Abstract
It is well known that bone fracture healing is delayed in diabetes mellitus, but the mechanism remains to be elucidated. Since several studies have demonstrated that diabetes causes abnormalities in bone marrow-derived cells, we used the streptozotocin (STZ)-induced diabetic mouse model after bone marrow transfer from green fluorescent protein (GFP) transgenic mice, and examined fracture healing. Compared with non-diabetic mice, diabetic mice at 3 weeks after fracture showed a decrease in mineralized callus, with the remainder consisting of cartilage. Bone formation parameters and mineralization rate were not altered in the STZ mice, but bone resorption parameters were significantly decreased. Therefore, the delayed bone formation in the STZ mice may have resulted from an impairment of cartilage resorption. Interestingly, we found that 80 % of the osteoclasts in the callus were derived from bone marrow and the sizes of the osteoclasts as well as the resorption pits formed were significantly smaller in the diabetic mice. Moreover, transcript analysis using RNA isolated by laser capture microdissection (LCM) showed that the expression of DC-STAMP, a putative pivotal gene for osteoclast fusion, was decreased in osteoclasts from diabetic mice. Since the sustainability of osteoclast function depends on the controlled renewal of multinuclear osteoclasts, impaired osteoclast function in diabetes may contribute to decreased cartilage resorption and delayed endochondral ossification.
Keywords: cell fusion, diabetes mellitus, fracture healing, green fluorescent protein, osteoclasts
Introduction
According to statistics from the International Diabetes Federation, diabetes is a world-wild problem and 380 million people will suffer from the disease by the year 2025 [1]. Apart from the well-characterized complications, i.e., neuropathy, retinopathy and renal damage, Albright described diabetic osteopenia in 1948 [2]. Diabetic osteoporosis has received increasing attention since then, and increased fracture risk and impairment of fracture repair have also been reported [3–5]. Despite the large number of studies dedicated to this theme, the detailed pathogenesis of these effects of diabetes so far remains ill defined [6–8].
During fracture healing, a variety of cells and factors participate in turn in the sequential, dynamic and intricate events of osteogenesis [9–11]. Following the inflammatory phase, a cartilage anlage is formed by chondrocytes, a crucial step for the acquisition of stability at an early stage of fracture healing. In order to achieve functional recuperation, the cartilage anlage must first become calcified and then replaced by woven bone [12, 13]. This process is a recapitulation of the embryonic growth of a long bone [14].
Osteoclasts play an important role in the replacement of cartilage by woven bone [15, 16]. However, so far it is not clear what kinds of impairments affect osteoclasts in diabetes. Osteoclasts mature as characteristic multinucleated giant cells, which are formed by fusion of their monocyte-lineage precursors. The major effect of multinucleation is obviously to increase cell size, which enables them to resorb larger areas of bone tissue [17]. The initial stage of osteoclast differentiation depends on receptor activator of nuclear factor-κB ligand (RANKL), as well as macrophage colony stimulating factor (M-CSF) [18]. Recent studies have shown that high glucose levels inhibit RANKL-mediated osteoclast differentiation and function [6, 19].
Our previous study has shown that diabetic neuropathy is partly the result of an unusual fusion activity of bone marrow-derived cells [20]. When cell fusion occurs between bone marrow-derived cells and nerve cells, the fusion cells begin producing TNF-α, a cytokine known to be involved in peripheral neuropathy. Moreover fusion cells in the liver may also be involved in the pathogenesis of diabetic liver diseases [21]. We hypothesize that bone marrow-derived monocyte-lineage osteoclast precursors may be similarly affected in diabetes. The abnormalities of osteoclast fusion may lead to osteoclast malfunction, leading to diabetic osteopenia.
In order to gain insight into the abnormal osteoclast function in diabetes, the present study evaluates fracture healing in the streptozotocin (STZ)-induced diabetic mouse model. We performed histological, functional, and molecular analysis of bone marrow-derived osteoclasts in mouse recipients of bone marrow cells from mice that expressed green fluorescent protein (GFP).
Materials and Methods
Animal model
Eight-week-old male C57BL/6 mice and male GFP transgenic C57BL/6 mice (CLEA Japan, Tokyo, Japan) were used for this study. For bone marrow transplantation (BMT), wild-type C57BL/6 mice were irradiated (9 Gy) and then injected with 4×106 bone marrow cells isolated from male GFP mice. We induced diabetes using STZ (150 mg/Kg) injected into the tail vein 4 weeks after BMT. Control mice were injected with citrate vehicle alone. Blood obtained from the tail vein was measured every week by Glutest-Ace (Sanwa kagaku, Nagoya, Japan) till the animals were euthanized. Diabetes was diagnosed at two weeks after STZ injection when blood glucose levels reached over 400 mg/dl. The fracture experiments were initiated immediately after the determination of blood glucose. The procedures were approved by the institutional animal care and use committee guidelines of Shiga University of medical science (Approval number; 2008-4-8).
Fracture model
Mice were anesthetized with sodium pentobarbital at 0.1 mg/100 g intraperitoneally, and a closed transverse fracture of the right femur was created as previously described [22]. Briefly, an incision was made on the medial aspect of the knee, and a 23-gauge needle was inserted into the right femur for internal fixation. After closing the incision, the mid-diaphysis of the pinned femur was fractured by blunt trauma. The traumatic force was applied by dropping a 300 g weight from a height of 5 cm. After surgery, the mice were permitted unrestricted, full weight bearing activity. Animals were euthanized at 1, 2, 3 and 5 weeks after fracture, and were evaluated as described below.
X-Ray analysis
Bone radiographs of the right femur were taken with a soft X-ray system (CMB-2, Softex, Osaka, Japan) at 4 mA, 40 sec, every week until the animals were euthanized (1 week: n = 20, 2 weeks: n = 15, 3 weeks: n = 10, 5 weeks: n = 5 for each group). The radiograms were taken under anesthesia.
Histology and bone histomorphometry
After euthanasia, the right femur was excised and fixed with 70 % ethanol after removal of the surrounding soft tissue. The specimens were then embedded in glycol methacrylate (Kureha Co, Tokyo, Japan). To identify new bone and cartilage formation, the sections were cut at 4 μm and stained for toluidine blue. The mean values were obtained by the measurement of 5 animals in each group.
