Abstract
Sleep fragmentation (SF) is prevalent in human sleep-related disorders. In rats, sustained SF has a potent suppressive effect on adult hippocampal dentate gyrus (DG) neurogenesis. Adult-generated DG neurons progressively mature over several weeks, and participate in certain hippocampal-dependent cognitive functions. We predicted that suppression of neurogenesis by sustained SF would affect hippocampal-dependent cognitive functions in the time window when new neurons would reach functional maturity.
Sprague-Dawley rats were surgically-prepared with EEG and EMG electrodes for sleep state detection. We induced sleep-dependent SF for 12 days, and compared SF animals to yoked SF controls (SFC), treadmill controls (TC) and cage controls (CC). Rats were injected with bromodeoxyuridine on treatment days 4 and 5. Rats were returned to home cages for 14 days. Cognitive performance was assessed in a Barnes maze with 5 days at a constant escape position followed by 2 days at a rotated position. After Barnes maze testing rats were perfused and DG sections were immunolabeled for BrdU and NeuN, a marker of mature neurons.
SF reduced BrdU-labeled cell counts by 32% compared to SFC and TC groups. SF reduced sleep epoch duration, but amounts of REM sleep did not differ between SF and SFC rats, and NREM was reduced only transiently. In the Barnes maze, SF rats exhibited a progressive decrease in escape time, but were slower than controls. SF animals used different search strategies. The use of a random, non-spatial search strategy was significantly elevated in SF compared to the SFC, TC and CC groups. The use of random search strategies was negatively correlated with NREM sleep bout length during SF.
Conclusions
Sustained sleep fragmentation reduced DG neurogenesis and induced use of a non-spatial search strategy, which could be seen 2 weeks after terminating the SF treatment. The reduction in neurogenesis induced by sleep fragmentation is likely to underlie the delayed changes in cognitive function.
Introduction
The subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) is one of two mammalian brain regions which, in adults, contain progenitor cells which proliferate and generate substantial numbers of new cells which can progressively mature into functional neurons. Adult DG neurogenesis occurs in several mammalian species, including humans (Gould and Gross, 2002;Ming and Song, 2005;Eriksson et al., 1998). Adult neurogenesis depends on a complex series of processes, including initiation of the cell cycle in progenitor cells, generation of a vascular niche, and expression of genes determining cell phenotype and supporting morphological maturation and migration (Ming and Song, 2005). Many newly-generated cells do not survive (Kemperman et al, 2003). Newly-generated hippocampal DG neurons have been shown to play a role in certain hippocampal-dependent cognitive functions. This has been shown previously by testing cognitive function after suppressing neurogenesis using manipulations including brain irradiation (Madsen et al., 2003;Raber et al., 2004;Saxe et al., 2006;Snyder et al., 2005;Winocur et al., 2006;Wojtowicz et al., 2008;Clelland et al., 2009), administration of anti-mitotic agents (Shors et al., 2001;Shors et al., 2002;Bruel-Jungerman et al., 2005;Garthe et al., 2009), or using transgenic manipulations(Zhang et al., 2008;Dupret et al., 2008;Jessberger et al., 2009;Deng et al., 2009). Newly generated cells require 3–4 weeks to reach functional maturity (Jessberger and Kempermann, 2003;Ge et al., 2007;Kee et al., 2007). If neurogenesis is suppressed, certain hippocampal-dependent cognitive deficits could be predicted beginning about 3–4 weeks after the suppressing treatment.
Each stage of adult neurogenesis is modulated, either positively or negatively, by physiological and behavioral events. We and others have shown that the proliferative stage is potently suppressed by 2–7 days sleep deprivation (Guzman-Marin et al., 2005;Tung et al., 2005;Roman et al., 2005;Mirescu et al., 2006), REM sleep deprivation (Guzman-Marin et al., 2008;Mueller et al., 2008) or sleep fragmentation (Guzman-Marin et al., 2007). Although stress can suppress the proliferative stage of adult neurogenesis (Gould et al., 1997;Gould et al., 1998;Pham et al., 2003), most evidence shows that stress does not account for the effects of sleep deprivation (Mueller et al., 2008) or fragmentation (Guzman-Marin et al., 2007), since these effects are preserved in adrenalectomized animals, with basal corticosterone replacement (but see Mirescu et al, (Mirescu et al., 2006)). Although the evidence is incomplete, sleep deprivation and fragmentation also may impair the ability of residual proliferating cells to achieve maturity (Guzman-Marin et al., 2005;Guzman-Marin et al., 2007). Sleep fragmentation (SF) is a predominant characteristic of prevalent sleep disorders in humans (Bonnet and Arand, 2003). Severe SF is a primary characteristic of obstructive sleep apnea, and varying degrees of SF, from mild to severe are often associated with major depressive disorder (MDD), periodic leg movements of sleep, primary insomnia, and normal aging (Bonnet and Arand, 2003). Cognitive changes after short term SF have been well-studied (McCoy et al., 2007;Jagasia et al., 2009;Tartar et al., 2006), but effects of more sustained SF, characteristic of human disease, are unknown.
Based on the evidence that SF potently suppresses adult neurogenesis, we hypothesized that sustained sleep fragmentation would result in hippocampal-dependent cognitive deficits in the time window when suppressed neurogenesis would be expected to have an impact. In the present study we maintained sleep fragmentation for 12 days, in an attempt to generate a substantial neurogenic deficit window. Our SF treatment was sleep-dependent, initiated by online detection of sleep using EEG and EMG properties of sleep, so spontaneous waking was not disturbed, as in human sleep disorders. Sleep was interrupted after 30 seconds of sustained sleep, to mimic the severe SF in human obstructive sleep apnea. Cognitive performance was assessed using the Barnes maze (Barnes, 1979), beginning 4 weeks after the beginning of the SF or control treatments, after animals had been maintained in home cages for 2 weeks.
Experimental Procedures
Animal Preparation
All protocols were conducted in accordance with the National Research council Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Animals Care and Use Committee at the V.A. Greater Los Angeles Healthcare System.Male Sprague-Dawley rats, 250–320 g, were used for this study. Animals were housed in a 12-h light/dark cycle (lights on at 05.00h) throughout the study. Rats were assigned to one of 4 groups: SF (n=14), sleep fragmentation control (SFC, n=14), treadmill control (TC, n=10) or cage control (CC, n=12). SF and SFC rats were surgically prepared for sleep-wake cycle monitoring in aseptic conditions using Ketamine 80 mg/Kg and xylazine 10 mg/Kg i.p to induce anesthesia. Six stainless steel gold-plated electrodes were implanted in the frontal and parietal bones of the skull for electroencephalogram (EEG) recording, and three stainless steel gold-plated wires were inserted into nuchal muscles for electromyogram (EMG) recording. All electrodes were then connected with a plug and fixed to the skull with dental cement. Post-surgically, the rats received 1 injection/day of 5.0 ml/kg Carprofen for 3 days. Rats were allowed between 10 and 12 days of post-surgical recovery before initiating treatments.
Sleep Fragmentation Method
To achieve sleep fragmentation, an intermittently-activated treadmill was used. Animals were housed in a 4-sided plastic enclosure (28 × 28 × 40 cm), the lower edges suspended 0.6 cm above the vinyl belt of a treadmill, the belt forming the floor of the cage. Two adjacent chambers were on the treadmill. Awakenings were induced by 3 s computer-controlled treadmill activation, after the detection of 30s of continuous NREM sleep (see below). Activation of the treadmill would wake the animals by lightly shifting them against the enclosure walls. Should the rat remain asleep after the first activation, the treadmill activation was repeated until arousal occurred. The treadmill speed was set at 7 cm/s. Treadmill direction changed every 5 min. The bottom edges of enclosures were rounded and weather stripping was use as bumpers to prevent injury. Every other day, the treadmill was scrubbed and wiped down with a cleaning solution and water. Food and water were available at all times.
For sleep–wake cycle recordings on the treadmill, SF and SFC rats were connected to amplifiers through light cables and a slip ring (Plastics One, Inc, Roanoke, VA) suspended overhead on a counterbalanced beam. Two channels of EEG and one channel of EMG activity were continuously recorded throughout the experiment. EEG and EMG signals were filtered at 1.0 and 30 Hz and 30–300 Hz, respectively, and digitized with the Cambridge Electronic Design (CED, Cambridge, Spike 2 V 5.0) recording system at 128 Hz and 256 Hz, respectively. On-line NREM sleep state detection was based on a Power Pass program derived from the Bergmann et al (Bergmann et al., 1978). NREM sleep detection was based on FFT EEG analysis yielding power in delta (0.5–4 Hz) and sigma (10–16 Hz) bands plus integrated EMG activity in each 2 sec epoch (cosine-tapered and 50% overlap). NREM sleep was identified by elevation of either delta or sigma activity above a threshold in at least 3 out of a set of 5 consecutive epochs, but was inhibited by elevated EMG activity, to exclude EEG artifacts during brisk body movements. Thresholds were individualized using frequency histograms of each EEG band and integrated EMG activity during baseline recordings.
