Summary
A fundamental component of signaling initiated by the BCR and CD19 is the activation of phosphoinositide 3-kinase (PI3K). Downstream of PI3K, the protein kinase AKT phosphorylates several substrates, including members of the Forkhead Box Subgroup O (Foxo) transcription factor family. Among the Foxo proteins, Foxo1 has unique functions in bone marrow B-cell development and peripheral B-cell function. Here we report a previously unrecognized role for Foxo1 in controlling the ratio of mature B-cell subsets in the spleen. Conditional deletion of Foxo1 in B cells resulted in an increased percentage of marginal zone B cells and a decrease in follicular B cells. In addition, Foxo1 deficiency corrected the absence of marginal zone B cells that occurs in CD19-deficient mice. These findings show that Foxo1 regulates the balance of mature B cell subsets and is required for the marginal zone B-cell deficiency phenotype of mice lacking CD19.
Keywords: B cell development, B cells, signal transduction, transcription factors
Introduction
BCR crosslinking activates PI3K, whose lipid products orchestrate the assembly of membrane-associated signaling complexes [1]. One group of proteins, termed the BCR signalosome, is responsible for maximal activation of phospholipase C-γ and subsequent phosphoinositide hydrolysis and Ca2+ mobilization. Another outcome of PI3K signaling is the activation of AKT. The AKT the serine/threonine kinases have numerous substrates, whose phosphorylation state controls diverse processes including proliferation, survival, metabolism and differentiation. The roles of most AKT substrates in B cell biology have not been defined.
CD19 is a transmembrane protein that enhances BCR signaling by multiple mechanisms [2, 3]. CD19 interacts with CD21 and CD81 to form the B cell co-receptor, which clusters with the BCR in response to complement-tagged antigens to augment signaling. CD19 can also associate with the BCR in the absence of CD21 to promote BCR signalosome assembly upon recognition of membrane-associated antigens [4]. The cytoplasmic tail of CD19 contains two canonical motifs for recruitment of PI3K (YXXM), and these are required for CD19 function [5]. Genetic evidence supports a functional role for AKT downstream of CD19, in that combined deletion of two AKT genes (Akt1 and Akt2) in mouse B cells confers a defect in marginal zone (MZ) B cell development [6] similar to the phenotype of CD19-deficient mice [5, 7]. However, it is not yet clear which AKT substrates regulate MZ-cell development.
Foxo transcription factors activate or suppress target genes in a cell type-specific and context-dependent manner [8, 9]. In resting lymphocytes, Foxo proteins are localized to the nucleus and activate genes that maintain quiescence as well as proper homing and recirculation [1]. Phosphorylation by AKT causes cytoplasmic sequestration and degradation of Foxo factors, inhibiting the Foxo gene expression program. The Foxo1 family member has been studied in lymphocytes by conditional deletion using Cre-lox systems. This work has identified unique roles for Foxo1 in several aspects of B cell function [10]. Deletion of the Foxo1 gene in early B cell progenitors using Mb1Cre caused a block at the pro-B cell stage. Deletion at a later stage with Cd19Cre caused a partial block at the pre-B cell stage. Deletion of Foxo1 in late transitional B cells with Cd21Cre blocked class-switch recombination. We have examined in more detail the phenotype of mature B cells in mice with Cd19Cre-mediated deletion of Foxo1. We find that these mice have fewer follicular (FO) B cells and a higher percentage of MZ cells. In mice homozygous for the Cd19Cre knock-in allele, which lack CD19 protein, MZ cells are absent as reported previously [5, 7] but this defect is reversed by the concomitant deletion of Foxo1. This genetic epitasis analysis suggests the possibility that CD19 negatively regulates Foxo1 to promote MZ B cell development.
