Summary
Experience-driven neuronal plasticity allows the brain to adapt its functional connectivity to recent sensory input. Here we use binocular rivalry [1], an experimental paradigm where conflicting images are presented to the individual eyes, to demonstrate plasticity in the neuronal mechanisms that convert visual information from two separated retinas into single perceptual experiences. Perception during binocular rivalry tended to initially consist of alternations between exclusive representations of monocularly defined images, but upon prolonged exposure, mixture percepts became more prevalent. The completeness of suppression, reflected in the incidence of mixture percepts, plausibly reflects the strength of inhibition that likely plays a role in binocular rivalry [2]. Recovery of exclusivity was possible, but required highly specific binocular stimulation. Documenting the prerequisites for these observed changes in perceptual exclusivity, our experiments suggest experience-driven plasticity at interocular inhibitory synapses, driven by the (lack of) correlated activity of neurons representing the conflicting stimuli. This form of plasticity is consistent with a previously proposed, but largely untested, anti-Hebbian learning mechanism for inhibitory synapses in vision [3, 4]. Our results implicate experience-driven plasticity as one governing principle in the neuronal organization of binocular vision.
Results
Perceptual advantages of binocular vision including stereopsis and enhanced contrast sensitivity through binocular summation require integration of initially separated monocular streams of information. Mechanisms responsible for binocular integration are shaped by activity-dependent neural development, both prenatally when ocular dominance columns are first established and for several years postnatally, when binocular mechanisms are refined based on visual experience [see 5]. Whereas the neuronal components subserving binocular integration may not change much after this critical period, the computational mechanisms, likely reflected in synaptic connectivity and efficacy, may be continuously recalibrated in response to modified sensory experience. This ongoing neuronal fine-tuning might in fact be the reason why some strabismus patients that have not adequately developed stereopsis during childhood can still acquire stereoscopic depth vision later in life through extensive visual therapy [for anecdotal evidence see 6].
Exposure to binocular rivalry stimuli [1] creates a well-controlled modified sensory context deviating from the system's ‘standard’ in the sense that the brain receives incompatible, non-matching inputs instead of matching ones. Under such conditions, binocular integration fails and, instead, observers tend to alternately perceive the monocular images. This perceptual cycling is commonly believed to arise from neural processes that include mutual inhibition between neuronal representations of the two images [1, 7, 8]. During smaller fractions of the time viewing rivalry, observers also transiently perceive various mixtures of both monocular images [2, 9, 10], the most common being transparent superimpositions of both images and patchwork-like zones of local monocular dominance termed ‘piecemeal’ (Fig. 1A). Mixtures suggest that even during rivalry periods of partial binocular integration occur. The absolute predominance of different mixture percepts depends on stimulus features including size [11], spatial frequency [2, 9, 12] and global context [13], and the incidence of these lapses in perceptual exclusivity plausibly depends on the strength of mutual inhibition [2], a notion supported by simulations with existing binocular rivalry models [14, 15] [Supplemental Information 1, Fig. S1].
Fig. 1.
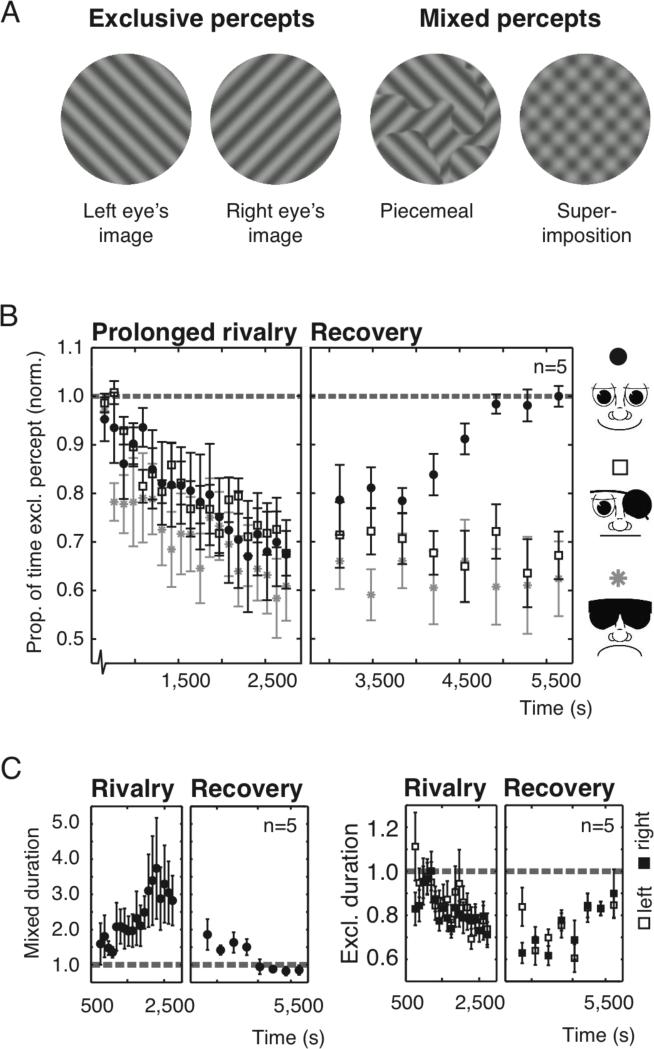