Experimental Design
Our experiment compared the effects of 12 days of SF or control treatments on neurogenesis and Barnes maze performance. Pairs of SF and SFC were placed on the treadmill, simultaneously. EEG/EMG signals from the designated SF animal controlled the treadmill. The SFC rat could sleep when the SF animal was spontaneously awake. As the SFC animal and the SF animal were on the same treadmill, they experienced identical treadmill movements. A 12-day TC treatment was used to evaluate whether the movement alone affected proliferation and subsequent behavior, independent of the role of sleep fragmentation. TC animals were placed on a treadmill identical to that of SF- SFC animals, but the treadmill was activated only in the dark phase, the predominately waking phase of the rat, for a total of 45 m in sets of 15 m (15 on: 15 off). This total duration of movement was matched to that measured in SF animals. One day of acclimation to the treadmill movement and one 24 h period of baseline sleep-wake recording, in SF and SFC rats, preceded the beginning of all treatments.
In subgroups of 8 SF and SFC and 10 TC animals, two BrdU injections (200mg/kg) were administered on days 4 and 5 of the treatments. One BrdU injection labels cells proliferating during about 11 hrs in the rat, including the 9 h duration of the S-phase of the cell cycle and a 2 h period of BrdU bioavailability (Cameron and McKay, 2001). Therefore, injections were given 12 hours apart (7 pm, day 4 and 7 am, day 5) to label most cells proliferating in a 24 h period. After SF and SFC treatments, rats were returned to their home cages for 14 days before beginning Barnes maze testing (see below). We chose 14 days so that the 7 days of Barnes maze testing would begin 26 days after beginning SF and control treatments, when neurons normally born during the time of treatments would exhibit functional maturity. Rats in all groups were sacrificed 34–35 days after initiating treatments, after completing Barnes maze testing.
Barnes maze apparatus and procedure
The apparatus used was similar to that originally described by Barnes (Barnes, 1979). The maze consisted of an elevated (82 cm) circular platform 122 cm in diameter, with 22 eight cm diameter circular holes evenly spaced around the perimeter. The platform could be rotated on its supporting pedestal. A dark escape box, supported by a structure invisible to the rats was located under one of the holes. Open space was below the other holes. The escape box was 12 cm in depth and contained a black wooden pull-out shelf to facilitate removing the rats. A covered cylinder that could be lifted by an overhead tether was placed in the center of the maze as the start-box. The maze was located in a white-walled room with prominent fixed black wall markings to serve as spatial cues. Three 100W lights in addition to the fluorescent lights illuminated the maze. A video recording system (model: KPC-s60NV, Kt & C, Korea) was placed 130 cm directly above the center of the maze to record rat activity.
The animals underwent 7 days of maze testing, 5 with a constant escape tunnel position followed by 2 with the escape tunnel position right-rotated 130°. Rats were given 5 trials per day, at the same time each day for a given animal, between ZT 8 and ZT 11. In each trial, the rat was placed in the start box facing a pre-selected randomly-varied direction for 30s. Once freed by lifting the start box, rats had a maximum of 4 min to locate the escape box. If the rat failed, it was placed in the escape box. Trials were separated by 1 minute in the escape box followed by 1 minute in their home cage. Between trials, with the escape position remaining fixed relative to room cues, the maze was rotated 130 ° always in the same direction to eliminate possible local cues such as odor.
Perfusion, immunohistochemistry, and cell counting
Subjects from SF, SFC, and TC groups were deeply anesthetized (Nembutal 100 mg/Kg and perfused transcardially with phosphate buffer (PB) 0.1 M followed by ice cold paraformaldehyde (4%); brains were removed and stored in 10% and 30% sucrose at 4 °C until they sank. Brains were cut in 40 µm coronal sections. Sections encompassing the hippocampus were preserved in a cryoprotectant solution containing sucrose, polyvinyl-pyrrolidone (PVP-40, Sigma, St. Louis, MO, USA) and ethylene glycol dissolved in PB pH 7.2, which provides long-term protection of the tissue. Sections were processed for BrdU immunolabeling to assess cell proliferation/survival or double immunofluorescence labeling for BrdU and NeuN to identify cell phenotype. As primary antibodies we used: rat anti-BrdU (1:100, Accurate, Westbury, NJ, USA), and mouse anti-NeuN (1:300, Chemicon, Temecula, CA, USA).
For single labeling, to visualize the expression of the BrdU alone we used the peroxidase method (ABC system, Vectastain, Vector Laboratories, Burlington, CA, USA). Immunohistochemistry was performed simultaneously on sections from all groups to maximize the reliability of comparisons across groups. Staining was done on slide-mounted sections on a one-in-six section series (~18 sections per animal). Slides were placed in pre-stirred boiling citric acid (0.01 M, pH = 6.0) for 40 min. After cooling, slides were rinsed in water (X5) and then PBS (X5 after this and each subsequent step unless otherwise specified). Sections were pretreated by DNA denaturation (2 M HCl at 37 °C for 30 min) followed by 10 min in borate buffer (pH 8.5). Sections were then incubated with a mouse anti-BrdU primary antibody in PBS containing 0.5% Tween 20 for 48 h at 4°C. Tissue from all groups was treated with aliquots from the same batch of antibodies. Sections were subsequently incubated with a biotinylated horse anti-mouse IgG (1:200, Vector Laboratories), then reacted with avidin–biotin complex (1:200, Vector Elite) and developed with diaminobenzidine tetrahydrochloride in hydrogen perioxide (DAB, Sigma kit). Absence of the primary antibody resulted in an absence of specific nuclear staining.
An individual blinded to the experimental conditions did all counting. One SF rat and 2 SFC rats had incomplete sets of sections and were not included in the counts. BrdU-positive cells were counted using a 60× objective (Nikon E600, Nikon, Tokyo, Japan) throughout the rostrocaudal extent of the DG granule cell layer (GCL). The optical fractionator method was used for counting as previously described (Guzman-Marin et al., 2005). Briefly, in every section, the contour of GCL/SGZ area was first delineated for counting using the tracing function of the MicroBrightfield Stereo Investigator stage control system software. Following this, the optical fractionator component was activated by entering parameters such as the grid size of the counting region, the thickness of the guard zone (3 µm) and the optical dissector height. Due to the sparse and sporadic distribution of BrdU-positive cells, we used exhaustive sampling, counting cells throughout the DG bilaterally to ensure that the counts were as accurate as possible. The StereoInvestigator software calculated the total number of BrdU positive cells per DG by utilizing the optical fractionator formula. The precision of estimates of the number of cells was expressed using the coefficient of error (CE). The stereological sampling scheme was considered acceptable only when CE Gunderson was less than 0.05 (West et al., 1991).
The phenotype of the newly generated BrdU-positive cells was determined using double immunolabeling with antibodies to BrdU and NeuN in a 1-in-12 series of sections in SF and SFC rats. Co-localization was assessed in at least 50 cells per animal (except in one SF rat 20 cells were studied). After pretreatment (see above) and blocking with goat serum and Triton-X 10% in TBS, sections were incubated in a mixture with antibodies against BrdU (monoclonal from rat) and NeuN (monoclonal from mouse) in TBS for 3 days at 4 °C. After rinsing with TBS and blocking for 1 h, sections were incubated in an antibody mixture of Alexa 488 goat anti-rat and Alexa 555 goat anti-mouse (both at 1:300, Molecular Probes, Eugene, OR, USA) in TBS for 2 h. Sections were mounted and coverslipped with Vectastain (Vector) mounting medium. Cell phenotype was assessed with a fluorescence microscope (Nikon E-400) confirmed by z-series analysis through the cell nucleus and three-dimensional reconstruction.
Barnes maze scoring
Barnes maze performance was quantified from video recordings. Barnes maze performance analysis included quantification of distance traveled, time to escape, the types of errors, and escape hole search strategy. Errors were head or nose pokes into incorrect holes. Repeats were head or nose pokes into previously visited incorrect holes. Distance traveled was calculated as the shortest linear distance between points. Time to escape was calculated from the time the cylinder was lifted to the time when then rat entered the tunnel entirely (tail excluded). The velocity was calculated by using the aforementioned distance and time values.
