Abstract
The endogenous retroviral envelope glycoprotein, gp70, implicated in murine systemic lupus erythematosus (SLE), has been considered to be a product of xenotropic, polytropic (PT) and modified PT (mPT) endogenous retroviruses. It is secreted by hepatocytes like an acute phase protein, but its response is under a genetic control. Given critical roles of TLR7 and TLR9 in the pathogenesis of SLE, we assessed their contribution to the acute phase expression of serum gp70, and defined a pivotal role of the Sgp3 (serum gp70 production 3) and Sgp4 loci in this response. Our results demonstrated that serum levels of gp70 were up-regulated in lupus-prone NZB mice injected with TLR7 or TLR9 agonist at levels comparable to those induced by injection of IL-1, IL-6 or TNF. In addition, studies of C57BL/6 Sgp3 and/or Sgp4 congenic mice defined the major roles of these two loci in up-regulated production of serum gp70 during acute phase responses. Finally, the analysis of Sgp3 congenic mice strongly suggests the presence of at least two distinct genetic factors in the Sgp3 interval, one of which controlled the basal-level expression of xenotropic, PT and mPT gp70 and the other which controlled the up-regulated production of xenotropic and mPT gp70 during acute phase responses. Our results uncovered an additional pathogenic role of TLR7 and TLR9 in murine lupus nephritis by promoting the expression of nephritogenic gp70 autoantigen. Furthermore, they revealed the involvement of multiple regulatory genes for the expression of gp70 autoantigen under steady-state and inflammatory conditions in lupus-prone mice.
Keywords: Toll-like receptor, Systemic lupus erythematosus, Endogenous retrovirus, Acute phase protein
1. Introduction
Relatively large amounts of the envelope glycoprotein, gp70, of endogenous retroviruses circulate free from any association with viral particles in the blood of virtually all strains of mice [1-3]. The demonstration of retroviral gp70 in immune deposits within diseased glomeruli of mice with systemic lupus erythematosus (SLE) indicates the pathogenic role of gp70-anti-gp70 immune complexes (gp70 IC) in the development of lupus nephritis [4, 5]. Notably, only lupus-prone (NZB × NZW)F1, MRL and BXSB mice spontaneously develop autoantibodies against serum gp70, detected as gp70 IC, and appearance and amounts of gp70 IC closely parallel the course of disease in each lupus-prone mouse [6-8].
Endogenous retroviruses are classified as ecotropic, xenotropic or polytropic according to the host range dictated by their respective envelope gp70 proteins [9]. Furthermore, based on differences in their gp70 nucleotide sequences [9, 10], the polytropic proviruses have been divided into two subgroups, termed polytropic (PT) and modified PT (mPT). Serological and tryptic peptide mapping analysis showed that the serum gp70 molecule most closely resembles, but is not identical to the gp70 protein of xenotropic viruses isolated from NZB mice [3, 11]. Recent analysis of the abundance of retroviral gp70 RNAs in different strains indicated that PT and mPT proviruses that encode gp70s closely related to xenotropic gp70 are additional important sources of serum gp70 [12].
Serum retroviral gp70 is secreted by hepatocytes in the blood circulation [13], and its expression is controlled by multiple structural and regulatory genes [12, 14]. Genetic studies identified at least two loci, Sgp3 (serum gp70 production 3) on mid chromosome 13 and Sgp4 on distal chromosome 4, which control basal serum levels of gp70 [8, 15-20] through the transcriptional regulation of multiple endogenous retroviral proviruses [12]. Significantly, the expression of serum gp70 corresponds to that of acute phase proteins, in which it is enhanced by agents, such as LPS, polyriboinosinic-polyribocytidylic acid or turpentine oil, with kinetics identical to those of acute phase proteins [13]. However, this response is strain-dependent, in which only mice having high basal levels of serum gp70 displayed an up-regulated expression of serum gp70 in response to LPS [13, 14, 21]. Increases in serum levels of gp70 in Sgp3 congenic mice injected with LPS suggest that the Sgp3 locus contributed to LPS-induced enhanced production of serum gp70 [12, 18]. However, it has not yet been determined if the basal-level expression of serum gp70 and the LPS-mediated enhanced production of serum gp70 are co-regulated by the same genetic element present in the Sgp3 locus or by different genes in the interval.
Toll-like receptors (TLRs) are a family of germ-line encoded receptors that recognize a diverse range of conserved molecular motifs commonly found in microbial pathogens. Recognition of microbial components by TLR is critical in host responses against pathogens [22]. Three TLRs act as receptors for nucleic acids in mice: TLR3 for double-stranded RNA, TLR7 for single-stranded RNA and TLR9 for unmethylated CpG DNA. Notably, nucleic acids can act as endogenous ligands for TLR7 and TLR9 [23], which both contribute to the development of autoimmune responses against nuclear autoantigens as well as serum retroviral gp70 in murine SLE [24-27]. Injection of TLR agonists, LPS for TLR4 and polyriboinosinic-polyribocytidylic acid for TLR3, induced high serum levels of gp70 in lupus-prone mice [13]. Similarly, the activation of TLR7 and TLR9 by apoptotic cells accumulated in lupus-prone mice or IgG IC containing nucleic acids might boost the production of serum gp70 during the course of the disease, thereby further accelerating the progression of lupus nephritis.
In the present study, we have implicated TLR7 and TLR9 in the production of serum gp70, and defined the contribution of the Sgp3 and Sgp4 loci to the acute phase response of serum gp70. Our results demonstrated that the stimulation of TLR7 and TLR9 enhanced the production of serum gp70, and that the Sgp3 and Sgp4 loci synergistically contributed to the LPS-induced serum gp70 response. Furthermore, the analysis of mPT transcripts in Sgp3 congenic mice provided evidence that the gene involved in the LPS-induced up-regulated transcription of mPT proviruses was distinct from that controlling the basal-level expression of xenotropic, PT and mPT proviruses. This strongly suggested the presence of multiple genes in the Sgp3 locus regulating the expression of different classes of endogenous retroviruses under physiological or inflammatory condition.
2. Materials and methods
2.1. Mice
NZB and BXSB mice were purchased from the Jackson Laboratory (Bar Harbor, ME). B6.NZB-Sgp3 (B6.Sgp3) and B6.NZB-Sgp4 (B6.Sgp4) congenic mice were generated by backcross procedures, as described previously [12, 16]. B6 mice double congenic for both Sgp3 and Sgp4 loci (B6.Sgp3/4) were generated by intercrossing F1 progeny from B6.Sgp3 and B6.Sgp4 mice, using marker-assisted selection for the NZB-derived Sgp3 and Sgp4 intervals, as described previously [12]. All studies presented were carried out in female mice. Animal studies described in the present study have been approved by the Ethics Committee for Animal Experimentation of the Faculty of Medicine, University of Geneva.