Dynamics of perceptual exclusivity. (A) Perceptual experiences during binocular rivalry. Exclusive percepts correspond entirely to one eye's stimulus. Mixed percepts resemble patch-like (piecemeal) or transparent superimpositions of the stimuli. (B) The average proportion of normalized exclusivity for five observers against time. In the recovery stage, observers experienced either normal binocular vision (solid black circles), monocular vision only (open squares), or no visual stimulation at all (gray asterisks). (C) The average epoch durations for mixed (left panel, solid circles), exclusive ‘left’, and ‘right’ percepts (right panel, open and black squares) of the two-eyes-stimulated condition. Dashed lines represent baseline levels. All error bars, s.e.m.
To test whether binocular integration is indeed a plastic mechanism that adapts to sensory experience, we presented the eyes with incompatible images for prolonged periods of time, sometimes interspersed with non-rival stimuli, while observers continuously reported whether they perceived either one of the exclusive monocular images or a mixture.
Experiment 1: Perceptual exclusivity and binocular rivalry
Observers viewed rival stimuli for prolonged durations while tracking periods of exclusive dominance and mixed percepts (Fig. 1A). The same rival images were presented to each eye throughout the experiment. If initial perceptual exclusivity in binocular rivalry were due to the ‘unnatural’ sensory context of dissimilar images in the two eyes causing strong mutual inhibition and preventing binocular integration, we would expect exclusivity to progressively decrease while experience with the modified sensory context accumulates. As expected from earlier results [16], our observations confirm this prediction (Fig. 1B), demonstrating a substantial decrease in exclusivity over 35 minutes of rivalry (Fig. 1B, Spearman rank correlation, R = -0.46, p << 0.001). Data points represent averaged data from 100 s rivalry trials, separated by 10 s rests. Data for individual observers was normalized by baseline values, determined in four rivalry trials (100 s rest) directly preceding the experiment.
The idea that the altered exclusivity in our experiment reflects experience-driven plasticity yields a second, more counterintuitive prediction: Exclusivity should not passively recover to baseline after having dropped during rivalry, but instead should require correspondence of visual signals from both eyes. In the second part of our experiment, immediately following the first, recovery of exclusivity was investigated with periods of exposure to various conditions of monocular or binocular stimulation. In one condition, observers walked around the laboratory with both eyes open. The matching, natural visual input to both eyes should cause a recalibration of the binocular mechanisms and re-strengthen the inhibition putatively weakened during rivalry. Because re-strengthening should be evidenced by increased perceptual exclusivity, the periods of free viewing were interspersed with brief rivalry trials. In a second condition free viewing was replaced by episodes without visual stimulation that should leave exclusivity unaltered. A third condition contained periods of monocular stimulation where one eye was patched during free viewing.
Significant increases in the proportion of exclusive dominance indeed occurred when two eyes received matched stimulation during free viewing (Fig. 1B, solid circles, Spearman rank, R = 0.75, p << 0.001), both because mixed percept epochs became briefer and exclusive percepts became longer (Fig. 1C, Spearman rank, Rmix = -0.68, pmix << 0.001; Rexcl = 0.45, pexcl < 0.01). Consistent with our prediction, no such recovery was observed throughout 48 minutes without visual stimulation (Fig. 1B, gray asterisks; Spearman rank, Rno_stim = -0.01, pno_stim = 0.97). Recovery was also entirely absent in the third, monocular stimulation condition (Fig. 1B, open squares; Spearman rank, Rpatched = -0.08, ppatched = 0.61) implying that binocular correspondence is essential for recalibration.
