Abstract
Long term function of human lung allografts is hindered by development of chronic rejection manifested as Bronchiolitis Obliterans Syndrome (BOS). We have previously identified the development of antibodies (Abs) following lung transplantation to K-α1-tubulin (KAT), an epithelial surface gap junction cytoskeletal protein, in patients who develop BOS. However, the biochemical and molecular basis of the interactions and signaling cascades mediated by KAT Abs are yet to be defined. In this report, we investigated the biophysical basis of the epithelial cell membrane surface interaction between KAT and its specific Abs. Towards this, we analyzed the role of the lipid raft domains in the membrane interactions which lead to cell signaling and ultimately increased growth factor expression. Normal human bronchial epithelial (NHBE) cells, upon specific ligation with Abs to KAT obtained either from the serum of BOS(+) patients or monoclonal KAT Abs, resulted in upregulation of growth factors VEGF, PDGF, and bFGF( 6.4 ± 1.1, 3.2 ± 0.9, and 3.4 ± 1.1 fold increase, respectively) all of which are important in the pathogenesis of BOS. To define the role for lipid raft in augmenting surface interactions, we analyzed the changes in the growth factor expression pattern upon depletion and enrichment with lipid raft following the ligation of the epithelial cell membranes with Abs specific for KAT. NHBE cells cultured in the presence of β-methyl cyclodextran (βMCD) had significantly reduced growth factor expression (1.3 ± 0.3,vs βMCD untreated being 6.4 ± 1.1 fold increase) upon stimulation with KAT Abs. Depletion of cholesterol on NHBE cells upon treatment with βMCD also resulted in decreased partitioning of caveolin in the membrane fraction indicating a decrease in raft-domains. In conclusion, our results demonstrate an important role for lipid raft-mediated ligation of Abs to KAT on the epithelial cell membrane, which results in the upregulation of growth factor cascades involved in the pathogenesis of BOS following human lung transplantation.
Keywords: Lipid rafts, K-α-tubulin, Chronic rejection, Lung Transplantation
1. Introduction
Lung transplantation is considered the definitive treatment for patients with end-stage lung diseases [1]. The long term survival of the transplanted lung allograft is limited by chronic rejection clinically diagnosed as Bronchiolitis Obliterans Syndrome (BOS). Chronic rejection is a fibroproliferative process that involves inflammation and fibrosis of the lamina propria and lumen resulting in progressive decline in pulmonary function and eventual allograft failure. The incidence of BOS is approximately 50% within 3 years of transplantation [2]. Furthermore, the median survival after the diagnosis of BOS is only 3 years. Current immunosuppressive regimens can only slow the progression of BOS and can't reverse the pathology [3].
Although the pathogenesis of BOS is not yet fully defined, several risk factors have been associated with the development of chronic rejection, including recurrent/refractory episodes of acute rejection, cytomegalo virus (CMV) and other respiratory viral infections, human leukocyte antigen (HLA) mismatches, primary graft dysfunction etc. In addition, several non-specific risk factors such as donor and recipient age, graft ischemic time, and bacterial/fungal/non-CMV viral infection have also been associated with decreased long term survival of the graft [4]. The development of Abs to donor HLA (Abs) has also been linked to lymphocytic bronchiolitis underscoring a potential role of humoral immunity in the development of chronic rejection [5]. Recent studies from our laboratory and others have also proposed a role for auto-Abs to self-antigens Collagen V (ColV) and K-α1 tubulin (KAT) in the pathogenesis of BOS following lung transplantation [6, 7] suggesting a cross-talk mechanism between alloimmune and autoimmune responses in the immunopathogenesis of BOS [8].
Studies have shown that the airway epithelial cells (AECs) are the main target for the immunologic insult during the pathogenesis of allograft rejection [9]. Activation of epithelial cells by Abs to HLA can result in the production of growth factors including epidermal growth factor (EGF), basic fibroblast growth factor (bFGF) and endothelin (ET)-1 [10, 11]. Exposure to these growth factors can lead to the activation and proliferation of fibroblasts and smooth muscle cells. More significantly, in vivo studies have revealed a temporal relationship between elevated levels of growth factors and significant fibroblast migration and proliferation within the small airways [12]. Although, previous studies from our laboratory and others have implicated that the development of Abs to donor HLA predisposes patients to the development of chronic rejection, there are many incidences of lung transplant recipients with BOS where Abs to mismatched donor HLA can't be readily demonstrated, thus suggesting a role for Abs to non-HLA antigens in the pathogenesis of BOS [13, 14].