2.2. Injection of TLR agonists or cytokines
TLR4 agonist, LPS from Escherchia coli 0111:B4 (Sigma-Aldrich, Saint Louis, MO), TLR7 agonist, 1V136 [28] (TLR7 Ligand II, Calbiochem, EMD Chemicals Inc., Darmstadt, Germany), TLR9 agonist, CpG-containing oligonucleotides (Type B CpG 1018; a kind gift of Dr. Eyal Raz, UCSD, San Diego, CA) or different cytokines were diluted to the desired concentrations with sterile PBS and i.p injected into 2-3 mo-old NZB, BXSB and B6 female mice. Recombinant human IL-1β, IL-6, TNFα and IFNβ were kindly provided by Dr Jean-Michel Dayer, University of Geneva, Switzerland. Livers and sera were collected 9 h after injection of LPS (25 μg), 1V136 (50 μg), CpG (50 μg), IL-1β (1 μg), IL-6 (5 μg), TNFα (5 μg), IFNβ (5 μg) or PBS.
2.3. Serological assays
Serum levels of retroviral gp70 from 2-3 mo-old female mice were determined by ELISA, as described previously [29]. Results are expressed as μg/ml of gp70 by referring to a standard curve obtained with a serum pool from NZB mice containing a known concentration of gp70.
2.4. Quantitative real-time RT-PCR
RNA from livers was purified with TRIzol reagent (Invitrogen AG, Basel, Switzerland) and treated with DNase I (Amersham Biosciences Corp., Piscataway, NJ). The abundance of xenotropic, PT and mPT env RNAs (genomic RNA and mRNA) was quantified by real-time RT-PCR, as described previously [30]. For the amplification of xenotropic gp70 cDNA, Xeno1098F forward and Xeno1298R reverse primers were used. For PT and mPT gp70 cDNA, a common PT/mPT730F forward primer, and PT892R and mPT880R reverse primers specific for PT and mPT viruses, respectively, were used. The two different deletion mutants (D1 and D2) env cDNAs were amplified using the following primers: mPT1115F forward and D1-R reverse primers for the D1 mutant; mPT1317F forward and D2-R reverse primers for the D2 mutant, as described [30]. Haptoglobin and arginine/serine-rich coiled-coil 1 (Rsrc1) mRNA levels were quantified using the following primers: haptoglobin; forward primer (5′-TGAACACAGTCGCTGGAGAG-3′) and reverse primer (5′-GCTGCCTTTGGCATCCATAG-3′), and Rsrc1; forward primer (5′-GCCACCCTGGTAGAACAAGTC-3′) and reverse primer (5′-GCACTTCACTTGGTTCTACTGC-3′). PCR was performed using the iCycler iQ Real-Time PCR Detection System (Bio-Rad, Philadelphia, PA) and iQ SYBR green Supermix (Bio-Rad). Results were quantified using a standard curve generated with serial dilutions of a reference cDNA preparation from NZB or BXSB liver and normalized using TATA-binding protein (TBP) mRNA.
2.5. RT-PCR
The gp70-p15E junction region of mPT env cDNAs was amplified with mPT-specific mPT858F gp70 forward primers and a common p15E-R reverse primer, as described [30]. For the amplification of wild-type (WT) and two deletion mutants (D1 and D2) of mPT env RNAs, an mPT858F forward primer and reverse primers specific for WT (mPT1447R) or deletion mutants (D1-R and D2-R) were used. Using these sets of primers, the abundance of three different species of mPT env RNAs was semi-quantified with 4-fold serially diluted cDNA templates. As a control, the abundance of GAPDH cDNA was semi-quantified. PCR products were visualized by staining with ethidium bromide after electrophoresis on 3.5% polyacrylamide or 2% agarose gels. Quantification was performed by densitometric analysis on the gels with the use of the ImageJ analysis program, and results of the intensity at each dilution of cDNAs are expressed as arbitrary units.
2.6. Statistical analysis
Unpaired comparison for hepatic levels of different RNAs, and paired comparison for serum levels of gp70 before and after injection of LPS were analyzed by Student's t test. Probability values <5% were considered significant.
3. Results
3.1. Increased levels of serum gp70 and hepatic gp70 RNAs in NZB mice after injection of 1V136, CpG or LPS
Injection of the TLR4 ligand, LPS, into lupus-prone NZB, NZW, BXSB and MRL mice promotes the production of serum gp70 [12-14]. In view of the critical involvement of TLR7 and TLR9 in the pathogenesis of SLE [24-27], we investigated whether the stimulation of TLR7 and TLR9 could enhance the production of serum gp70 in NZB mice. 9 h after injection of 50 μg of TLR7 agonist, 1V136, or TLR9 agonist, CpG, NZB mice displayed significant 3.2- and 4.6-fold increases of serum concentrations of gp70, respectively (P < 0.001), as compared with preinjection levels (Table 1). Time course studies confirmed that kinetics of the serum gp70 response elicited by 1V136 or CpG were comparable to that induced with LPS (data not shown). We quantified the changes in abundance of xenotropic, PT and mPT retroviral gp70 RNAs in livers after injection of 1V136 or CpG, in comparison with those after injection of LPS. Injection of 1V136 or CpG in NZB mice led to substantial up-regulation of xenotropic gp70 RNA levels (P < 0.0001), which were similar to those observed in LPS-injected NZB mice (Table 2). As noted previously [12], NZB mice treated with LPS also displayed modest increases in mPT gp70 RNA (P < 0.0005), while no statistically significant elevation of mPT gp70 RNA was observed in mice administered with 1V136 or CpG. In contrast, levels of PT gp70 RNA was not up-regulated in NZB mice injected with 1V136, CpG or LPS. Notably, the injection of either TLR agonist induced 5-6-fold increases in hepatic haptoglobin mRNA levels (P < 0.0001). It should be also stressed that the injection of 1V136 or CpG failed to enhance serum levels of gp70 in B6 mice having low serum levels of gp70 (data not shown), as in the case of B6 mice injected with LPS [13, 14].
Table 1.
