Abstract
Background
Because the development of donor-specific anti-HLA antibodies (DSA) after lung transplantation has been associated with acute and chronic rejection we implemented a clinical protocol to screen all recipients for DSA after transplantation and preemptively treat those who develop DSA with rituximab and intravenous immune globulin (IVIG) or IVIG alone.
Methods
We conducted a prospective observational study of this protocol and used the LABScreen® Single Antigen assay to detect DSA after transplantation. We compared the incidence of acute rejection, lymphocytic bronchiolitis, and bronchiolitis obliterans syndrome (BOS) between those who developed DSA and those who did not using Cox proportional hazards models and compared freedom from BOS and survival between those who had persistent DSA and those who had successful depletion of DSA using the Kaplan-Meier method.
Results
Among 116 recipients screened, 65 developed DSA during the study period. Those who developed DSA and received antibody-directed therapy had a similar incidence of acute rejection, lymphocytic bronchiolitis, and BOS as those who did not develop DSA. Furthermore, recipients who had successful depletion of DSA had greater freedom from BOS and better survival than those who had persistent DSA. Finally, those treated for DSA had a similar incidence of infectious complications as those who did not develop DSA.
Conclusions
The development of DSA is surprisingly common after lung transplantation. Antibody-directed therapy may reduce the risk of rejection associated with DSA, but a randomized controlled trial is necessary to critically evaluate the efficacy of this treatment protocol.
INTRODUCTION
Lung transplantation has become a conventional definitive treatment for patients with end-stage lung disease. However, long-term outcomes remain disappointing, and the median survival after transplantation is approximately 5 years (1). Bronchiolitis obliterans syndrome (BOS) has emerged as the primary obstacle to better long-term outcomes. Indeed, the incidence of BOS is approximately 40–50% within three years of transplantation (2, 3). More importantly, the median survival after the diagnosis of BOS is only three years (4). While the pathogenesis of BOS remains elusive, several risk factors have been identified. Among these, the development of antibodies to human leukocyte antigens (HLA) is increasingly recognized as an important risk factor for BOS (5–7). In addition, the development of anti-HLA antibodies has been linked to persistent, recurrent, and high-grade acute rejection as well as lymphocytic bronchiolitis (8, 9), further underscoring the potential role of humoral immunity in graft rejection.
An in vitro study demonstrated that class I anti-HLA antibodies can induce airway epithelial cell proliferation, the release of fibrogenic growth factors, and epithelial cell apoptosis (10). Furthermore, studies in a murine model have shown that anti-HLA antibodies can induce airway obliteration when administered systemically in a heterotopic tracheal transplant model (11) and small airway obliteration when delivered intrabronchially into native lungs (12). These findings strongly suggest that anti-HLA antibodies have a direct pathogenic effect on airway epithelium and are not merely an epiphenomenon of the cellular immune response.
In light of these clinical and experimental data, we developed a protocol to screen for the development of anti-HLA antibodies and instituted a preemptive antibody-directed treatment protocol for patients transplanted at our program. The purpose of this study is to review our findings.
METHODS
Study design and patients
We conducted a prospective observational study of the clinical protocol we adopted in July 2006. Between 7/1/2006 and 7/31/2008, 122 adults underwent lung transplantation at our program. Before transplantation, all patients were screened for pre-formed anti-HLA antibodies using the LABScreen® Single Antigen assay (One Lambda Inc., Canoga Park, CA) every 3 months. Donor lungs were accepted only if a virtual crossmatch with all previously identified antibodies was compatible. At transplantation, all recipients had a direct crossmatch using serum obtained the day of surgery, and the results were available post-operatively. After transplantation, recipients were screened for donor-specific anti-HLA antibodies (DSA) using the LABScreen® Single Antigen assay at the same time points as our surveillance bronchoscopy schedule and when there was clinical evidence of graft dysfunction. In addition, we performed follow-up testing for DSA every 3 months for those who developed DSA. Because of fluctuations in mean fluorescence intensity (MFI) between positive controls, our center’s HLA lab calculates a ratio of sample MFI to positive control MFI and defines a ratio ≥ 0.2 as positive (13). The study was approved by the Washington University School of Medicine Institutional Review Board for human studies.
Management
All recipients were treated with induction immunosuppression, but some patients were enrolled in the EZ-2053 double-blinded randomized controlled trial (ClinicalTrials.gov Identifier NCT00105183) and may have received placebo. Otherwise, recipients were treated with basiliximab if they were cytomegalovirus (CMV) mismatched (i.e. recipient seronegative and donor seropositive) or if they had severe primary graft dysfunction (PGD), bleeding complications, coagulopathy, or were otherwise hemodynamically unstable. All other recipients were treated with equine anti-thymocyte globulin. All recipients were treated with a maintenance immunosuppressive regimen consisting of tacrolimus, azathioprine 2 mg/kg daily, and prednisone. CMV mismatched recipients were treated with valganciclovir for prophylaxis for the first 6 months after transplantation. All recipients were screened for CMV infection with a PCR assay weekly for the first 12 weeks, monthly thereafter, and when clinically indicated.
