Abstract
Nucleus accumbens mu-opioid receptor activation can strongly stimulate intake of high-fat food in satiated rats, and one of the mechanisms involves activation of lateral hypothalamic orexin neurons and orexin receptor-1 signaling in the mesolimbic dopamine system. Here, we tested the potential contribution of NPY/Y1R and α-MSH/MC3/4R-signaling to accumbens-induced high-fat feeding. Prior administration of the selective Y1R antagonist 1229U91 or the MC3/4R agonist MTII into the lateral ventricle (LV) dose-dependently decreased high-fat intake induced by nucleus accumbens injection of the mu-opioid receptor agonist DAMGO. Both drugs also decreased high-fat feeding induced by switching rats from regular chow to high-fat diet, but less efficiently than when DAMGO-induced. Administration of 1229U91 directly into the PVH also suppressed DAMGO-induced high-fat intake, but a higher dose was required. The results suggest that NPY/Y1R signaling in the PVH and other forebrain sites is necessary for accumbens DAMGO to elicit high-fat intake, and that forebrain MC3/4R signaling can suppress it.
Introduction
The rewarding aspect of food is thought to be an important factor contributing to increased energy consumption and prevalence of obesity in the modern world, but the mechanisms of reward-driven eating are not well understood. Chemical manipulation of the nucleus accumbens has been demonstrated to induce strong feeding responses in rats (Kelley, 2004). Activation of nucleus accumbens mu-opioid receptors with DAMGO selectively increases intake of fat but not carbohydrate when given a choice (Zhang et al., 1998; Zhang and Kelley, 2000) and this happens even in rats previously satiated on the same high-fat diet (Zheng et al., 2007). Furthermore, chronic suppression of nucleus accumbens mu-opioid receptors attenuates intake of palatable food and diet-induced obesity, suggesting that endogenous MOR signaling in this brain area is required for the full effect of high-fat diet to induce obesity (Lenard et al., 2010). Therefore, the accumbens DAMGO paradigm is an excellent model for studying reward-driven food intake.
Previous research indicated that DAMGO-induced high-fat intake depends on normal functioning of a number of brain areas including the lateral and dorsomedial hypothalamus, the ventral tegmental area in the midbrain, and the NTS in the caudal brainstem (Will et al., 2003; Zheng et al., 2007). Of particular interest are projections from the nucleus accumbens to the hypothalamus (Groenewegen and Russchen, 1984; Otake and Nakamura, 2000; Sano and Yokoi, 2007; Usuda et al., 1998), as they might provide a link between hedonic and homeostatic regulatory circuits (Berthoud, 2007). In a series of experiments, we and others have shown that DAMGO administration to the nucleus accumbens preferentially activates lateral hypothalamic orexin neurons and that blocking orexin receptor-1 signaling in the ventral tegmental area almost completely abolishes the DAMGO effect on fat intake (Zheng et al., 2007). Together with the observations that ICV orexin administration preferentially enhances fat intake in a choice situation (Clegg et al., 2002) and that orexin administration to the ventral tegmental area (VTA) excites dopamine neurons (Borgland et al., 2006), these findings suggest that an accumbens-hypothalamic pathway recruiting orexin neurons might be crucial for reward-driven food intake.
However, direct and indirect projections from the nucleus accumbens are not exclusively directed to orexin neurons - we have previously shown a functional connection with arcuate nucleus NPY and POMC neurons (Zheng et al., 2003), and chow intake induced by GABAA-agonist administration to the nucleus accumbens shell was blocked by pretreatment of the third ventricle with a selective Y1R antagonist (Stratford and Wirtshafter, 2004). Therefore, the aim of the present study was to examine the role of Y1R and MC3/4R signaling in high-fat diet intake induced by nucleus accumbens administration of DAMGO.
Materials and Methods
Animals and housing
Adult male Sprague Dawley rats (Harlan Industries, Indianapolis, IN) weighing 280 – 320 g at the time of surgery were housed individually in hanging wire mesh cages in a climate controlled room (22±2°C) on a 12:12 h light cycle with lights on at 0700 h and lights off at 1900 h. Food and water were available ad libitum except as specified below. All experimental protocols were approved by the Institutional Animal Care and Use Committee (Pennington Biomedical Research Center) and were conducted in compliance with USDA regulations and APS principles for research involving animals.