We have recently demonstrated that Abs to KAT expressed on the epithelial cell surface play an important role in the pathogenesis of human lung transplant recipients diagnosed with BOS [7]. However, the molecular basis of the interaction of Abs to KAT and AECs and the mechanisms by which KAT Abs mediate fibroproliferation remain ill defined. In this report, we demonstrate that lipid rafts present on the surface of the epithelial cells are the critical first step leading to enhanced growth factor cascade, which is essential in the pathogenesis of BOS.
2. Materials and Methods
2.1 Cell cultures
Normal human bronchial epithelial (NHBE) cells were obtained from the American Type Culture Collection (CRL-2503, ATCC, Manassas, VA) and cultured in small airway cell basal medium (CC-3119, Lonza, USA) supplemented by SAGM™ provided by the company (CC-4124, Lonza, USA). Cell lines were frozen at 70°C until use. Upon thawing, cells were maintained in sterile 5% CO2 incubator in the growth media at 37°C.
2.2 Detection of KAT Abs by ELISA
The patients sera were tested for the development of Abs to KAT by enzyme linked immunosorbent assay (ELISA) developed in our laboratory. Recombinant human KAT (NM_006082) was purified from Ecoli expression vector stock. E.coli was cultured overnight at 37 °C with kanamycin and IPG (each at concentration of 1ng/mL). The bacteria were centrifuged, lysed and protein purified on Ni-NTA column following manufacturer's instructions (PrepEase kits, Affymetrix/USB Corporation, OH). To perform ELISA, 96-well plates (Nunc, NY) were coated with 1 ug/mL purified KAT in phosphate-buffer solution (PBS) and incubated overnight at 4°C. The antigen coated wells were blocked with 1% bovine serum albumin for 2 hours. Sera were tested at dilutions of 1:500 for presence of Abs against KAT. Commercially available anti-KAT Abs ((Santacruz Biotechnology, CA) were used as positive controls. For detection of specific binding, anti-human IgG, IgM bound to horseradish peroxidase (Jackson ImmunoResearch Laboratory, PA) was utilized and developed with tetramethylbenzidine substrate (Millipore, CA). Immunoabsorbance was detected at 460nm and concentration of Abs was calculated based on a standard curve using the binding of known concentration of commercial anti-KAT Abs. The titers of Abs to self-antigens was determined using values obtained from calculating two standard deviations from the mean concentration of KAT Abs in healthy control subjects.
2.3 Modulation of cholesterol level on epithelial cells
The NHBE cells were enriched with or depleted of cholesterol by incubating them with methyl-β-cyclodextrin (βMCD) saturated with cholesterol or with βMCD alone (non-complexed with cholesterol) respectively[15]. Briefly, the cholesterol stock solution in chloroform:methanol (1:1, vol/vol) was added to a glass tube and the solvent was evaporated. Then, 5 mM βMCD solution in RPMI medium without serum was added to the dried cholesterol. The tube was vortexed, sonicated, and incubated overnight in a shaking bath at 37°C. βMCD was saturated with cholesterol at a βMCD/cholesterol molar ratio of 8:1. In preparation for an experiment, cells were washed three times with serum-free RPMI medium. Cells were then incubated with cholesterol-saturated βMCD solution or with βMCD solution containing no cholesterol, or with a mixture of these for 120 min. During the incubation, cells were maintained in a humidified CO2 incubator at 37°C. After exposure to βMCD, cells were washed three times with serum-free media and incubated in serum-free media for at least 24 h, providing the time window for the electrophysiological recordings. To attain the intermediate cellular levels of cholesterol, cells were exposed to various mixtures of 5 mM MβCD saturated with cholesterol and 5 mM MβCD. MβCD and cholesterol were purchased from Sigma Chemical (St. Louis, MO).