To further examine the failure of recovery with monocular stimulation, the two first authors subjected themselves to an extended the period of continuous eye-patch wearing for 24 hours. Remarkably, decreased exclusivity levels barely recovered during this day of patching, yet recovery started immediately after both eyes received matched stimulation during free viewing [Supplemental Information 2, Fig. S2]. The longevity of decreased exclusivity in the absence of binocular input is reminiscent of the enduring time-course of contingent adaptation effects [e.g. 17] and perhaps ‘storage’ of non-contingent after-effects [18-20]. The slow decay of adaptation in all these cases could have a common cause: neurons encoding a specific adapting stimulus may retain their adapted state so as long as they are shielded from novel sensory experience, thereby precluding recalibration [17, 18, 20].
The results of these first experiments support experience-driven plasticity in the connectivity between neuronal representations involved in binocular rivalry by implying both the weakening and re-strengthening of inhibition in the anticipated conditions. While the necessity of binocular stimulation is clear, several remaining questions regarding the exact prerequisites for the observed changes in exclusivity prompted the following experiments.
Experiment 2: Decrease of perceptual exclusivity
To establish the prerequisites of decreasing exclusivity, two variations of our first experiment were performed in which we temporarily inverted the stimulus-eye configuration on every fifth trial (‘opposite-configuration trials’) so that the same monocular stimuli were presented to the opposite eyes. While this manipulation leaves the global competition between binocular stimulus representations unaffected, it does activate different monocular neurons on those specific opposite-configuration trials. Figure 2A demonstrates the results using the same stimuli as in Experiment 1. The opposite-configuration trials (white squares) have significantly higher levels of exclusivity than their temporal neighbors (Fig. 2A, paired t-test, p < 0.05). Additionally, exclusivity decreases only for the eye-stimulus configuration used in the majority of trials (Spearman rank, Rmajority = -0.48, pmajority << 0.001; Ropposite-configuration = -0.22, popposite-configuration = 0.36).
Fig. 2.
Prerequisites for decreasing exclusivity. (A) The average proportion of exclusive grating percepts over time for five observers. The eye-stimulus configuration was the same for most trials (‘Majority trials’, solid black circles), but was switched on some interleaved trials (‘Opposite configuration trials’, open squares). (B) Similar to (A) but here the monocular images were complex pictures of a house and a face, not orthogonal sinusoidal gratings. Dashed lines represent baseline exclusivity; Statistics asterisks, p < 0.05; All error bars, s.e.m.
Whereas superimposition mixture percepts may be readily understood in terms of weak inhibitory gain, the occurrence of piecemeal mixtures more likely reflects weak inhibitory spatial coherence or weak excitatory lateral connectivity [13, 21]. We repeated the experiment using images of a house and a face as rival targets to establish whether changes of exclusivity also occur with more complex images for which spatial coherence is particularly strong. Again, exclusivity decreased for the major eye-stimulus configuration, but not for the opposite-configuration trials (Fig. 2B, Spearman rank: Rmajority = -0.65, pmajority << 0.001; Ropposite-configuration = - 0.15, popposite-configuration = 0.54). An additonal control experiment designed to disentangle the relative contributions of superimposition and piecemeal percepts further suggested that decreases in exclusivity are predominantly caused by increases in the incidence of superimposition [Supplementary Information 3, Fig. S3].
The opposite-configuration results support the idea that inhibitory connections involved in experience-driven plasticity are at least partially interocular, promoting inhibition between representations of rivaling images comprising eye-of-origin information. If eye-of-origin information were not involved, the stimuli on the majority of trials and the opposite-configuration trials should be equivalent and yield equivalent results. The eye-specificity is consistent with current thinking about binocular rivalry as a hierarchical process involving multiple stages of visual processing [22, 23].