Implantation and verification of chronic injection cannulas
Rats were anesthetized with ketamine/acepromazine/ xylazine (80/1.6/5.4 mg/kg, s.c.) and given atropine (1mg/kg, i.p.). Bilateral 24 GA stainless steel guide cannulas were aimed either at the medial nucleus accumbens core (AP: 1.2 mm, ML: 1.5 mm, DV: − 7.4 mm) or at the nucleus accumbens shell (AP: 1.2 mm, ML: 1.0mm, DV: − 7.4mm) and in some rats an additional unilateral cannula was aimed at the right lateral ventricle (AP: 0.9 mm; ML: 1.6 mm; DV: −3.5 mm) or bilateral cannulas at the paraventricular nucleus of the hypothalamus (AP: −1.8 mm; ML: 0.5 mm; DV: −8.0 mm). Twelve to fifteen days were allowed for recovery from surgery, at which time animals were given one saline injection with 31 GA injectors extending 2.5 mm beyond the tip of guide cannulas for intra-accumbens and PVH injections and 0.6mm for lateral ventricle injections to avoid potential side effects of initial penetration to the injection site on the behavioral response.
Lateral ventricular cannulas for the Y1R antagonist experiments were verified by injecting angiotensin II (15 ng/3µl saline; Sigma, St Louis,MO) and measuring water intake. Only rats that drank with a latency < 5 min at least 5 ml of water in 30 min were used for the study. For MTII experiments, only rats that suppressed high-fat intake at least 30% by the highest dose (0.5 nmol/3µl saline) were used for analysis, and one rat failed this criterion. Accumbens and PVH injection sites were histologically verified after injection of a blue dye (Chicago Blue, Sigma)at the end of the experiments (Fig. 7).
Fig. 7.
Summary of histological verification of injection sites. A: Injection sites in the nucleus accumbens core for Experiment 2 with PVH injection of the Y1R antagonist (filled circles), and in the nucleus accumbens shell for Experiment 3 with LV injections of the MC3/4R agonist MTII (open circles). B: Injection sites in the PVH.
Experimental protocol and measurement of food intake
Three experiments were carried out in separate groups of rats. The effect of the Y1R-antagonist 1229U91 injected into the lateral ventricle was tested in Experiment 1, and its effect when injected into the PVH was tested in Experiment 2. The effect of the MC3/4R agonist MTII was tested in Experiment 3. The effect of the ligands on palatability contrast-induced high-fat intake and on DAMGO-induced high-fat intake was measured in separate trials in the same animals in Experiment 1 and different animals in Experiment 3 (Fig. 1).
Fig. 1.
Schematic overview of experimental design. Rats had ad libitum access to regular chow, except for the two test periods when high-fat diet intake was measured. The effects of the Y1R and MC3/4R receptor ligands injected into the lateral ventricle (LV) or paraventricular hypothalamus (PVH) on high-fat intake induced by the palatability contrast or by nucleus accumbens DAMGO administration were measured on separate test days.
Rats maintained on regular lab chow were trained to eat high-fat diet (60% energy from fat, D12492, Research Diets, New Brunswick, NJ) for 2h every other day between 9:00 and 11:00 during recovery from surgery. On experimental days, chow was replaced with high-fat diet for 1 h from 09:00 to 10:00. Thirty minutes later, high-fat diet was made available again for 2 h (Fig. 1). To assess the effects of the ligands on palatability contrast-induced high-fat intake, rats were administered 1229U91 (0, 0.5, 1.0, or 10.0 µg/3 µl; Experiment 1) or MTII (0, 0.02, 0.1, 0.5 nmol/3µl; Experiment 3), with saline as control, into the lateral ventricle, 15 minutes before access to the high-fat diet for 1 h. The Y1R antagonist 1229U91 and MTII (both from Sigma) were dissolved in sterile saline.
To assess the effects of the ligands on DAMGO-induced high-fat intake, rats were pre-satiated for one hour on high-fat diet and then pretreated with either 1229U91 (0, 0.5, 1.0 µg/3 µl) into the lateral ventricle (Experiment 1) or 1229U91 (2 × 0, 0.5, 2.5 µg/300 nL) bilaterally into the PVH (Experiment 2), or MTII (0, 0.02, 0.5 nmol/3µl) into the lateral ventricle (Experiment 3). Fifteen min later, DAMGO (0 or 0.25µg/500 nL) was injected into the nucleus accumbens, and 2 h high-fat intake was measured.
