Abstract
Detection and quantitation of biomolecules is one of the most commonly performed measurements in biomedical research and clinical diagnostics. There is a high demand for convenient, rapid and sensitive biomolecule detection methodologies. In this review we discuss a family of sensors that have been developed in our laboratory that share a common simple biophysical mechanism of action and that are capable of rapid detection of a diverse range of biological targets. The sensors generate fluorescence signal in the presence of the target molecule through target-induced association of short fluorochrome-labeled complementary oligonucleotides that are attached to target recognition elements of the sensors (antibodies, aptamers, etc.) via nanometer scale flexible linkers. This sensor design can be used for detecting proteins, antibodies, nucleic acids and whole cells. The assays using these sensors require only adding a sample to the sensor mix followed by simple fluorescence intensity readout. The simplicity, the speed of detection and the potential for miniaturization are the main assets of these sensors.
Keywords: biosensor, fluorescence, FRET, immunodetection
Technologies that allow detection, quantitation and imaging of biomolecules play essential roles in biomedical research and clinical diagnosis. While ELISA remains the gold standard for biomolecule detection and quantitation [1–5], its relative complexity, low throughput and long time required to perform the assay provided strong motivation for continuing development of improved biomolecule detection methodologies. These efforts emphasize improvements in high throughput capabilities of assays (for example, [6]), improvements in sensitivity (for example, [7]), and simplification of assay protocols (for example, [8]).
In the past several years our laboratory has developed a family of sensors capable of detecting a wide range of biomolecules [9–12]. These sensors exhibit many of the characteristics that are desired in improved biomolecule detection technologies. These sensors are extremely simple to use since they requires only the addition of the sample to the sensor mix followed by fluorescence intensity readout. This homogeneous nature of the sensors makes them well suited for high-throughput applications. They are fast and can be multiplexed. It is possible to miniaturize them to the extent limited only by technical capabilities of the instrumentation used to detect the fluorescence. All these sensors utilize a common simple biophysical mode of action illustrated in Fig. 1. Sensor recognition elements (DNA, aptamers, antibodies, peptides, etc.) are labeled with short complementary oligonucleotides via nanometer scale flexible linkers. The length (hybridization energy) of these signaling oligonucleotides is designed such that at the concentrations used in the assay (typically in nM range) very little association between the oligonucleotides occurs. Association of sensor recognition elements with the target biomolecule triggers the annealing of the signaling oligonucleotides (Fig. 1). This is because upon association of the two sensor recognition elements with the target, local effective concentration of signaling oligonucleotides is greatly increased since their sphere of diffusion will be limited and defined by the length of flexible liners that connect them to sensor recognition elements. We used a simple model system to determine the range of effective local concentrations that could be expected in the system depicted in Fig. 1. With linkers up to ~ 50 nm length local effective concentrations were in micromolar range (typically, in tens of micomolar range) [13]. Thus, association of the target recognition elements with the target produces ~ 3 orders of magnitude increase (from nanomolar to micromolar range) of effective local concentrations of signaling oligonucleotides. As long as the dissociation constant of the signaling oligonucleotides duplex is in low micromolar to hundreds on nanomolar range, effective target-induced association of signaling oligonucleotides should be observed (Fig. 1). Very long nanometer size linkers that are used for attaching signaling oligonucleotides assure that large targets could be accommodated by the design shown in Fig. 1.
Fig. 1.
Design of sensors utilizing target-induced annealing of complementary oligonucleotides for signaling. A and B; target recognition elements; c; flexible nanometer scale linkers; d and e; complementary signaling oligonucleotides; T; target biomolecule.
Target-induced annealing of the signaling oligonucleotides provides the means for generating observable signal reporting the presence of the target. For example, labeling each of the signaling oligonucleotides with fluorochromes that could participate in Fluorescence Resonance Energy Transfer (FRET) [14] allows using FRET signal for target detection. It should be emphasized that FRET signaling can be incorporated into design illustrated in Fig. 1 without the challenges usually associated with designing FRET-based assays. For efficient FRET, the donor and acceptor probes have to be brought to a close proximity (~ 5nm) that can be difficult to achieve especially if interacting partners are large. In the case of sensor design depicted in Fig. 1, regardless of the size of the target, donor and acceptor probe are brought the same distance that can be tuned to any desired value using well-established geometry of DNA duplex. Signaling oligonucleotides also contribute to the sensor function in another way by providing additional favorable free energy component that enhances the stability of the complex between the target and target recognition elements. This contributes to the sensitivity of the detection and also to the specificity of the sensors since the signal in the presence of the target is generated due to an obligate coincidence of three molecular events: the recognition of the target by each of the two target recognition elements and the association of complementary signaling oligonucleotides.