Experiment 3: Recovery of perceptual exclusivity
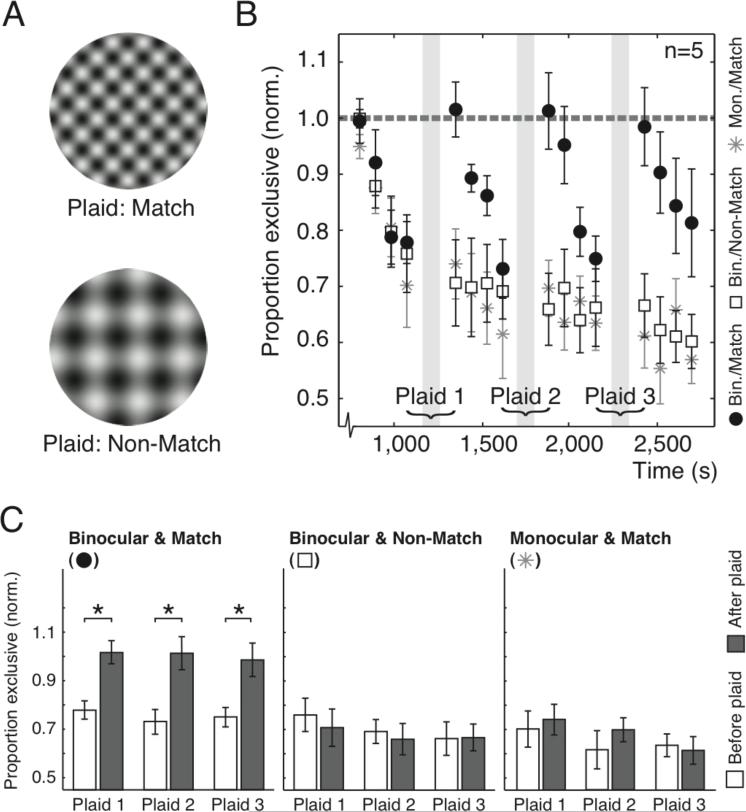
We next investigated the requirements for re-strengthening of inhibition more closely. When binocular free viewing in Experiment 1 caused recovery, both eyes received matching naturalistic input containing a broad range of orientations and spatial frequencies, presumably including those of our rivalry targets. To investigate whether recovery merely requires binocular correspondence or specific binocular correspondence of the rivaling stimulus features, we performed experiments in which we replaced our rivalry gratings with a high contrast plaid stimulus on every fifth trial. “Matching” plaids with the same spatial frequency and orientations as the rivaling gratings (Fig. 3A) were presented to two eyes simultaneously (Fig. 3B, black circles) or one eye at a time, alternating between eyes every few seconds (Fig. 3B, gray asterisks). Plaids with different spatial frequency and orientations (Fig. 3A) were also presented to two eyes simultaneously (Fig. 3B, white squares). Figure 3C compares the exclusivity levels between trials directly preceding and following plaid presentations. Only binocularly presented, matching plaids evoked a significant recovery of exclusivity (Paired t-tests, pBinocular_Match << 0.001; pMonocular_Match = 0.10; pBinocular_NoMatch = 0.35) supporting the hypothesis that re-strengthening of inhibition only occurs during coinciding activity of eye-specific, orientation- and spatial frequency-tuned neurons. It also argues against an alternative hypothesis of reduced exclusivity through contrast adaptation. In principle, such adaptation might reduce exclusivity by lowering the activity of suppressing neurons. However, during presentation of matching plaid stimuli, when the same stimulus features are present as during rivalry, contrast adaptation should continue casuing exclusivity to further reduce, not recover like we observed.
Fig. 3.
Prerequisites for recovery of decreased exclusivity. (A) The plaid stimuli that were interleaved with rivalry trials. Matching plaids had the same components as the rivaling gratings while non-matching plaids’ components had different spatial frequencies and orientations. (B) The average proportion exclusivity over time for five observers. Rivalry trials were interleaved with plaid presentations (gray areas). Matching plaids were presented to two eyes (solid black circles) or one eye (gray asterisks) simultaneously. Non-matching plaids were always presented to two eyes (open squares). The dashed line represents baseline exclusivity. (C) Exclusivity, compared between rivalry trials that directly preceded (white bars) and followed (gray bars) plaid presentation. Statistics asterisks, p < 0.05; All error bars, s.e.m.
Experiment 4: Replay-Rivalry
Our results suggest that prolonged binocular rivalry weakens interocular inhibition through recalibration of binocular integration mechanisms in response to cumulative experience with non-fusible input. If such experience-driven binocular plasticity is a generic property of visual perception, the choice for rivalry stimuli should not be essential. Monocular, non-rivaling, stimulation might also weaken inhibition if it activates neurons corresponding to one eye without simultaneously activating their counterparts belonging to the other eye. We tested this prediction using the reported percept durations of baseline rivalry trials to create ‘replay-trials’ where individual eyes were alternately presented with their corresponding monocular images. This manipulation provides the required activity patterns without evoking rivalry (Fig. 4A). Observers viewed three sets of two monocular replay-trials followed by a single rivalry trial to measure exclusivity. The decreasing exclusivity following replay-trials depicted in Figure 4B (t-tests, p < 0.05) favors an interpretation where experience-driven plasticity is not restricted to rivalry, but serves as a generic principle of binocular vision.