All injections were made over a period of 1 min and the injector was left in place for an additional 1 min to prevent backflow. In Experiments 1 and 2, each rat received all combinations of treatments with 3–5 days between tests. In Experiment 3, separate groups of rats served for the assessment of MTII effects on palatability contrast and DAMGO-induced fat intake. Within each group, each rat received all treatment combinations and doses, with 4–5 days between tests. To prevent receptor desensitization with repeated DAMGO injections we minimized the exposure to DAMGO to a given injection site by injecting it unilaterally and alternating between left and right accumbens. Previous studies in our laboratory showed that unilateral (+ 6.2 g on average) and bilateral (+ 5.8 g) injection of DAMGO into the medial core of the nucleus accumbens produced similar effects (Lenard et al., 2010; Zheng et al., 2007). Furthermore, left and right injections of DAMGO into the medial core also produced similar effects [left: + 6.4 + 0.4 g; right: + 6.0 + 0.4 g, n = 22; (Lenard et al., 2010)]
Statistical analysis
The effects of the Y1R antagonist on high-fat intake were analyzed as follows: one-way ANOVA for palatability contrast-induced intake, and repeated measure ANOVA for DAMGO-induced intake. The effects of Y1R antagonist on percent of suppression of high-fat intake was analyzed with two-way ANOVA. Direct comparisons were made after ANOVA with either Tuckey’s or Bonferroni’s multiple comparison tests. Significance level was set at p < 0.05.
Results
1. Experiment 1: Effects of Y1R antagonist 1229U91 injected into the lateral ventricle
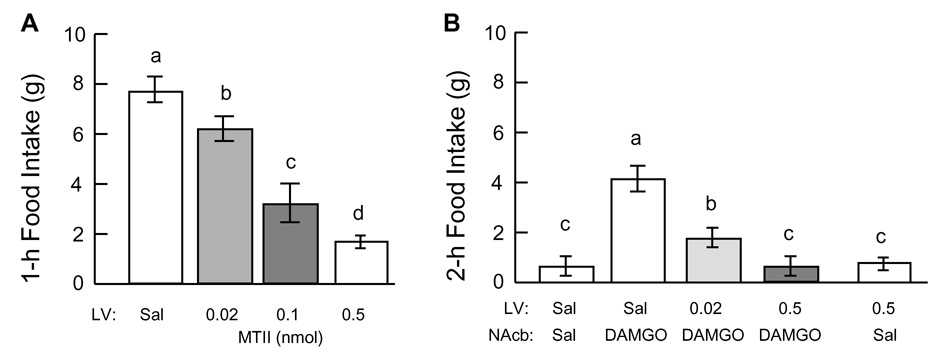
During the first 1-h access to high-fat diet (after ad lib access to regular chow), rats typically consumed about 7–8 g across experiments, whether there was prior injection of saline into the lateral ventricle or no injection. This palatability contrast-induced high-fat intake was dose-dependently decreased by prior injection of 1229U91 (F[3,31] = 11.1, P < 0.001; Fig. 2A). The two lower doses (0.5 and 1.0 µg) did not have a significant effect, but the highest dose (10µg) reduced 1-h high fat intake significantly (P < 0.01) by about 70%.
Fig. 2.
Effect of Y1R antagonist 1229U91 injected into the lateral ventricle (LV) on high-fat intake induced by palatability contrast (switching from chow to high-fat diet, A) or induced by DAMGO injection into the nucleus accumbens (NAcb, B). A: Rats were administered different doses of 1229U91 into the LV in random order and 1-h intake of high-fat diet was measured. B: The same rats were allowed to eat high-fat diet for 1 h as in A (without blocker administration) and were then administered different doses of 1229U91 into the LV and 15 minutes later DAMGO or saline into the NAcb; 2-h intake of high-fat diet was then measured. Bars that do not share the same letter are significantly (P < 0.05) different from each other (based on ANOVA followed by Bonferroni-adjusted multiple comparisons.
During the 2-h period following pre-satiation, rats consumed very little (< 1g) additional high-fat diet when they received vehicle into the lateral ventricle and saline into the nucleus accumbens (Fig. 2B). However, injection of DAMGO in LV vehicle-pretreated rats induced another highly significant (P < 0.01) bout of high-fat intake of 8.3 ± 1.2 g. This response was dose-dependently reduced by LV pretreatment with 1229U91 (F[2,20] = 13.2, P < 0.001). Even the lowest dose (0.5µg) reduced accumbens-induced intake by more than 50%, and the 1 µg dose abolished it completely. Injection of 1229U91 (1µg) in rats with saline injected into the NAcb did not have a significant effect on the low baseline intake. No adverse behavioral effects of the antagonist were noted at any dose.