Serum levels of gp70 in NZB mice injected with 1V13, CpG or LPS
| Serum gp70 | ||
|---|---|---|
| Stimuli | Before | After |
| 1V136 | 63.2 ± 3.7 | 199.4 ± 21.4 (3.2) |
| CpG | 61.1 ± 11.4 | 270.0 ± 20.2 (4.6) |
| LPS | 62.4 ± 11.7 | 449.2 ± 122.4 (7.3) |
| PBS | 62.2 ± 9.0 | 63.5 ± 11.1 (1.0) |
Serum levels of gp70 (μg/ml; mean ± SD of 4 mice) in 2-3 mo-old NZB female mice before and 9 h after an i.p. injection of 1V136 (50 μg), CpG (50 μg), LPS (25 μg) or PBS. Fold increases in serum levels of gp70 after injection of CpG, 1V136, LPS or PBS are indicated in parentheses.
Table 2.
Fold increases of gp70 RNAs and haptoglobin mRNA in livers of NZB mice injected with 1V136, CpG or LPS relative to those in PBS-injected NZB mice
| Stimuli | Xeno gp70 | PT gp70 | mPT gp70 | Haptoglobin |
|---|---|---|---|---|
| 1V136 | 3.53 ± 0.49 | 0.99 ± 0.20 | 1.31 ± 0.46 | 5.24 ± 0.79 |
| CpG | 4.66 ± 0.36 | 1.25 ± 0.63 | 1.47 ± 0.43 | 6.49 ± 0.55 |
| LPS | 5.49 ± 0.67 | 1.23 ± 0.12 | 2.95 ± 0.34 | 6.09 ± 0.71 |
| PBS | 1.00 ± 0.04 | 1.00 ± 0.14 | 1.00 ± 0.14 | 1.00 ± 0.19 |
Levels of gp70 RNAs and haptoglobin mRNA (mean ± SD of 4 mice) in livers of 2-3 mo-old NZB female mice 9 h after an i.p. injection of 1V136 (50 μg), CpG (50 μg), LPS (25 μg) or PBS were quantified relative to a standard curve generated with serial dilutions of a reference cDNA preparation and normalized using TBP mRNA. Results are expressed as fold increases of each transcript relative to PBS-injected NZB mice.
3.2. Increased levels of serum gp70 and hepatic gp70 RNAs in NZB mice after injection of IL-1β, IL-6 or TNFα
The effect of IL-1, IL-6 and TNF on the production of serum gp70 in NZB mice was next assessed, as these cytokines are well known inducers for acute phase proteins in the liver. Consistent with the idea that serum gp70 behaves as an acute phase protein, NZB mice displayed significant 2-3-fold increases in serum concentrations of gp70 9h after injection of recombinant human IL-1β (1 μg), IL-6 (5 μg) or TNFμ (5 βg) (IL-1β, P < 0.005; IL-6, P < 0.005; TNFα, P < 0.001; Table 3). These increases paralleled 3.3- to 4.5-fold up-regulation of xenotropic gp70 RNA levels (P < 0.0005 for all three cytokines) in livers of NZB mice (Table 4). In contrast, no statistically significant increases in the abundance of PT and mPT gp70 RNAs were observed after injection of the cytokines. As expected, the injection of these three inflammatory cytokines induced substantial increases in hepatic haptoglobin mRNA levels (IL-1β, P < 0.05; IL-6, P < 0.05; TNFα, P < 0.01). Notably, the extent of elevation in haptoglobin mRNA induced by each cytokine was essentially identical to that observed for xenotropic gp70 RNA.
Table 3.
Serum levels of gp70 in NZB mice injected with IL-lβ, IL-6, TNFα or IFNβ
| Serum gp70 | ||
|---|---|---|
| Stimuli | Before | After |
| IL-1β | 55.6 ± 7.4 | 182.7 ± 33.2 (3.3) |
| IL-6 | 64.2 ± 10.6 | 111.5 ± 19.9 (1.7) |
| TNFα | 62.1 ± 8.7 | 211.8 ± 28.9 (3.4) |
| IFNβ | 58.3 ± 6.9 | 60.1 ± 9.9 (1.0) |
| PBS | 60.9 ± 6.3 | 63.3 ± 3.5 (1.1) |
Serum levels of gp70 (μg/ml; mean ± SD of 4 mice) in 2-3 mo-old NZB female mice before and 9 h after an i.p. injection of IL-lβ (1 μg), IL-6 (5 μg), TNFα (5 μg), IFNβ (5 μg) or PBS. Fold increases in serum levels of gp70 after injection of cytokines or PBS are indicated in parentheses.
Table 4.
Fold increases of gp70 RNAs and haptoglobin mRNA in livers of NZB mice injected with IL-lβ, IL-6, TNFα or IFNβ relative to those in PBS-injected NZB mice
| Stimuli | Xeno gp70 | PT gp70 | mPT gp70 | Haptoglobin |
|---|---|---|---|---|
| IL-1β | 3.93 ± 0.58 | 1.12 ± 0.54 | 1.47 ± 0.59 | 4.45 ± 1.29 |
| IL-6 | 3.28 ± 0.56 | 1.05 ± 0.26 | 1.26 ± 0.30 | 3.38 ± 1.14 |
| TNFα | 4.50 ± 1.22 | 1.39 ± 0.26 | 1.94 ± 0.70 | 4.51 ± 1.33 |
| IFNβ | 1.03 ± 0.31 | 0.85 ± 0.22 | 0.81 ± 0.18 | 0.98 ± 0.28 |
| PBS | 1.00 ± 0.19 | 1.00 ± 0.11 | 1.00 ± 0.18 | 1.00 ± 0.34 |
Levels of gp70 RNAs and haptoglobin mRNA (mean ± SD of 4 mice) in livers of 2-3 mo-old NZB female mice 9 h after an i.p. injection of IL-lβ (1 μg), IL-6 (5 μg), TNFα (5 μg), IFNβ (5 μg) or PBS were quantified relative to a standard curve generated with serial dilutions of a reference cDNA preparation and normalized using TBP mRNA. Results are expressed as fold increases of each transcript relative to PBS-injected NZB mice.
The stimulation of plasmacytoid dendritic cells by TLR7 and TLR9 is known to trigger the secretion of relatively large amounts of type I interferon [31]. Therefore, we assessed the possible effect of IFNβ on the stimulation of serum gp70 production. Injection of recombinant human IFNβ (5 μg) failed to increase the synthesis of serum gp70 and the abundance of hepatic retroviral gp70 RNAs and haptoglobin mRNA in NZB mice (Tables 3 and 4). This finding indicated that the increase in the synthesis of serum gp70 corresponds to an acute phase response rather than a secondary response to IFNβ.