All recipients underwent surveillance bronchoscopy with bronchoalveolar lavage and transbronchial lung biopsies 1, 2, 3, 6, and 12 months after transplantation. Bronchoscopy was also performed when clinically indicated and 3–6 weeks after an episode of acute rejection to exclude persistent rejection. All episodes of acute rejection and lymphocytic bronchiolitis were diagnosed histologically according to the International Society for Heart and Lung Transplantation (ISHLT) criteria (14). Spirometry was measured weekly for the first 12 weeks then monthly thereafter, and BOS was diagnosed and staged according to the ISHLT criteria (15).
Antibody-directed therapy
According to the clinical protocol, recipients who developed DSA after transplantation were treated preemptively with either intravenous immune globulin (IVIG) and rituximab or IVIG alone depending on the following clinical factors: recipients who were infected or colonized with multi-drug resistant bacterial organisms, or had a history of recurrent infections, or had chronic leukopenia were treated with IVIG alone. Otherwise, patients were treated with IVIG and rituximab. We treated recipients with IVIG 500 mg/kg monthly for at least 6 months if the follow-up DSA screen became negative and continued monthly treatment if follow-up screening remained positive. We used a single dose of rituximab at 375 mg/m2 for patients who met the criteria to receive it.
Statistical analysis
We evaluated patient demographics between the two groups using the chi-squared test. To avoid assigning risk before the development of DSA, we compared post-transplant outcomes between the two groups using Cox proportional hazards models with DSA as a time-dependent variable. To assess the cumulative burden of rejection between groups, we defined the cumulative acute rejection A score as the sum of all A scores for each subject and evaluated the cumulative lymphocytic bronchiolitis B score similarly. We compared freedom from BOS and survival between those who had persistent DSA and those who cleared the DSA using the Kaplan-Meier method. We conducted the statistical analysis using SPSS 17.0 (SPSS Inc., Chicago, IL) and considered p values < 0.05 statistically significant.
RESULTS
Patients and DSA development
Follow-up was complete through 7/31/2009, and the study included 204 patient-years of follow-up with a mean follow-up (± standard deviation) of 1.67 ± 0.82 years per patient. The baseline characteristics of the cohort are shown in Table 1. The distribution of diagnoses spans the spectrum of common end-stage lung diseases. The majority (94%) of recipients had a bilateral transplant, and 47.5% were women. Of note, 46 of the 122 (37.7%) recipients had pre-formed antibodies (allosensitized) before transplantation, and the mean number of donor-recipient HLA mismatches at loci A, B, and DR was 5. It is also noteworthy that all recipients had a negative direct crossmatch with the donor at the time of transplantation. After transplantation, 53 (43%) recipients were treated with equine anti-thymocyte globulin, 53 (43%) with basiliximab, and 17 (14%) with the EZ-2053 study drug for induction immunosuppression. All recipients were treated with tacrolimus, azathioprine, and prednisone for maintenance immunosuppression.
Table 1.
Baseline characteristics for the entire cohort and for those who developed donor-specific anti-HLA antibodies (DSA) and those who did not.