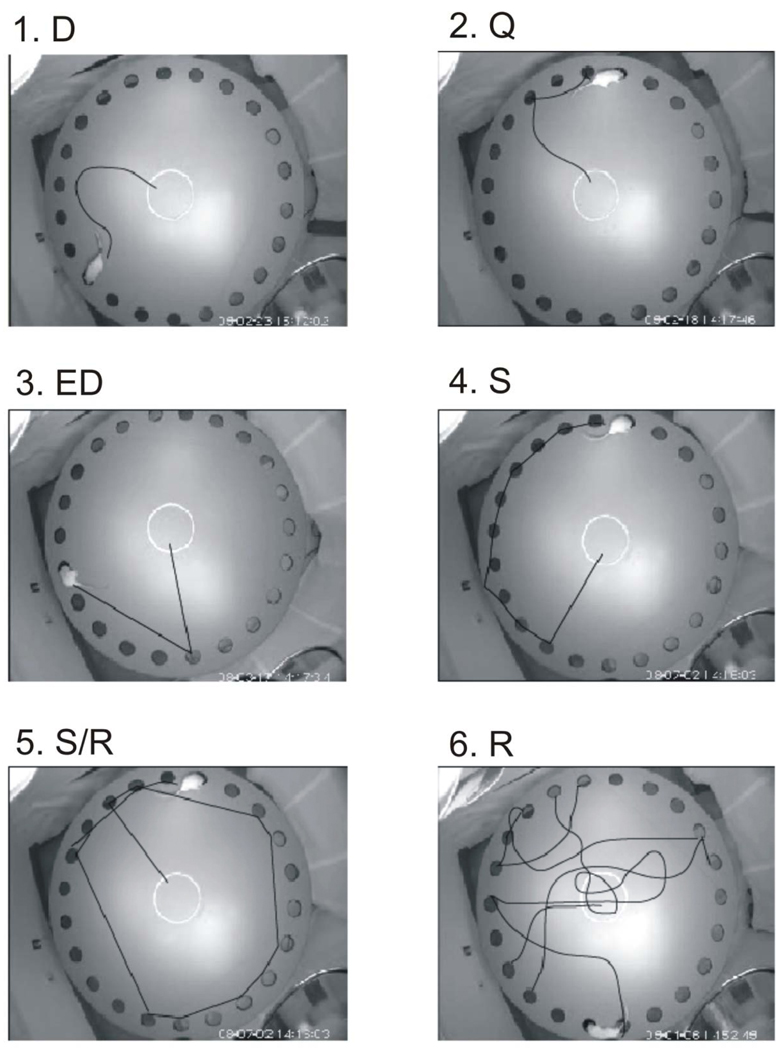
To quantify search strategy, we classified the rat’s escape path in each trail into the following categories: Direct (D), the rats go directly to the escape hole or an adjacent hole, Error direct (ED), rats go to an incorrect hole, but no more than 2 holes from the correct hole and then directly to the escape hole, Quadrant (Q), rats go directly to the correct quadrant (within 3 holes) of the escape hole, then to the escape hole, Serial (S), rats go from one hole to the next, but not starting in the correct quadrant, Random (R), rats go between non-adjacent holes, not starting in the correct quadrant, Serial/Random (S/R), rats used a serial pattern but then skip sections of the maze (See Fig 1). Assuming that movement in the direction of escape hole represented a spatial strategy, and that random or serial movements represented a non-spatial strategy, an overall strategy score was generated as follows: R = 1, S/R = 2, S = 3, ED = 4, Q = 5, D = 6. For this purpose, serial paths were weighted higher than random paths because they indicated an organized search strategy.
Fig. 1.
Samples of alternative search strategies derived from video recordings, in which the prior path of the rat has been drawn. 1. Direct (D). Rat goes directly to the correct escape hole. 2. Quadrant (Q). Rat goes to the correct quadrant, then to the correct hole. 3. Error Direct (ED). The rat goes to an incorrect hole outside of the correct quadrant, then directly to the correct hole. 4. Serial (S). Rat goes sequentially around the perimeter, not starting in the correct quadrant. 5. Serial Random (S/R). Rat follows a path including both serial and apparently random strategies. 6. Random (R). Rat follows a path without either serial or quadrant-related elements.
Sleep-wake state analysis
Sleep–wake states were scored in 10 s epochs on the basis of the predominant state within the epoch. Wake was identified by low-voltage high-frequency EEG activity and sustained neck muscle tone. High-amplitude low frequency EEG with decreased muscle activity defined non-rapid eye movement (NREM) sleep whereas rapid eye movement (REM) sleep was defined by EEG similar to waking with dominant theta frequency (4–8 Hz), combined with low muscle tone. The percentages of each state were calculated for 4 24-hour periods (Baseline, day 2, day 7, and day 11) of the recording process. In addition to this, we measured the duration of sleep bouts to access the level of SF.
EEG spectral power was determined using SleepSign (Kissei Comtec, Japan), as follows. EEG data were digitally low-pass filtered at 20 Hz and epochs containing artifacts were excluded. Spectral analyses were conducted using the fast Fourier transformation on 10 sec epochs tapered by Hanning window. The integral EEG spectral power for wake, NREM and REM sleep was calculated for the spectral range from 0.50–20.0 Hz. Spectral data for each of the three stages, averaged every 3hrs, was calculated for three frequency bands: - lower delta (0.50 – 2.0 Hz), upper delta (2.1– 4 Hz) and theta (4.5– 8 Hz). Changes in spectral power induced by sleep fragmentation were calculated as a percentage difference of power from the baseline day in the respective frequency bands at 3 h intervals.
Statistical analysis
To assess the impact of the SF paradigm on different sleep–wake cycle parameters between and within groups, a two-way repeated-measures ANOVA was carried out, with days of treatment as the repeated-measures variable followed by Fischer’s or Holm-Sidak post hoc test in the case of no significant interaction. Otherwise, a simple one-way ANOVA was used to further analyze the data. Effects of SF on EEG spectral power was also assessed using a two-way repeated measures ANOVA, followed by the Holm-Sidak post-hoc test. Unless otherwise indicated, repeated measures ANOVAs and Fischer’s post-hoc test were also used to assess Barnes maze data. To measure correlations between and within different behavioral and sleep data sets we used the Spearman R correlation test. Correlations with NREM bout length were based on day 7 of SF or SFC sleep measurements. Cell count differences between groups were assessed with Student’s t-test. A P value of <0.05 was adopted for significance.
Results
Effect of Sleep Fragmentation on Proliferating Cell Survival and Phenotype
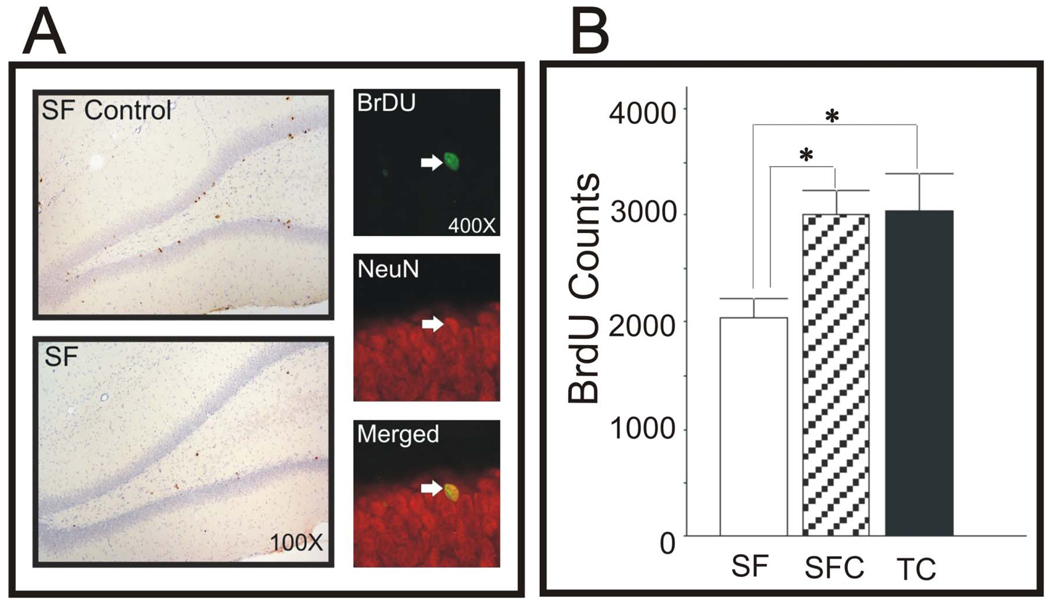
The numbers of BrdU-labeled cells was counted in animals sacrificed following Barnes maze testing, 30 days after BrdU administration. Representative sections are shown in Fig 2A. The BrdU-labeled cell count was reduced by 32% in SF animals compared to both SFC and TC groups (Fig 2B, both, p< 0.05). The assessment of cell phenotype was based on coincidence and merging of red (NeuN) and green (BrdU) fluorescent labeling (Fig 2A,right). There were no significant differences between SF and SFC groups in the percentages of BrdU-labeled cells also expressing NeuN (SF: 83%, SFC: 89%, p >0.4).
Fig. 2.
A. Left. Examples of BrdU-immunostained cells from DG in SFC and SF animals. A. Right. Examples of fluorescent images of cells stained for BrdU (green), NeuN (red), and merged images, to confirm neuronal phenotype of proliferating cells. B. Counts of BrdU-labeled cell in SF, SFC, and TC groups. SF reduced significantly reduced cell counts by 32% compared to both control groups (*, both p < 0.05).