3.3. Synergistic effect of the Sgp3 and Sgp4 loci to LPS-induced up-regulated expression of serum gp70 in B6 mice
Earlier studies indicated that LPS-induced serum gp70 responses were strain-dependent [14]. Studies with B6.Sgp3 and B6.Sgp4 congenic mice have shown only modest (~2-fold) increases of serum gp70 in response to LPS [12, 18]. As basal serum levels of gp70 are regulated by multiple genes, it may be possible that stronger serum gp70 responses upon the injection of LPS might be elicited through the combination of Sgp3 and Sgp4 acting synergistically. To address this question, we generated B6.Sgp3/4 double congenic mice and assessed their ability to produce serum gp70 in response to LPS. When compared to B6 and B6 mice congenic for only the Sgp3 or Sgp4 locus, untreated B6.Sgp3/4 mice had substantially higher basal levels of serum gp70. Basal levels observed for the B6.Sgp3/4 mice were 14.9-, 3.6- and 2.0-fold higher than those observed with B6 (P < 0.0001), B6.Sgp4 (P < 0.0005) and B6.Sgp3 (P < 0.01) mice, respectively (Table 5). Injection of LPS led to a greater increase in serum concentrations of gp70 in B6.Sgp3/4 congenic mice than increases observed with B6 mice congenic for either Sgp3 or Sgp4 alone (B6.Sgp3, P < 0.005: B6.Sgp4, P < 0.0005; Table 5). Notably, mean increases in serum gp70 concentrations after injection of LPS in double congenic mice (96.1 μg/ml) were 3.2 times more than added values obtained by these two single congenic mice (29.7 μg/ml). These data indicated that Sgp3 and Sgp4 synergistically acted to promote the production of serum gp70 in response to LPS.
Table 5.
Serum levels of gp70 in B6 Sgp congenic mice injected with LPS
| Serum gp70 | ||
|---|---|---|
| Stimuli | Before | After |
| B6 | 2.4 ± 0.5 | 2.8 ± 0.8 (1.2) |
| B6.Sgp4 | 10.0 ± 1.0 | 17.8 ± 2.5 (1.8) |
| B6.Sgp3 | 18.2 ± 5.7 | 40.1 ± 10.7 (2.2) |
| B6.Sgp3/4 | 35.8 ± 8.7 | 131.9 ± 19.0 (3.8) |
Serum levels of gp70 (μg/ml; mean ± SD of 5 mice) in 2-3 mo-old B6 or B6 Sgp congenic female mice before and 9 h after an i.p. injection of 25 μg of LPS. Fold increases in serum levels of gp70 after injection of LPS are indicated in parentheses.
3.4. Differential effect of TLR agonists or inflammatory cytokines on the abundance of three different species of mPT env RNAs in NZB, BXSB and B6.Sgp3 mice
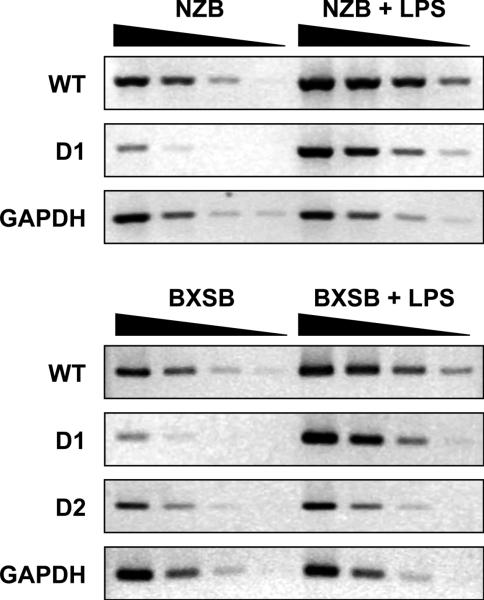
We have recently shown the presence in B6 mice of not only intact WT mPT env transcripts but also two defective (D1 and D2) mPT env transcripts which carry a deletion in the env sequence [30]. Furthermore, all four lupus-prone mice (NZB, NZW, BXSB and MRL) predominantly expressed WT mPT env transcripts rather than the defective env transcripts. Since the Sgp3, but not Sgp4 locus derived from lupus-prone mice was responsible for the selective expression of the WT mPT env RNA, we examined LPS-induced increases in mPT env RNA levels to determine if the up-regulation was restricted only to proviruses carrying WT mPT env genes. The abundance of the two defective (D1 and D2) and the WT mPT env RNAs were semi-quantified by RT-PCR specific for the three different mPT env sequences in BXSB as well as NZB mice, as only BXSB mice carry the D2 mutant provirus and express D2 mPT env transcripts among four different lupus-prone mice [30]. The use of semi-quantified RT-PCR was necessary since we are unable to design real-time RT-PCR primers specific for WT mPT env cDNA due to the remarkable homology in the gp70-p15E junction region between mPT and PT env genes [30]. The analysis with serially diluted cDNA samples from NZB and BXSB mice injected with LPS showed moderate and robust increases in WT and D1 mPT env RNAs, respectively, as compared to PBS-injected mice (Fig. 1 and Table S1). In contrast, no appreciable increases in D2 mPT env RNA were observed in BXSB mice.
Figure 1.
Semi-quantitative RT-PCR analysis for WT, D1 and D2 mPT env RNAs in NZB and BXSB mice.
Semi-quantitative RT-PCR analysis for WT, D1 and D2 mPT env RNAs with reverse primers specific for the three different mPT env genes (mPT1447R, D1-R and D2-R) and a common forward mPT-specific primer (mPT858F) was carried out with 4-fold serially diluted cDNAs from 2-3 mo-old female mice. As a control, the abundance of GAPDH mRNA was assessed in parallel. For the analysis of WT mPT env transcripts in NZB and BXSB mice, the first dilution of cDNA was 1:40, and four serial dilutions of cDNAs were examined, while for D1/D2 mPT env RNAs and GAPDH mRNA the first dilution was 1:10. Intensities of different RT-PCR products at each dilution of cDNAs, expressed as arbitrary units, are given in Table S1. Representative results of three individual mice analyzed are shown.