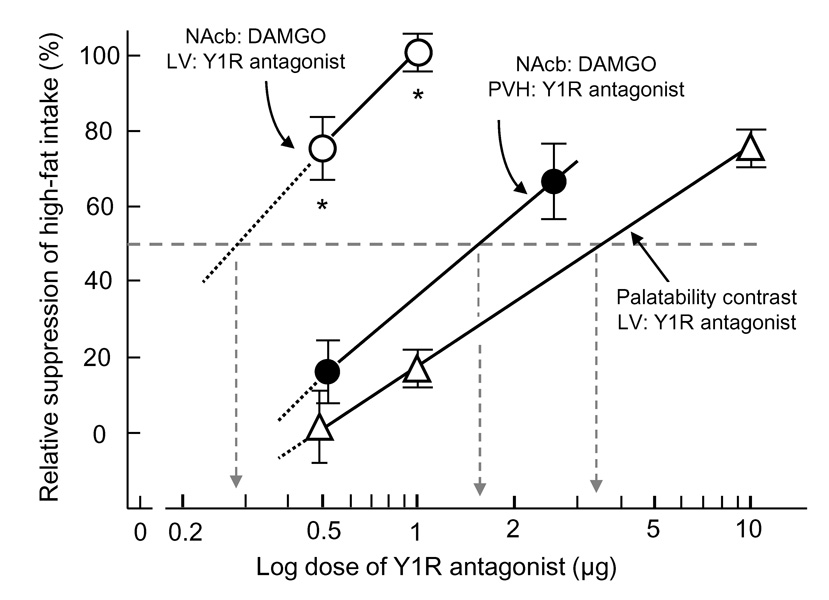
Direct comparison of the relative suppression of high-fat intake by 1229U91 showed that DAMGO-induced intake was about 10-fold, significantly more sensitive to the blocker than palatability contrast-induced intake (F[2,48] = 27.9, P <0.0001), with an estimated ED50 of ~0.3 µg and 3.0 µg, respectively (Fig. 4)
Fig. 4.
Relative suppression of DAMGO-induced (circles) and palatability contrast-induced (triangles) high-fat intake with different doses of Y1R antagonist 1229U91 administered either into the lateral ventricle (LV, open symbols) or the paraventricular nucleus of the hypothalamus (PVH, closed circles). LV administration of the antagonist was significantly more potent in suppressing DAMGO-induced intake compared with PVH administration and with palatability contrast-induced intake. * P < 0.05 compared with both other conditions (based on ANOVA followed by Bonferroni-adjusted preplanned comparisons).
2. Experiment 2: Effects of Y1R antagonist 1229U91 injected into the PVH
As in Experiment 1, rats consumed about 8g of high-fat diet during the 1-h pre-satiation period in the absence of any treatment (Fig. 3A), and in this experiment we did not assess the effect of 1229U91 on this palatability contrast-induced eating. And again, under vehicle/saline conditions these fully satiated rats consumed little additional high-fat diet, while under vehicle/DAMGO conditions they consumed another large amount (P < 0.01) of high-fat diet (Fig. 3B). Pretreatment of the PVH with the lower dose (0.5µg) of 1229U91 had no significant effect, but the higher dose (2.5µg) decreased high-fat intake by about 50%. Compared to lateral ventricular administration, PVH administration of 1229U91 was significantly less potent in suppressing DAMGO-induced high-fat intake (P < 0.001), with an estimated ED50 of ~1.5 µg, similar to the potency for suppression of palatability contrast-induced high-fat intake (Fig. 4).
Fig. 3.
Effect of Y1R antagonist 1229U91 injected into the paraventricular nucleus of the hypothalamus (PVH) on high-fat intake induced by DAMGO injection into the nucleus accumbens (NAcb, B). A: 1-h intake of high-fat diet induced by palatability contrast (no blocker administered). B: The same rats were then administered different doses of 1229U91 into the LV and 15 minutes later DAMGO or saline into the NAcb, and 2-h intake of high-fat diet was measured. Bars that do not share the same letter are significantly (P < 0.05) different from each other (based on ANOVA followed by Bonferroni-adjusted multiple comparisons.