Sleep-wake parameters
A two-way repeated measures ANOVA with procedure as a between subjects factor and day as a repeated measures factor was performed to analyze the differences in sleep parameters between SF and SFC animals and between the baseline and selected days of treatment (2nd,7th, and 11th). Significant interactions between the factors were found for all the parameters studied except REM sleep parameters. In the presence of a significant interaction, analyses for simple main effects (one-way ANOVAs) were then applied to the data.
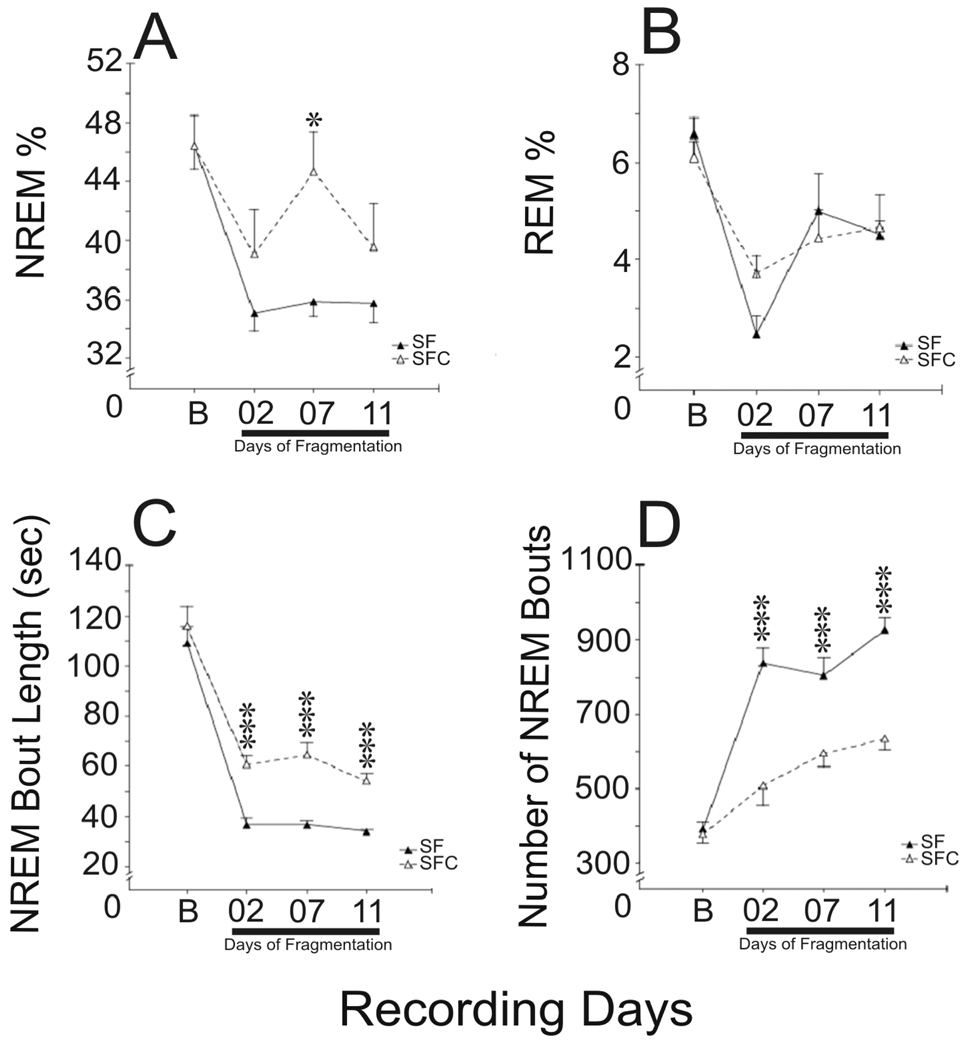
SF animals compared to SFC did not exhibit statistically significant differences in the percentages of sleep-wake states during the baseline, or days 2 and 11 of the SF/SFC procedure (Fig.3). On day 7, SF rats, compared to SFC spent about 17% more time awake (F(1, 23)=7.5, p<0.05, one-way ANOVA) exclusively at the expense of NREM sleep (F(1, 23)=10.2, p<0.01, one-way ANOVA). Although total sleep stage amounts were little changed, the SF group exhibited a higher number of NREM sleep bouts, compared to SFC rats on the 2nd/7th/11th days of the procedure [[Fig 3 C–D, [F(1,23)=30.7/14.8/53.9, p<0.001, one-way ANOVA]], and NREM bouts were shorter in duration (F(1,23)=25,1/ 31.0/43.1, respectively, p<0.001, one-way ANOVA).
Fig 3.
Sleep parameters in SF and SFC groups during baseline (B) and on days 2, 7, and 11 of the treatments. SF had few effects of sleep stage amounts. % NREM was reduced only on day 7 (A), and % REM was not significantly changed (B). However, SF consistently reduced NREM bout length (C), and increased the number of bouts (D) [***, all p, 0.001). Both SF and SFC groups has reductions in %NREM and % REM compared to baseline [p, 0.001, indicated by solid line under Days of Fragmentation].
In SF and SFC group the effect of the repeated measures factor day was statistically significant for both the bout length (F(3, 36)=121.4 and F(3, 33)=41.8, respectively, p<0.001, one-way repeated measures ANOVA) and bout number (F(3, 36)=70.131 and F(3, 33)=12.8, respectively, p<0.001, one-way repeated measures ANOVA). On each selected day of the procedure, SF rats and their yoked controls both exhibited a significant increase in NREM sleep bout number and decrease in bout length compared to the baseline (p<0.001, Fisher LSD post-hoc test). NREM sleep bout length and the number of bouts did not differ between SF and SFC animals during the baseline.
The REM sleep bout length and number did not differ between SF and SFC rats during the baseline and throughout the procedure (F(1, 23)=0.3 and F(1, 23)=4.0, respectively, p>0.05, two-way repeated measures ANOVA). The factor day significantly affected both duration and number of REM sleep bouts (F(3, 69)=2.8, p<0.05 and F(3, 69)=5.7, p<0.01, respectively, two-way repeated measures ANOVA). The REM sleep bout length was lower on day 7 compared to the baseline and day 11 (p<0.05 and p<0.01, Fisher LSD post-hoc test). The number of REM sleep bouts was lower on day 2 compared to baseline, 7th and 11th day of the procedure (p<0.05–0.01 ,Fisher LSD post-hoc test).
During SF/SFC procedures, both SF and SFC groups exhibited mild increases in % waking and decreases in %NREM and % REM sleep, compared to baseline (Fig 3A–B). The effect of the repeated measures factor day was significant in both SF and SFC groups for each sleep-wake state (%waking, F(3, 36)=39.7, p<0.001 and F(3, 33)=7.2, p<0.01, respectively, one-way repeated measures ANOVA; %NREM sleep, F(3, 36)=40.5, p<0.001 and F(3, 33)=6.0, p<0.01, respectively, one-way repeated measures ANOVA; and %REM sleep, F(3, 69)=17.2, p<0.001, two-way repeated measures ANOVA). NREM changes were significant for each day during SF/SFC procedures (Fig 3A, p<0.01–0.001, Fisher LSD post-hoc test) except on Day 7 for the SFC group. %REM was also reduced on each day of the SF and SFC procedure compared to baseline (Fig 3B., p<0.001–0.05, Fisher LSD post-hoc test). SF animals exhibited a significant increase in %REM sleep on days 7 and 11 compared to day 2 (p<0.001 and p<0.01, respectively).