Semi-quantitative RT-PCR analysis suggested that injection of LPS in NZB and BXSB mice more efficiently induced transcription of the D1 mPT env gene than those of WT mPT env genes. Indeed, real-time RT-PCR analysis confirmed 12.0- and 15.4-fold increases in D1 mPT env RNA levels in NZB and BXSB mice, respectively (Table 6). These results contrasted to only 3-fold increases in the abundance of total mPT env RNAs, which include WT, D1 and D2 transcripts, and no changes in the levels of D2 mPT env RNA. Notably, similar results were obtained in LPS-injected B6.Sgp3 congenic mice, while levels of the three mPT env transcripts were not elevated in B6 mice in response to LPS (Table 6). These results indicated that LPS induction influenced the expression of each of these transcripts in distinct manners with strong, modest and no up-regulated expression of D1, WT and D2 mPT proviruses, respectively, in mice bearing the Sgp3 locus derived from lupus-prone mice. This contrasted to the selectively increased expression of WT mPT env gene by Sgp3 observed under steady-state condition [30]. It was further confirmed that injection of 1V136, CpG and inflammatory cytokines (IL-1β, IL-6 and TNFα) resulted in 3.5- to 10.0-fold elevation of D1 env RNA levels in livers of NZB mice (Fig. 2).
Table 6.
Fold increases of Dl and D2 mPT env RNAs in livers of NZB, BXSB, B6.Sgp3 and B6 mice injected with LPS relative to those in PBS-injected mice
| Mice | Stimuli | Totala | Pb | D1a | Pb | D2a |
|---|---|---|---|---|---|---|
| NZB | LPS | 2.95 ± 0.34 | *** | 12.03 ± 2.29 | *** | NDc |
| NZB | PBS | 1.00 ± 0.14 | 1.00 ± 0.14 | ND | ||
| BXSB | LPS | 3.34 ± 0.04 | **** | 15.35 ± 3.96 | ** | 1.08 ± 0.35 |
| BXSB | PBS | 1.00 ± 0.15 | 1.00 ± 0.15 | 1.00 ± 0.08 | ||
| B6.Sgp3 | LPS | 2.36 ± 0.33 | * | 4.79 ± 1.00 | ** | 1.10 ± 0.15 |
| B6.Sgp3 | PBS | 1.00 ± 0.27 | 1.00 ± 0.21 | 1.00 ± 0.12 | ||
| B6 | LPS | 1.18 ± 0.16 | 1.70 ± 0.38 | 0.94 ± 0.22 | ||
| B6 | PBS | 1.00 ± 0.23 | 1.00 ± 0.17 | 1.00 ± 0.10 |
Levels of total (WT, Dl and D2), Dl and D2 mPT env RNAs (mean ± SD of 4 mice) in livers of 2-3 mo-old NZB, BXSB, B6.Sgp3 or B6 female mice 9 h after an i.p. injection of different stimulators or PBS were quantified relative to a standard curve generated with serial dilutions of a reference cDNA preparation and normalized using TBP mRNA. Results are expressed as fold increases of each transcript relative to PBS-injected NZB, BXSB, B6.Sgp3 or B6 mice.
P value of comparison between LPS- and PBS-injected mice.
P < 0.01
P < 0.005
P < 0.0005
P < 0.0001
Not detectable.
Figure 2.
Quantitative real-time RT-PCR analysis of D1 mPT env RNA in livers of NZB mice injected with 1V136, CpG, IL-1β, IL-6, TNFα, IFNβ or PBS.
Levels of D1 mPT env RNA (mean ± SEM of 4 mice) in livers of 2-3 mo-old NZB female mice 9 h after an i.p. injection of different stimulators or PBS were quantified relative to a standard curve generated with serial dilutions of a reference cDNA preparation and normalized using TBP mRNA. Results are expressed as fold increases of D1 mPT env RNA relative to PBS-injected NZB mice. P value of comparison with PBS-injected mice. * P < 0.05; ** P < 0.01; *** P < 0.0001
The markedly enhanced levels of D1 mPT env RNA in NZB, BXSB and B6.Sgp3 mice during acute phase responses can be due to a unique integration site of this particular provirus. BLAST search analysis revealed that the D1 mPT mutant provirus is integrated in the right transcription direction within the 4th intron of the Rsrc1 (arginine/serine-rich coiled 1) gene in B6 chromosome 3. However, the levels of Rsrc1 mRNA were comparable in livers of untreated B6 and B6.Sgp3 mice (means of 3 mice ± SD: B6, 0.77 ± 0.13; B6.Sgp3, 0.85 ± 0.23), and the injection of LPS failed to enhance, but rather down-regulated levels of Rsrc1 mRNA in these mice (means of 3 mice ± SD: B6, 0.27 ± 0.06; B6.Sgp3, 0.32 ± 0.04). These results thus argued against the possibility that the enhanced expression of the D1 mPT env gene in LPS-injected B6.Sgp3 mice was a result of co-regulated transcription of the LPS-responsive host gene, in which the D1 provirus is integrated.
4. Discussion
Endogenous retroviral gp70 has been shown as one of the major nephritogenic autoantigens in murine SLE. Its expression is under a polygenic control and regulated by inflammatory stimuli, as it behaves as an acute phase protein. In the present study, we have shown that TLR7 and TLR9 were also involved in the enhanced production of serum gp70 during acute phase responses. In addition, our results demonstrated that the Sgp3 and Sgp4 loci play critical roles in up-regulated expression of serum gp70 under inflammatory conditions as well as in its steady-state expression. Moreover, analysis of B6.Sgp3 congenic mice revealed that the Sgp3 locus contains at least two distinct genetic factors; one of which controls the basal-level expression of serum gp70 and the other the up-regulated production of serum gp70 during systemic inflammation.
Induction of high serum levels of gp70 in NZB mice injected with TLR7 and TLR9 agonists (1V136 for TLR7 and CpG for TLR9) is likely to be mediated by cellular and molecular mechanisms responsible for the induction of acute phase responses in livers, based on the following findings. First, levels of serum gp70 and of hepatic haptoglobin mRNA in NZB mice injected with 1V136 or CpG were similarly up-regulated, as in the case of NZB mice injected with IL-1β, IL-6 and TNFα, known to be a potent inducer of acute phase responses. Second, kinetics of serum gp70 responses induced by 1V136 or CpG was essentially identical to that induced by LPS or IL-1β. Notably, activation of TLR7 and TLR9 in monocytes/macrophages induced the secretion of IL-6 and TNFα [32, 33], while no serum gp70 or haptoglobin responses were induced by the type I interferon, which is a unique cytokine abundantly secreted by plasmacytoid dendritic cells upon stimulation of TLR7 or TLR9. Furthermore, the pattern of up-regulated expression of three different classes of endogenous retroviral gp70 RNAs in NZB mice injected with 1V136 or CpG was essentially identical to that observed after injection of inflammatory cytokines. As discussed previously [12], the lack of up-regulated expression of PT proviruses can be in part related to the absence of an IL-6-responsive element (IL6-RE), common to genes encoding acute phase protein [34], in the U3 regulatory region of the long terminal repeat (LTR). In addition, higher responses of xenotropic gp70 RNA can be explained by the presence of NF-kB-binding motif in the U3 region of xenotropic viruses [35], as NF-kB is involved in one of the several distinct signaling pathways leading to the synthesis of acute phase proteins [36].