Fig. 4.
The effect of monocular replay-rivalry. (A) While during rivalry both eyes are simultaneously stimulated with conflicting images, replay-rivalry consists of alternating monocular stimulations with a temporal structure based on individual perceptual reports during real rivalry. (B) The average proportion of exclusivity in three rivalry trials that were each preceded by two replay trials (gray areas). The dashed line represents baseline exclusivity; Statistics asterisks, p < 0.05; Error bars, s.e.m.
Discussion
Experience literally changes our view of the world. Neuronal processes converting retinal images to conscious perception constantly adapt to changing sensory contexts. Our results demonstrate that upon prolonged exposure to binocular rivalry stimuli, the nature of the accompanying perceptual experience progressively changes. Where observers initially perceive mostly alternations between exclusive representations of monocular images, mixtures of the two images [16] become more prevalent over time. Building upon the idea that binocular rivalry involves inhibition between neuronal populations representing competing images [1, 7, 8], we suggest that the rise in predominance of mixed percepts is caused by weakening of inhibitory efficacy [2].
Anti-Hebbian plasticity
A theoretical framework for inhibitory plasticity in vision has been constructed around so-called ‘anti-Hebbian’ inhibitory synapses [3, 4]. Hebbian synapses are well known as a neuronal principle for experience-driven plasticity. The basic idea is that when a presynaptic excitatory neuron participates in successfully activating a postsynaptic neuron, their synaptic bond is strengthened and the correlation between their response patterns increases. While there is abundant biological evidence for Hebbian learning in synapses mediating excitatory interactions [24, 25], the related principle for inhibitory connections has received far less attention. Extending Hebb's postulate, Barlow and Földiák have proposed that inhibitory interactions are similarly strengthened and weakened by coinciding pre- and postsynaptic activity or a lack thereof [3]. Since such a plasticity-scheme decorrelates pre- and postsynaptic activity, it is sometimes dubbed ‘anti-Hebbian’ [26](a term also used for several other decorrelating synaptic mechanisms [27]). Anti-Hebbian plasticity is inherent in several models of unsupervised neuronal learning [e.g. 26, 28] and an indirect route via inhibitory interneurons has been physiologically demonstrated in several species and brain structures [29, 30the , 31, 32]. However, plasticity rules for direct inhibitory synapses appear to be more variable [25] and while such anti-Hebbian learning has been suggested in the context of contingent visual after-effects [3, 4], pattern-adaptation [33] and center-surround suppression [34], direct behavioral evidence is sparse.
Our binocular rivalry results are consistent with anti-Hebbian learning mechanisms for interocular inhibition in binocular vision. Assuming that perceptual dominance of one rival image indicates successful suppression of the competing neuronal representation, dominance may entail activity in presynaptic neurons representing the dominant image without equivalent activity in the postsynaptic neurons encoding the (suppressed) opposite image. These are exactly the conditions for which anti-Hebbian weakening of inhibitory efficacy was postulated, explaining why initially high perceptual exclusivity should progressively decrease with viewing time. Furthermore, the anti-Hebbian principle predicts that (re-)strengthening of inhibition would require simultaneous activation of the same neurons involved in rivalry. This can arguably be achieved by presenting binocularly corresponding stimuli with features to which those specific neurons are tuned. Our experiments demonstrate both the predicted drop in perceptual exclusivity and the expected dependence of recovery on stimulus features.
Plasticity and rivalry
Previously demonstrated changes in perceptual experience with prolonged or repeated rivalry include short-term slowing of perceptual switch-rates during single binocular rivalry trials [35, 36] and long-term speeding of switch rates when sessions are repeated over many days [36]. While short-term effects were explained by contrast adaptation build-up [35, 36], long-term effects were attributed to plasticity in neuronal responses and/or connectivity within multiple brain areas [36]. Because none of the abovementioned studies included the dynamics of mixture percepts in their binocular rivalry evaluation, it is difficult to unify the changes in switch-rate with our changes of binocular integration. However, one emerging notion is that the adult visual system seems more plastic than previously realized and future studies of binocular rivalry need to appreciate that exposure to rival stimuli may cause plastic changes in the very neuronal mechanisms targeted for study.