Results and Discussion
Deletion of Foxo1 affects B cell development
We generated a conditional Foxo1 allele by inserting LoxP sites flanking the first exon of Foxo1 [11]. Mice homozygous for the Foxo1-flox allele are denoted Foxo1f/f herein. We bred Foxo1f/f mice with Cd19Cre mice in which the Cre recombinase is knocked into the Cd19 locus [12]. Splenic B cells from Foxo1f/fCd19Cre mice expressed no detectable Foxo1 protein as determined by immunoblot, while Foxo3a expression was unchanged (Supporting Information Fig. 1A). Several aspects of B cell development in these mice were altered in a manner similar to the phenotype of another strain of Foxo1f/fCd19Cre mice reported by Dengler and colleagues [10]. In particular, our Foxo1f/fCd19Cre mice had fewer IgM+ bone marrow B cells (Supporting Information Fig. 1B), and a population of peripheral B220+ cells lacking surface expression of IgM or IgD (Supporting Information Fig. 1C). Furthermore, Foxo1f/fCd19Cre mice had markedly fewer lymph node (LN) B cells and an increase in peripheral blood B cells (Supporting Information Fig. 1D). The paucity of LN B cells correlated with reduced surface expression of CD62L (L-selectin), the LN homing receptor (Supporting Information Fig. 1E). The mice also had a reduced percentage of CD5+ B cells in the peritoneal cavity (Supporting Information Fig. 1F).
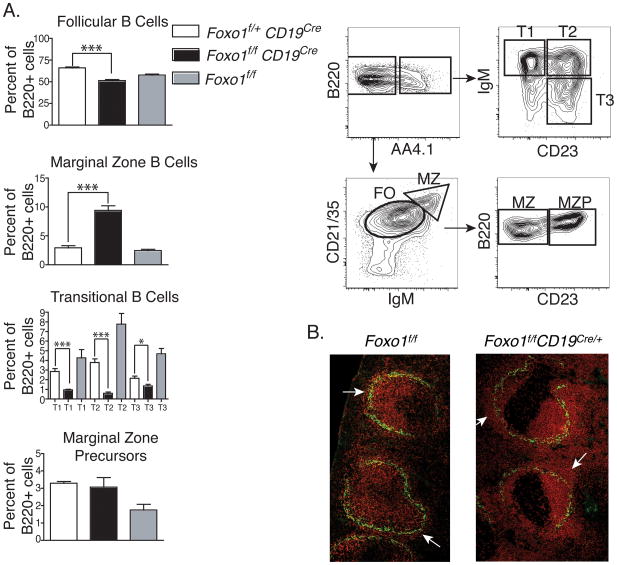
The report from Dengler et al. did not examine the developmental status or function of peripheral B220+IgM+ cells in Foxo1f/fCd19Cre mice [10]. We stained splenocytes from our Foxo1f/fCd19Cre mice and controls with antibody combinations that distinguish two mature subsets (FO, MZ) and four transitional B cell subsets (T1, T2, T3 and marginal zone precursor (MZP)) [13]. When compared to control Foxo1f/+Cd19Cre mice, Foxo1f/fCd19Cre mice displayed a consistent and statistically significant increase in the percentage of MZ cells, defined as B220+AA4.1−IgMhiCD21hiCD23lo (Fig. 1A). In contrast, the percentage of FO cells (B220+AA4.1−IgMloCD21intCD23hi) was reduced (Fig. 1A). A normal percentage of MZP cells was present in Foxo1f/fCd19Cre mice, despite reduced percentages of T1 and T2 cells; this suggests that immature transitional cells might commit preferentially to the MZP stage. The absolute numbers of splenocytes were equivalent between Foxo1f/fCd19Cre mice and controlmice (data not shown). Increased abundance of B220+ cells in the splenic marginal zone and other extrafollicular regions was also apparent by immunofluorescent staining of spleen sections (Fig. 1B). The percentages of mature FO and MZ cells were comparable in the two control groups (Foxo1f/+Cd19Cre and Foxo1f/f) (Fig. 1A), and other experiments showed a consistently greater population of MZ cells (B220+CD21hiCD23lo) in Foxo1f/fCd19Cre compared to Foxo1f/f mice (data not shown). Therefore, we used Foxo1f/f mice as controls in Figure 1B and in other experiments to simplify breeding schemes.
Figure 1.