It should be stressed that as in the case of LPS-induced gp70 responses, 1V136- and CpG-induced gp70 responses were also strain-dependent, as B6 mice having low serum levels of gp70 failed to display any increases in serum gp70 after injection of either 1V136 or CpG. Because of only modest gp70 responses in B6.Sgp3 and B6.Sgp4 single congenic mice injected with LPS [12], it was considered that the contribution of the Sgp loci to LPS-induced serum gp70 responses was relatively limited. However, substantial, synergistic increases in serum levels of gp70 in B6.Sgp3/4 double congenic mice injected with LPS indicated that the Sgp loci do play a major role in the up-regulated expression of serum gp70 during acute phase responses. Notably, serum levels of gp70 in LPS-injected B6.Sgp3/4 mice were comparable to those observed with LPS-injected BXSB mice and even higher than those of LPS-injected MRL mice [12, 14]. However, increases in serum gp70 were still less than those in LPS-injected NZB and NZW mice.
Basal levels of serum gp70 in B6.Sgp3/4 congenic mice were comparable to those of BXSB and MRL mice, but still lower than those in NZB and NZW mice. This could be accounted for if NZB and NZW mice carry an additional Sgp locus. Indeed, the genetic analysis involving BALB/c mice revealed a strong linkage of serum gp70 levels with a locus on proximal chromosome 12 of both NZB and NZW mice [20]. Preliminary studies in B6 mice bearing the proximal chromosome 12 interval derived from NZB mice showed modest, but significant increases in serum gp70, similar to those observed in BALB/c mice congenic for this putative Sgp locus derived from NZW mice [20]. The involvement of multiple loci in the up-regulated expression of serum gp70 during acute phase responses is consistent with the finding that the extent of serum gp70 responses after injection of LPS was highly variable among different strains of mice [14, 21].
Strikingly, the analysis of three different species of mPT env RNAs in NZB, BXSB and B6.Sgp3 mice revealed that inducers of acute phase responses up-regulated the abundance of the D1 mPT env RNA more strongly than that of WT mPT env RNA, and that levels of D2 mPT env RNA were not modulated. This suggested that the expression of only a fraction of mPT proviruses was selectively enhanced during the acute phase response. BLAST search analysis revealed the presence in the B6 genome of 11 mPT proviruses carrying the WT env gene, in addition to the D1 and D2 mPT proviruses on chromosome 3 and 5, respectively. Notably, the presence of the IL6-RE is not a determinant of the differential expression we have observed, since this motif is conserved in the U3 sequence of all mPT proviruses, including the D2 mPT provirus, present in B6 mice. However, we noted considerable microheterogeneity in their U3 sequences. Notably, the D1 mutant carries two unique mutations in the U3 regulatory region [30]: a substitution of G (guanine) with A (adenine) in an SV40 core-like motif (GTGATCA instead of GTGGTCA) and an insertion of T (thymine) in the UCR (upstream conserved region), which negatively regulates the expression of endogenous retroviruses [37]. It remains to be determined whether these two mutations contribute to the up-regulated transcription of the D1 mPT provirus by the presence of inflammatory stimuli. Alternatively, the site of integration of mPT proviruses may play a critical role in these responses. This possibility seems unlikely for the D1 mPT provirus, since we observed that LPS failed to enhance the expression of the Rsrc1 gene which contains the D1 mPT provirus in the correct orientation. Another plausible explanation is that the enhancer element(s) of the U3 region, implicated in the increased expression in response to inflammatory stimuli, may be selectively methylated in certain proviruses, as the expression of retroviral sequences is strongly affected by the state of DNA methylation [38-40].
In contrast to the enhanced expression of the D1 mPT env RNA in LPS-injected B6.Sgp3 congenic mice, the basal-level expression of this transcript was the same as in WT B6 mice [30]. Since D1 mPT env RNA levels were enhanced in B6.Sgp3, but not in WT B6 mice following injection of LPS, the Sgp3 locus by itself is responsible for LPS-induced increases in this transcript. Thus, the simplest explanation would be that the Sgp3 locus harbors at least two distinct genetic elements, which control respectively the basal-level transcription of xenotropic, PT and mPT retroviral sequences and the up-regulated expression of xenotropic and mPT retroviral sequences during acute phase responses. In view of the remarkable differences in the U3 region of LTR among xenotropic, PT and mPT retroviruses [35], the presence of several genetic factors which differentially control the expression of individual classes of retroviruses under steady-state or inflammatory condition might not be surprising. In this regard, it is noteworthy that the Gv1 locus derived from the 129 strain was reported to regulate the transcription of PT, but not mPT proviruses [41], and that Gv1 controls the expression of gp70 in a semi-dominant fashion [42]. Consistent with these findings, our on-going studies on Sgp3 homozygous and heterozygous mice revealed that the basal-level expression of xenotropic, PT and mPT viral sequences was regulated in a dominant, semi-dominant and recessive manner, respectively. All these data underline the complexity for the genetic control of the expression of different classes of endogenous retroviruses in mice.
Our on-going studies have narrowed down the Sgp3 locus within a ~9.0 Mb NZB interval flanked by markers D13Mit283 (63.4 Mb from the centromere) and D13Mit26 (72.4 Mb). Notably, this region contains approximately 20 Zfp genes, which encode KRAB (Krüppel-associated box) zinc-finger proteins, but the target genes regulated by most of these Zfp genes are still unknown [43]. The KRAB transcription repressor domain has been shown to suppress lentivirus proviral transcription by inducing heterochromatization in the lentiviral integration sites [44]. More recently, it has also been shown that ZFP809 recognizes integrated retroviral DNAs and silences them through the recruitment of TRIM28 (tripartite motif-containing 28) in embryonic stem cells [45]. Thus, it may be possible that several of the Zfp genes present in the Sgp3 locus are involved in the regulation of the expression of endogenous retroviruses under physiological and inflammatory conditions in mice.