The many similarities and differences in the dynamics of binocular rivalry and other forms of perceptual rivalry [7, 22, 35, 37-39] have promoted the idea that different types of rivalry, while perhaps resolved at different processing stages, may share common computational components in their rivalry-resolving mechanisms [7]. Since mutual inhibition is conceivably one of those components [7], it would be interesting to know whether plasticity of inhibitory efficacy also influences other forms of rivalry. The reduced exclusivity observed in our study proved to be specific to eye-stimulus configuration, locating the proposed plasticity mechanism at a stage of binocular rivalry processing that includes eye-of-origin information. [22, 23]. Still, this does not entirely preclude the possibility of inhibitory plasticity in other forms of rivalry or at other processing levels. Furthermore, it implies that plastic interocular inhibition may be a general mechanism of binocular vision, raising the intriguing question what might happen if exposure to rival stimulation were prolonged for hours or days, impractical though it might be to find out.
Conclusions
Our findings suggest experience-driven (anti-Hebbian) plasticity as one governing principle in the neuronal organization of binocular vision. It is tempting to envision this mechanism as a means for interocular gain-control during binocular combination. It could balance monocular signals so that binocular contrast and surface lightness are not noticeably different from monocular viewing [40]. On this view, our binocular rivalry experiments reveal the operation of such inhibitory mechanism and its dynamic modification. The experience-driven plasticity we demonstrate may provide important clues towards solving the longstanding question of how rivalry and stereopsis can emerge from a single neuronal organization of binocular vision [40-44].
Experimental Procedure
Observers viewed stimuli through a mirror stereoscope in a quiet, darkened room. Rival stimuli were surrounded by an alignment-ring to facilitate binocular fusion. Observers continuously reported perceptual experience by pressing buttons on a keyboard. One of two buttons was held while observers exclusively perceived the corresponding monocular stimulus. Both buttons were released when mixtures were perceived. The basic experimental paradigm consisted of a baseline determination followed by two stages differing in the timing of stimulus presentation. During baseline determination, individual observers’ levels of exclusivity were established with stimulus presentations lasting 100 s, separated by 100 s rests during which observers viewed the alignment frame only. During the first part of the actual experiment, stimulus presentations also lasted 100s, but rests were reduced to 10 s. In Experiment 1, a second part comprised stimulus presentations of 60 s and rests of 300 s. These long rests consisted of 240 s of predefined visual input (depending on the condition) and 60 s of uniform field adaptation during which observer's viewed a gray screen. For all rivalry trials we calculated proportions of exclusivity as the sum of all exclusive percept durations divided by the total trial duration. These proportions were normalized by the average baseline proportion of exclusivity for each observer. A more detailed description of the Experimental Procedure is available as Supplemental Experimental Procedure.
Supplementary Material
Acknowledgements
We thank our observers for their perseverance during these long, demanding experiments, Helmut Kröger for sharing his code for our model simulations, and Sidney Lehky, Martin Lankheet, Tomas Knapen and André Noest for commenting on earlier versions of this manuscript. This work was supported by a VIDI (RvW) and a Rubicon grant from the Netherlands Organisation for Scientific Research (JB), an Utrecht University High Potential grant (CK & RvW), NIH grant EY13358 (RB), and a grant (R32-10142) from the World Class University program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (RB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Binocular rivalry reveals plasticity in binocular fusion
- Perceptual coherence deteriorates during extended visual rivalry
- Psychophysical evidence for plasticity in human vision