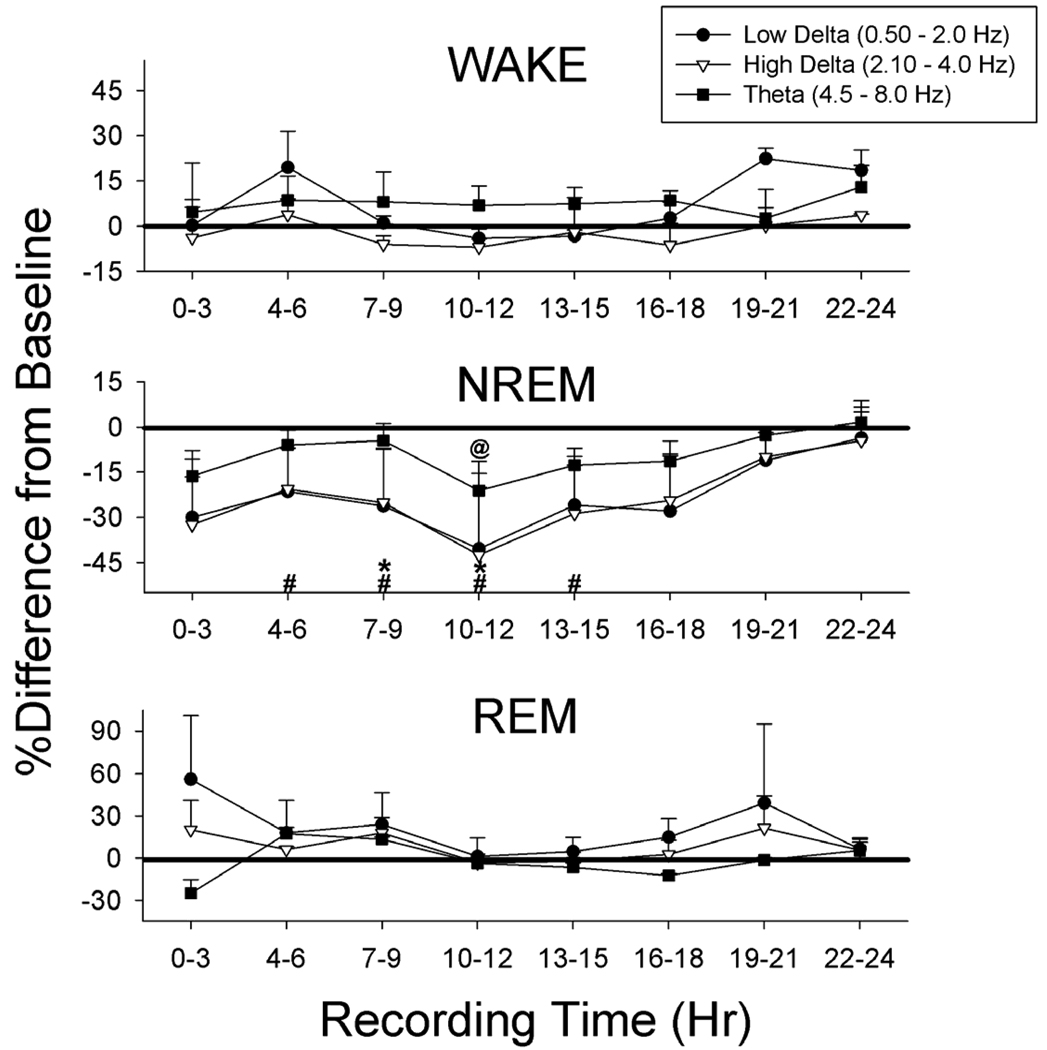
EEG patterns
Effects of SF on low and high delta and theta EEG frequency bands were assessed in wake, NREM and REM, in successive 3 hr intervals across the 24h of day 11, compared to baseline (Fig 4). ANOVA with repeated measures (day and time of day) showed significant day by time interactions [F(1,4) = 7.9–10.8, p, 0.05)]. Within NREM sleep high frequency delta was reduced during four successive three-hour periods and low frequency delta was reduced during 6 hrs. EEG theta activity was reduced in NREM during one 3h period, but was not significantly changed in wake or REM. Neither delta band nor theta were significantly changed in wake or REM.
Fig. 4.
Effects of SF on sleep-related EEG frequency bands, compared to baseline, in 3 hr blocks across the 24 h recording period. During NREM sleep higher frequency delta was significantly reduced during 4 3-h blocks and low frequency delta was reduced during 2 blocks. Reductions in delta occurred during the light phase, which began at hour 4. Theta was reduced during one 3-hr block. There were no significant changes in these bands during wake or REM. Note that the scale is changed for REM sleep. (#, low frequency delta, *, higher frequency delta, @, theta, all p < 0.05)
In summary, the SF treatment compared to the yoked SFC effectively fragmented NREM sleep, reducing bout lengths and increasing bout frequency. NREM and REM sleep % were mildly reduced in both SF and SFC groups, compared to baseline, but sleep state amounts differed little between these groups. NREM delta EEG activity was reduced, compared to baseline in the SF group.
Barnes maze performance
A two-way repeated measures ANOVA with training day as a within-subjects factor and procedure as a between-subjects factor was used to compare Barnes maze performance in SF, SFC, CC and TC animals across the training days. The factor training day significantly affected the escape time (F(6, 276)=73.5, p<0.001), number of errors (F(6, 276)=8.0, p<0.001) and repeats (F(6, 276)=15.6, p<0/001). No significant procedure × training day interaction was found for each of these parameters. Performance progressively improved in all groups over the course of training (Fig. 5). Escape time, number of errors and repeats gradually decreased and reached their minimum values on day 5, significantly increased on day 6 with escape box relocation (p<0.05–0.001, Fisher’s LSD post-hoc test), and significantly decreased again on day 7 (p<0.05–0.001, Fisher’s LSD post-hoc test). The differences between the groups in the number of errors and repeats were insignificant.
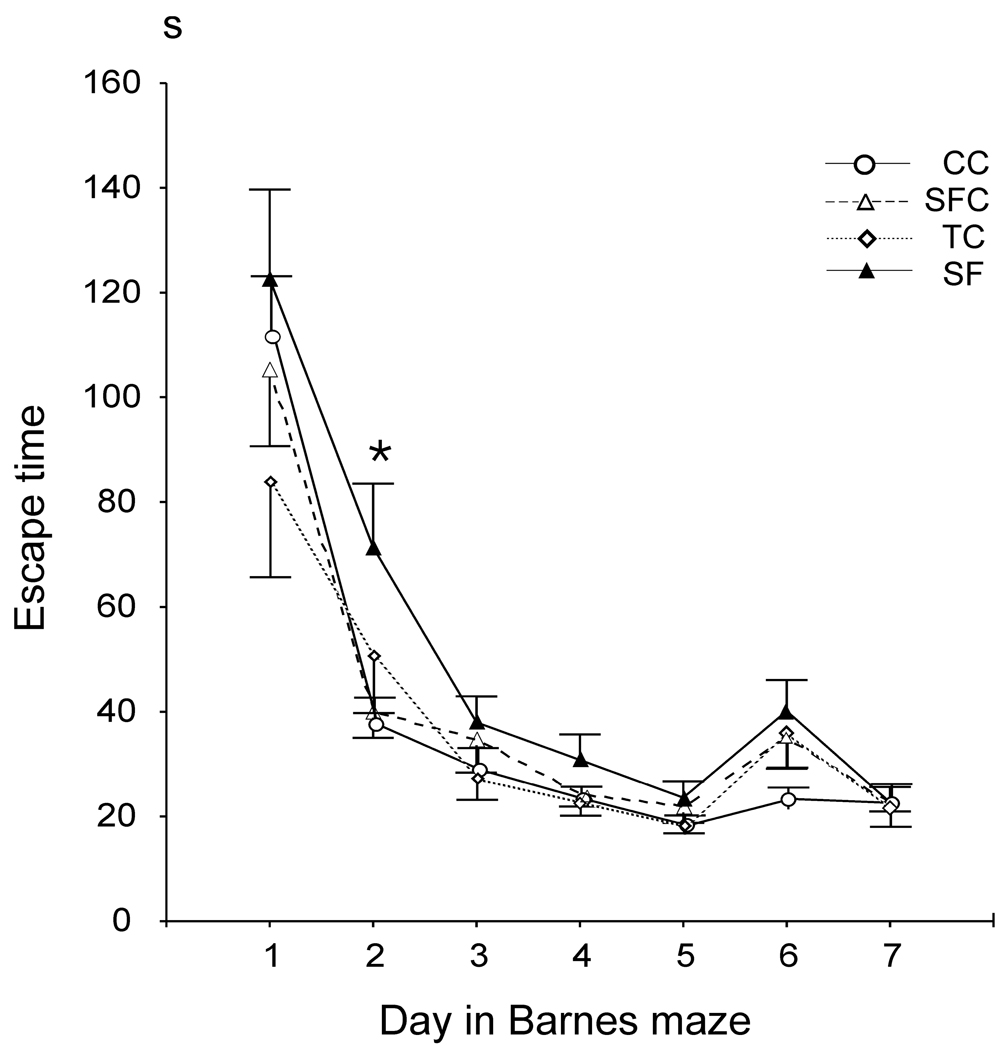
Fig. 5.
Barnes maze escape time in seconds (S) across days of testing, including 5 days with a constant escape hole location and 2 days (days 6 and 7) with a new escape location. All groups exhibited progressively shorter escape times across the first 5 days, and were longer on day 6. Escape time was significant longer in the SF compared to the SFC and CC groups only on day 2 (*, p < 0.05).
Across all training days the escape time in SF animals tended to be longer compared to SFC (p=0.08) and was significantly longer compared to both CC and TC animals (p<0.05, Fisher LSD post-hoc test). Pair-wise comparisons for each training day confirmed that escape time was longer in SF compared to SFC and CC rats on day 2 (Fig. 5., p<0.05, Fisher LSD post-hoc test).