Bone histomorphometry of the callus area of undecalcified sections was performed using specimens from animals euthanized at 2 and 3 weeks postoperatively. Subcutaneous injection of calcein (8 mg/kg) was carried out 7 days and 2 days before euthanasia. The right femur was excised, fixed with 70 % ethanol after removal of the surrounding soft tissue, and embedded in glycol methacrylate. Sections were cut at 4 μm and stained for tartrate resistant acid phosphatase (TRAP)/toluidine blue (Kureha Co, Tokyo, Japan). We determined three points of callus area as previously described in each animal [23, 24], and calculated the mean values of 5 animals in each group.
Immunohistochemistry
Immunohistochemical staining of the callus area was performed using specimens from animals euthanized at 3 weeks postoperatively. After euthanasia, mice were perfused and fixed with 4 % paraformaldehyde (PFA). After excision of the right femur, the specimen was immersion-fixed with 4 % PFA overnight at 4 °C. The intramedullary metal was removed and the specimens were decalcified in ethylenediamine tetraacetic acid disodium (EDTA) for 1 week at 4 °C. The specimens were then dehydrated through a graded ethanol series, embedded in paraffin, sectioned at 4 μm and prepared for staining. The decalcified sections were stained with an anti-osteocalcin (OC) antibody (sc-18322, Santa Cruz Biotechnology, Santa Cruz, CA) and an anti-RANK antibody (sc-9072, Santa Cruz Biotechnology) at 3 weeks postoperatively. In addition, nuclear staining with To-Pro 3 (Invitrogen, CA) was performed on each section. Stained samples were examined by confocal laser scanning microscopy (LSM510, Leica, Germany) (n = 5 for each group).
Resorption-pit assay
The skull bones of wild type C57BL/6 were excised and the periosteum was removed [25]. The cortical bone plates were fixed and dehydrated with 100% ethanol, then were cut into 2 × 2 mm squares to put into 96 well dishes. Bone marrow cells were isolated from both the STZ and the control groups, and then one cortical bone plate and 3 × 106 marrow cells were cultured together in a well of a 96-well dish. Culture was performed in DMEM (Invitrogen Japan, Tokyo, Japan) supplemented with 10 % fetal bovine serum (MP Biomedicals, Irvine, CA) under 5 % CO2 and 95 % air at 37 °C, and floating cells were removed 2 hours after the initial inoculation. The cells remaining on the cortical bone plates were cultured with the medium changed every 3 days [26]. The cortical bone plates and cells were cultured for 3, 5, or 7 days. At the end of the culture period, the attached cells were removed with an ultrasonic processor (XL2020, MISONIX, Farmingdale, NY) in NH4OH, then dehydrated through a graded ethanol series. The cortical bone plates were coated with ioncoter (IB-3, Eiko-Seiki, Japan) and examined for formation of resorption pits with a scanning electron microscope (SEM, JSM-7505, JEOL, Tokyo, Japan) at 25 kV (n = 12 for each group).
Laser capture microdissection and quantitative real-time PCR
The right femur was extracted, and the surrounding soft tissue was removed at 2 weeks after fracture. The specimens were immediately embedded in SCEM (Leica Microsystems, Tokyo Japan), frozen, and sectioned at 8 μm (CM3050, Leica, Germany). Then, the specimens were subjected to laser capture microdissection (LCM) and quantitative real-time PCR as previously described [21, 27]. In summary, thin sectioned specimens were fixed and dehydrated on non-coated glass and then used for LCM (Pixcell IIe; Arcturus BioScience, Mountain View, CA). Fifty to 100 GFP-positive cells were captured using CapSure HS LCM Caps (Arcturus BioScience). After mRNA extraction using a PicoPure RNA isolation kit (Arcturus BioScience), cDNA was synthesized using a commercial kit (Superscript III, Invitrogen, Tokyo Japan) according to the manufacturer’s instructions.
Q-PCR was performed on the LightCycler System (Roche Diagnostics, Indianapolis, IN) using a LightCycler Fast Start DNA Master SYBR green I kit (Roche Diagnostics) following the manufacturer’s protocol. The reaction was performed in a 20 μl mixture containing 2 μl of the above-synthesized cDNA and 18 μl master mix. For matrix metalloproteinase-9 (MMP9), Cathepsin-K, receptor activator of NF-κB (RANK), dendritic cell-specific transmembrane protein (DC-STAMP), RANK-ligand (RANKL), osteoprotegerin (OPG), osteocalcin (OC) and glyceraldehyde-3- phosphate dehydrogenase (GAPDH), each cDNA sample was amplified using specific primers (Table 1, Hokkaido system science, Sapporo, Japan). After an initial denaturation step at 95 °C for 10 min, amplification was performed using 60 cycles of denaturation (95 °C for 10 s), annealing (60 °C for 15 s) and extension (72 °C for 10 s). For each run, a standard curve was generated from purified cDNA of the total bone marrow cells. Gene expression of MMP9, Cathepsin-K, RANK, DC-STAMP, RANKL, OPG and OC were normalized to GAPDH (n = 6 for each group).
TABLE 1.
Sequences of the Primer Pairs for PCR
| Gene | Forward | Reverse |
|---|---|---|
| MMP9 | 5′-GCCCTGGAACTCACACGACA-3′ | 5′-TTGGAAACTCACACGCCAGAAG-3′ |
| CathepsinK | 5′-CAGCAGAACGGAGGCATTGA-3′ | 5′-CCTTTGCCGTGGCGTTATAC-3′ |
| RANK | 5′-CCAGGACAGGGCTGATGAGAA-3′ | 5′-TGGCTGACATACACCACGATGA-3′ |
| DC-STAMP | 5′-CCGCTGTGGACTATCTGCTG-3′ | 5′-CTCAATGGCTGCTTTGATCG-3′ |
| RANKL | 5′-CAGAAGATGGCACTCACTGCA-3′ | 5′-CACCATCGCTTTCTCTGCTCT-3′ |
| OPG | 5′-GGAACCCCAGAGCGAAATACA-5′ | 5′-CCTGAAGAATGCCTCCTCACA-3′ |
| Osteocalcine | 5′-AAGCCTTCATGTCCAAGCAGG-3′ | 5′-TTTGTAGGCGGTCTTCAAGCC-3′ |
| GAPDH | 5′-AACGACCCCTTCATTGAC-3′ | 5′-TCCACGACATACTCAGCAC-3′ |
Measurement and Statistics
The callus area on soft X-ray radiographs and the resorption-pit area on SEM were measured and analyzed with Image J (National Institutes of Health, Bethesda, MD). Histological callus area and bone histomorphometry were measured and analyzed with Image pro plus (Media cybernetics, Bethesda, MD). All statistical analyses were performed using Student’s t-test.