- Efficacy of interocular inhibition depends on recent perceptual history
References
- 1.Alais D, Blake R, editors. Binocular Rivalry. MIT Press; Cambridge, MA: 2005. [Google Scholar]
- 2.Hollins M. The effect of contrast on the completeness of binocular rivalry suppression. Percept Psychophys. 1980;27:550–556. doi: 10.3758/bf03198684. [DOI] [PubMed] [Google Scholar]
- 3.Barlow HB, Földiák P. Adaptation and Decorrelation in the Cortex. In: Miall C, Durbin RM, Mitchison GJ, editors. The Computing Neuron. Addison-Wesley; Wokingham, England: 1989. pp. 54–72. [Google Scholar]
- 4.Barlow HB. A theory about the functional role and synaptic mechanism of visual after-effects. In: Blakemore CB, editor. Vision: coding and efficiency. Cambridge University Press; Cambridge: 1990. pp. 363–375. [Google Scholar]
- 5.Katz LC, Crowley JC. Development of cortical circuits: lessons from ocular dominance columns. Nat Rev Neurosci. 2002;3:34–42. doi: 10.1038/nrn703. [DOI] [PubMed] [Google Scholar]
- 6.Barry SR. Fixing my gaze: A scientist's journey into seeing in three dimensions. First Edition Basic Books; New York, New York, USA: 2009. [Google Scholar]
- 7.Klink PC, van Ee R, van Wezel RJA. General Validity of Levelt's Propositions Reveals Common Computational Mechanisms for Visual Rivalry. PLoS ONE. 2008;3:e3473. doi: 10.1371/journal.pone.0003473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson HR. Minimal physiological conditions for binocular rivalry and rivalry memory. Vision Res. 2007;47:2741–2750. doi: 10.1016/j.visres.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Rose D, Blake R. On the variety of percepts associated with dichoptic viewing of dissimilar monocular stimuli. Perception. 1992;21:47–62. doi: 10.1068/p210047. [DOI] [PubMed] [Google Scholar]
- 10.Brascamp JW, van Ee R, Noest AJ, Jacobs R, van den Berg AV. The time course of binocular rivalry reveals a fundamental role of noise. J Vis. 2006;6:1244–1256. doi: 10.1167/6.11.8. [DOI] [PubMed] [Google Scholar]
- 11.Blake R, O'Shea RP, Mueller TJ. Spatial zones of binocular rivalry in central and peripheral vision. Vis Neurosci. 1992;8:469–478. doi: 10.1017/s0952523800004971. [DOI] [PubMed] [Google Scholar]
- 12.O'Shea RP, Sims AJ, Govan DG. The effect of spatial frequency and field size on the spread of exclusive visibility in binocular rivalry. Vision Res. 1997;37:175–183. doi: 10.1016/s0042-6989(96)00113-7. [DOI] [PubMed] [Google Scholar]
- 13.Kovacs I, Papathomas TV, Yang M, Feher A. When the brain changes its mind: interocular grouping during binocular rivalry. Proc Natl Acad Sci U S A. 1996;93:15508–15511. doi: 10.1073/pnas.93.26.15508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson HR, Blake R, Lee S-H. Dynamics of travelling waves in visual perception. Nature. 2001;412:907–910. doi: 10.1038/35091066. [DOI] [PubMed] [Google Scholar]
- 15.Noest AJ, van Ee R, Nijs MM, van Wezel RJA. Percept-choice sequences driven by interrupted ambiguous stimuli: a low-level neural model. J Vis. 2007;7:10, 11–14. doi: 10.1167/7.8.10. [DOI] [PubMed] [Google Scholar]
- 16.Hollins M, Hudnell K. Adaptation of the binocular rivalry mechanism. Invest Ophthalmol Vis Sci. 1980;19:1117–1120. [PubMed] [Google Scholar]
- 17.Vul E, Krizay E, MacLeod DIA. The McCollough effect reflects permanent and transient adaptation in early visual cortex. J Vis. 2008;8:4, 1–12. doi: 10.1167/8.12.4. [DOI] [PubMed] [Google Scholar]
- 18.Thompson PG, Movshon JA. Storage of spatially specific threshold elevation. Perception. 1978;7:65–73. doi: 10.1068/p070065. [DOI] [PubMed] [Google Scholar]
- 19.Verstraten FA, Fredericksen RE, Grüsser OJ, van de Grind WA. Recovery from motion adaptation is delayed by successively presented orthogonal motion. Vision Res. 1994;34:1149–1155. doi: 10.1016/0042-6989(94)90297-6. [DOI] [PubMed] [Google Scholar]
- 20.van de Grind WA, van der Smagt MJ, Verstraten FAJ. Storage for free: a surprising property of a simple gain-control model of motion aftereffects. Vision Res. 2004;44:2269–2284. doi: 10.1016/j.visres.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Alais D, Melcher D. Strength and coherence of binocular rivalry depends on shared stimulus complexity. Vision Res. 2007;47:269–279. doi: 10.1016/j.visres.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Blake R, Logothetis NK. Visual competition. Nat Rev Neurosci. 2002;3:13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- 23.Wilson HR. Computational evidence for a rivalry hierarchy in vision. Proc Natl Acad Sci USA. 2003;100:14499–14503. doi: 10.1073/pnas.2333622100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sejnowski TJ. The once and future of Hebb synapse. Canadian Psychology. 2003;44:17–20. [Google Scholar]