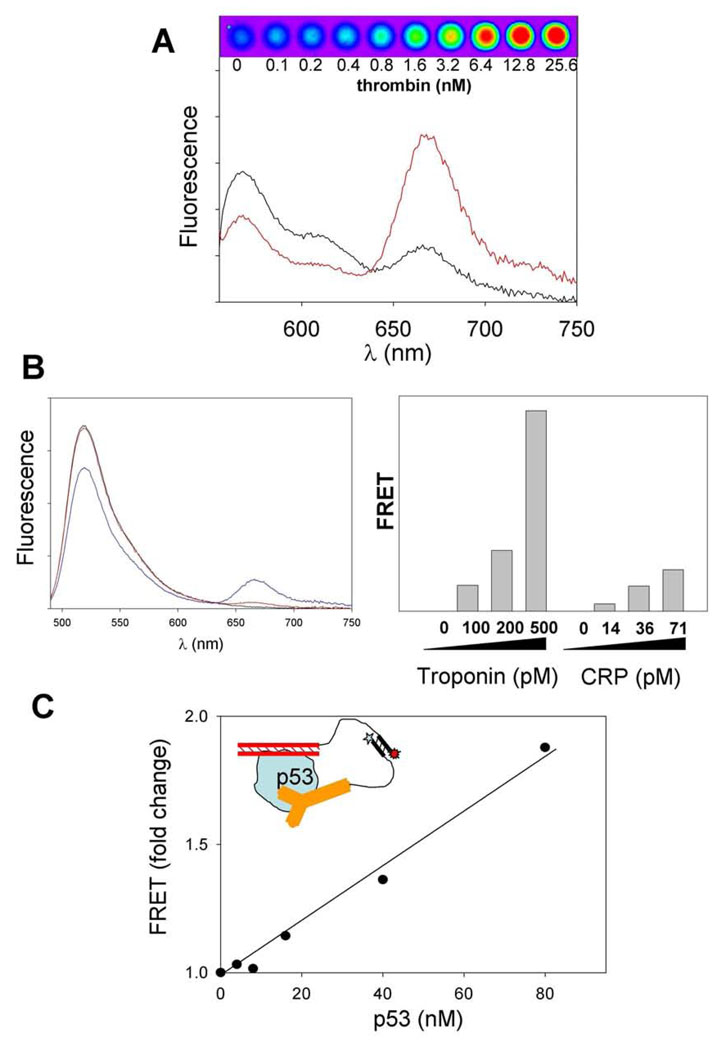
We have demonstrated the feasibility of sensor design shown in Fig. 1 using aptamers, antibodies and ds DNA as target recognition elements (Fig. 2). A pair of thrombin-specific aptamers that bind thrombin at two non-overlapping epitopes was used to prepare thrombin sensor according to design illustrated in Fig. 1 [9]. Thrombin concentration dependent increase in FRET signal was observed (Fig. 2A) that allowed detection of thrombin down to ~ 50 pM concentration. The sensor was highly specific for thrombin since, for example, it could discriminate between thrombin and prothrombin[9]. The sensor also could detect thrombin in the context of complex samples such as cell extracts or plasma. The functionality of the sensor design with antibodies as target recognition elements was demonstrated using cardiac troponin and C-reactive protein as targets [10]. Large target protein dependent FRET signal was observed (Fig. 2B) allowing specific detection of target proteins with limits of detection of ~ 40 pM for troponin and ~ 17 pM for C-reactive protein. About 10 min incubation time was necessary for the FRET signal to reach its maximum value after addition of the target protein. Target proteins could be detected in complex samples (plasma). The functionality of sensor design utilizing ds DNA as recognition element was demonstrated using p53 protein as a target (Fig. 2C) [10]. In this case, the sensor was composed of two heterologous target recognition elements: ds DNA and the antibody. P53 protein is a sequence-specific DNA binding protein and ds DNA fragment used contained high affinity p53 binding site. The generality of sensor design depicted in Fig. 1 is thus well supported by the examples in Fig. 2 as well as by seven additional yet unpublished sensors that were successfully developed in our laboratory.