Comparison of splenic B cell subsets by FACS and histology. (A) Flow cytometry was used to distinguish FO, MZ and immature (T1, T2, T3 and MZP) subsets. Spleen cells were stained with antibodies to B220, AA4.1, IgM, CD21 and CD23. The gating strategy is shown on the right for a representative control spleen. Graphs on the left depict mean cell percentages + SD. n = 4 Foxo1f/+Cd19Cre and Foxo1f/fCd19Cre; n = 2 Foxo1f/f. *p<0.05; ***p<0.001, unpaired two-tailed t-test. (B) Immunofluorescent staining of spleen sections with antibodies to B cells (anti-B220; red) and macrophages lining the marginal zone (anti-MOMA-1, green) shows increased population of MZ B cells (white arrows) in Foxo1f/fCd19Cre mice. Confocal images (10X magnification; scale bar = 100μM) are representative of at least 10 follicles from 2 mice of each genotype.
The altered balance of FO and MZ cells in Foxo1f/fCd19Cre mice was not observed in analyses of mice with Foxo1-deficient B cells generated using Cd21Cre [10]. A likely explanation is that Cd21Cre drives deletion of Foxo1 at a time point after transitional B cells commit to either the FO or MZ lineage, whereas Cd19Cre deletion is complete by this stage. Interestingly, Foxo1f/fCd21Cre mice [10] shared the reduced LN B cell population and CD62L expression observed here in Foxo1f/fCd19Cre mice. This could be explained by a requirement for Foxo1 in CD62L gene expression in mature B cells, after Cd21Cre-mediated deletion is completed.
Altered proliferative responses in Foxo1-deficient B cells
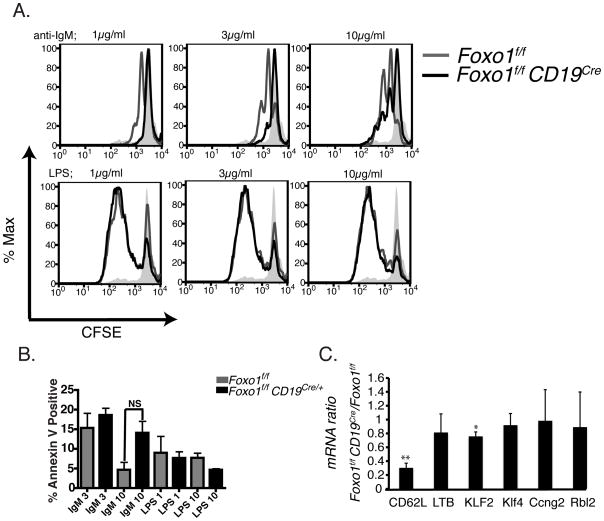
We purified splenic B cells and activated them in vitro with titrated doses of either a BCR stimulus (anti-IgM) or a TLR stimulus (LPS). We measured cell proliferation and survival by cell division tracking using CFSE. B cells from Foxo1f/fCd19Cre proliferated more weakly to anti-IgM, compared to B cells from Foxo1f/f mice (Fig. 2A). There was no significant difference in apoptosis in Foxo1f/fCd19Cre B cells stimulated with anti-IgM or LPS (Fig. 2B). The altered response to anti-IgM could arise from a decreased FO/MZ ratio, since BCR engagement causes proliferation of FO cells but apoptosis of MZ cells [14, 15]. However, reduced anti-IgM-mediated proliferation was also observed in B cells from Foxo1f/fCd21Cre mice in which no changes in FO/MZ ratios were reported [10], and is consistent with the presence of a prominent IgM- B cell population (Supporting Information Fig. 1C). Measuring live cell number using a metabolic dye conversion (MTS) assay confirmed the finding of impaired anti-IgM response in Foxo1f/fCd19Cre B cells (Supporting Information Fig. 2A). The LPS response in Foxo1f/fCd19Cre B cells was increased when measured using MTS assays (Supporting Information Fig. 2A), but not using the CFSE assay (Fig. 2A). This might indicate that LPS-stimulated B cells have altered metabolism when Foxo1 is absent, leading to increased MTS conversion despite equivalent cell number.
Figure 2.