2.4 Growth factor assay
Expression profiles of intracellular signal genes in the isolated NHBE were analyzed using the FAm-labeled RT-PCR primers for VEGF, PDGF, bFGF (Applied Biosystems, Foster City, CA) as per the manufacturer's recommendation. Briefly, total RNA was extracted from 106 cells using TRIzol reagent (Sigma-Aldrich). The RNA was reverse-transcribed and Real-time PCR was performed in a final reaction volume of 20 μL using iCycler 480 Probes Master (Roche Diagnostics). Each sample was analyzed in triplicate. Cycling conditions consisted of an initial denaturation of 95°C for 15 min, followed by 40 cycles of 95°C for 30s, followed by 61°C for 1 min.
2.5 Western blot
Protein level of caveolin and α-tubulin were analyzed using the western blot. The retrieved cells from the matrigels on day 30 were lysed using 4% SDS cell lysis buffer supplemented with protease inhibitor cocktail and EDTA. The lysates were boiled for 20 minutes in sample buffer (200 mmol/L Tris (pH6.8), 20% glycerol, 2% SDS, 0.1% bromophenol blue, and 10% β-mercaptoethanol and centrifuged for 30 minutes and run on 4-12% gradient Bis-Tris denaturing gel (Nupage, Invitrogen). The gel was transferred onto nitrocellulose membrane, and blocked overnight with 5% non-fat milk in PBS-T (0.1% Tween 20). Thereafter, the membrane was incubated for 1 hour at room temperature with the appropriate Abs. After labeling with HRP-labeled secondary Ab (1:10,000 dilution, R&D systems), the membrane was developed using the chemiluminescence kit (Millipore) and analyzed on using Bio-Rad Universal Hood II (Hercules, CA). Morphometric analysis was done using the software provided by the company.
2.6 Statistical Analysis
Data are expressed as mean ± SE. Statistical differences between means were analyzed using a paired or unpaired Student's t test. A value of P less than 0.05 was considered significant. All data analysis was obtained using Origin 6 software (Origin Labs, Northampton, MA).
3. Results
3.1 Upregulaion of growth factor production (VEGF, PDGF, and bFGF) in NHBE cells by ligation of KAT with serum from BOS+ lung transplant recipient
To determine the effect of KAT Abs on the AECs we incubated NHBE cells with serum from patients who developed BOS following lung transplantation. Sera from normal subjects were used as negative controls, while commercial Abs to KAT were used as positive controls. The cell cultures were incubated with the appropriate sera or pure Abs for 72 hrs. The cells were collected and mRNA was analyzed for the levels of growth factors, VEGF, PDGF and bFGF. Results demonstrate that incubation of NHBE cells with serum (at 1:250 dilution) from BOS(+) KAT(+) patient cohort caused a 6.4 ± 1.1 fold increase in the VEGF level (Figure 1A). While, incubation with BOS(+) KAT(-) sera at an identical dilution resulted only in 1.1 ± 0.3 fold increase in VEGF, which is similar to the levels noted with normal human sera. Incubation of NHBE cells with BOS(+) KAT(+) serum (at 1:250 dilution) or with a monocloncal Ab to KAT also caused a 6.7 ± 1.2 fold and 6.6 ± 1.3 fold increase of VEGF mRNA expression respectively. A similar pattern of increased expression has been observed for the other growth factors PDGF and bFGF (Figure 1A). Furthermore, kinetic analysis by serial dilutions demonstrated that there was an increased growth factor expression with increasing concentration of serum (Figure 1B). These results demonstrate that ligation of KAT on the epithelial cells by its specific Abs induces enhanced growth factor expression.
Figure 1.