Fig. 2.
Examples of applications of sensor design shown in Fig. 1. (A) Sensor for thrombin utilizing thrombin-specific aptamers as target recognition elements. Reprinted with permission from [9]. Copyright 2005 American Chemical Society. Fluorescence spectra of Cy3 and Cy5-labeled sensor mix in the absence (black) and presence (red) of thrombin. False color FRET fluorescence image of microplate wells containing sensor and indicated concentrations of thrombin is also shown. (B) Sensors for cardiac troponin and C-reactive protein (CRP) utilizing target-specific antibodies as target recognition elements. Reprinted with permission from [10]. Copyright 2008 American Chemical. Society. Left panel: Fluorescence spectra of fluorescein and Cy5-labeled sensor mix in the absence (red) and presence (blue) of troponin. Right panel: FRET signals observed at low target concentrations. (C) Sensor for p53 utilizing ds DNA and antibody as target recognition elements. Reprinted with permission from [10]. Copyright 2008 American Chemical. Society.
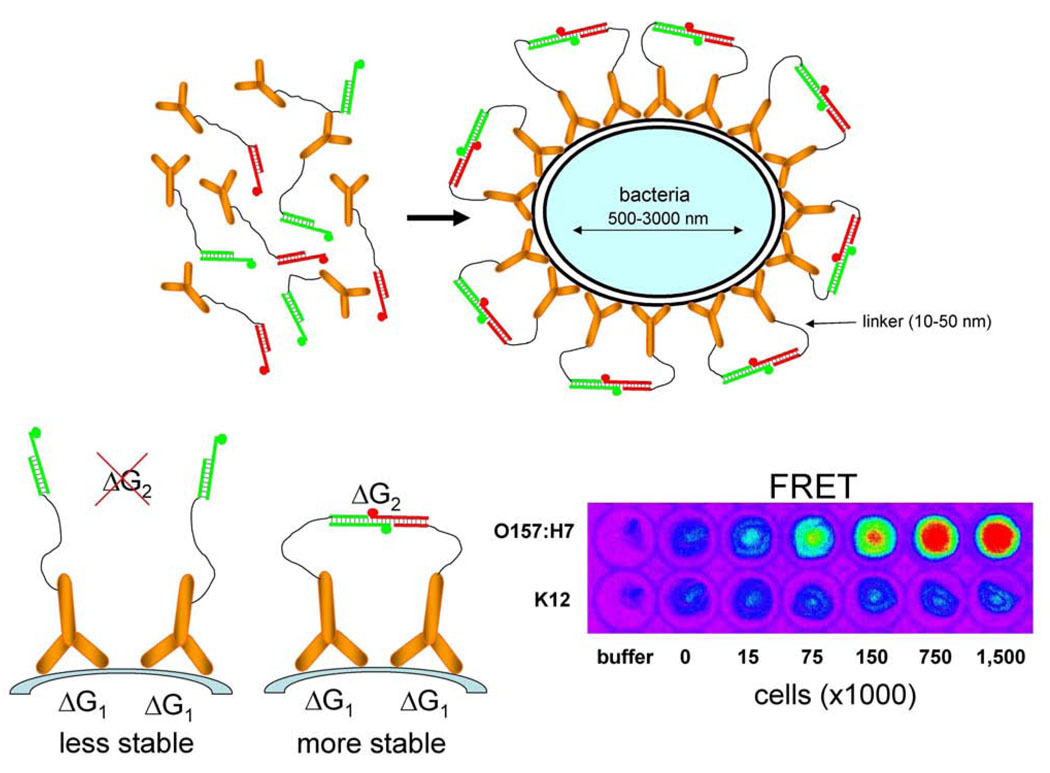
Sensor design in Fig. 1 is not limited to protein targets only. Any mechanism that could bring signaling oligonucleotide-labeled target recognition elements to the proximity compatible with the length of flexible linkers could in principle be used for generating FRET signal necessary for target detection. This concept is well illustrated by the design of sensors for detecting pathogenic bacteria (Fig. 3) [12]. In this case, taking into account relative dimensions of the bacteria and the length of flexible linkers in target recognition elements, the binding of the target recognition element (in this case an antibody) to the cell surface epitopes will likely bring the antibodies to the proximity necessary for FRET signaling even if the density of the epitopes on the cell surface would be only moderate. The sensor for pathogenic bacteria employs an additional simple biophysical “trick”. In the case of this sensor only a single antibody (labeled with donor-modified or acceptor-modified signaling oligonucleotides) recognizing cell surface epitope was used. This in principle should produce a sensor exhibiting diminished performance since only a fraction of labeled antibodies should bind in a configuration that allows FRET signal generation (i.e. donor-labeled antibody binding in the vicinity of acceptor-labeled antibody). The majority of antibodies should be bound in a configuration that would not produce FRET signal (i.e. donor-labeled antibody binding in the vicinity of another donor-labeled antibody or acceptor-labeled antibody binding in the vicinity of another acceptor-labeled antibody). However, the additional favorable free energy derived from the hybridization of complementary signaling oligonucleotides when donor-labeled antibody binds near acceptor-labeled antibody (Fig. 3) makes this configuration more stable and more preferable. Thus, the sensor design shown in Fig. 3 has a built-in self-organizing process that assures maximum FRET signal in the presence of the target. Sensors specific for Escherichia coli O157:H7 and Salmonella typhimurium according to this design has been successfully developed. These sensors exhibited high specificity, short response time and were capable of detecting ~ 300 cells.