Comparison of proliferation, apoptosis and gene expression. (A) Purified B cells from Foxo1f/fCD19Cre and control Foxo1f/f mice were labeled with CFSE, then stimulated with anti-IgM or LPS at the indicated concentrations for 66 h at 37 °C. Filled gray histogram shows unstimulated control cells. Similar results were obtained in 4 separate experiments. (B) In 3 of the CFSE labeling experiments, cells were stained at 66 hr with Annexin V to quantitate apoptotic cells among the divided population. Data show mean + SD. Unpaired two-tailed t-tests showed no significant differences between genotypes (p>0.05 for all comparisons; p = 0.055 for comparison labeled “NS”). (C) Quantitative real-time PCR (qRT-PCR) data using RNA extracted from B cells of Foxo1f/fCD19Cre and control Foxo1f/f mice. The numbers indicate the ratio of each gene in Foxo1f/fCD19Cre as compared to Foxo1f/f mice, after normalization to βactin. Data show mean + SD of three separate experiments. *p<0.05; * p<0.01; comparing ratio of each gene to 1.0 using a one-tailed t-test.
Transforming growth factor-beta (TGF-β) is a cytokine with potent anti-proliferative effects in lymphocytes [16]. TGF-β signaling activates Smad transcription factors, which in several cellular systems cooperate with Foxo proteins to activate target promoters [17, 18]. Furthermore, the TGF-β/Smad signaling axis regulates marginal zone B cell development [19]. Although we obtained evidence for functional cooperation of Foxo1 and Smad transcription factors in B cells (Supporting Information Fig. 2B, C), Foxo1 was not required for TGF-β-mediated suppression of B cell proliferation triggered by anti-IgM or LPS (Supporting Information Fig. 2A).
Gene expression changes in Foxo1-deficient B cells
CD62L mRNA was consistently reduced about 3-fold in Foxo1f/fCd19Cre B cells (Fig. 2C), indicating that lower CD62L protein expression on the surface of these cells is at least partly due to reduced steady state mRNA levels, resulting from altered transcription and/or RNA processing. Foxo1 also controls expression of the Sell gene encoding CD62L in T cells [20–22]. Another Foxo target gene, Klf2, regulates CD62L expression in T cells and might be a link between Foxo1 and CD62L [20, 21, 23–25]. Klf2 mRNA expression was also significantly reduced in Foxo1-deficient B cells, though less prominently than the reduction in Sell mRNA (Fig. 2C). Previously, we identified Ccng2, Rbl2 and Klf4 as Foxo target genes in B cells [26, 27]. By various criteria, including reporter assays, electrophoretic mobility shift assays and chromatin immunoprecipitation, these genes were regulated similarly by Foxo1 and Foxo3a [26, 27]. RNA measurements using quantitative real-time PCR showed that none of these genes were differentially expressed in Foxo1-deficient B cells (Fig. 2C), further suggesting that Foxo1 and Foxo3a have redundant functions at these target promoters.
Foxo1 deficient complements MZ phenotype in CD19 knockout mice
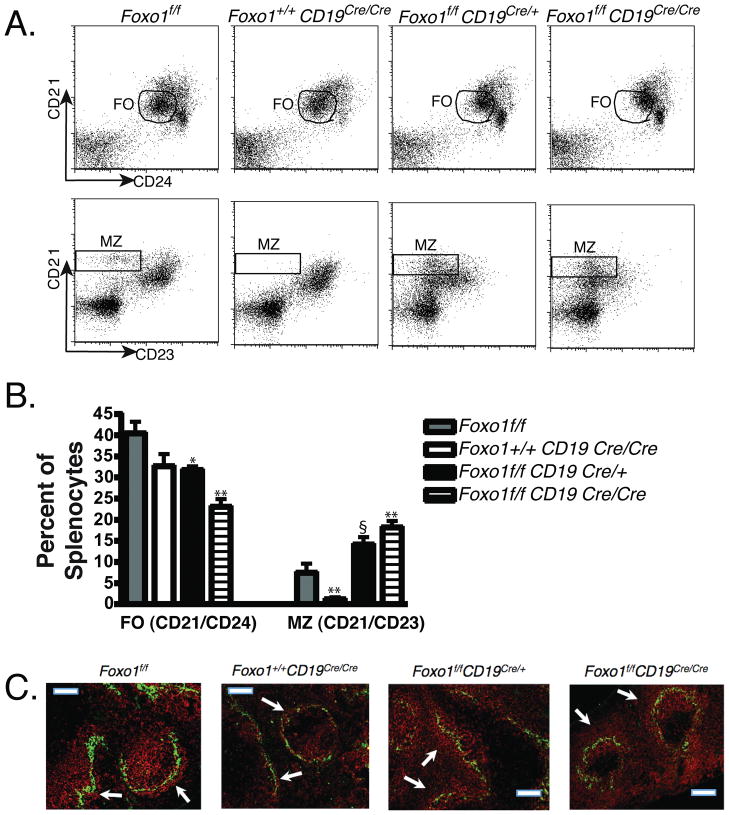