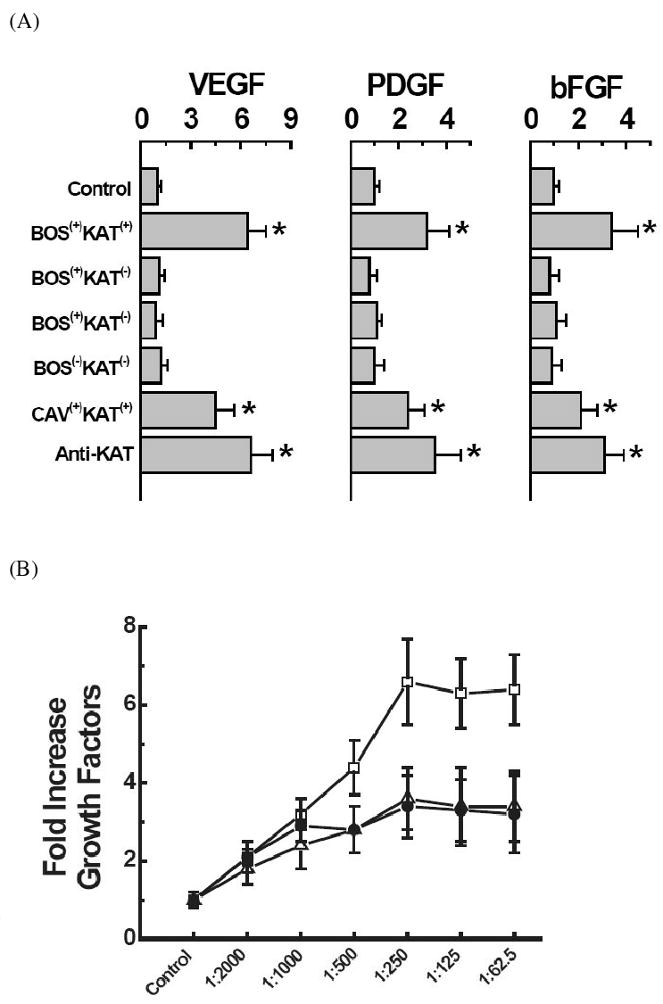
Effect of KAT Abs on the upregulation of growth factor expression. (A) mRNA expression profile of VEGF, PDGF, bFGF; (*) p-value < 0.05. (B) Dose dependent changes in the growth factor expression; (□) VEGF, (Δ) PDGF, (●) bFGF.
3.2 Depletion in the cellular cholesterol level inhibits KAT Abs mediated upregulation of growth factor expression
To determine the effect of cholesterol on growth factor upregulation by KAT Abs on epithelial cells, the cholesterol levels in NHBE cell cultures were modulated by incubation with βMCD or βMCD saturated with cholesterol at 1:8 (βMCD-cholesterol) at its saturation limit [16]. βMCD is a known chelator of cholesterol from the cell membrane surface [17]. Conversely, βMCD-cholesterol enhances the cholesterol content on the cell membrane. Results presented in Figure 2A demonstrate that incubation of cell cultures with BOS(+) KAT(+) serum at 1:250 dilution under cholesterol depleted condition caused no increase in the VEGF expression (1.3 ± 0.3, control being 6.4 ± 1.1).. However, there is an increase in VEGF expression (8.6 ± 1.4, p-value <0.001 with respect to depleted; and >0.5 with respect to non-treated) upon enrichment of the membrane with cholesterol by addition of βMCD-cholesterol. Similar findings were noted with the expression pattern of PDGF and bFGF (Figure 2A).
Figure 2.
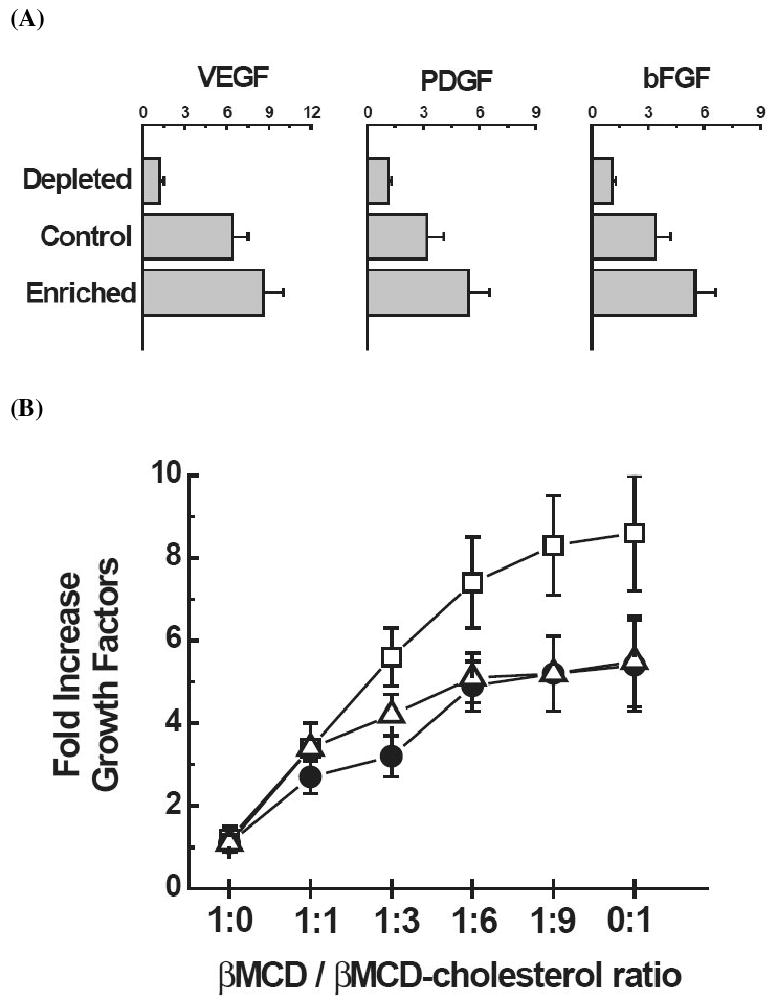
Effect of membrane cholesterol on the KAT Abs induced growth factor expression with and without treatment of βMCD. (A) mRNA expression profile of VEGF, PDGF, bFGF. (B) Dose dependent change of cholesterol saturation on the growth factor expression; (□) VEGF, (Δ) PDGF, (●) bFGF.