| Variable | Entire cohort (n = 122) |
DSA1 positive (n = 65) |
DSA negative (n = 51) |
p value |
|---|---|---|---|---|
| Age (range; mean ± std dev2) |
19 – 69; 49.6 ± 14.7 |
19 – 68; 48.1 ± 15.8 |
21 – 69; 50.7 ± 13.9 |
0.36 |
| Female gender | 58 (47.5%) | 30 (46%) | 24 (47%) | 0.90 |
| Diagnosis | 0.65 | |||
| COPD or A1E3 | 41 (33.6%) | 19 (29%) | 18 (35%) | |
| Cystic fibrosis | 24 (19.7%) | 13 (20%) | 11 (21.5%) | |
| Pulmonary fibrosis | 36 (29.5%) | 20 (31%) | 14 (27.5%) | |
| Pulmonary hypertension | 3 (2.5%) | 3 (5%) | 0 (0%) | |
| Re-transplant | 6 (5%) | 4 (6%) | 2 (4%) | |
| Other diagnosis | 12 (10%) | 6 (9%) | 6 (12%) | |
| Bilateral lung transplant | 115 (94%) | 62 (95%) | 48 (94%) | 0.64 |
| CMV mismatch (R−/D+) | 33 (27%) | 14 (21.5%) | 17 (33%) | 0.15 |
| CMV at risk (R+ or D+) | 100 (82%) | 53 (81.5%) | 42 (82%) | 0.90 |
| HLA A, B, DR mismatch (range; mean ± std dev) |
2 – 6; 4.9 ± 0.96 |
3 – 6; 5.0 ± 0.8 |
2 – 6; 4.7 ± 1.1 |
0.17 |
| Allosensitized pre-transplant | 46 (37.7%) | 28 (43%) | 16 (31%) | 0.19 |
| Study follow-up time in yrs (mean ± std dev) |
1.67 ± 0.82 | 1.64 ± 0.77 | 1.91 ± 0.69 | 0.10 |
| Number of lung biopsies (mean ± std dev) |
5.3 ± 1.7 | 5.2 ± 1.5 | 5.3 ± 1.9 | 0.65 |
| Number of DSA screens (mean ± std dev) |
6.0 ± 2.1 | 6.5 ± 2.2 | 5.4 ± 1.9 | < 0.01 |
DSA: donor-specific anti-HLA antibodies.
std dev: standard deviation.
A1E: alpha-1 antitrypsin deficiency emphysema.
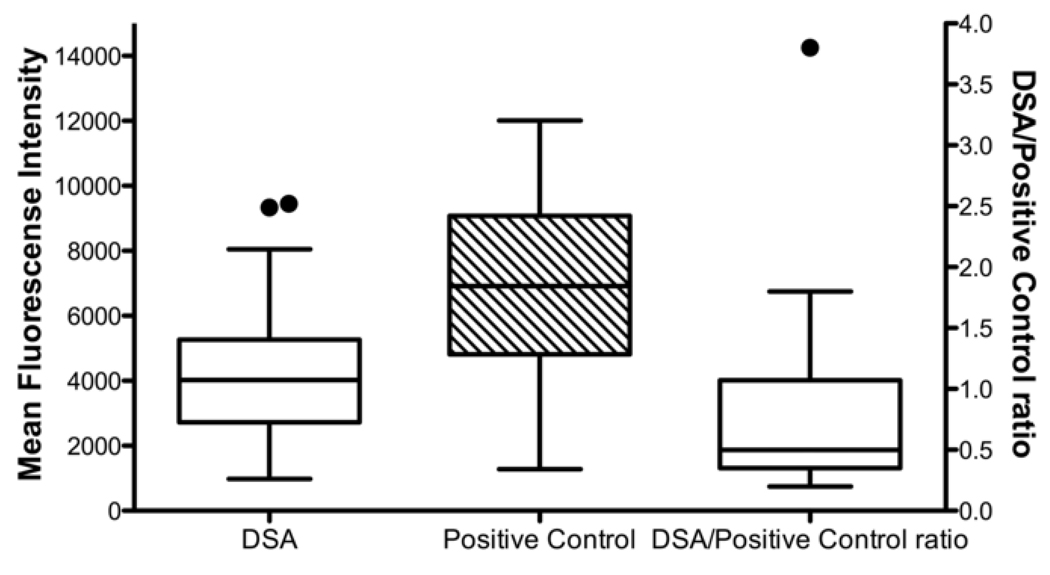
Among the 122 patients in this cohort, 6 died within 30 days of transplantation and were not screened for the de novo development of DSA; the remaining 116 patients were screened at least once. Among these, 65 (56%) developed DSA, and 52 of these did so in the first 90 days after transplantation, while 51 (44%) did not develop DSA during the study period. The mean DSA MFI (± standard deviation) was 4358 ± 2233, and the mean DSA MFI to positive control MFI ratio was 0.75 ± 0.67 (Figure 1). Furthermore, 10 of the 65 recipients who developed DSA developed antibodies only to class I antigens, 41 developed antibodies only to class II antigens, and 14 developed antibodies to both class I and class II antigens. There was no significant association between pre-transplant allosensitization and the post-transplant development of DSA (p = 0.20).
Figure 1.
Distributions of mean fluorescence intensity (MFI) and ratio of donor-specific anti-HLA antibody (DSA) MFI to positive control MFI for recipients who developed DSA. Boxes extend from the 25th to the 75th percentiles and whiskers extend to the largest and smallest values within 1.5 box lengths. The line within the boxes represents the median. The solid circles represent outlier cases between 1.5 and 3 box lengths from the upper edge of the boxes.
