growth Factor: Direct Neuroprotective Effect In In Vitro Ischemia. Proc Natl Acad Sci U S A. 2000;97:10242–7. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck PJ, et al. Vascular Permeability Factor, An Endothelial Cell Mitogen Related To PDGF. Science. 1989;246:1309–12. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- Kilic E, et al. The Phosphatidylinositol-3 Kinase/Akt Pathway Mediates VEGF's Neuroprotective Activity And Induces Blood Brain Barrier Permeability After Focal Cerebral Ischemia. FASEB J. 2006;20:1185–7. doi: 10.1096/fj.05-4829fje. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed Neuronal Death. Neuropathology. 2000;20(Suppl):S95–7. doi: 10.1046/j.1440-1789.2000.00306.x. [DOI] [PubMed] [Google Scholar]
- Kuvin JT, et al. Effects Of Extended-Release Niacin On Lipoprotein Particle Size, Distribution, And Inflammatory Markers In Patients With Coronary Artery Disease. Am J Cardiol. 2006;98:743–5. doi: 10.1016/j.amjcard.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Le DA, et al. Caspase Activation And Neuroprotection In Caspase-3 Deficient Mice After In Vivo Cerebral Ischemia And In Vitro Oxygen Glucose Deprivation. Proc Natl Acad Sci U S A. 2002;99:15188–93. doi: 10.1073/pnas.232473399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DW, et al. Vascular Endothelial Growth Factor Is A Secreted Angiogenic Mitogen. Science. 1989;246:1306–9. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Li F, et al. Cell Life Versus Cell Longevity: The Mysteries Surrounding The NAD+ Precursor Nicotinamide. Curr Med Chem. 2006;13:883–95. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. Neuronal Damage And Plasticity Identified By Microtubule-Associated Protein 2, Growth-Associated Protein 43, And Cyclin D1 Immunoreactivity After Focal Cerebral Ischemia In Rats. Stroke. 1998;29:1972–80. doi: 10.1161/01.str.29.9.1972. Discussion 1980–1. [DOI] [PubMed] [Google Scholar]
- Lin SH, et al. Prevention Of Nitric Oxide-Induced Neuronal Injury Through The Modulation Of Independent Pathways Of Programmed Cell Death. J Cereb Blood Flow Metab. 2000;20:1380–91. doi: 10.1097/00004647-200009000-00013. [DOI] [PubMed] [Google Scholar]
- Lu M, et al. Global Test Statistics For Treatment Effect Of Stroke And Traumatic Brain Injury In Rats With Administration Of Bone Marrow Stromal Cells. J Neurosci Methods. 2003;128:183–90. doi: 10.1016/s0165-0270(03)00188-2. [DOI] [PubMed] [Google Scholar]
- Lunn JS, et al. Vascular Endothelial Growth Factor Prevents G93ASOD1-Induced Motor Neuron Degeneration. Dev Neurobiol. 2009;69:871–84. doi: 10.1002/dneu.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, et al. Induction Of Caspase-Activated Deoxyribonuclease Activity After Focal Cerebral Ischemia And Reperfusion. J Cereb Blood Flow Metab. 2002;22:15–20. doi: 10.1097/00004647-200201000-00002. [DOI] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ. Nicotinamide: Necessary Nutrient Emerges As A Novel Cytoprotectant For The Brain. Trends Pharmacol Sci. 2003;24:228–32. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- Manabat C, et al. Reperfusion Differentially Induces Caspase-3 Activation In Ischemic Core And Penumbra After Stroke In Immature Brain. Stroke. 2003;34:207–13. doi: 10.1161/01.STR.0000047101.87575.3C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers CD, et al. Niacin Therapy In Atherosclerosis. Curr Opin Lipidol. 2004;15:659–65. doi: 10.1097/00041433-200412000-00006. [DOI] [PubMed] [Google Scholar]
- Mokudai T, et al. Delayed Treatment With Nicotinamide (Vitamin B(3)) Improves Neurological Outcome And Reduces Infarct Volume After Transient Focal Cerebral Ischemia In Wistar Rats. Stroke. 2000;31:1679–85. doi: 10.1161/01.str.31.7.1679. [DOI] [PubMed] [Google Scholar]
- Morales JR, et al. Activation Of Liver X Receptors Promotes Neuroprotection And Reduces Brain Inflammation In Experimental Stroke. Circulation. 2008;118:1450–9. doi: 10.1161/CIRCULATIONAHA.108.782300. [DOI] [PubMed] [Google Scholar]