The increased population of MZ B cells in Foxo1f/fCd19Cre mice was intriguing, since Foxo factors are turned off by the PI3K/AKT pathway and the opposite phenotype occurs in mice lacking PI3K genes [28–30]. CD19-deficient mice, which display reduced PI3K/AKT signaling in B cells, also have a greatly reduced MZ population [5, 7], as do chimeric mice generated with Akt1/Akt2-deficient progenitors [6]. Conversely, loss of the PTEN phosphatase that opposes PI3K signaling expands the MZ subset and overcomes the loss of CD19 [31]. Like the MZ cell increase in Foxo1f/fCd19Cre mice, the MZ cell decreases in mice lacking PI3K, Akt1/Akt2 or CD19 are B cell-intrinsic [6, 7, 32]. We therefore considered the possibility that Foxo1 inactivation is central to MZ lineage choice promoted by CD19/PI3K. It was convenient to test this possibility for CD19 in our system, since breeding of the Cd19Cre knock-in allele to homozygosity generates mice lacking CD19 expression. As expected, homozygous Cd19Cre/Cre mice had a profound reduction in the MZ population as determined by CD21/CD23 staining (Fig. 3A, B) and immunofluorescent staining of spleen sections (Fig. 3C). In CD19/Foxo1 double-deficient mice (genotype = Foxo1f/fCd19Cre/Cre), the frequency of MZ B cells was restored to the levels seen in Foxo1f/fCd19Cre mice, again elevated relative to Foxo1f/f mice (Fig. 3A, B). Therefore, loss of Foxo1 has a dominant effect on MZ lineage choice and is sufficient to complement the MZ B cell defect arising in CD19-deficient mice. Interestingly, CD19/Foxo1 double-deficient mice had a greater reduction of FO B cells than either Foxo1f/fCd19Cre or Cd19Cre/Cre mice (Fig. 3A, B). Further work is required to investigate whether this phenomenon results from impaired development or survival of CD19/Foxo1 double-deficient FO cells.
Figure 3.
Assessment of complementation of the MZ B cell defect in CD19-deficient mice. (A) Flow cytometry was used to compare FO and MZ B cell percentages from representative mice. B220-gated cells were analyzed for CD21 and CD24 to quantitate FO cells (CD21intCD24int), or CD21 and CD23 to quantitate MZ cells (CD21hiCD23lo). (B) The percentages of FO and MZ cells in the spleens from mice of each genotype are shown. Data represent mean ± SD, n = 3-5 mice. *p<0.05; **p<0.01 vs. Foxo1f/f, using unpaired, two-tailed t-test. §p = 0.051 vs. Foxo1f/f. (C) Immunofluorescent staining of spleen sections was done as in Figure 1B.
Concluding Remarks
CD19 is essential for proper B cell development and activation, and most of these functions require the PI3K binding sites in the cytoplasmic tail of CD19 [5] and are opposed by PTEN [31]. One phenotype shared by mice lacking CD19 or PI3K/AKT signaling components is a near absence of MZ B cells. Other work has shown that the MZ lineage choice is promoted by a low level of self-antigen [33] and that CD19 associates with BCR signaling clusters and promotes activation even in the absence of complement fragments and co-receptor action [4]. Together these observations suggest a model in which CD19 promotes MZ development by enhancing self antigen-triggered BCR signaling and PI3K activation. CD19 and PI3K augment Ca2+ mobilization, in part through membrane recruitment and activation of the tyrosine kinase BTK [34]. However, mice lacking BTK have a normal MZ B cell compartment [29, 35]. Recent findings indicate that AKT, a well known downstream target of PI3K, is a relevant effector for MZ B cell lineage choice [6]. The results presented here suggest that of the many downstream sequelae of AKT activation, the inactivation of Foxo1 is integral to the developmental choice between FO and MZ B cell lineages. Along with previous findings demonstrating stage-specific functions of Foxo1 in B cells and T cells [10, 21, 22], these findings further illustrate the complex and context-specific role of forkhead transcription factors such as Foxo1 in lymphocyte development, differentiation, and activation.