Antibody-directed therapy and rejection
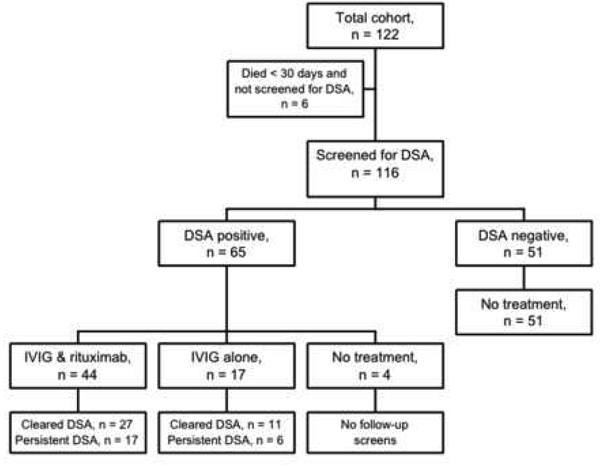
Based on our clinical protocol, recipients who had a history of recurrent infections or leukopenia were treated with IVIG alone when they developed DSA, while others were treated with IVIG and rituximab. However, 4 patients developed DSA but did not receive any antibody-directed therapy because of concurrent severe critical illness, and all died within 30 days of DSA detection (Figure 2). Of the 65 patients who developed DSA, 44 were treated with IVIG and rituximab and 17 were treated with IVIG alone (Figure 2). Treatment was instituted a mean (± standard deviation) 96 ± 131 days after transplantation. Among those treated with IVIG and rituximab, 27 cleared the DSA, and 17 had persistent DSA on follow-up testing (Figure 2). Similarly, among those treated with IVIG alone, 11 cleared the DSA and 6 had persistent DSA (Figure 2).
Figure 2.
Flowchart of the study, treatments, and possible outcomes.
Among those who developed DSA, 36 (55%) developed at least one episode of acute rejection grade ≥ A2 compared to 20 of the 51 (39%) recipients who did not develop DSA (p = 0.07; Table 2). However, of the 36 recipients who developed DSA and acute rejection, 16 developed both concurrently (within a 7 day time period), 10 developed acute rejection first (a mean 94 ± 97 days before they developed DSA), and 10 developed DSA first (a mean 94 ± 70 days before they developed acute rejection). To avoid assigning risk before the development of DSA, we evaluated the impact of DSA on acute rejection using a Cox proportional hazards model with DSA as a time-dependent variable. This demonstrated that DSA was not a significant risk factor for acute rejection grade ≥ A2 (HR = 1.15; 95% CI: 0.63 – 2.11, p = 0.6). Furthermore, there was no significant difference in the incidence of acute rejection grade A1, A2, or A3 between those who developed DSA and those who did not or in the cumulative acute rejection A score between the two groups (Table 2). Similarly, there was no significant difference in the incidence of lymphocytic bronchiolitis between the two groups. Ten of 65 (15%) recipients who developed DSA, and 7 of 51 (14%) who did not develop DSA had at least one episode of lymphocytic bronchiolitis grade ≥ B2 (p = 0.78). Furthermore, DSA evaluated as a time-dependent variable was not a significant risk factor for lymphocytic bronchiolitis in a Cox proportional hazards model (HR = 0.96; 95% CI: 0.31 – 3.0, p = 0.95). Lastly, there was no association between DSA class and acute rejection or lymphocytic bronchiolitis (Table 3).
Table 2.
Acute rejection profile for recipients who developed donor-specific anti-HLA antibodies (DSA) and those who did not*.
| DSA positive (n = 65) |
DSA negative (n = 51) |
p value |
|
|---|---|---|---|
| At least 1 episode of acute rejection grade ≥ A1, n (% of total) |
44 (68%) | 30 (59%) | 0.27 |
| At least 1 episode of acute rejection grade ≥ A2, n (% of total) |
36 (55%) | 20 (39%) | 0.07 |
| At least 1 episode of acute rejection grade ≥ A3, n (% of total) |
8 (12%) | 3 (6%) | 0.24 |
| Number of episodes of acute rejection grade ≥A1, mean ± SD |
1.13 ± 1.06 | 1.18 ± 1.32 | 0.82 |
| Number of episodes of acute rejection grade ≥A2, mean ± SD |
0.70 ± 0.75 | 0.59 ± 0.88 | 0.45 |
| Number of episodes of acute rejection grade ≥ A3, mean ± SD |
0.14 ± 0.39 | 0.06 ± 0.24 | 0.19 |
| Cumulative acute rejection score, mean ± SD |
2.00 ± 1.90 | 1.82 ± 2.20 | 0.65 |
There were no episodes of acute rejection grade A4 during the study period in either group.
Table 3.