3. Experiment 3: Effects of MC3/4R agonist MTII injected into the lateral ventricle
The magnitude of the 1-h palatability contrast-induced high-fat intake was similar to the other experiments and was dose-dependently reduced by LV pretreatment with MTII (Fig. 5A). The highest dose (0.5 nmol) significantly (P < 0.01) reduced 1-h intake by about 77%.
Fig. 5.
Effect of MC3/4R agonist MTII injected into the lateral ventricle (LV) on high-fat intake induced by palatability contrast (switching from chow to high-fat diet, A) or induced by DAMGO injection into the nucleus accumbens (NAcb, B). A: Rats were administered different doses of MTII into the LV in random order and 1-h intake of high-fat diet was measured. B: A separate group of rats was allowed to eat high-fat diet for 1 h as in A (without blocker administration) and were then administered different doses of MTII into the LV and 15 minutes later DAMGO or saline into the NAcb; 2-h intake of high-fat diet was then measured. Bars that do not share the same letter are significantly (P < 0.05) different from each other (based on ANOVA followed by Bonferroni-adjusted multiple comparisons.
During the 2-h period following pre-satiation, rats consumed very little (< 1g) additional high-fat diet when they received saline into the lateral ventricle and nucleus accumbens (Fig. 5B). Unilateral injection of DAMGO into the NAcb induced a significant feeding response (P < 0.01) of about 4g that was dose-dependently decreased by LV pretreatment with MTII. The high dose of MTII (0.5 nmol completely abolished DAMGO-induced intake.
Direct comparison of the relative suppression of high-fat intake by MTII showed that DAMGO-induced intake was significantly more sensitive to the blocker than palatability contrast-induced intake (F[3,28] = 16.8, P <0.001), with an estimated ED50 of < 0.01 µg and ~0.05 µg, respectively (Fig. 6). However, because the DAMGO-induced feeding response was only about half of the palatability contrast-induced response, the absolute suppression by the two MTII doses was similar for the two conditions.
Fig. 6.
Relative suppression of DAMGO-induced (circles) and palatability contrast-induced (triangles) high-fat intake with different doses of MC3/4R agonist MTII administered into the lateral ventricle. MTII was significantly more potent to suppress DAMGO-induced high-fat intake compared with palatability contrast-induced intake. * P < 0.05 (based on ANOVA followed by Bonferroni-adjusted preplanned comparisons).
Discussion
Reward-driven food intake is thought to play an important role in the increased prevalence of obesity around the globe, but the underlying neural mechanisms are far from clear. The easy availability of energy-dense and often fat-rich tasty foods together with ever-present cues associated with such foods is suspected to lead to hyperphagia and eventually body weight gain in many susceptible individuals. Here we use a rat model, capitalizing on the strong stimulation of fatty foods by chemically manipulating the nucleus accumbens, a key area in reward functions, to shed light on the underlying neural mechanisms and pathways.
We find that the strong accumbens-induced feeding response is dose-dependently reduced by either inhibiting endogenous Y1R-signaling or by stimulating MC3/4 melanocortin receptor-signaling in the forebrain. Both manipulations also inhibit feeding in response to switching chow-maintained rats to palatable high-fat diet, but less efficiently. Furthermore, in an attempt to localize a critical site of Y1R signaling, we found that although accumbens-induced high-fat feeding was reduced by bilateral antagonist injection into the paraventricular nucleus of the hypothalamus, a higher dose was required to obtain the same suppression. We conclude that endogenous Y1R signaling in forebrain areas that may include the PVH is a necessary downstream signaling step for the accumbens-induced high-fat feeding response as well as for the “natural” palatability contrast-induced response. In addition, the MTII effects suggest that although reward-driven eating can override satiety signals, it is no match for artificially stimulated melanocortin-signaling at the MC3/4 receptor.