Materials and methods
Mice
Foxo1f/f mice were reported previously [11] and were used here in a mixed genetic background. CD19-Cre C57Bl/6 mice were purchased from the Jackson Laboratory. CD19-Cre C57Bl/6 mice were bred to Foxo1f/f mice and the progeny were intercrossed to generate mice of the different genotypes used in this study. Control mice were littermates or relatives in a similar mixed background. All animal protocols were approved by the Institutional Animal Care and Use Committee of University of California, Irvine.
Flow cytometry
Single cell suspensions were obtained from the spleens, lymph nodes, bone marrow and peritoneal lavages of 6–8 week-old mice. Cell suspensions from spleen and bone marrow were depleted of RBCs by hypotonic lysis. Approximately 1 million cells were used for antibody staining. All antibodies were purchased from eBioscience. Data from at least 20, 000 total events were acquired and analyzed (FACSCalibur and CellQuest software, BD Biosciences; FlowJo software, Treestar).
Immunohistochemistry
Immunohistochemistry was carried out as previously described [3] with slight modification. Mouse spleens were harvested, embedded in OCT medium (Sakura, Torrance, CA) and frozen in 2-methylbutane precooled by liquid nitrogen. Eight-micrometer sections were cut and mounted on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA). Slides were cleared with CitriSolv (Fisher), fixed with Acetone in −20°C for 20 min, and blocked with 10% goat serum (Vector Laboratories, Burlingame, CA) in PBS for 30 min at room temperature (25°C). Immunohistochemical staining was done sequentially with rat anti-mouse B220 (BD Biosciences), goat anti-rat IgG Alexa Fluor 594 (Molecular Probes) and FITC-conjugated rat anti-mouse metallophilic macrophage (MOMA-1, MCA947F; Serotec) each diluted in PBS, for 1 h at room temperature, and followed by 3×5 min wash in PBS after each staining. All images shown were acquired at 10× magnification using Olympus Fluoview FV1000 Laser Scanning Confocal Microscope.
B cell proliferation and survival assays
Purified B cells were cultured in RPMI 1640 supplemented with 10% heatinactivated FBS, 5 mM HEPES, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 50 μM 2-ME at 37°C incubator with 5% CO2. For cell division tracking, B cells were labeled with carboxyfluorescein succinimidylester (CFSE) as described previously [2, 4, 5]. Labeled cells were stimulated in 48-well dishes with goat anti-mouse IgM (F(ab′)2,; Jackson ImmunoResearch Laboratories, West Grove, PA) or LPS (serotype 0127:B8; Sigma-Aldrich, St Louis, MO). After 66 hr, cells were harvested, stained with Annexin-V-PE and analyzed by FACS. For the MTS assay format, B cells were first treated with or without TGF-β (Sigma) at 5ng/ml for 15 minutes, and were stimulated in triplicate in 100 μl of total volume in 96-well flat bottom dishes, using mitogens as described for the CFSE assays. After an incubation period of 66 hr, twenty microliters of CellTiter 96 Aqueous One Solution Reagent (Promega) were added to each well, incubated for 3-4 hours at 37°C, and then absorbance at 490 nm was determine in a plate reader.
qRT-PCR
RNA was prepared from purified B cells of Foxo1f/fCd19Cre and Foxo1f/f mice using Trizol reagent (Invitrogen) as previously described [1, 2]. cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). Primers for genes of interest (Klf2, Klf4, Ccng2, Rbl2, Sell and Ltb) and housekeeping gene (β-actin) were optimized to amplify products between 75 and 200 nucleotides. Primer sequences are available on request. Q-PCR was performed with SyBr green as previously described [1, 2].