- Namura S, et al. Activation And Cleavage Of Caspase-3 In Apoptosis Induced By Experimental Cerebral Ischemia. J Neurosci. 1998;18:3659–68. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawashiro H, et al. Inhibition Of Tumor Necrosis Factor And Amelioration Of Brain Infarction In Mice. J Cereb Blood Flow Metab. 1997;17:229–32. doi: 10.1097/00004647-199702000-00013. [DOI] [PubMed] [Google Scholar]
- Osaki M, et al. PI3K-Akt Pathway: Its Functions And Alterations In Human Cancer. Apoptosis. 2004;9:667–76. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- Rabuffetti M, et al. Inhibition Of Caspase-1-Like Activity By Ac-Tyr-Val-Ala-Asp-Chloromethyl Ketone Induces Long-Lasting Neuroprotection In Cerebral Ischemia Through Apoptosis Reduction And Decrease Of Proinflammatory Cytokines. J Neurosci. 2000;20:4398–404. doi: 10.1523/JNEUROSCI.20-12-04398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, et al. CNS Plasticity And Assessment Of Forelimb Sensorimotor Outcome In Unilateral Rat Models Of Stroke, Cortical Ablation, Parkinsonism And Spinal Cord Injury. Neuropharmacology. 2000;39:777–87. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Schulz JB, et al. Caspases As Treatment Targets In Stroke And Neurodegenerative Diseases. Ann Neurol. 1999;45:421–9. doi: 10.1002/1531-8249(199904)45:4<421::aid-ana2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Sehara Y, et al. Decreased Focal Inflammatory Response By G-CSF May Improve Stroke Outcome After Transient Middle Cerebral Artery Occlusion In Rats. J Neurosci Res. 2007;85:2167–74. doi: 10.1002/jnr.21341. [DOI] [PubMed] [Google Scholar]
- Shohami E, et al. Dual Role Of Tumor Necrosis Factor Alpha In Brain Injury. Cytokine Growth Factor Rev. 1999;10:119–30. doi: 10.1016/s1359-6101(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Storkebaum E, et al. Treatment Of Motoneuron Degeneration By Intracerebroventricular Delivery Of VEGF In A Rat Model Of ALS. Nat Neurosci. 2005;8:85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- Sun FY, Guo X. Molecular And Cellular Mechanisms Of Neuroprotection By Vascular Endothelial Growth Factor. J Neurosci Res. 2005;79:180–4. doi: 10.1002/jnr.20321. [DOI] [PubMed] [Google Scholar]
- Sun Y, et al. VEGF-Induced Neuroprotection, Neurogenesis, And Angiogenesis After Focal Cerebral Ischemia. J Clin Invest. 2003;111:1843–51. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, et al. Ambivalent Aspects Of Interleukin-6 In Cerebral Ischemia: Inflammatory Versus Neurotrophic Aspects. J Cereb Blood Flow Metab. 2009;29:464–79. doi: 10.1038/jcbfm.2008.141. [DOI] [PubMed] [Google Scholar]
- Swanson RA, et al. A Semiautomated Method For Measuring Brain Infarct Volume. J Cereb Blood Flow Metab. 1990;10:290–3. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Ungerstedt JS, et al. Nicotinamide Is A Potent Inhibitor Of Proinflammatory Cytokines. Clin Exp Immunol. 2003;131:48–52. doi: 10.1046/j.1365-2249.2003.02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. Inhibition Of Tumor Necrosis Factor-Alpha-Converting Enzyme By A Selective Antagonist Protects Brain From Focal Ischemic Injury In Rats. Mol Pharmacol. 2004;65:890–6. doi: 10.1124/mol.65.4.890. [DOI] [PubMed] [Google Scholar]
- Wu BJ, et al. Evidence That Niacin Inhibits Acute Vascular Inflammation And Improves Endothelial Dysfunction Independent Of Changes In Plasma Lipids. Arterioscler Thromb Vasc Biol. doi: 10.1161/ATVBAHA.109.201129. [DOI] [PubMed] [Google Scholar]
- Yang J, et al. Nicotinamide Therapy Protects Against Both Necrosis And Apoptosis In A Stroke Model. Pharmacol Biochem Behav. 2002a;73:901–10. doi: 10.1016/s0091-3057(02)00939-5. [DOI] [PubMed] [Google Scholar]
- Yang ZJ, et al. Role Of Vascular Endothelial Growth Factor In Neuronal DNA Damage And Repair In Rat Brain Following A Transient Cerebral Ischemia. J Neurosci Res. 2002b;70:140–9. doi: 10.1002/jnr.10380. [DOI] [PubMed] [Google Scholar]
- Yin W, et al. Preconditioning Suppresses Inflammation In Neonatal Hypoxic Ischemia Via Akt Activation. Stroke. 2007;38:1017–24. doi: 10.1161/01.STR.0000258102.18836.ca. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, et al. VEGF Enhances Angiogenesis And Promotes Blood-Brain Barrier Leakage In The Ischemic Brain. J Clin Invest. 2000;106:829–38. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]