To determine the dose dependent effect of growth factor expression as a function of changing cholesterol concentration, the ratio of βMCD and βMCD-cholesterol is sequentially changed in BOS(+) KAT(+) sera treated individual cell culture (i.e., 1:0, 1:1, 1:3, 1:6, 1:9 and 0:1). As shown in Figure 2B, enriching the membrane with cholesterol, increased the expression of all 3 growth factors, VEGF, PDGF, and bFGF, following a dose-dependent pattern. Similar pattern of results were noticed with changing the sera from various patient cohorts (shown in figure 1). These results demonstrate that the cholesterol present on the membrane influences the efficient surface ligation of KAT Abs.
3.3 Enhanced lipid raft formation with increases in membrane cholesterol concentration
To investigate further the potential increase of lipid rafts on cell membranes with an increase in the surface cholesterol concentration, we quantified the membrane caveolin level, a marker for raft formation [18]. The cells cultured in the presence of BOS(+) KAT(+) sera with changing cholesterol levels as described above were collected and total membrane fraction was analyzed by western blot analysis. As shown in Figure 3, caveolin levels in the membrane fraction of cells depleted with cholesterol were significantly (3 fold) decreased. However, there was no statistically significant increase (p-value > 0.7) in the membrane localization of caveolin among the untreated and enriched cell membrane components. It is of interest that there is no change of the KAT concentration in the membrane fraction of cholesterol depleted cells. Therefore, results presented in figures 2 and 3 strongly suggest that increased lipid raft formation on the membrane surface results in improved ligation of the KAT Abs leading to upregulation of growth factor expression.
Figure 3.
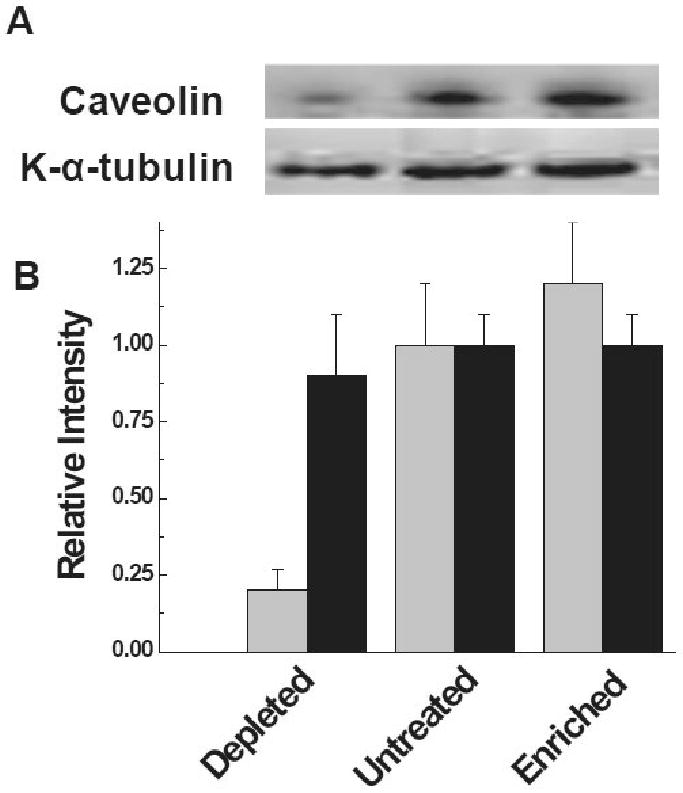
Membrane localization of K-α-tubulin under changing membrane lipid raft domains. (A) Localization of K-α-tubulin and caveolin in the membrane fraction with and without treatment of βMCD. (B) Semi-quantitative morphometric analysis of the K-α-tubulin and caveolin; (light filled), represents caveolin and (dark filled), represents K-α-tubulin.