Statistics
A two-tailed student’s t-test was used for all comparisons. The specifics of each test (one vs. two-tailed) are indicated in the figure legends.
Supplementary Material
Acknowledgments
This work was supported in part by a Research Scholar Grant from the American Cancer Society (to DAF) and by NIH grants AI057471 and AI061478 (to SLP). We thank Craig Walsh and Aimee Edinger for helpful discussions, Lomon So for technical assistance, and Christine McLaren for statistical analysis.
Abbreviations
- PI3K
phosphoinositide 3-kinase
- Foxo
Forkhead Box Subgroup O
- FO
follicular
- MZ
marginal zone
- Tr
transitional
- TGF-β
Transforming growth factor-beta
Footnotes
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Fruman DA, Bismuth G. Fine tuning the immune response with PI3K. Immunol Rev. 2009;228:253–272. doi: 10.1111/j.1600-065X.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- 2.Carter RH, Wang Y, Brooks S. Role of CD19 signal transduction in B cell biology. Immunol Res. 2002;26:45–54. doi: 10.1385/IR:26:1-3:045. [DOI] [PubMed] [Google Scholar]
- 3.Del Nagro CJ, Otero DC, Anzelon AN, Omori SA, Kolla RV, Rickert RC. CD19 function in central and peripheral B-cell development. Immunol Res. 2005;31:119–131. doi: 10.1385/IR:31:2:119. [DOI] [PubMed] [Google Scholar]
- 4.Depoil D, Fleire S, Treanor BL, Weber M, Harwood NE, Marchbank KL, Tybulewicz VL, Batista FD. CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nat Immunol. 2008;9:63–72. doi: 10.1038/ni1547. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Brooks SR, Li X, Anzelon AN, Rickert RC, Carter RH. The physiologic role of CD19 cytoplasmic tyrosines. Immunity. 2002;17:501–514. doi: 10.1016/s1074-7613(02)00426-0. [DOI] [PubMed] [Google Scholar]
- 6.Calamito M, Juntilla MM, Thomas M, Northrup DL, Rathmell J, Birnbaum MJ, Koretzky G, Allman D. Akt1 and Akt2 promote peripheral B cell maturation and survival. Blood. 2009 doi: 10.1182/blood-2009-09-241638. Epub Dec. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.You Y, Zhao H, Wang Y, Carter RH. Cutting edge: Primary and secondary effects of CD19 deficiency on cells of the marginal zone. J Immunol. 2009;182:7343–7347. doi: 10.4049/jimmunol.0804295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng SL. Foxo in the immune system. Oncogene. 2008;27:2337–2344. doi: 10.1038/onc.2008.26. [DOI] [PubMed] [Google Scholar]
- 9.van der Vos KE, Coffer PJ. FOXO-binding partners: it takes two to tango. Oncogene. 2008;27:2289–2299. doi: 10.1038/onc.2008.22. [DOI] [PubMed] [Google Scholar]
- 10.Dengler HS, Baracho GV, Omori SA, Bruckner S, Arden KC, Castrillon DH, DePinho RA, Rickert RC. Distinct functions for the transcription factor Foxo1 at various stages of B cell differentiation. Nat Immunol. 2008;9:1388–1398. doi: 10.1038/ni.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gubbels Bupp MR, Edwards B, Guo C, Wei D, Chen G, Wong B, Masteller E, Peng SL. T cells require Foxo1 to populate the peripheral lymphoid organs. Eur J Immunol. 2009;39:2991–2999. doi: 10.1002/eji.200939427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allman D, Pillai S. Peripheral B cell subsets. Curr Opin Immunol. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donahue AC, Fruman DA. Distinct signaling mechanisms activate the target of rapamycin in response to different B-cell stimuli. Eur J Immunol. 2007;37:2923–2936. doi: 10.1002/eji.200737281. [DOI] [PubMed] [Google Scholar]
- 15.Oliver AM, Martin F, Gartland GL, Carter RH, Kearney JF. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur J Immunol. 1997;27:2366–2374. doi: 10.1002/eji.1830270935. [DOI] [PubMed] [Google Scholar]