Class of donor-specific anti-HLA antibodies (DSA) and acute rejection and lymphocytic bronchiolitis.
| DSA negative |
Class I DSA |
Class II DSA |
Class I&II DSA |
p value |
|
|---|---|---|---|---|---|
| At least 1 episode of acute rejection grade ≥ A2, n (% of total) |
20 (39%) | 5 (50%) | 23 (56%) | 8 (57%) | 0.32 |
| Cumulative acute rejection A score, mean ± SD |
1.8 ± 2.2 | 2.0 ± 1.9 | 1.8 ± 1.7 | 2.5 ± 2.5 | 0.65 |
| At least 1 episode of lymphocytic bronchiolitis grade ≥ B2, n (% of total) |
7 (14%) | 2 (20%) | 4 (10%) | 4 (29%) | 0.38 |
| Cumulative lymphocytic bronchiolitis B score, mean ± SD |
1.5 ± 1.9 | 1.1 ± 1.6 | 1.1 ± 1.2 | 2.0 ± 1.6 | 0.24 |
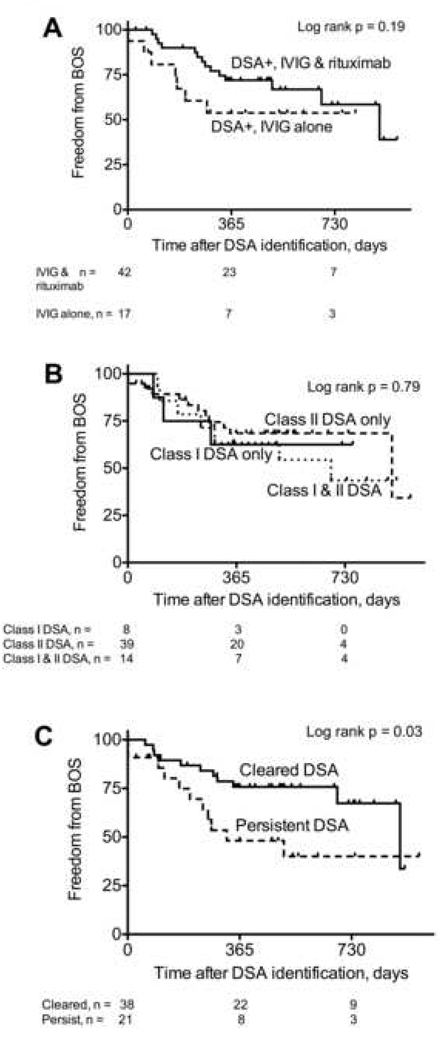
One hundred and eleven recipients (61 DSA positive and 50 DSA negative) survived 90 days and were evaluable for BOS. Among these, 23 of the 61 (38%) who developed DSA developed BOS, and 23 of the 50 (46%) who did not develop DSA developed BOS (p = 0.34). Two recipients developed DSA after the diagnosis of BOS, but 59 developed DSA and were preemptively treated with antibody-directed therapy before the diagnosis of BOS. To evaluate the impact of DSA on BOS without assigning risk before DSA occurred, we analyzed DSA as a time-dependent variable in a Cox proportional hazards model, and DSA was not associated with an increased risk of BOS (HR = 1.08; 95% CI: 0.61 – 1.93, p = 0.79). Furthermore, there was no significant difference in freedom from BOS from the time of DSA identification between those treated with IVIG alone and those treated with IVIG and rituximab (log rank p = 0.19; Figure 3A). Likewise, there was no association between DSA class and freedom from BOS (log rank p = 0.79; Figure 3B). However, recipients who had persistent DSA were significantly more likely to develop BOS than those who cleared the DSA (log rank p = 0.03; Figure 3C).
Figure 3.
Freedom from bronchiolitis obliterans syndrome (BOS). There was no significant difference in freedom from BOS between those who developed DSA and were treated with intravenous immune globulin (IVIG) and rituximab and those who were treated with IVIG alone (A). There was no significant difference in freedom from BOS between those who developed DSA to Class I, Class II, or both Class I and Class II antigens (B). Recipients who had persistent DSA were significantly more likely to develop BOS than those who cleared the DSA (C).
Infections and other adverse events
There were no significant differences in the number of episodes of CMV viremia, the incidence of CMV pneumonia, bacterial bronchitis, bacterial pneumonia, community-acquired respiratory viral (CARV) infection, or Aspergillus airway colonization between those who developed DSA and those who did not (Table 4). One recipient who did not have DSA developed invasive pulmonary aspergillosis during the study period; there were no other cases of invasive fungal disease.
Table 4.