Results
Bone radiographs
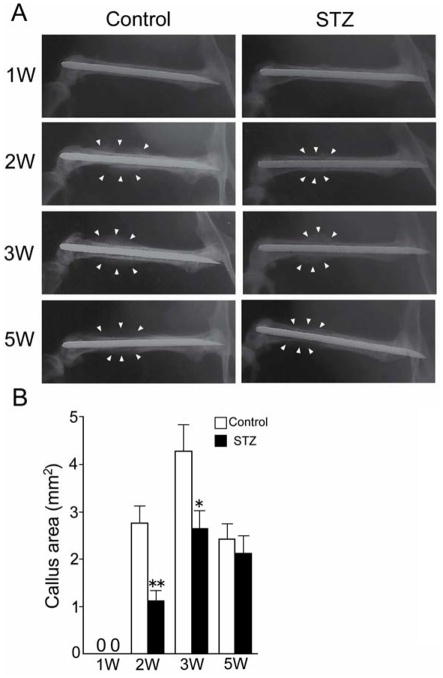
At 1 week after fracture, the contact radiogram did not show the presence of callus in either the control group or the STZ group (1W, Fig. 1A). At 2 and 3 weeks after fracture, the contact radiogram clearly showed callus in both the control and the STZ group (2W and 3W, Fig. 1A). The size of the radiographically depicted callus was larger in the control group than in the STZ group (2W and 3W, Fig. 1B). For both the control group and the STZ group, however, the callus was reduced in size by the 5th week. The reduction of the callus during weeks 3 to 5 was attributable to bone remodeling.
FIG. 1.
Callus area on bone radiographs. (A) A 23-gauge needle was inserted into the right femur of the mouse for internal fixation; the bone was then fractured by blunt trauma. Bone radiographs of the right femur were taken every week after fracture. Left panels show the control group, right panels show the streptozotocin (STZ) group. Radiographs are shown of one mouse from each group at each time point. Arrowheads indicate bone callus. (B) The callus areas on bone radiographs were measured by Image J. Data are mean ± SEM. *: p < 0.05. **: p < 0.005.
Histology
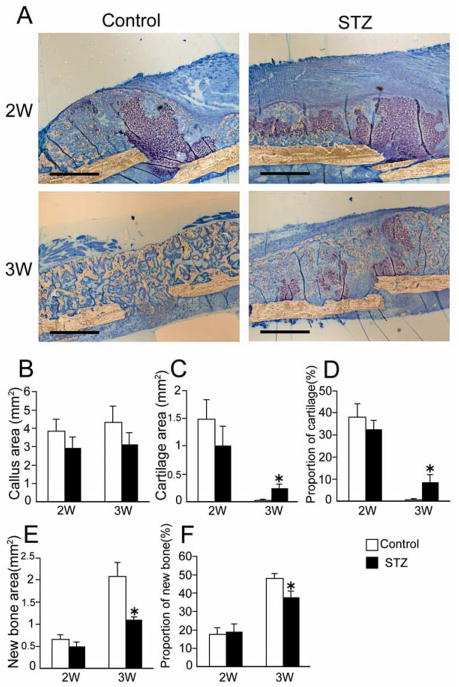
Histological investigation of the fracture site revealed that the repair process in the STZ mice was distinctive as compared to that of the control mice. At 2 weeks after fracture, cartilage tissue was detected in both the control group and the STZ group. However, the cartilage tissue disappeared from the control group at 3 weeks after fracture, while, in contrast, cartilage tissue remained in the STZ group (Fig. 2A).
FIG. 2.
The proportion of new bone and cartilage. (A) The undecalcified fracture site of the femur was sectioned at 4 μm, and stained with toluidine blue. Cartilage was stained purple, ossified tissue was stained light brown. The upper panels show the fracture site at 2 weeks after fracture; the lower panels show the fracture site at 3 weeks after fracture. Bars = 500 μm. (B) The callus area was measured. (C, D) The cartilage area and proportion of cartilage (cartilage area divided by callus area) were measured. (E, F) The new bone area and proportion of new bone were measured. All measurements were done by Image pro plus. Data are mean ± SEM. *: p < 0.05.
Undecalcified specimens were used to investigate the proportion of new bone and cartilage within the callus. The proportion of new bone and cartilage was depicted as each area versus total callus area (area within the peripheral fibrous capsule, excluding the original cortical bone). At 1 week after fracture, histological examination did not reveal any ossified area in the callus in either the control group or the STZ group (data not shown). The size of the callus area was not significant difference between the control and STZ group at 2 and 3 weeks after fracture (Fig. 2B). The cartilage area and new bone area were also not different between the control and STZ groups at 2 weeks after fracture (2W, Fig. 2C, 2E). The proportions of cartilage of control and STZ mice accounted for 30 – 40 % of the total callus area at 2 weeks after fracture (2W, Fig. 2D). Subsequently, the cartilage tissue disappeared from the callus of control mice, indicating rapid replacement by ossified tissue (Fig. 2F). Intriguingly, we found a large amount of cartilage remaining at 3 weeks (3W, Fig. 2C, 2D) and the formation of smaller amount of new bone (3W, Fig. 2E, 2F) in the STZ group as compared with the nondiabetic group at 3 weeks after fracture. The cartilage component eventually disappeared from the STZ group by the 5th week, suggesting delayed remodeling of the ossified tissue.