The pioneering work of Ann E. Kelly and her colleagues has brought to light the crucial role of the nucleus accumbens in food reward mechanisms (for a review of much of her work see (Baldo and Kelley, 2007; Kelley et al., 2002). Using a model with limited accessibility of high-fat diet only during 3-h daily test sessions, they showed that mu-opioid receptor stimulation within the nucleus accumbens selectively enhances intake of high-fat food (Zhang et al., 1998). Even rats preferring high-carbohydrate diet over high-fat diet under basal conditions and even 24-h food-deprived rats showed a preferential increase in high-fat diet after accumbens DAMGO administration (Zhang et al., 1998). The effect of DAMGO was remarkably strong with some rats eating in excess of 17g (~100 Kcal),or about 80% of their daily need of high-fat diet within one hour (Zhang et al., 1998). Opioid stimulation of a subarea of the nucleus accumbens also increases the hedonic impact of taste or ‘liking’ (Pecina and Berridge, 2000; Pecina and Berridge, 2005; Pecina, 2008), as measured in the taste-reactivity test (Grill and Norgren, 1978). Furthermore, increased mu-opioid signaling in the nucleus accumbens has been shown to selectively enhance consumption of preferred palatable foods exclusively on flavor cues (Woolley et al., 2006; Woolley et al., 2007). Together, as suggested by Ann Kelley: “We hypothesize that opioid-mediated mechanisms within the ventral striatal medium spiny neurons mediate the affective or hedonic response to food (‘liking’ or food ‘pleasure’) and “that they encode positive affect induced by tasty and/or calorically dense foods (such as sugar and fat), and promotes behaviors associated with this enhanced palatability. It is proposed that this brain mechanism was beneficial in evolutionary development for ensuring the consumption of relatively scarce, high-energy food sources. However, in modern times, with unlimited supplies of high-calorie food, it has contributed to the present epidemic of obesity” (Kelley et al., 2002), p 265).
Given these credentials and the robustness of the feeding response, we used it as a model for reward-driven palatable food intake and have begun “dissecting” its effector pathways. We and others have identified one effector pathway involving lateral hypothalamic orexin neurons with projections to the ventral tegmental area (Baldo et al., 2004; Zheng, 2007) and a role for these orexin projections for behavior selection by integrating metabolic, external, and emotional information is emerging (Adamantidis and de Lecea, 2009; Boutrel et al., 2009; Harris et al., 2005). However, there are undoubtedly other effector pathways as indicated by studies looking at accumbens DAMGO-induced neural activation (Zhang and Kelley, 2000) and the effect of neural inhibition in some of these areas (Will et al., 2003). Within the hypothalamus, other than orexin-expressing neurons in the perifornical, dorsomedial, medial preoptic, and supramammillary nuclei are activated by DAMGO (Zhang and Kelley, 2000; Zheng, 2007).
NPY/AGRP- and POMC/CART-expressing neurons in the arcuate nucleus have been shown to play key roles in the control of food intake and homeostatic regulation of body weight, but the downstream neural network leading to the behavioral, autonomic, and endocrine effects is less well defined. Specifically, the relationship between these metabolically driven effector pathways with reward-driven pathways for elaboration of ingestive behavior are not known. Here we show that DAMGO-induced high-fat intake can be completely abolished by lateral ventricular pretreatment with a specific Y1R antagonist, suggesting that downstream Y1R-signaling in areas accessible to lateral ventricular diffusion of the drug is necessary for the DAMGO effect.
To better distinguish DAMGO-induced from palatability contrast-induced high-fat intake, we modified the Kelley protocol by adding a 1-h pre-satiation period, so that the control intake during the following DAMGO-test period is negligible, and we assessed the effect of the Y1R antagonist separately, on both periods. The results indicate that the DAMGO-induced was more sensitive than the palatability contrast-induced high-fat diet intake, which is presumably mediated by endogenous opioid release in the nucleus accumbens (Will et al., 2007). Several interpretations for this differential sensitivity to Y1R blockade are possible. It could simply reflect the difference in satiation and the presence of satiety factors; NPY-signaling is expected to be lower and suppressible by a lower dose of antagonist after the 1-h pre-satiation period. To further investigate this possibility, an additional condition with DAMGO-administration in continuously high-fat fed rats should be helpful. Alternatively, the site of natural opioid-signaling during the palatability contrast-induced response is likely different from the site affected by DAMGO and could therefore activate different effector pathways.
To narrow down the potential site(s) of Y1R antagonism, we injected the blocker bilaterally into the PVH, as this nucleus was shown to be a major recipient of arcuate nucleus NPY projections. The rationale for implicating a specific brain site for drug action is that the dose required for an effect should be considerably lower than when the drug is injected into the brain ventricles. This was not the case for our Y1R antagonist injections, suggesting that although the PVH may participate, other, unidentified areas, that are accessible to diffusion from the lateral ventricle, are more important for the blockade of DAMGO-induced high-fat intake.