Infectious complications.
| Infection | IVIG alone (n = 17) |
IVIG & rituximab (n = 44) |
DSA negative (n = 51) |
p value |
|---|---|---|---|---|
| Number of episodes of CMV viremia, (mean ± SD) |
1.2 ± 1.6 | 0.9 ± 1.0 | 1.2 ± 1.3 | 0.32 |
| CMV pneumonia, n | 0 | 1 | 5 | 0.44 |
| Number of episodes of bacterial bronchitis, (mean ± SD) |
2.2 ± 2.5 | 1.5 ± 1.6 | 1.3 ± 1.4 | 0.20 |
| Bacterial pneumonia, n | 6 | 11 | 15 | 0.72 |
| CARV1 infection, n | 3 | 3 | 6 | 0.45 |
| Aspergillus colonization, n | 8 | 16 | 14 | 0.30 |
CARV: community-acquired respiratory virus.
Survival and causes of death
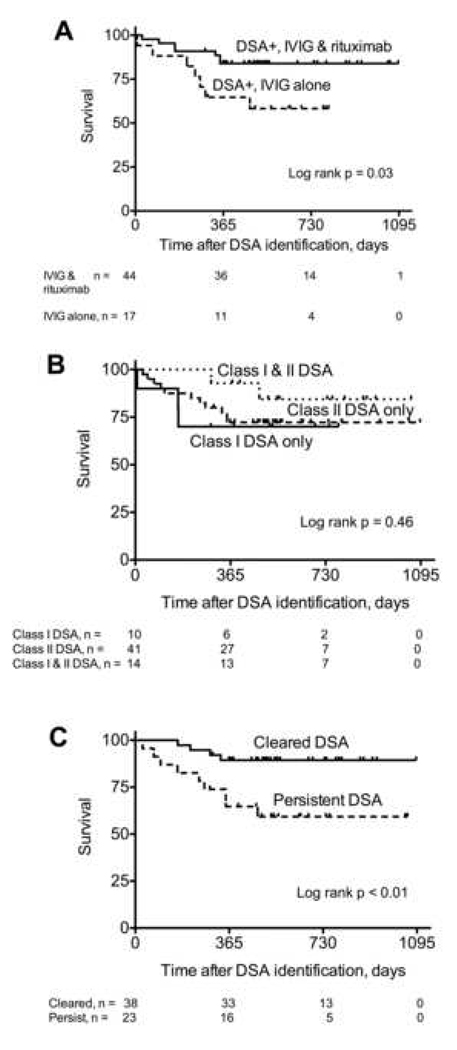
We evaluated the impact of DSA on survival using a Cox proportional hazards model with DSA as a time-dependent variable and found no statistically significant association between DSA and survival (HR = 1.89; 95% CI: 0.79 – 4.51, p = 0.15). During the study period, 17 of the 65 (26%) recipients who developed DSA died compared to 9 of the 51 (18%) who did not develop DSA (p = 0.28). Among the 17 recipients who developed DSA and died, 4 did not receive antibody-specific treatment because of a concurrent severe critical illness, 6 were treated with IVIG and rituximab, and 7 were treated with IVIG alone. Recipients treated with IVIG alone had a significantly worse survival after the detection of DSA than those treated with IVIG and rituximab (log rank p = 0.03; Figure 4A). However, it should be emphasized that patients were not randomized to these treatment regimens and clinical factors that influenced the treatment decision may have impacted survival. There was no association between DSA class and survival (log rank p = 0.46; Figure 4B). Finally, recipients who had persistent DSA had a significantly worse survival than those who cleared the DSA (log rank p < 0.01; Figure 4C). The most common cause of death was BOS, accounting for 7 of the 17 deaths in the DSA positive group and 6 of the 9 deaths in the DSA negative group (Figure 5). The second leading cause of death was pneumonia, accounting for 6 of the 17 deaths in the DSA positive group and 3 of the 9 deaths in the DSA negative group (Figure 5).
Figure 4.
Survival. Recipients who developed DSA and were treated with IVIG alone had a significantly worse survival than those treated with IVIG and rituximab (A). There was no significant survival difference between those who developed DSA to Class I, Class II, or both Class I and Class II antigens (B). Recipients who had persistent DSA had a significantly worse survival than those who cleared the DSA (C).
Figure 5.