Bone histomorphometry
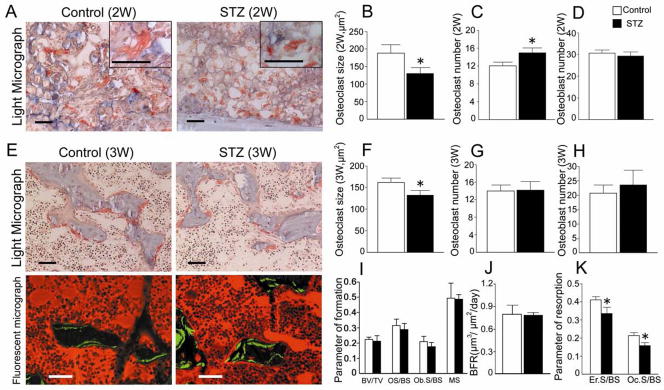
The callus area of undecalcified specimens was stained with TRAP and toluidine blue for standard bone histomorphometry. Numerous osteoclasts were detected in the vicinity of the hypertrophic chondrocytes in both groups at 2 weeks after fracture (Fig. 3A). Intriguingly, the STZ group appeared to contain an increased number of osteoclasts that were significantly smaller (Fig. 3A the panel insert) in size compared with those in the control group (Fig. 3B, 3C). Active deposition of osteoid tissue by the osteoblasts was detected in both control and STZ groups at 3 weeks after fracture (Light micrograph, Fig. 3E). Bone resorption by the osteoclasts appeared to proceed in parallel with bone formation (Light micrograph, Fig. 3E), as expected for normal bone remodeling. However, the size of the osteoclasts in the STZ group remained significantly smaller than the control group at 3 weeks after fracture (Fig. 3F), though the number of osteoclasts was not different between the control and STZ groups at 3 weeks after fracture (Fig. 3G). In addition, the number of osteoblasts was not different between the control and STZ groups at 2 and 3 weeks after fracture (Fig. 3D, 3H).
FIG. 3.
Bone histomorphometric parameters and osteoclast size. (A) The undecalcified fracture site of the femur was sectioned at 4 μm and stained with TRAP and toluidine blue. Osteoclasts stained red, calcified osteoid stained light blue, uncalcified osteoid was stained light pink. Panels show the fracture site at 2 weeks after fracture. The panel insert in each group shows representative osteoclasts at higher magnification. All bars = 50 μm. (B, C, D) The osteoclast number and size and the osteoblast number were measured by Image pro plus at 2 weeks after fracture. (E) Light micrographs (upper panels) and calcein labeled fluorescent micrographs (lower panels) of the fracture site at 3 weeks after fracture. Bars = 50 μm. (F, G, H) The osteoclast number and size and the osteoblast number were measured by Image pro plus at 3 weeks after fracture. (I, J, K) Bone histomorphometry was performed at 3 weeks after fracture. I, J: The bone formation parameters [bone volume/total tissue volume (BV/TV), osteoid surface/bone surface (OS/BS), osteoblast surface/bone surface (Ob.S/BS), mineralizing surface (MS), bone formation rate (BFR)]. K: The bone resorption parameters [erosion surface/bone surface (Er.S/BS), osteoclast surface/bone surface (Oc.S/BS)]. Data are mean ± SEM. *: p < 0.05.
Since the calceine double labels had not yet formed at 2 weeks after fracture, the double labeling test was investigated using the 3-week specimens. The bone formation parameters [bone volume/total tissue volume (BV/TV), osteoid surface/bone surface (OS/BS), osteoblast surface/bone surface (Ob.S/BS), mineralizing surface (MS)] of the STZ group were not significantly different from those of the nondiabetic controls at 3 weeks (Fig. 3I). Calcein labeling showed the calcification front (Fluorescent micrograph, Fig. 3E) the mineralizing surface (MS, Fig. 3I), and the bone formation rate (BFR, Fig. 3J) were not altered either. However, bone resorption parameters [erosion surface/bone surface (ES/BS), osteoclast surface/bone surface (Oc.S/BS)] were significantly decreased in the STZ group as compared to the control group at 3 weeks (Fig. 3K).
Immunohistochemistry
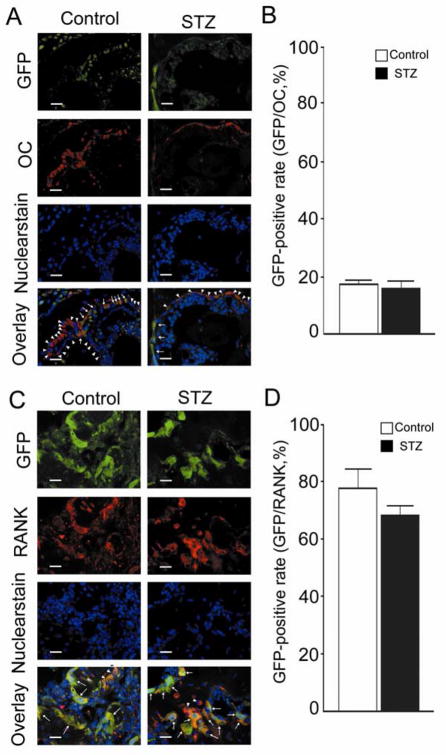
In order to determine whether the osteoblasts and osteoclasts were of bone marrow origin, we performed immunohistochemical staining. After BMT, all bone marrow-derived cells would be GFP-labeled. Decalcified specimens at 3 weeks after fracture were immunohistochemically stained using anti-OC (osteoblast-specific) or anti-RANK (osteoclast-specific) antibodies. Apparent overlap expression of GFP was detected on the OC-positive cells (i.e., osteoblasts) as well as the RANK-positive cells (i.e., osteoclasts). Osteoblasts and osteoclasts co-expressing GFP were considered to be of bone marrow origin (Fig. 4A, 4C arrows), whereas those without co-expression were likely to have originated from radiation-resistant somatic cells (Fig. 4A, 4C arrowheads), e.g, periosteal lining cells for osteoblasts. Enumeration of co-expressing cells revealed that approximately 80% of the RANK-positive cells were GFP-positive (Fig. 4D), whereas only 20% of the OC-positive cells were GFP-positive (Fig. 4B).