4. Discussion
Growing evidence suggests that membrane proteins are not uniformly distributed over the cell membrane, but rather are often localized in certain cholesterol-enriched domains of the membrane called lipid rafts [19]. Even though the exact nature of these membrane-domains such as composition, solubility, and stability is still not clear, lipid rafts are widely acknowledged to serve as scaffolding platforms for the association of signaling molecules and compartmentalization of cellular processes. It is well recognized that cholesterol-rich membrane domains play an important role in the regulation of membrane-cytoskeleton-dependent processes controlling the cellular biomechanics [20]. PIP2, a lipid component involved in actin-membrane linkage, partitions into the cholesterol-caveolin rich (lipid raft) fraction of the plasma membrane [21]. It has also been shown that partitioning of Rac1 to lipid rafts is essential for the Rho family GTPases and integrins functioning towards Rho-induced changes in the organization of the cytoskeleton regulated cell polarization, trafficking and proliferation [22]. Furthermore, studies on T-cell activation point out the importance of the raft-cytoskeleton mediated signaling for T cell antigen receptor and raft mediated cytokine signaling cascades [23, 24]. Put together, all these studies strongly favor the importance of raft-domains in several physiological processes.
Previous studies from our lab have demonstrated that the development of Abs to self antigen KAT, an epithelial gap junction localizing protein, correlated significantly with development of chronic rejection following human lung transplantation[7]. Using Gene array and Luminex assays we have previously demonstrated that binding of KAT Abs to AECs causes an up-regulation of PKC-driven calcium maintenance pathway that is regulated by HSPs 27 and 90 [7]. These pathways are known to induce cellular mitosis, proliferation, and growth factor production [7]. Abs against tubulin have also been reported in small-cell lung cancer [25] breast cancer [26], as well as in postcardiac transplant fatal cardiomyopathy [27], strongly suggesting that this protein is immunogenic under selected pathologic circumstances. In this report we have shown that ligation of KAT with its specific Abs on the NHBE cell lines caused an upregulation of growth factors such as VEGF, PDGF and bFGF in a dose dependent manner (Figure 1). All of these growth factors have been shown to play a crucial role in the pathogenesis of obliterative airway disease, thus suggesting that KAT Abs are most likely involved in the immunopathogenesis of chronic rejection following human lung transplantation[28].
Studies by Dudez et al. have demonstrated that activation of lipid rafts in gap junctions causes cystic fibrosis transmembrane conductance regulator (CFTR) association with c-Src and TNFR1, and forms a responsive signaling complex to mediate cytokine signaling [29]. Similarly, a role for protein kinase Cγ in regulation of gap junction activity through lipid rafts has also been proposed (29). These studies provide strong evidence for the potential role of lipid rafts in the function of gap junctions. Based on these we hypothesized that lipid rafts may regulate the ligation of KAT Abs to K-α1-tubulin, an epithelial gap junction protein, resulting in induction of signals for growth factor production which play an important role in the pathogenesis of the BOS.