The role of TLR7 and TLR9 for the development of autoimmune responses against nuclear autoantigens and retroviral gp70, both of which are implicated in murine lupus nephritis, has been established [24-27]. In addition, our present results demonstrated that they are also involved in the enhanced production of nephritogenic gp70 antigens during the course of SLE, possibly through the activation of monocytes/macrophages in response to DNA- or RNA-containing IgG IC. Thus, TLR7 and TLR9 display dual effects on the development of SLE. On one hand, they promote autoimmune responses against nuclear and retroviral antigens through the activation of autoreactive B cells as well as dendritic cells, and on the other hand, they enhance the production of serum gp70 in the presence of the Sgp loci, thereby providing an additional source for antigenic stimulation and for nephritogenic IC formation. Increased levels of serum gp70 during the course of SLE, in association with increases in serum levels of gp70 IC and accelerated development of lupus nephritis, have previously been demonstrated in lupus-prone BXSB mice [46]. The contribution of TLR7 to the production of anti-gp70 antibodies also suggests the implication of endogenous retroviruses in murine SLE. The eventual identification of the Sgp genes will help elucidate a molecular basis responsible for the expression of endogenous retroviruses implicated in murine SLE, and will enable us to address the relevance of their human counterparts, thus providing a clue for the potential role of endogenous retroviruses in human SLE.
Supplementary Material
Acknowledgments
We thank Mr Guy Brighouse and Ms Montserrat Alvarez for their excellent technical assistance. This work was supported by a grant from the Swiss National Foundation for Scientific Research. L.H.E. was supported by the intramural research program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lerner RA, Wilson CB, Villano BC, McConahey PJ, Dixon FJ. Endogenous oncornaviral gene expression in adult and fetal mice: quantitative, histologic, and physiologic studies of the major viral glycorprotein, gp70. J Exp Med. 1976;143:151–66. doi: 10.1084/jem.143.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strand M, August JT. Oncornavirus envelope glycoprotein in serum of mice. Virology. 1976;75:130–44. doi: 10.1016/0042-6822(76)90012-x. [DOI] [PubMed] [Google Scholar]
- 3.Elder JH, Jensen FC, Bryant ML, Lerner RA. Polymorphism of the major envelope glycoprotein (gp70) of murine C-type viruses: virion associated and differential antigens encoded by a multi-gene family. Nature. 1977;267:23–28. doi: 10.1038/267023a0. [DOI] [PubMed] [Google Scholar]
- 4.Yoshiki T, Mellors RC, Strand M, August JT. The viral envelope glycoprotein of murine leukemia virus and the pathogenesis of immune complex glomerulonephritis of New Zealand mice. J Exp Med. 1974;140:1011–27. doi: 10.1084/jem.140.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izui S, McConahey PJ, Theofilopoulos AN, Dixon FJ. Association of circulating retroviral gp70-anti-gp70 immune complexes with murine systemic lupus erythematosus. J Exp Med. 1979;149:1099–116. doi: 10.1084/jem.149.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vyse TJ, Drake CG, Rozzo SJ, Roper E, Izui S, Kotzin BL. Genetic linkage of IgG autoantibody production in relation to lupus nephritis in New Zealand hybrid mice. J Clin Invest. 1996;98:1762–72. doi: 10.1172/JCI118975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haywood MEK, Vyse TJ, McDermott A, Thompson EM, Ida A, Walport MJ, et al. Autoantigen glycoprotein 70 expression is regulated by a single locus, which acts as a checkpoint for pathogenic anti-glycoprotein 70 autoantibody production and hence for the corresponding development of severe nephritis, in lupus-prone BXSB mice. J Immunol. 2001;167:1728–33. doi: 10.4049/jimmunol.167.3.1728. [DOI] [PubMed] [Google Scholar]
- 9.Stoye JP, Coffin JM. The four classes of endogenous murine leukemia virus: structural relationships and potential for recombination. J Virol. 1987;61:2659–69. doi: 10.1128/jvi.61.9.2659-2669.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamont C, Culp P, Talbott RL, Phillips TR, Trauger RJ, Frankel WN, et al. Characterization of endogenous and recombinant proviral elements of a highly tumorigenic AKR cell line. J Virol. 1991;65:4619–28. doi: 10.1128/jvi.65.9.4619-4628.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izui S, Elder JH, McConahey PJ, Dixon FJ. Identification of retroviral gp70 and anti-gp70 antibodies involved in circulating immune complexes in NZB × NZW mice. J Exp Med. 1981;153:1151–60. doi: 10.1084/jem.153.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baudino L, Yoshinobu K, Morito N, Kikuchi S, Fossati-Jimack L, Morley BJ, et al. Dissection of genetic mechanisms governing the expression of serum retroviral gp70 implicated in murine lupus nephritis. J Immunol. 2008;181:2846–54. doi: 10.4049/jimmunol.181.4.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hara I, Izui S, Dixon FJ. Murine serum glycoprotein gp70 behaves as an acute phase reactant. J Exp Med. 1982;155:345–57. doi: 10.1084/jem.155.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara I, Izui S, McConahey PJ, Elder JH, Jensen FC, Dixon FJ. Induction of high serum levels of retroviral env gene products (gp70) in mice by bacterial lipopolysaccharide. Proc Natl Acad Sci USA. 1981;78:4397–401. doi: 10.1073/pnas.78.7.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tucker RM, Vyse TJ, Rozzo S, Roark CL, Izui S, Kotzin BL. Genetic control of gp70 autoantigen production and its influence on immune complex levels and nephritis in murine lupus. J Immunol. 2000;165:1665–72. doi: 10.4049/jimmunol.165.3.1665. [DOI] [PubMed] [Google Scholar]
- 16.Kikuchi S, Fossati-Jimack L, Moll T, Amano H, Amano E, Ida A, et al. Differential role of three major NZB-derived loci linked with Yaa-induced murine lupus nephritis. J Immunol. 2005;174:1111–17. doi: 10.4049/jimmunol.174.2.1111. [DOI] [PubMed] [Google Scholar]