The importance of hypothalamic POMC neurons in the control of food intake and energy balance has been well established (Schwartz, 2001). Stimulation of MC3/4R-signaling with the natural ligand α-MSH can reduce food intake acutely (McMinn et al., 2000) and its stable analog MTII can inhibit food intake and body weight gain for longer periods, even when rats are fed a palatable diet (Hamilton and Doods, 2002). However, compared to normal chow diet, the anorexic effect of intracerebroventricular MTII is reduced in rats fed high-fat diet (Clegg et al., 2003), and when meal initiation is learned by presenting food in scheduled meals (Benoit et al., 2003). This latter observation suggests that cognitively driven food intake may be partially resistant to melanocortin-induced anorexia, and we wanted to see whether this is also the case for reward-driven food intake.
Although we did not directly compare intake of regular chow and high-fat diet, our results show that MTII very potently suppressed intake of high-fat diet, whether it was driven naturally or by opioid-stimulation of the nucleus accumbens. Even our lowest dose of 20 pmol reduced DAMGO-induced high-fat intake by about 50%, and the highest dose of 0.5 nmol completely abolished it. Compared with other studies (Benoit et al., 2003; Hagan et al., 2000; Hansen et al., 2005; Thiele et al., 1998), these are among the lowest doses of MTII to produce a given decrease in food intake.
As for Y1R antagonism, palatability contrast-induced high-fat intake was slightly less sensitive to MC3/4 agonism with MTII than DAMGO-induced high-fat intake when considering relative suppression. Absolute suppression of intake by MTII was, however, similar. Previous research has clearly demonstrated that MTII is more potent to inhibit food intake in food-restricted rats fed a high-fat diet compared with ad libitum fed rats (Benoit et al., 2003). Therefore, one would expect that during the palatability-contrast-induced 1-h period, when they were deprived of high-fat diet, our rats would be more, rather than less, sensitive to MTII. That this was not the case may have several explanations. One explanation is that the site of natural opioid-signaling during the palatability contrast-induced response is likely different from the site affected by DAMGO and could therefore activate different effector pathways, differentially affected by MTII.
Unlike for the Y1R-antagonist experiments, where endogenous signaling is blocked, artificially stimulating the MC3/4R with exogenous MTII, does not allow conclusions regarding the mechanism of interaction between the two (MTII and DAMGO) manipulations. We can conclude that the melanocortin pathway is dominant over palatability-driven intake, but it remains to be determined where in the brain these opposing influences come together to determine intake.
In conclusion, both Y1R antagonism and MC3/4 agonism are very effective in counteracting reward-driven intake of palatable high-fat diet, at least in the acute situation. More challenging longer-term experiments are needed to demonstrate usefulness of these two neuropeptide signaling pathways in potential drug therapies to fight obesity.
Acknowledgments
Research supported by National Institutes of Health grant DK47348 and DK071082.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamantidis A, de Lecea L. The hypocretins as sensors for metabolism and arousal. J Physiol. 2009;587:33–40. doi: 10.1113/jphysiol.2008.164400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE. Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci. 2004;19:376–386. doi: 10.1111/j.1460-9568.2004.03093.x. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Clegg DJ, Barrera JG, Seeley RJ, Woods SC. Learned meal initiation attenuates the anorexic effects of the melanocortin agonist MTII. Diabetes. 2003;52:2684–2688. doi: 10.2337/diabetes.52.11.2684. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Interactions between the "cognitive" and "metabolic" brain in the control of food intake. Physiol Behav. 2007;91:486–498. doi: 10.1016/j.physbeh.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Cannella N, de Lecea L. The role of hypocretin in driving arousal and goal-oriented behaviors. Brain Res. 2009 doi: 10.1016/j.brainres.2009.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Air EL, Woods SC, Seeley RJ. Eating elicited by orexin-a, but not melanin-concentrating hormone, is opioid mediated. Endocrinology. 2002;143:2995–3000. doi: 10.1210/endo.143.8.8977. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Benoit SC, Air EL, Jackman A, Tso P, D'Alessio D, Woods SC, Seeley RJ. Increased dietary fat attenuates the anorexic effects of intracerebroventricular injections of MTII. Endocrinology. 