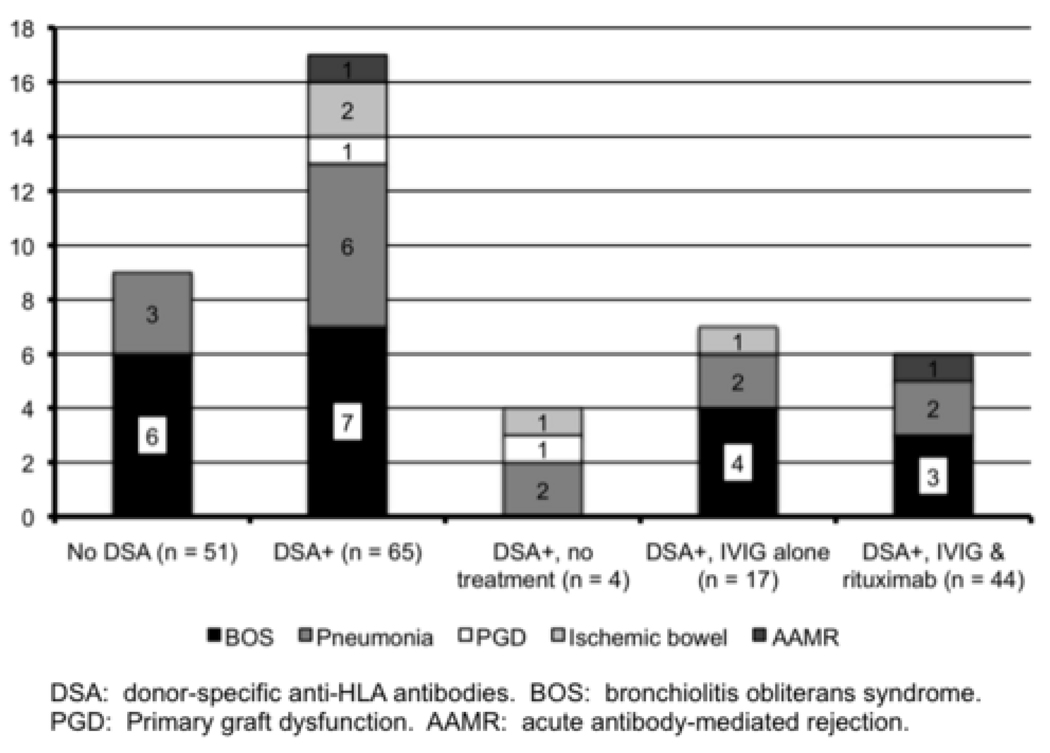
Causes of death. BOS was the most frequent cause of death, followed by pneumonia.
DISCUSSION
In this study, we evaluated the incidence of DSA after lung transplantation and the potential role for preemptive antibody-directed treatment. Our findings demonstrate that the development of DSA is surprisingly common. Indeed, over 50% of recipients developed DSA, and this typically occurred early after transplantation. While the incidence of DSA after lung transplantation in previous studies has varied between 10–27% (5–9), we attribute the higher incidence in this study to a very sensitive antibody detection assay (16) and a rigorous, prospective screening protocol. Furthermore, our data suggest that preemptive antibody-directed treatment may reduce the risk of rejection associated with DSA. However, the lack of a control group in this cohort limits our understanding of the impact of antibody-directed therapy and underscores the need for a randomized controlled trial.
The relationship between acute rejection and DSA is complex; however, when we evaluated DSA as a time-dependent variable, it was not a significant risk factor for acute rejection. Nevertheless, the co-occurrence of acute rejection and DSA in some patients emphasizes the link between cellular and humoral immunity. Previous studies identified the development of DSA as a significant risk factor for persistent and recurrent acute rejection, lymphocytic bronchiolitis, and BOS (5–9). However, we did not find an association between treated DSA and acute rejection, lymphocytic bronchiolitis, or BOS in this study, and propose that early antibody-directed treatment may mitigate this risk of rejection. In addition, recipients who had successful depletion of DSA had greater freedom from BOS and better survival than those who had persistent DSA. Our data also suggest that treatment with one dose of rituximab and monthly IVIG in conjunction with maintenance immunosuppression is reasonably safe after lung transplantation. While recipients treated with IVIG alone had significantly worse survival than those treated with IVIG and rituximab, this may be due to confounding variables that influenced the decision to treat with IVIG alone since patients were not randomized to the treatment assignments.
There are several limitations inherent to this study’s design. First, this was not a randomized controlled trial, and the cohort does not include a control group who developed DSA but was not treated with antibody-directed therapy. This makes any conclusion about the potential benefit of antibody-directed treatment and identifying the optimal regimen difficult. Nonetheless, our results provide an accurate assessment of the incidence of DSA development after lung transplantation, and this can be used to calculate the necessary sample size in future studies. Furthermore, our clinical protocol and findings will be useful in planning randomized controlled trials focused on the efficacy of antibody-directed treatment. Additionally, the development of DSA was surprisingly common, and this may have been influenced by the definition of positive used by our center’s HLA lab. Unfortunately, there is no widely accepted standardized definition of DSA positivity, and different centers use different definitions. Indeed, using our center’s definition, the mean DSA MFI was 4358 in this cohort. Finally, we are unable to evaluate the clinical significance of different thresholds of DSA positivity using these data because all patients with the exception of 4 who died within 30 days of DSA detection were treated with antibody-directed therapy based on our center’s definition.