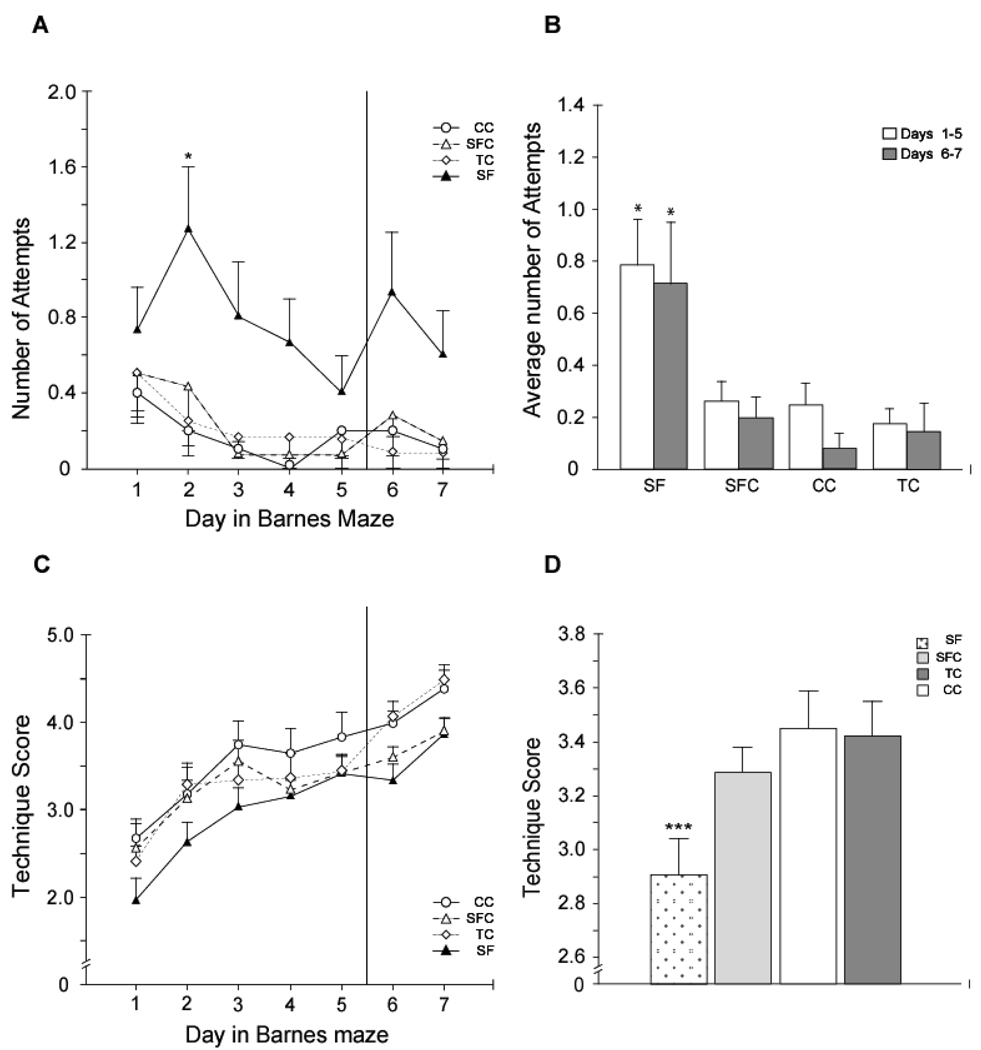
Analysis of search strategies showed that over the course of training, the number of random search attempts changed significantly (F(6, 276)=3.0, p<0.01, two-way repeated measures ANOVA). The number of R attempts decreased with training in all groups and was minimal on day 5 differing significantly from days 1 and 2 (p<0.01 and p<0.05, respectively), The changes in the number of R attempts on days 6 (escape box relocation) and 7 were insignificant. No significant procedure by training day interaction was found.
The effect of the procedure was highly significant (F(3, 46)=7.4, p<0.001). SF animals exhibited significantly more random search attempts compared to all control groups across the training days (Fig. 6, p<0.001, Fisher LSD post-hoc test). No significant differences between control groups were found. There were no significant differences between groups in S, S/R, ED, or D strategies. Following escape box rotation, on days 6 and 7, both the SF and SFC groups had significantly lower number of Q attempts compared to the CC (Day 6, SF: p <0.02, SFC: p<0.05, and Day 7 SF: p<0.02, SFC: p<0.005, two-way ANOVA followed by Fisher LSD post-hoc test). This implies that both SF and SFC rats had more difficulty adjusting to the new tunnel position compared to CC group.
Fig. 6.
SF animals exhibited different escape strategies in the Barnes maze. A–B. SF animals exhibited significantly more random attempts across the days 1–5 and days 6–7 (B, *, both p< 0.001). Day by day analysis showed more random attempts on day 2 (*, p < 0.05). C–D. The overall technique score analysis, which weighted direct and quadrant strategies over serial and random strategies showed that SF had lower scores on days 1–5 of testing, compared to SFC, TC, and CC groups (***, p < 0.001).
Overall escape search strategy scores significantly improved in all groups over the course of training (F(6, 276)=27.2, p<0.001). The effect of the factor procedure was significant (F(3, 46)=4.5, p<0.01). SF animals had significantly lower overall escape hole search strategy scores compared to SFC, CC, and TC group (Fig. 6, p<0.05, p<0.01, and p<0.01, respectively, Fisher LSD post-hoc test). No significant interaction between factors day and procedure was found.
Correlations with Sleep
There were strong links between NREM bout length and technique score, especially the use of random search strategies. Combining groups, the overall technique score for days 1–5 was positively correlated with NREM bout length (r = 0.49, p<0.05). The use of random strategy on test days 2,3,4, and 6 of Barnes maze testing was negatively correlated with NREM bout length (day 2: r =−.46, p < 0.05, day 3: r = −0.51, p < 0.05, day 4: r = −0.51, p < 0.05, day 6: r = −0.45, p < 0.05).
Static Behavior and Appearance
On the Barnes maze, 37.5 percent of the SF group, but only 13% of SFC and one TC or CC animals displayed “static” activity. Rats moved to the edge of the maze and were nearly motionless near one escape hole throughout the duration of the trial. SF and SFC animals’ weight increased 0.7 and 11.7 g, respectively, on the treadmill. After the SF treatment, both groups of animals gained 25 g/week. After one week on the treadmill approximately equal numbers of SF and SFC animals exhibited porphyrin staining around eyes and nostrils and matted fur.
Discussion
Our study showed that sustained SF resulted in changes in spatial learning in the Barnes maze in assessments started 2 weeks after termination of the SF treatment, when all animals had been maintained in a standard cage environment. This result confirmed our prediction that delayed hippocampal-dependent cognitive changes could occur following the 12 day SF treatment, based on the adult neurogenesis model. Newly-generated neurons require 3–4 weeks to mature (Kee et al., 2007;Fariola-Vecchioli et al., 2008) and participate in new spatial learning or other hippocampal-dependent forms of learning (Shors et al., 2002;Bruel-Jungerman et al., 2007). Delayed deficits of several types were shown previously after suppressing neurogenesis using manipulations such as brain irradiation (Madsen et al., 2003;Raber et al., 2004;Snyder et al., 2005;Saxe et al., 2006;Winocur et al., 2006;Clelland et al., 2009;Wojtowicz et al., 2008), administering anti-mitotic agents such as methylazoxymethanol (Shors et al., 2001;Shors et al., 2002;Bruel-Jungerman et al., 2005) or temozolomide (Garthe et al., 2009), or using transgenic manipulations (Dupret et al., 2008;Deng et al., 2009;Zhang et al., 2008;Fariola-Vecchioli et al., 2008;Jessberger et al., 2009), but not after treatments such as sleep fragmentation mimicking chronic abnormalities commonly encountered by adult humans. Although the above methods used to suppress neurogenesis were designed to more selectively affect proliferating cells, and SF produces other brain functional changes, acutely (7055}, the delayed changes we observed, particularly the change in search strategies, are reasonably attributed to changes in neurogenesis. Changes in sleep after sustained total sleep deprivation last only a few days (Rechtschaffen and Bergmann, 1995). Given that both SF and control animals were housed in home cages for 14 days before testing, without disturbance except for normal servicing, is unlikely that changes in sleep directly affected Barnes maze performance. However, we cannot exclude the possibility that sustained sleep-dependent sleep fragmentation produces other long-lasting functional abnormalities that could affect spatial learning strategies. Stress or elevation of corticosterone also non-selectively suppress neurogenesis (reviewed (Lucassen et al., 2010)), and can impair hippocampal-dependent cognitive functions, including Barnes maze performance (Mclay et al., 1998). However, to our knowledge, a delayed cognitive deficit following stress, like that produced by SF, has not been demonstrated.
By continuously monitoring EEG and EMG activity and using automated sleep detection, we could initiate treadmill movements only after a pre-selected duration of sustained sleep. This method permits the use of yoked controls that experience the identical treadmill movement, but can sleep when SF animals are spontaneously awake. Previously we used this treadmill system coupled with automatic state detection for REM sleep deprivation, with yoked controls (Guzman-Marin et al., 2008). Sleep-dependent total sleep deprivation, REM deprivation, and slow wave deprivation, have been studied previously using the disk-over-water method (Everson, 1995;Rechtschaffen et al., 1999). An innovation of the present study was the first use of sleep-dependent sleep fragmentation with a treadmill, avoiding sustained water exposure or restraint. The primary effects of the SF treatment on sleep compared to the yoked control treatment were a reduction in NREM bout length bout length; there were no significant differences between SF and SFC groups in REM sleep amounts, and NREM was reduced by SF only in day 7 data.