Fig. 3.
Design of homogeneous sensors for detecting pathogenic bacteria (upper panel) [12]. Increased stability of the pair of antibodies bound to neighboring sites on the surface of the cell due to additional favorable free energy derived from hybridization of complementary signaling oligonucleotides (lower left panel). Fluorescence FRET images of 96-well microplate wells containing indicated amounts of E. coli O157:H7 cells or control cells (E. coli K12) (lower right panel). Reprinted from Analytical Biochemistry, 396, Heyduk, E. and Heyduk, T., Fluorescent homogenous immunosensors for detecting pathogenic bacteria, 298–303, 2010, with permission from Elsevier.
Sensor design depicted in Fig. 1 can be also employed for detecting antibodies (Fig. 4) [11]. In this case, the bivalent character of the antibody is utilized in sensor design. Peptide corresponding to the antigen recognized by the antibody is used as the target recognition element this case and is conjugated with fluorochrome-labeled signaling oligonucleotides via long flexible linkers. As in the case of sensors for pathogenic bacteria, this sensor also has a built-in self-organizing process that assures maximum FRET signal in the presence of the target antibody (Fig. 4B). The functionality of sensors for detecting antibodies was demonstrated using two cardiac troponin-specific antibodies. Picomolar concentrations of target antibody could be easily detected (Fig. 4C). The sensors exhibited excellent specificity and were able to detect target antibody in complex samples (serum). If the antigen peptide used to construct the sensor is also accessible to the antibody in the context of the intact protein, antibody sensor can be used for detecting the protein containing antigen peptide employing competitive assay design as demonstrated with cardiac troponin. Another interesting application of the antibody sensor is to use it as an assay for post-translational modifications reactions that target the antigen peptide sequence. Modification of the peptide (for example, phosphorylation) is likely going to abolish its binding to the antibody resulting in disappearance of FRET signal. This application of antibody sensors has been demonstrated using phosphorylation of troponin peptide by PKA kinase as a model [11]. Alternatively, antibody specific to the modified form of the peptide could be used to prepare sensors that would produce FRET signal in response to peptide modification.
Fig. 4.
(A) Design of antigen peptide-based sensors for detecting antibodies [11]. (B) Enhanced stability of the complex between the antibody and the peptide-oligonucleotide conjugates containing donor and acceptor fluorochromes-labeled complementary signaling oligonucleotides. (C) Response of the antibody sensor to indicated concentrations of the antibody measured at two concentrations of sensor components. Reprinted with permission from [11]. Copyright 2009 American Chemical. Society.