In conclusion, our results demonstrate that the development of DSA is surprisingly common after lung transplantation, and a randomized controlled trial is necessary to evaluate the efficacy of antibody-directed therapy and identify the optimal regimen.
Acknowledgments
This study was supported in part by NIH HL 056643-13A1 (RRH, TM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
REFERENCES
- 1.Christie JD, Edwards LB, Aurora P, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-fifth official adult lung and heart/lung transplantation report – 2008. J Heart Lung Transplant. 2008;27:957–969. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Burton CM, Carlsen J, Mortensen J, Andersen CB, Milman N, Iversen M. Long-term survival after lung transplantation depends on development and severity of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2007;26:681–686. doi: 10.1016/j.healun.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Lingaraju R, Pochettino A, Blumenthal NP, et al. Lung transplant outcomes in white and African American recipients: Special focus on acute and chronic rejection. J Heart Lung Transplant. 2009;28:8–13. doi: 10.1016/j.healun.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Valentine VG, Robbins RC, Berry GJ, et al. Actuarial survival of heart-lung and bilateral sequential lung transplant recipients with obliterative bronchiolitis. J Heart Lung Transplant. 1996;15:371–383. [PubMed] [Google Scholar]
- 5.Sundaresan S, Mohanakumar T, Smith MA, et al. HLA-A locus mismatches and development of antibodies to HLA after lung transplantation correlate with the development of bronchiolitis obliterans syndrome. Transplantation. 1998;65(5):648–653. doi: 10.1097/00007890-199803150-00008. [DOI] [PubMed] [Google Scholar]
- 6.Smith MA, Sundaresan S, Mohanakumar T, et al. Effect of development of antibodies to HLA and cytomegalovirus mismatch on lung transplantation survival and development of bronchiolitis obliterans syndrome. J Thorac Cardiovac Surg. 1998;116(5):812–820. doi: 10.1016/S0022-5223(98)00444-9. [DOI] [PubMed] [Google Scholar]
- 7.Palmer SM, Davis RD, Hadjiliadis D, et al. Development of an antibody specific to major histocompatibility antigens detectable by flow cytometry after lung transplant is associated with bronchiolitis obliterans syndrome. Transplantation. 2002;74(6):799–804. doi: 10.1097/00007890-200209270-00011. [DOI] [PubMed] [Google Scholar]
- 8.Girnita AL, McCurry KR, Iacono AT, et al. HLA-specific antibodies are associated with high-grade and persistent-recurrent lung allograft acute rejection. J Heart Lung Transplant. 2004;23:1135–1141. doi: 10.1016/j.healun.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 9.Girnita AL, Duquesnoy R, Yousem SA, et al. HLA-specific antibodies are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant. 2005;5:131–138. doi: 10.1111/j.1600-6143.2004.00650.x. [DOI] [PubMed] [Google Scholar]
- 10.Jaramillo A, Smith CR, Maruyama T, Zhang L, Patterson GA, Mohanakumar T. Anti-HLA class I antibody binding to airway epithelial cells induces production of fibrogenic growth factors and apoptotic cell death: a possible mechanism for bronchiolitis obliterans syndrome. Hum Immunol. 2003;64:521–529. doi: 10.1016/s0198-8859(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 11.Maruyama T, Jaramillo A, Narayanan K, Higuchi T, Mohanakumar T. Induction of obliterative airway disease by anti-HLA class I antibodies. Am J Transplant. 2005;5:2126–2134. doi: 10.1111/j.1600-6143.2005.00999.x. [DOI] [PubMed] [Google Scholar]
- 12.Fukami N, Ramachandran S, Saini D, et al. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182:309–318. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris GP, Phelan DL, Jendrisak MD, Mohanakumar T. Virtual crossmatch by identification of donor-specific anti-human leukocyte antigen antibodies by solid-phase immunoassay: a 30-month analysis in living donor kidney transplantation. Hum Immunol. 2010;71:268–273. doi: 10.1016/j.humimm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Yousem SA, Berry GJ, Cagle PT, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15:1–15. [PubMed] [Google Scholar]
- 15.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 16.Tait BD, Hudson F, Cantwell L, et al. Review article: luminex technology for HLA antibody detection in organ transplantation. Nephrology. 2009;14:247–254. doi: 10.1111/j.1440-1797.2008.01074.x. [DOI] [PubMed] [Google Scholar]