In the present study, 30 days after BrdU administration, the number of cells labeled on days 4 and 5 of the SF treatment was reduced by 32%, compared to both SFC and TC groups. Previously we showed that 4 or 7 days sleep fragmentation suppressed the proliferative stage of DG neurogenesis (Guzman-Marin et al., 2007). The design of the present study does not allow us to differentiate between effects of SF on initial proliferation or subsequent survival of labeled cells. To achieve sleep-dependent SF and SFC, animals occupy an individual treadmill chamber for 14 days and undergo continuous polygraphic recording, with individualized sleep detection and computer-controlled treadmill movement, so completing separate studies of proliferation and survival was not attempted.
Although stress is a potent inhibitor of neurogenesis, we showed previously that the reduction in proliferation by 4 days SF was not affected by adrenalectomy (ADX) with basal corticosterone replacement (Guzman-Marin et al., 2007). Further ADX did not prevent the anti-proliferative effects of 4 days sleep deprivation using the small-platform-over-water method (Mueller et al., 2008). Although corticosterone levels may be elevated for the initial 6 hr-2 days of sleep deprivation or fragmentation (Hairston et al, 2005, Tartar et al, 2009), they are usually not different from controls after more sustained treatments (Cirelli and Tononi, 2000;Guzman-Marin et al., 2008;Everson and Crowley, 2004). Sustained sleep restriction blunts the CRH response to stress without affecting the corticosterone response (Novati et al, 2008). These findings argue that it is unlikely that the effects of sustained SF on neurogenesis were due to elevation of corticosterone or CRH to stress levels, although this possibility was not explicitly excluded in the present study.
An additional feature of glucocorticoid secretory pattern, distinct from stress effects, was found to modulate neurogenesis, and could be affected by sleep fragmentation. In rats, flattening of the diurnal rhythm of corticosterone using sustained release pellets prevents the pro-proliferative effects of chronic administration of the nitric oxide synthase inhibitor, L-NAME, the 5-HT-1A agonist, 8-OH-DPAT, fluoxetine, and local hippocampal administration of the neurotrophic factor, brain derived neurotrophc factor (Pinnock and Herbert, 2008, Huang and Herbert, 2005). Normally, glucocorticoid secretion is minimal before and during the first portion of the primary sleep period in both rodents and humans. The low corticosterone level associated with the beginning of the light phase in rats was prevented by sleep deprivation, as measured in hippocampus (Linthorst and Reul, 2008). In humans, acute sleep deprivation prevents the low cortisol levels normally induced at sleep onset (e.g., Born et al, 1988), and insomnia patients with sleep fragmentation exhibit elevation of cortisol levels during the sleep period (Vgontzas et al, 2001). As peak wake-associated levels are not changed by these procedures, by preventing low levels during sleep, sleep deprivation or fragmentation have the effect of reducing or flattening the amplitude of the diurnal variation of glucocorticoid levels. As noted above, this flattening could restrict stimulation of proliferation. On the other hand, corticosterone re-supply after ADX by pellets to only basal awake levels reduced sleep amounts in rats (Bradbury et al, 1998). Administration of glucocorticoids also suppresses human sleep (e.g., Born et al, 1991). Thus, available evidenced suggests that sustained sleep is required for low glucocorticoid secretion and a low glucocorticoid level is required for sustained sleep. These two mechanisms might synergistically facilitate stimulation of cell proliferation, but specification of their separate and combined roles requires further study.
The intermittent treadmill procedure is not comparable to exercise as total treadmill movement, about 300 m/day, is much less than spontaneous running wheel distance (often 4 Km/day), and was at “walking” rate for the rat (7 cm/sec).
In our study, SF had only transient effects on the time required to reach the escape hole location in the Barnes maze (day 2), but resulted in altered strategy to locate the escape hole. SF led to an increased number of what we classified as random search patterns in the Barnes maze. NREM sleep bout length was negatively correlated with use of a random escape hole search strategy during Barnes maze testing. Our findings are in agreement with a previous study showing that performance in the Barnes maze was impaired following irradiation-induced suppression of neurogenesis, primarily by affecting escape search strategies and the use of random search patterns (Raber et al., 2004). A role for adult-born DG neurons in learning strategy was also found in a recent study using the Morris water maze (MWM), in which mice with TMZ- suppressed neurogenesis were most impaired in adapting to a new platform location because inflexible search strategies (Garthe et al., 2009). Studies of the effects of suppressed adult DG neurogenesis on cognitive function have been somewhat inconsistent, but most studies have found deficits in either acquisition or retention of spatial tasks or contextual fear conditioning, in agreement with general concepts of DG information processing. Some studies failed to find initial acquisition deficits in MWM after suppression of neurogenesis (Shors et al., 2002;Saxe et al., 2006;Raber et al., 2004;Wojtowicz et al., 2008;Jessberger et al., 2009), but deficits may be revealed when more challenging test protocols are used (Zhang et al., 2008). The Barnes maze test readily reveals alternative forms of behavioral deficits. Our study suggests that animals with deficits do not choose strategies that depend on spatial navigation. We would predict that humans with deficits in neurogenesis would tend to choose strategies that do not depend on hippocampal-dependent functions.
These findings indicate that sleep continuity or the associated enhancement of cortical EEG slow wave activity provide a critical benefit to the brain. Sleep fragmentation rather than sleep deprivation is commonly associated with prevalent human disease and aging. Bonnet and co-workers and several subsequent studies (Bonnet, 1986) showed that if human sleep was fragmented by brief awakenings after each one minute of sleep, daytime performance on addition and vigilance tasks was impaired the next day to virtually the same extent as after total sleep deprivation. Awakenings at 10 min. intervals did not have this effect. It is important to note that experimental sleep fragmentation in humans had small effects on NREM or REM amounts, as in our study. Several studies have confirmed that short-term sleep fragmentation acutely impairs cognitive performance in animals (McCoy et al., 2007;Tartar et al., 2006). Short term REM sleep restriction may also acutely alter strategies used in a spatial task (Bjorness et al., 2005). It is reasonable to speculate that sustained sleep episodes, as contrasted with fragmented sleep episodes, provide support for anabolic processes including brain protein synthesis that underlie both acute forms of brain plasticity and longer-term processes such as DG cell proliferation and maturation (Ramm and Smith, 1990;Nakanishi et al., 1997). For optimum expression of plastic mechanisms, some minimum duration of a stable cellular state, without impinging perturbations, might be required.
Acknowledgements
Supported by NIH MH 075076 and the Research Service of the Veterans Administration. The authors thank Ramya Davis, Maple Schrader, and Keng-Tee Chew for technical assistance.
Abbeviations
- ADX
adrenalectomy
- ANOVA
analysis of variance
- BrdU
bromodeoxyuridine
- DG
dentate gyrus
- EEG
electroencephalogram
- EMG
electromyogram
- FFT
Fast Fourier Transform
- GCL
granule cell layer
- MDD
major depressive disorder
- MWM
Morris water maze
- NeuN
neuronal nuclear antigen
- NREM
non-rapid eye movement sleep
- OSA
obstructive sleep apnea
- REM
rapid eye movement sleep
- SF, SFC
sleep fragmentation, sleep fragmentation control
- SGZ
subgranular zone
- TC
treadmill control
- ZT
zeitgeber (time-giver) time, environmental circadian time. By convention, ZT0 is the beginning of the light period, ZT12 is the beginning of the dark period in a 12:12 L:D Schedule.