- 16.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 17.Gomis RR, Alarcon C, He W, Wang Q, Seoane J, Lash A, Massague J. A FoxO-Smad synexpression group in human keratinocytes. Proc Natl Acad Sci U S A. 2006;103:12747–12752. doi: 10.1073/pnas.0605333103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seoane J, Le HV, Shen L, Anderson SA, Massague J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–223. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- 19.Klein J, Ju W, Heyer J, Wittek B, Haneke T, Knaus P, Kucherlapati R, Bottinger EP, Nitschke L, Kneitz B. B cell-specific deficiency for Smad2 in vivo leads to defects in TGF-beta-directed IgA switching and changes in B cell fate. J Immunol. 2006;176:2389–2396. doi: 10.4049/jimmunol.176.4.2389. [DOI] [PubMed] [Google Scholar]
- 20.Fabre S, Carrette F, Chen J, Lang V, Semichon M, Denoyelle C, Lazar V, Cagnard N, Dubart-Kupperschmitt A, Mangeney M, Fruman DA, Bismuth G. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J Immunol. 2008;181:2980–2989. doi: 10.4049/jimmunol.181.5.2980. [DOI] [PubMed] [Google Scholar]
- 21.Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, Hedrick SM. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin receptor. Nat Immunol. 2009;10:176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouyang W, Beckett O, Flavell RA, Li MO. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity. 2009;30 :358–371. doi: 10.1016/j.immuni.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 24.Sebzda E, Zou Z, Lee JS, Wang T, Kahn ML. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nat Immunol. 2008;9:292–300. doi: 10.1038/ni1565. [DOI] [PubMed] [Google Scholar]
- 25.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Yusuf I, Andersen HM, Fruman DA. FOXO transcription factors cooperate with delta EF1 to activate growth suppressive genes in B lymphocytes. J Immunol. 2006;176:2711–2721. doi: 10.4049/jimmunol.176.5.2711. [DOI] [PubMed] [Google Scholar]
- 27.Yusuf I, Kharas MG, Chen J, Peralta RQ, Maruniak A, Sareen P, Yang VW, Kaestner KH, Fruman DA. KLF4 is a FOXO target gene that suppresses B cell proliferation. Int Immunol. 2008;20:671–681. doi: 10.1093/intimm/dxn024. [DOI] [PubMed] [Google Scholar]
- 28.Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, Humphries LA, Rawlings D, Reynolds H, Vigorito E, Turner M. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med. 2002;196:753–763. doi: 10.1084/jem.20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donahue AC, Hess KL, Ng KL, Fruman DA. Altered splenic B cell subset development in mice lacking phosphoinositide 3-kinase p85alpha. Int Immunol. 2004;16:1789–1798. doi: 10.1093/intimm/dxh180. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki H, Matsuda S, Terauchi Y, Fujiwara M, Ohteki T, Asano T, Behrens TW, Kouro T, Takatsu K, Kadowaki T, Koyasu S. PI3K and Btk differentially regulate B cell antigen receptor-mediated signal transduction. Nat Immunol. 2003;4:280–286. doi: 10.1038/ni890. [DOI] [PubMed] [Google Scholar]
- 31.Anzelon AN, Wu H, Rickert RC. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat Immunol. 2003;4:287–294. doi: 10.1038/ni892. [DOI] [PubMed] [Google Scholar]
- 32.Oak JS, Chen J, Peralta RQ, Deane JA, Fruman DA. The p85beta regulatory subunit of phosphoinositide 3-kinase has unique and redundant functions in B cells. Autoimmunity. 2009;42:447–458. doi: 10.1080/08916930902911746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen L, Brill-Dashoff J, Shinton SA, Asano M, Hardy RR, Hayakawa K. Evidence of marginal-zone B cell-positive selection in spleen. Immunity. 2005;23:297–308. doi: 10.1016/j.immuni.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Donahue AC, Fruman DA. PI3K signaling controls cell fate at many points in B lymphocyte development and activation. Semin Cell Dev Biol. 2004;15:183–197. doi: 10.1016/j.semcdb.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 35.Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, Lamers MC, Carsetti R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.