FIG. 4.
Imunohistochemical stain and percentage of GFP-positive cells. (A, C) Sections of decalcified femur were stained with immunohistochemical anti-osteocalcin antibody (OC) or anti-receptor activator of NF-κB (RANK) antibody. OC/Control: stained with OC in the control group. OC/STZ: stained with OC in the STZ group. RANK/Control: stained with RANK in the control group. RANK/STZ: stained with RANK in the STZ group. (B) Percentage of GFP-positive cells among the OC positive cells. (D) Percentage of GFP-positive cells among the RANK positive cells. Bars = 20 μm. Data are mean ± SEM.
Resorption-pit assay
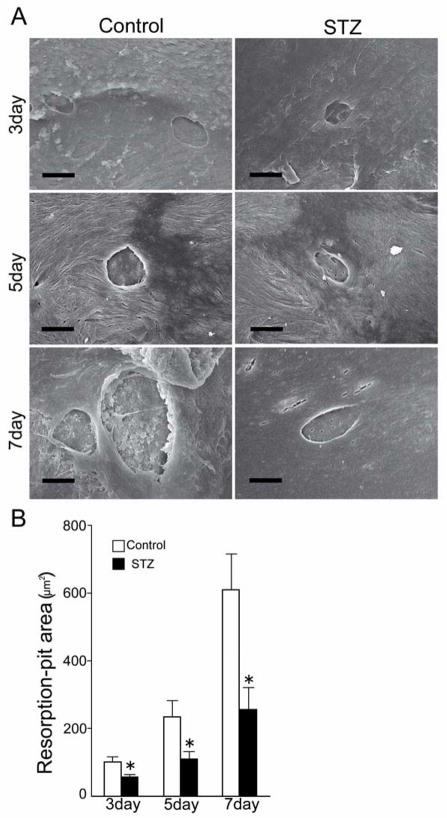
Before the determination of the resorbing function of mature osteoclasts, we performed TRAP staining to count the osteoclasts that came from bone marrow-derived cells in both STZ and control mice, and have found no difference between the two (data not shown). After culturing the adherent cells on cortical bone plates, we observed the surface of the plates by scanning electron microscopy and found well demarcated resorption pits produced by the osteoclasts on the cortical bone plates (Fig. 5A). By morphometric assessment, we could easily distinguish cracks and vascular foramens from resorption pits. The area of the resorption pits became progressively larger from day 3 to day 7. Computer-assisted measurement of the resorption-pit area revealed that the total resorption-pit area of the STZ group was significantly smaller than that observed in the control group (Fig. 5B).
FIG. 5.
Area of the resorption pits. (A) Bone marrow cells adhering to the cortical bone plates were cultured. After culture, the cortical bone plates were observed by scanning electron microscopy. The panels show 1, 3 and 5 days of the control group and the STZ group. Bars = 10 μm. (B) The area of the resorption pits was measured by Image J. Data are mean ± SEM (n=12 for each group). *: p < 0.05.
Quantitative real-time PCR
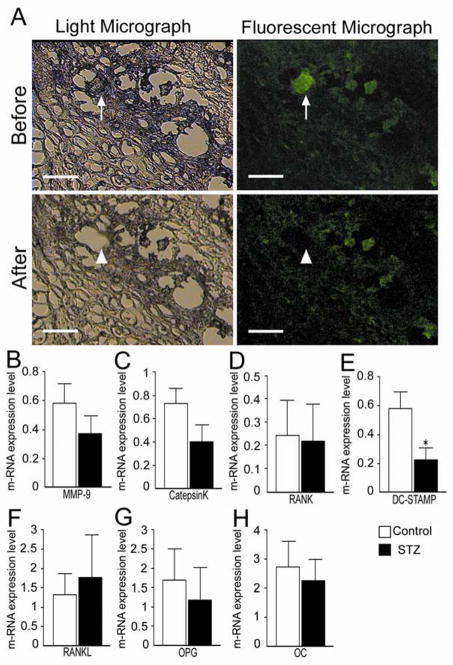
We captured 50 to 100 GFP-positive osteoclast-like cells within the callus tissue by LCM (see Fig. 6A), isolated the RNA and quantified mRNA expression by real-time qPCR. We found no difference in the level of mRNA for MMP9, Cathepsin-K and RANK, markers of mature osteoclasts, between the control and STZ group (Fig. 6B–D). In contrast, the expression level of DC-STAMP, a gene of presumed importance for osteoclast fusion, was significantly decreased in the STZ group compared to the control groups (Fig. 6E). Fifty to 100 GFP-positive osteoblast-like cells within the callus tissue were captured for analysis of the level of other mRNAs. We found no difference in the mRNA levels of RANKL, OPG or osteocalcin between the control and STZ groups (Fig. 6F–H).
FIG. 6.
Expression levels of m-RNA in GFP-positive cells. (A) GFP-positive osteoclasts in the callus tissue were captured by laser capture microdissection (arrow to arrowhead) Bar = 50 μm. (B–E) The expression level of mRNA in the captured osteoclasts was measured. The quantity of metalloproteinase-9 (MMP9), Cathepsin K (Cate-K), RANK and dendritic cell-specific transmembrane protein (DC-STAMP) were calibrated to the expression level of GAPDH. (F–H) The expression level of mRNA in the captured every GFP-positive cell was measured. The quantity of RANKL, osteoprotegerin (OPG) and osteocalcin (OC) were normalized to the expression level of GAPDH. Data are mean ± SEM *: p < 0.05.
Discussion
The callus plays a pivotal role in fracture repair and is the site in which cartilage tissue is first formed. The cartilage is then resorbed and replaced by non-mineralized osteoid which, through the process of mineralization, grows into ossified bone. In the present study, radiographic analysis first demonstrated that the size of the mineralized callus was smaller in the STZ group than in the control group. The size difference was most prominent during the 2 to 3 weeks after fracture (Fig. 1A, 1B). However, histological examination revealed that the total callus size of STZ mice was not significantly different between the diabetic and control groups (Fig. 2B), indicating that production of cartilage tissue was not affected in the STZ group. These observations also support the finding that osteoblast function was not altered in the present animal model.