Search Strategies
- D
direct
- E
error
- E/D
error/direct
- R
random
- S
serial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psych. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann BM, Winter JB, Rosenberg RS, Rechtschaffen A. NREM sleep with low voltage EEG in the rat. Sleep. 1978;10:1–11. doi: 10.1093/sleep/10.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Bjorness TE, Riley BT, Tysort MK, Poe GR. REM restriction persistently alters strategy used to solve a spatial task. Learning and Memory. 2005;12:352–359. doi: 10.1101/lm.84805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnet MH. Performance and sleepiness as a function of frequency and placement of sleep disruption. Psychophysiol. 1986;23:263–271. doi: 10.1111/j.1469-8986.1986.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet MH, Arand DL. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Medicine Reviews. 2003;7:297–310. doi: 10.1053/smrv.2001.0245. [DOI] [PubMed] [Google Scholar]
- 6.Born J, Muth S, Fehm HL. the significance of sleep onset and slow wave sleep for nocturnal release of growth hormone (GH) and cortisol. Psychoneuroendocrinology. 1988;13:233–342. doi: 10.1016/0306-4530(88)90021-2. [DOI] [PubMed] [Google Scholar]
- 7.Born J, DeKloet ER, Wenz H, Kern W, Fehm HL. Gluco- and antimeralocorticoid effects on human sleep: a role of central corticosteroid receptors. Am J Physiol. Endocrinol Metab. 1991;260:E183–E188. doi: 10.1152/ajpendo.1991.260.2.E183. [DOI] [PubMed] [Google Scholar]
- 8.Bradbury MJ, Dement WC, Edgar DM. Effects of adrenalectomy and subsequent corticosterone replacement on rat sleep state and EEG power spectra. Am. J. Physiol., Reg. Integr. Comp. Physiol. 1998;275:R555–R565. doi: 10.1152/ajpregu.1998.275.2.R555. [DOI] [PubMed] [Google Scholar]
- 9.Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- 10.Bruel-Jungerman E, Rampon C, Laroche S. Adult hippocampal neurogenesis, synaptic plasticity, and memory: facts and hypotheses. Rev Neurosci. 2007;18:93–114. doi: 10.1515/revneuro.2007.18.2.93. [DOI] [PubMed] [Google Scholar]
- 11.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 12.Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–321. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- 13.Clelland CD, Choi M, Romberg C, Clemenson JGD, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325 doi: 10.1126/science.1173215. 210213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupret D, Revest J-M, Koehl M, Ichas F, DeGiorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLos One. 2008;3:1–14. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn A-M, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;11:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 716.Everson CA. Functional consequences of sustained sleep deprivation in the rat. Behav Brain Res. 1995;69:43–54. doi: 10.1016/0166-4328(95)00009-i. [DOI] [PubMed] [Google Scholar]
- 18.Everson CA, Crowley WR. Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats. Amer J Physiol Endocrinol Metab. 2004;286:E1060–E1070. doi: 10.1152/ajpendo.00553.2003. [DOI] [PubMed] [Google Scholar]
- 19.Fariola-Vecchioli S, Saraulli D, Costanzi M, Pacioni S, Cina I, Aceti M, Micheli L, Bacci A, Cestari V, Tirone F. The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. PLos Biology. 2008;6:2188–2204. doi: 10.1371/journal.pbio.0060246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow theflexible use of spatially precise learning strategies. PLOS 4. 2009:1–13. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge S, Yang C-H, Hsu K-S, Ming G-L, Song H. A critical period for enhanced syanptic palstricity of newly generted neurons of the sdult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gould E, Gross CG. Neurogenesis in adult animals: Some progress and problems. J Neurosci. 2002;22:619–623. doi: 10.1523/JNEUROSCI.22-03-00619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor stimulation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci. 1998;95:3168–3177. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guzman-Marin R, Bashir T, Suntsova N, Szymusiak R, McGinty D. Hippocampal neurogenesis is reduced by sleep fragmentation in the adult rat. Neurosci. 2007;148:325–333. doi: 10.1016/j.neuroscience.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guzman-Marin R, Suntsova N, Bashir T, Nienhuis R, Szymusiak R, McGinty D. Rapid eye movement sleep deprivation contributes to reduction of neurogenesis in the hippocampal dentate gyrus of the adult rat. Sleep. 2008;31:167–175. doi: 10.1093/sleep/31.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guzman-Marin R, Suntsova N, Methippara M, Greiffenstein R, Szymusiak R, McGinty D. Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. European Journal of Neuroscience. 2005;22:2111–2116. doi: 10.1111/j.1460-9568.2005.04376.x. [DOI] [PubMed] [Google Scholar]
- 28.Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, Lie DC. GABA-cAMP response element binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci. 2009;29:7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learning and Memory. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Europ J Neuroscience. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- 31.Hairstion IS, Little MTL, Scanlon MD, Barakat MT, Palmer TD, Sapolsky RM, Heller HC. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol. 2005;94:4224–4233. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- 32.Huang G-J, Herbert J. Stimulation of neurogenesis in the hippocampus of the adult rat by fluoxetine requires rhythmic change in corticosterone. Biol. Psychiat. 2006;59:619–624. doi: 10.1016/j.biopsych.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 34.Kee N, Teixeira CA, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule celss into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 35.Linthorst ACE, Ruel JM. Stress and the brain: solving the puzzle using microdialysis. Pharm Biochem Behav. 2008;90:163–173. doi: 10.1016/j.pbb.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 36.Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, Oomen CA, Czeh B. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation; implications for depression and antidepressant action. Eur Neuropsychopharm. 2010;20:1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Madsen TM, Kristjansen PEG, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal fucntion following fractionated brain irradiation in the adult rat. Neurosci. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 38.McCoy JG, Tartar JL, Bebis AC, Ward CP, McKenna JT, Baxter MG, McGaughy J, McCarley RW, Strecker RE. Experimental sleep fragmentation impairs attentional set-shifting in rats. Sleep. 2007;30:52–60. doi: 10.1093/sleep/30.1.52. [DOI] [PubMed] [Google Scholar]
- 39.Mclay RN, Freeman SM, Zadine JE. Chronic corticosterone impairs memory performance in the Barnes maze. Physiol and Behav. 1998;63:933–937. doi: 10.1016/s0031-9384(97)00529-5. [DOI] [PubMed] [Google Scholar]
- 40.Ming G-L, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 41.Mirescu C, Peters JD, Noiman L, Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc Natl Acad Sci. 2006;103:19170–19175. doi: 10.1073/pnas.0608644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mueller A, Pollock MS, Lieblich SE, Epp JR, Galea LAM, Mistlberger RE. Sleep deprivation can inhibit adult hippocampal neurogenesis independent of adrenal stress hormones. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1693–R1703. doi: 10.1152/ajpregu.00858.2007. [DOI] [PubMed] [Google Scholar]
- 43.Nakanishi H, Sun Y, Nakamura RK, Mori K, Ito M, Suda S, namba H, Storch FI, Dang TP, Mendelson W, Mishkin M, Kennedy C, Gillin JC, Smith CB, Sokoloff L. Positive correlations between cerebral protein synthesis rates and deep sleep in Macca mulatta. Europ J Neuroscience. 1997;9:271–279. doi: 10.1111/j.1460-9568.1997.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 44.Novati A, Roman V, Cetin T, Hagewoud R, den Boer JA, Luiten PG, Meerlo P. Chronically restricted sleep leads to depression-like changes in neurotransmitter receptor sensitivity and neuroendocrine stress reactivity in rats. Sleep. 2008;31:1579–1585. doi: 10.1093/sleep/31.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pham K, Nacher J, Hof PR, McEwen BS. Repeated restrain stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- 46.Pinnock SB, Herbert J. Brain-derived neurotrophic factor and neurogenesis in the adult dentate gyrus: interactions with corticosterone. Eur J Neurosci. 2008;27:2493–2500. doi: 10.1111/j.1460-9568.2008.06250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raber J, Rola R, Lefevour A, Morhardt D, Curely J, Mizumatsu S, Vandenberg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiation Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 48.Ramm P, Smith CT. Rates of cerebral protein synthesis are linked to slow wave sleep in the rat. Physiol Behav. 1990 doi: 10.1016/0031-9384(90)90220-x. [DOI] [PubMed] [Google Scholar]
- 49.Rechtrschaffen A, Bergmann BM. Sleep deprivation in the rat by the disk-over-water method. Behav Brain Res. 1995;69:55–63. doi: 10.1016/0166-4328(95)00020-t. (1995) [DOI] [PubMed] [Google Scholar]
- 50.Rechtschaffen A, Bergmann BM, Gilliland MA, Bauer K. Effects of method, duration, and sleep stage on rebounds from sleep deprivation in the rat. Sleep. 1999;22:11–31. doi: 10.1093/sleep/22.1.11. [DOI] [PubMed] [Google Scholar]
- 51.Roman V, van der Borght K, Leemburg SA, van der Zee EA, Meerlo P. Sleep restriction by forced activity reduces hippocampal cell proliferation. Brain Res. 2005;1065:53–59. doi: 10.1016/j.brainres.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 52.Saxe MD, Battaglia F, Wang J-W, Malleret G, David DJ, Monckton JE, Garcia ADR, Sofroniew MV, Kandel ER, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 54.Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neurosci. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 56.Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, Brown RE, Strecker RE. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Europ J Neuroscience. 2006;23:2739–2748. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tartar JL, Ward CP, Cordeira JW, Largare SL, Blanchette AJ, McCarley RW, Strecker RE. Experimental sleep fragmentation and sleep deprivation increases exploration in an a open field test of anxiety while increasing plasma corticosterone levels. Behav BrainRes. 2009;197:450–453. doi: 10.1016/j.bbr.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tung A, Takase L, Fornal C, Jacobs BL. Effects of sleep deprivation and recovery sleep upon cell proliferation in adult rat dentate gyrus. Neurosci. 2005;134:721–723. doi: 10.1016/j.neuroscience.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 59.Vgontzas AN, Bixler EO, Lin H-M, Prolo P, Mastorakos G, Velo-Bueno A, Kales A, Chrousos GP. Chronic insomnia is associated with Nyctochemeral activation of the hypothalamic-pituitary axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787–3794. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 60.West MJ, Slomianka L, Gunderson HL. Unbiased stereological estimation of the total numbers of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 61.Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 62.Wojtowicz JM, Askew ML, Winocur G. The effects of running and inhibiting adult neurogenesis on learning and memory in rats. Eur J Neurosci. 2008;27:1494–1502. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- 63.Zhang C-I, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behavior. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]