2003;144:2941–2946. doi: 10.1210/en.2002-0218. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 1978;143:281–297. doi: 10.1016/0006-8993(78)90569-3. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Russchen FT. Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: a tracing and immunohistochemical study in the cat. J Comp Neurol. 1984;223:347–367. doi: 10.1002/cne.902230303. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, Van Der Ploeg LH, Woods SC, Seeley RJ. Long-term orexigenic effects of AgRP-(83---132) involve mechanisms other than melanocortin receptor blockade. Am J Physiol Regul Integr Comp Physiol. 2000;279:R47–R52. doi: 10.1152/ajpregu.2000.279.1.R47. [DOI] [PubMed] [Google Scholar]
- Hamilton BS, Doods HN. Chronic application of MTII in a rat model of obesity results in sustained weight loss. Obes Res. 2002;10:182–187. doi: 10.1038/oby.2002.28. [DOI] [PubMed] [Google Scholar]
- Hansen MJ, Schioth HB, Morris MJ. Feeding responses to a melanocortin agonist and antagonist in obesity induced by a palatable high-fat diet. Brain Res. 2005;1039:137–145. doi: 10.1016/j.brainres.2005.01.063. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Lenard NR, Zheng H, Berthoud HR. Chronic suppression of mu-opioid receptor signaling in the nucleus accumbens attenuates development of diet-induced obesity in rats. Int J Obes (Lond) 2010 doi: 10.1038/ijo.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMinn JE, Wilkinson CW, Havel PJ, Woods SC, Schwartz MW. Effect of intracerebroventricular alpha-MSH on food intake, adiposity, c-Fos induction, and neuropeptide expression. Am J Physiol Regul Integr Comp Physiol. 2000;279:R695–R703. doi: 10.1152/ajpregu.2000.279.2.R695. [DOI] [PubMed] [Google Scholar]
- Otake K, Nakamura Y. Possible pathways through which neurons of the shell of the nucleus accumbens influence the outflow of the core of the nucleus accumbens. Brain Dev. 2000;22 Suppl 1:S17–S26. doi: 10.1016/s0387-7604(00)00142-x. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic 'liking' for food: map based on microinjection Fos plumes. Brain Res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S. Opioid reward 'liking' and 'wanting' in the nucleus accumbens. Physiol Behav. 2008;94:675–680. doi: 10.1016/j.physbeh.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Sano H, Yokoi M. Striatal medium spiny neurons terminate in a distinct region in the lateral hypothalamic area and do not directly innervate orexin/hypocretin- or melanin-concentrating hormone-containing neurons. J Neurosci. 2007;27:6948–6955. doi: 10.1523/JNEUROSCI.0514-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW. Brain pathways controlling food intake and body weight. Exp Biol Med (Maywood) 2001;226:978–981. doi: 10.1177/153537020122601103. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. NPY mediates the feeding elicited by muscimol injections into the nucleus accumbens shell. Neuroreport. 2004;15:2673–2676. doi: 10.1097/00001756-200412030-00024. [DOI] [PubMed] [Google Scholar]
- Thiele TE, van Dijk G, Yagaloff KA, Fisher SL, Schwartz M, Burn P, Seeley RJ. Central infusion of melanocortin agonist MTII in rats: assessment of c- Fos expression and taste aversion. Am J Physiol. 1998;274:R248–R254. doi: 10.1152/ajpregu.1998.274.1.R248. [DOI] [PubMed] [Google Scholar]
- Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res. 1998;797:73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23:2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will MJ, Vanderheyden WM, Kelley AE. Striatal opioid peptide gene expression differentially tracks short-term satiety but does not vary with negative energy balance in a manner opposite to hypothalamic NPY. Am J Physiol Regul Integr Comp Physiol. 2007;292:R217–R226. doi: 10.1152/ajpregu.00852.2005. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Lee BS, Fields HL. Nucleus accumbens opioids regulate flavor-based preferences in food consumption. Neuroscience. 2006;143:309–317. doi: 10.1016/j.neuroscience.2006.06.067. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Lee BS, Taha SA, Fields HL. Nucleus accumbens opioid signaling conditions short-term flavor preferences. Neuroscience. 2007;146:19–30. doi: 10.1016/j.neuroscience.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther. 1998;285:908–914. [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR. Peptides that Regulate Food Intake: Appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1436–R1444. doi: 10.1152/ajpregu.00781.2002. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud H-R. Orexin-signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007 doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]