High dose radiation may have a significant effect on fracture risk and repair [28]. In this experiment, we have used 9 Gy of radiation, which, according to previous reports, has no significant effect on the diabetic status [20, 21]. Furthermore, we had performed a pilot study to examine fracture healing without radiation/BMT both in control and diabetes, and found no significant effect of radiation on the time course of fracture healing.
A number of studies have shown that osteoblast function is impaired in diabetes patients, model animals, and cell culture experiments in the presence of high glucose concentration [3, 29–31]. However, histological and morphometrical analyses in this study suggest that osteoblast function is not impaired in the STZ mice with 14-day diabetic duration. In the animal experiments, tight glycaemic control was found to be critical for restoring fracture healing back to that seen in control [32]. However, there is a paucity of reports on the effect of diabetes duration on fracture healing [33]. It was reported that osteoblast function is time-dependently decreased in STZ-rat (diabetic duration 0–12 weeks). In a clinical study, it was reported that the longer duration of diabetes is a significant risk factor for the incidence of malunion, nonunion of the bone repair [34]. Although the extent of osteoblast malfunction depends on the severity of the diabetes, the role of the duration of diabetes on osteoblast function remains poorly defined and should be addressed in future studies.
We calculated the percentages of bone marrow-derived cells in the osteoblast population in STZ and control mice. Using osteocalcin (OC) as an osteoblast marker and its co-expression with GFP would provide an estimation on the number of osteoblasts that have originated from bone marrow-derived cells. Approximately 20% of OC-positive cells co-expressed GFP, and the remaining 80% of OC-positive cells were negative for GFP. These OC-positive and GFP-negative cells were thought to have originated from the radiation-resistant somatic cells, i.e., the periosteal lining cells. The periosteal lining cells act as a self-renewal reservoir for osteoblasts, and become activated at the time of fracture healing [9].
During the replacement of cartilage by osteoid tissue, the cartilage tissue is resorbed by osteoclasts. Firstly, histological analysis demonstrated that a large proportion of the callus still consisted of cartilage in the STZ group at 3 weeks after fracture (Fig. 2C, 2D). Secondly, bone morphometry demonstrated that the bone resorption parameters of the STZ group were significantly decreased as compared to those of the control group (Fig. 3K). Furthermore, bone formation parameters and mineralizing rate were not altered in the STZ group (Fig. 3I, 3J). Therefore, the delayed bone formation in the STZ group likely resulted from an impairment of cartilage resorption. The functional alteration of osteoclasts in diabetes remains controversial. [35–37]. A previous study showed that osteoclasts number and cartilage resorption rate were increased at 16 days after fracture [35]. Although we did not determine changes in fracture healing at 16 days, we observed that the osteoclast number was actually increased at 14 days in diabetic mice (Figure 3C). We tried to measure the resorption parameters at 14 days, but we could not obtain reliable data. Since the osteoclast size was decreased both at 14 days and 21 days after the fracture and the osteoclast function was decreased at 21 days, we concluded that decreased osteoclast function played major roles in the abnormal fracture healing in diabetes mellitus. The differences between a previous report [35] and our findings might be related to differences in the details of the animal models. In an attempt to characterize the osteoclast malfunction, we measured the size of osteoclasts and found that it was significantly smaller in the STZ group than in the control group at both 2 and 3 weeks (Fig. 3B, 3F). Scanning electron microscopy demonstrated that the size of resorption pits was significantly smaller in the STZ group than in the control group (Fig. 5A, 5B). It has long been known that sustainability of osteoclast function depends on the controlled renewal of multinucleated osteoclasts [17].
There is considerable interest in the molecular mechanism of osteoclast fusion [38, 39]. Although we observed no difference in mRNA expression level of a number of mature osteoclast markers between diabetes and control (Fig. 6B–D), we did find a significant difference in DC-STAMP expression. DC-STAMP is a transmembrane protein that seems to play a pivotal role in osteoclast fusion [40, 41]. Intriguingly, PCR analysis demonstrated that the expression of DC-STAMP is decreased in osteoclasts isolated from the STZ group compared to those of the control group (Fig. 6E). Collectively, the decreased expression of DC-STAMP is likely to result not only in smaller osteoclasts but also impaired bone resorption through attenuated renewal of the multinucleated osteoclasts. Recently, it was reported that GM-CSF induced fusion of prefusion osteoclasts to form multinucleated osteoclasts, making the osteoclast capable of bone resorption [41]. Since GM-CSF production in the wounds of diabetics was decreased and the exogenous GM-CSF substantially enhanced wound healing in diabetic mice [42], the decreased DC-STAMP expression may play an important role in the poor fracture healing observed in diabetes.
Since RANK is an osteoclast marker, its co-expression with GFP is only seen in osteoclasts that came from bone marrow-derived cells. Most of the RANK-positive cells co-expressed GFP. Our previous study suggested that malfunction of bone marrow-derived cells may underlie different diabetic complications [20, 21]. The impaired osteoclast function in the STZ group may have resulted from malfunction of the bone marrow stem cells.
In summary, we investigated the mechanism of osteoclast malfunction in diabetes mellitus using the STZ-induced diabetic mouse model. In the STZ group, the smaller cell sizes as well as smaller resorption pit areas suggest an impairment in the fusion and function of mononucleated osteoclasts. We found reduced expression of DC-STAMP, a pivotal molecule for mononuclear fusion, which may have contributed to the impaired osteoclast fusion. The resulting impairment in osteoclast function may be the cause of decreased cartilage resorption as well as delayed endochondral ossification. An in depth investigation into the progenitor cells of osteoclasts in the future may help elucidate the pathogenesis of osteoclast malfunction in diabetes mellitus.
Acknowledgments
The authors thank Prof. Mineko Fujimiya (University of Sapporo medical Science) for suggesting the basis of the method, Mrs. Yoko Uratani, Takefumi Yamamoto, and Yasuhiro Mori for skillful technical assistance, and Dr. Hiroshi Urabe and Dr. Yoshinori Takemura for their help and advice.