Concluding remarks
Sensor design illustrated in Fig. 1 provides an example of how simple biophysical concepts can be put into practical use. These sensors exhibit several desirable characteristics that are likely to facilitate their practical applications. The homogenous mix-and-read nature of the assay simplifies greatly their use, shortens time of the assay and will be especially beneficial in situations where the access to highly trained personnel or sophisticated instrumentation is limited. For example, our sensor design should facilitate development of point-of-care assays and the deployment of the assays in developing world. As already demonstrated by applying sensors for detecting a wide range of biomolecule targets, their design is not specific to any target and is only limited by the availability of appropriate target recognition elements. Sensors producing different colors of fluorescence signal could be employed for simple multiplexing. They can be miniaturized to the smallest volume compatible with fluorescence instrumentation used to read the signal. Current limits of detection (tens of pM) observed when good quality recognition elements are available will be sufficient for many targets but there will be also important targets that would need to be detected at lower concentrations. One of the goals of the ongoing research in our laboratory is to improve detection sensitivity of the sensors to further widen their applicability.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Engvall E, Perlman P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971;8:871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- 2.Van Weemen BK, Schuurs AH. Immunoassay using antigen-enzyme conjugates. FEBS Lett. 1971;15:232–236. doi: 10.1016/0014-5793(71)80319-8. [DOI] [PubMed] [Google Scholar]
- 3.Reen DJ. Enzyme-linked immunosorbent assay (ELISA) Methods Mol Biol. 1994;32:461–466. doi: 10.1385/0-89603-268-X:461. [DOI] [PubMed] [Google Scholar]
- 4.Porstmann T, Kiessig ST. Enzyme immunoassay techniques. An overview. J Immunol Methods. 1992;150:5–21. doi: 10.1016/0022-1759(92)90061-w. [DOI] [PubMed] [Google Scholar]
- 5.Self CH, Cook DB. Advances in immunoassay technology. Curr Opin Biotechnol. 1996;7:60–65. doi: 10.1016/s0958-1669(96)80096-6. [DOI] [PubMed] [Google Scholar]
- 6.Haab BB, Dunham MJ, Brown PO. Protein microarrays for highly parallel detection and quantitation of specific proteins and antibodies in complex solutions. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-2-research0004. RESEARCH0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schweitzer B, Wiltshire S, Lambert J, O'Malley S, Kukanskis K, Zhu Z, Kingsmore SF, Lizardi PM, Ward DC. Inaugural article: immunoassays with rolling circle DNA amplification: a versatile platform for ultrasensitive antigen detection. Proc Natl Acad Sci U S A. 2000;97:10113–10119. doi: 10.1073/pnas.170237197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Zhang Y, Wang S. Development of flow-through and dip-stick immunoassays for screening of sulfonamide residues. J Immunol Methods. 2008;337:1–6. doi: 10.1016/j.jim.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Heyduk E, Heyduk T. Nucleic acid-based fluorescence sensors for detecting proteins. Anal Chem. 2005;77:1147–1156. doi: 10.1021/ac0487449. [DOI] [PubMed] [Google Scholar]
- 10.Heyduk E, Dummit B, Chang YH, Heyduk T. Molecular pincers: antibody-based homogeneous protein sensors. Anal Chem. 2008;80:5152–5159. doi: 10.1021/ac8004154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian L, Heyduk T. Antigen peptide-based immunosensors for rapid detection of antibodies and antigens. Anal Chem. 2009;81:5218–5225. doi: 10.1021/ac900845a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heyduk E, Heyduk T. Fluorescent homogeneous immunosensors for detecting pathogenic bacteria. Anal Biochem. 2010;396:298–303. doi: 10.1016/j.ab.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian L, Heyduk T. Bivalent ligands with long nanometer-scale flexible linkers. Biochemistry. 2009;48:264–275. doi: 10.1021/bi801630b. [DOI] [PubMed] [Google Scholar]
- 14.Selvin PR. Fluorescence resonance energy transfer. Methods Enzymol. 1995;246:300–334. doi: 10.1016/0076-6879(95)46015-2. [DOI] [PubMed] [Google Scholar]