- 17.Santiago ML, Mary C, Parzy D, Jacquet C, Montagutelli X, Parkhouse RME, et al. Linkage of a major quantitative trait locus to Yaa gene-induced lupus-like nephritis in (NZW × C57BL/6)F1 mice. Eur J Immunol. 1998;28:4257–67. doi: 10.1002/(SICI)1521-4141(199812)28:12<4257::AID-IMMU4257>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 18.Laporte C, Ballester B, Mary C, Izui S, Reininger L. The Sgp3 locus on mouse chromosome 13 regulates nephritogenic gp70 autoantigen and predisposes to autoimmunity. J Immunol. 2003;171:3872–77. doi: 10.4049/jimmunol.171.7.3872. [DOI] [PubMed] [Google Scholar]
- 19.Rankin J, Boyle JJ, Rose SJ, Gabriel L, Lewis M, Thiruudaian V, et al. The Bxs6 locus of BXSB mice is sufficient for high-level expression of gp70 and the production of gp70 immune complexes. J Immunol. 2007;178:4395–401. doi: 10.4049/jimmunol.178.7.4395. [DOI] [PubMed] [Google Scholar]
- 20.Rigby RJ, Rozzo SJ, Gill H, Fernandez-Hart T, Morley BJ, Izui S, et al. A novel locus regulates both retroviral glycoprotein 70 and anti-glycoprotein 70 antibody production in New Zealand mice when crossed with BALB/c. J Immunol. 2004;172:5078–85. doi: 10.4049/jimmunol.172.8.5078. [DOI] [PubMed] [Google Scholar]
- 21.Maruyama N, Lindstrom CO, Sato H, Dixon FJ. Serum gp70 production regulated by a gene on murine chromosome 7. Immunogenetics. 1983;18:365–71. doi: 10.1007/BF00372469. [DOI] [PubMed] [Google Scholar]
- 22.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 23.Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–39. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–28. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–35. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–51. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 27.Santiago-Raber ML, Dunand-Sauthier I, Wu T, Li QZ, Uematsu S, Akira S, et al. Critical role of TLR7 in the acceleration of systemic lupus erythematosus in TLR9-deficient mice. J Autoimmun. 2010;34:339–48. doi: 10.1016/j.jaut.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi T, Gray CS, Chan M, Tawatao RI, Ronacher L, McGargill MA, et al. Prevention of autoimmune disease by induction of tolerance to Toll-like receptor 7. Proc Natl Acad Sci USA. 2009;106:2764–969. doi: 10.1073/pnas.0813037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merino R, Fossati L, Lacour M, Lemoine R, Higaki M, Izui S. H-2-linked control of the Yaa gene-induced acceleration of lupus-like autoimmune disease in BXSB mice. Eur J Immunol. 1992;22:295–99. doi: 10.1002/eji.1830220202. [DOI] [PubMed] [Google Scholar]
- 30.Yoshinobu K, Baudino L, Morito N, Dunand-Sauthier I, Morley BJ, Evans LH, et al. Selective up-regulation of intact, but not defective env RNAs of endogenous modified polytropic retrovirus by the Sgp3 locus of lupus-prone mice. J Immunol. 2009;182:8094–103. doi: 10.4049/jimmunol.0900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 32.Heikenwalder M, Polymenidou M, Junt T, Sigurdson C, Wagner H, Akira S, et al. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat Med. 2004;10:187–92. doi: 10.1038/nm987. [DOI] [PubMed] [Google Scholar]
- 33.Uematsu S, Sato S, Yamamoto M, Hirotani T, Kato H, Takeshita F, et al. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha} induction. J Exp Med. 2005;201:915–23. doi: 10.1084/jem.20042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tolosano E, Altruda F. Hemopexin: structure, function, and regulation. DNA Cell Biol. 2002;21:297–306. doi: 10.1089/104454902753759717. [DOI] [PubMed] [Google Scholar]
- 35.Tomonaga K, Coffin JM. Structures of endogenous nonecotropic murine leukemia virus (MLV) long terminal repeats in wild mice: implication for evolution of MLVs. J Virol. 1999;73:4327–40. doi: 10.1128/jvi.73.5.4327-4340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramadori G, Christ B. Cytokines and the hepatic acute-phase response. Semin Liver Dis. 1999;19:141–55. doi: 10.1055/s-2007-1007106. [DOI] [PubMed] [Google Scholar]
- 37.Flanagan JR, Becker KG, Ennist DL, Gleason SL, Driggers PH, Levi BZ, et al. Cloning of a negative transcription factor that binds to the upstream conserved region of Moloney murine leukemia virus. Mol Cell Biol. 1992;12:38–44. doi: 10.1128/mcb.12.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harbers K, Schnieke A, Stuhlmann H, Jahner D, Jaenisch R. DNA methylation and gene expression: endogenous retroviral genome becomes infectious after molecular cloning. Proc Natl Acad Sci USA. 1981;78:7609–13. doi: 10.1073/pnas.78.12.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groudine M, Eisenman R, Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981;292:311–17. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- 40.Niwa O, Sugahara T. 5-Azacytidine induction of mouse endogenous type C virus and suppression of DNA methylation. Proc Natl Acad Sci USA. 1981;78:6290–94. doi: 10.1073/pnas.78.10.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliver PL, Stoye JP. Genetic analysis of Gv1, a gene controlling transcription of endogenous murine polytropic proviruses. J Virol. 1999;73:8227–34. doi: 10.1128/jvi.73.10.8227-8234.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stockert E, Old LJ, Boyse EA. The GIX system. A cell surface allo-antigen associated with murine leukemia virus; implications regarding chromosomal integration of the viral genome. J Exp Med. 1971;133:1334–55. doi: 10.1084/jem.133.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krebs CJ, Larkins LK, Price R, Tullis KM, Miller RD, Robins DM. Regulator of sex-limitation (Rsl) encodes a pair of KRAB zinc-finger genes that control sexually dimorphic liver gene expression. Genes Dev. 2003;17:2664–74. doi: 10.1101/gad.1135703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bulliard Y, Wiznerowicz M, Barde I, Trono D. KRAB can repress lentivirus proviral transcription independently of integration site. J Biol Chem. 2006;281:35742–46. doi: 10.1074/jbc.M602843200. [DOI] [PubMed] [Google Scholar]
- 45.Wolf D, Goff SP. Embryonic stem cells use ZFP809 to silence retroviral DNAs. Nature. 2009;458:1201–04. doi: 10.1038/nature07844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Izui S, Hara I, Hang LM, Elder JH, McConahey PJ, Dixon FJ. Association of elevated serum glycoprotein gp70 with increased gp70 immune complex formation and accelerated lupus nephritis in autoimmune male BXSB mice. Clin Exp Immunol. 1984;56:272–80. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.