Funding sources
This work was supported by a Grant-in-Aid (#18390100 to H. Kojima) for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan, by the President’s Discretionary Fund from Shiga University of Medical Science (#1515503L to H. Kojima), and by a grant (HL-51586) from the US National Institutes of Health (to L. Chan).
Footnotes
All authors have declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Toshiyuki Kasahara, Email: tkasa@belle.shiga-med.ac.jp.
Sinji Imai, Email: simai@belle.shiga-med.ac.jp.
Hideto Kojima, Email: kojima@belle.shiga-med.ac.jp.
Miwako Katagi, Email: katagi@belle.shiga-med.ac.jp.
Hiroshi Kimura, Email: kimurah@belle.shiga-med.ac.jp.
Lawrence Chan, Email: lchan@bcm.tmc.edu.
Yoshitaka Matsusue, Email: matsusue@belle.shiga-med.ac.jp.
References
- 1.Federation ID. Diabetes Atlas. 3. Brussels: International Diabetes Federation; 2006. [Google Scholar]
- 2.Albright F. Parathyroid glands and metabolic bone disease. Baltiomore: The williams and Wilkins Company; 1948. [Google Scholar]
- 3.Kemink SA, Hermus AR, Swinkels LM, Lutterman JA, Smals AG. Osteopenia in insulin-dependent diabetes mellitus; prevalence and aspects of pathophysiology. J Endocrinol Invest. 2000;23:295–303. doi: 10.1007/BF03343726. [DOI] [PubMed] [Google Scholar]
- 4.Inzerillo AM, Epstein S. Osteoporosis and diabetes mellitus. Rev Endocr Metab Disord. 2004;5:261–8. doi: 10.1023/B:REMD.0000032415.83124.20. [DOI] [PubMed] [Google Scholar]
- 5.Gaston MS, Simpson AH. Inhibition of fracture healing. J Bone Joint Surg Br. 2007;89:1553–60. doi: 10.1302/0301-620X.89B12.19671. [DOI] [PubMed] [Google Scholar]
- 6.Wittrant Y, Gorin Y, Woodruff K, Horn D, Abboud HE, Mohan S, Abboud-Werner SL. High d(+)glucose concentration inhibits RANKL-induced osteoclastogenesis. Bone. 2008;42:1122–30. doi: 10.1016/j.bone.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamada Y, Kitazawa S, Kitazawa R, Fujii H, Kasuga M, Fukagawa M. Histomorphometric analysis of diabetic osteopenia in streptozotocin-induced diabetic mice: a possible role of oxidative stress. Bone. 2007;40:1408–14. doi: 10.1016/j.bone.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 8.Valcourt U, Merle B, Gineyts E, Viguet-Carrin S, Delmas PD, Garnero P. Non-enzymatic glycation of bone collagen modifies osteoclastic activity and differentiation. J Biol Chem. 2007;282:5691–703. doi: 10.1074/jbc.M610536200. [DOI] [PubMed] [Google Scholar]
- 9.Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998:S7–21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 10.Ford JL, Robinson DE, Scammell BE. Endochondral ossification in fracture callus during long bone repair: the localisation of ‘cavity-lining cells’ within the cartilage. J Orthop Res. 2004;22:368–75. doi: 10.1016/j.orthres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Eriksen EF, Eghbali-Fatourechi GZ, Khosla S. Remodeling and vascular spaces in bone. J Bone Miner Res. 2007;22:1–6. doi: 10.1359/jbmr.060910. [DOI] [PubMed] [Google Scholar]
- 12.Rahn BA, Gallinaro P, Baltensperger A, Perren SM. Primary bone healing. An experimental study in the rabbit. J Bone Joint Surg Am. 1971;53:783–6. [PubMed] [Google Scholar]
- 13.Taguchi K, Ogawa R, Migita M, Hanawa H, Ito H, Orimo H. The role of bone marrow-derived cells in bone fracture repair in a green fluorescent protein chimeric mouse model. Biochem Biophys Res Commun. 2005;331:31–6. doi: 10.1016/j.bbrc.2005.03.119. [DOI] [PubMed] [Google Scholar]
- 14.Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40:46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Schenk RK, Spiro D, Wiener J. Cartilage resorption in the tibial epiphyseal plate of growing rats. J Cell Biol. 1967;34:275–91. doi: 10.1083/jcb.34.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engsig MT, Chen QJ, Vu TH, Pedersen AC, Therkidsen B, Lund LR, Henriksen K, Lenhard T, Foged NT, Werb Z, Delaisse JM. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol. 2000;151:879–89. doi: 10.1083/jcb.151.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishii M, Saeki Y. Osteoclast cell fusion: mechanisms and molecules. Mod Rheumatol. 2008;18:220–7. doi: 10.1007/s10165-008-0051-2. [DOI] [PubMed] [Google Scholar]
- 18.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–64. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 19.Kim JM, Jeong D, Kang HK, Jung SY, Kang SS, Min BM. Osteoclast precursors display dynamic metabolic shifts toward accelerated glucose metabolism at an early stage of RANKL-stimulated osteoclast differentiation. Cell Physiol Biochem. 2007;20:935–46. doi: 10.1159/000110454. [DOI] [PubMed] [Google Scholar]
- 20.Terashima T, Kojima H, Fujimiya M, Matsumura K, Oi J, Hara M, Kashiwagi A, Kimura H, Yasuda H, Chan L. The fusion of bone-marrow-derived proinsulin-expressing cells with nerve cells underlies diabetic neuropathy. Proc Natl Acad Sci U S A. 2005;102:12525–30. doi: 10.1073/pnas.0505717102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujimiya M, Kojima H, Ichinose M, Arai R, Kimura H, Kashiwagi A, Chan L. Fusion of proinsulin-producing bone marrow-derived cells with hepatocytes in diabetes. Proc Natl Acad Sci U S A. 2007;104:4030–5. doi: 10.1073/pnas.0700220104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2:97–101. doi: 10.1002/jor.1100020115. [DOI] [PubMed] [Google Scholar]