- 25.Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- 26.Földiák P. Forming sparse representations by local anti-Hebbian learning. Biological Cybernetics. 1990;64:165–170. doi: 10.1007/BF02331346. [DOI] [PubMed] [Google Scholar]
- 27.Nelson SB. Hebb and anti-Hebb meet in the brainstem. Nat Neurosci. 2004;7:687–688. doi: 10.1038/nn0704-687. [DOI] [PubMed] [Google Scholar]
- 28.Deco G, Obradovic D. Decorrelated Hebbian Learning for Clustering and Function Approximation. Neural Comput. 1995;7:338–348. [Google Scholar]
- 29.Bell CC, Han VZ, Sugawara Y, Grant K. Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature. 1997;387:278–281. doi: 10.1038/387278a0. [DOI] [PubMed] [Google Scholar]
- 30.Lamsa KP, Heeroma JH, Somogyi P, Rusakov DA, Kullmann DM. Anti-Hebbian long-term potentiation in the hippocampal feedback inhibitory circuit. Science. 2007;315:1262–1266. doi: 10.1126/science.1137450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci. 2004;7:719–725. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- 32.Yazaki-Sugiyama Y, Kang S, Câteau H, Fukai T, Hensch TK. Bidirectional plasticity in fast-spiking GABA circuits by visual experience. Nature. 2009;462:218–221. doi: 10.1038/nature08485. [DOI] [PubMed] [Google Scholar]
- 33.Carandini M, Barlow HB, O'Keefe LP, Poirson AB, Movshon JA. Adaptation to contingencies in macaque primary visual cortex. Philos Trans R Soc Lond B Biol Sci. 1997;352:1149–1154. doi: 10.1098/rstb.1997.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falconbridge M, Badcock DR. Implicit exploitation of regularities: novel correlations in images quickly alter visual perception. Vision Res. 2006;46:1331–1335. doi: 10.1016/j.visres.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 35.van Ee R. Dynamics of perceptual bi-stability for stereoscopic slant rivalry and a comparison with grating, house-face, and Necker cube rivalry. Vision Res. 2005;45:29–40. doi: 10.1016/j.visres.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki S, Grabowecky M. Long-term speeding in perceptual switches mediated by attention-dependent plasticity in cortical visual processing. Neuron. 2007;56:741–753. doi: 10.1016/j.neuron.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 37.Brascamp JW, van Ee R, Pestman W, van den Berg AV. Distributions of alternation rates in various forms of bistable perception. J Vis. 2005;5:287–298. doi: 10.1167/5.4.1. [DOI] [PubMed] [Google Scholar]
- 38.Pearson J, Clifford CWG. When your brain decides what you see: grouping across monocular, binocular, and stimulus rivalry. Psychol Sci. 2005;16:516–519. doi: 10.1111/j.0956-7976.2005.01566.x. [DOI] [PubMed] [Google Scholar]
- 39.Klink PC, van Ee R, Nijs MM, Brouwer G, Noest AJ, van Wezel RJA. Early interactions between neuronal adaptation and voluntary control determine perceptual choices in bistable vision. J Vis. 2008;8:16, 11–18. doi: 10.1167/8.5.16. [DOI] [PubMed] [Google Scholar]
- 40.Ding J, Sperling G. A gain-control theory of binocular combination. Proc Natl Acad Sci USA. 2006;103:1141–1146. doi: 10.1073/pnas.0509629103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrews TJ, Purves D. Similarities in normal and binocularly rivalrous viewing. Proc Natl Acad Sci USA. 1997;94:9905–9908. doi: 10.1073/pnas.94.18.9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blake R, Yang YD, Wilson HR. On the coexistence of stereopsis and binocular rivalry. Vision Res. 1991;31:1191–1203. doi: 10.1016/0042-6989(91)90044-6. [DOI] [PubMed] [Google Scholar]
- 43.Livingstone MS. Differences between stereopsis, interocular correlation and binocularity. Vision Res. 1996;36:1127–1140. doi: 10.1016/0042-6989(95)00175-1. [DOI] [PubMed] [Google Scholar]
- 44.Grossberg S, Yazdanbakhsh A, Cao Y, Swaminathan G. How does binocular rivalry emerge from cortical mechanisms of 3-D vision? Vision Res. 2008;48:2232–2250. doi: 10.1016/j.visres.2008.06.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.