- 23.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 24.Morinobu M, Nakamoto T, Hino K, Tsuji K, Shen ZJ, Nakashima K, Nifuji A, Yamamoto H, Hirai H, Noda M. The nucleocytoplasmic shuttling protein CIZ reduces adult bone mass by inhibiting bone morphogenetic protein-induced bone formation. J Exp Med. 2005;201:961–70. doi: 10.1084/jem.20041097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amano S, Hanazawa S, Kawata Y, Ohta K, Kitami H, Kitano S. An assay system utilizing devitalized bone for assessment of differentiation of osteoclast progenitors. J Bone Miner Res. 1992;7:321–8. doi: 10.1002/jbmr.5650070312. [DOI] [PubMed] [Google Scholar]
- 26.Hattersley G, Dorey E, Horton MA, Chambers TJ. Human macrophage colony-stimulating factor inhibits bone resorption by osteoclasts disaggregated from rat bone. J Cell Physiol. 1988;137:199–203. doi: 10.1002/jcp.1041370125. [DOI] [PubMed] [Google Scholar]
- 27.Claudino M, Garlet TP, Cardoso CR, de Assis GF, Taga R, Cunha FQ, Silva JS, Garlet GP. Down-regulation of expression of osteoblast and osteocyte markers in periodontal tissues associated with the spontaneous alveolar bone loss of interleukin-10 knockout mice. Eur J Oral Sci. 118:19–28. doi: 10.1111/j.1600-0722.2009.00706.x. [DOI] [PubMed] [Google Scholar]
- 28.Cannon CP, Lin PP, Lewis VO, Yasko AW. Management of radiation-associated fractures. J Am Acad Orthop Surg. 2008;16:541–9. [PubMed] [Google Scholar]
- 29.Inaba M, Terada M, Koyama H, Yoshida O, Ishimura E, Kawagishi T, Okuno Y, Nishizawa Y, Otani S, Morii H. Influence of high glucose on 1,25-dihydroxyvitamin D3-induced effect on human osteoblast-like MG-63 cells. J Bone Miner Res. 1995;10:1050–6. doi: 10.1002/jbmr.5650100709. [DOI] [PubMed] [Google Scholar]
- 30.Gopalakrishnan V, Vignesh RC, Arunakaran J, Aruldhas MM, Srinivasan N. Effects of glucose and its modulation by insulin and estradiol on BMSC differentiation into osteoblastic lineages. Biochem Cell Biol. 2006;84:93–101. doi: 10.1139/o05-163. [DOI] [PubMed] [Google Scholar]
- 31.Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR. Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology. 2005;146:3622–31. doi: 10.1210/en.2004-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beam HA, Parsons JR, Lin SS. The effects of blood glucose control upon fracture healing in the BB Wistar rat with diabetes mellitus. J Orthop Res. 2002;20:1210–6. doi: 10.1016/S0736-0266(02)00066-9. [DOI] [PubMed] [Google Scholar]
- 33.Katayama Y, Akatsu T, Yamamoto M, Kugai N, Nagata N. Role of nonenzymatic glycosylation of type I collagen in diabetic osteopenia. J Bone Miner Res. 1996;11:931–7. doi: 10.1002/jbmr.5650110709. [DOI] [PubMed] [Google Scholar]
- 34.Jones KB, Maiers-Yelden KA, Marsh JL, Zimmerman MB, Estin M, Saltzman CL. Ankle fractures in patients with diabetes mellitus. J Bone Joint Surg Br. 2005;87:489–95. doi: 10.1302/0301-620X.87B4.15724. [DOI] [PubMed] [Google Scholar]
- 35.Kayal RA, Tsatsas D, Bauer MA, Allen B, Al-Sebaei MO, Kakar S, Leone CW, Morgan EF, Gerstenfeld LC, Einhorn TA, Graves DT. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res. 2007;22:560–8. doi: 10.1359/jbmr.070115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCabe LR. Understanding the pathology and mechanisms of type I diabetic bone loss. J Cell Biochem. 2007;102:1343–57. doi: 10.1002/jcb.21573. [DOI] [PubMed] [Google Scholar]
- 37.He H, Liu R, Desta T, Leone C, Gerstenfeld LC, Graves DT. Diabetes causes decreased osteoclastogenesis, reduced bone formation, and enhanced apoptosis of osteoblastic cells in bacteria stimulated bone loss. Endocrinology. 2004;145:447–52. doi: 10.1210/en.2003-1239. [DOI] [PubMed] [Google Scholar]
- 38.Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Yano K, Morinaga T, Higashio K. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem Biophys Res Commun. 1998;253:395–400. doi: 10.1006/bbrc.1998.9788. [DOI] [PubMed] [Google Scholar]
- 39.Bar-Shavit Z. The osteoclast: a multinucleated, hematopoietic-origin, bone-resorbing osteoimmune cell. J Cell Biochem. 2007;102:1130–9. doi: 10.1002/jcb.21553. [DOI] [PubMed] [Google Scholar]
- 40.Yagi M, Ninomiya K, Fujita N, Suzuki T, Iwasaki R, Morita K, Hosogane N, Matsuo K, Toyama Y, Suda T, Miyamoto T. Induction of DC-STAMP by alternative activation and downstream signaling mechanisms. J Bone Miner Res. 2007;22:992–1001. doi: 10.1359/jbmr.070401. [DOI] [PubMed] [Google Scholar]
- 41.Lee MS, Kim HS, Yeon JT, Choi SW, Chun CH, Kwak HB, Oh J. GM-CSF regulates fusion of mononuclear osteoclasts into bone-resorbing osteoclasts by activating the Ras/ERK pathway. J Immunol. 2009;183:3390–9. doi: 10.4049/jimmunol.0804314. [DOI] [PubMed] [Google Scholar]
- 42.Fang Y, Shen J, Yao M, Beagley KW, Hambly BD, Bao S. Granulocyte-macrophage colony-stimulating factor enhances wound healing in diabetes via upregulation of proinflammatory cytokines. Br J Dermatol. 2010;162:478–86. doi: 10.1111/j.1365-2133.2009.09528.x. [DOI] [PubMed] [Google Scholar]