To test for the role of the lipid rafts in mediating the surface ligation of the KAT Abs to the cell membrane leading to cell signals for growth factor production, we designed experiments to measure the two important markers of lipid rafts, namely, cholesterol and caveolin [30]. Results presented in Fig 2A clearly demonstrate that depleting the cholesterol content from the membrane by βMCD resulted in a decreased response from all three growth factors. This indicates an important role for lipid rafts in mediating efficient ligation of KAT Abs to membrane surface leading to signaling and growth factor up regulation. Dose dependent studies presented in Fig 2B also show decreased growth factor expression under cholesterol depleted conditions. However, no significant increase in growth factor expression was observed in untreated vs enriched conditions (Figure 2B). This suggests that under basal conditions KAT Abs binding kinetics has attained a saturation phase. Furthermore, quantitative analysis in the βMCD treated cell cultures also demonstrated significantly reduced amount caveolin levels (Figure 3) which indicate a loss of lipid rafts upon drug treatment. However, K α-tubulin levels largely remained unchanged in the membrane fraction of all three groups namely, depleted, untreated and enriched. Thus our data strongly favors the idea that the non-raft protein expression and localization is unaltered in the experimental conditions employed in our study.
In conclusion, in this report we demonstrate that the signaling events leading to growth factor upregulation by AECs upon ligation of KAT by its specific Abs is biochemically dependent on the raft-domain formation on the cell membrane. Our current findings strongly suggest that lipid raft targeting drugs may be of value in preventing and or treating patients with chronic rejection following human lung transplantation.
Research Highlights
Addition of KAT Abs (+) sera to NHBE culture causes upregulation of growth factors.
Cholesterol depletion causes down regulation of growth factor expression.
Cholesterol depletion is accompanied by loss of membrane bound caveolin.
Thus, we demonstrate lipid raft are critical for efficient ligation of the KAT Abs.
Acknowledgments
All authors have no conflict of interest to report. This work was supported by an ARRA Award HL056643 from the National Institutes of Health/National Heart Lung Blood Institute, HL66452 NIH/SAT/NHLBI, HL092514 NIH/NHLBI/NIAID, and the Barnes-Jewish Hospital Foundation (TM). NA is the recipient of JDRF 3-2009-218 Postdoctoral Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, Taylor DO, Kucheryavaya AY, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult lung and heart/lung transplantation report--2008. J Heart Lung Transplant. 2008;27:957–969. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Burton CM, Carlsen J, Mortensen J, Andersen CB, Milman N, Iversen M. Long-term survival after lung transplantation depends on development and severity of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2007;26:681–686. doi: 10.1016/j.healun.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Valentine VG, Robbins RC, Berry GJ, Patel HR, Reichenspurner H, Reitz BA, Theodore J. Actuarial survival of heart-lung and bilateral sequential lung transplant recipients with obliterative bronchiolitis. J Heart Lung Transplant. 1996;15:371–383. [PubMed] [Google Scholar]
- 4.Al-Githmi I, Batawil N, Shigemura N, Hsin M, Lee TW, He GW, Yim A. Bronchiolitis obliterans following lung transplantation. Eur J Cardiothorac Surg. 2006;30:846–851. doi: 10.1016/j.ejcts.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 5.Nath DS, Basha HI, Mohanakumar T. Antihuman leukocyte antigen antibody-induced autoimmunity: role in chronic rejection. Curr Opin Organ Transplant. 15:16–20. doi: 10.1097/MOT.0b013e3283342780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, Gopalakrishnan B, Cai J, Brand DD, Yoshida S, Cummings OW, Wilkes DS. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180:4487–4494. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiriveedhi V, Weber J, Seetharam A, Mohanakumar T. Cross-talk of alloimmune response and autoimmunity: role in pathogenesis of chronic rejection. Discov Med. 9:229–235. [PubMed] [Google Scholar]
- 9.Zissel G, Ernst M, Rabe K, Papadopoulos T, Magnussen H, Schlaak M, Muller-Quernheim J. Human alveolar epithelial cells type II are capable of regulating T-cell activity. J Investig Med. 2000;48:66–75. [PubMed] [Google Scholar]
- 10.Jaramillo A, Smith CR, Maruyama T, Zhang L, Patterson GA, Mohanakumar T. Anti-HLA class I antibody binding to airway epithelial cells induces production of fibrogenic growth factors and apoptotic cell death: a possible mechanism for bronchiolitis obliterans syndrome. Hum Immunol. 2003;64:521–529. doi: 10.1016/s0198-8859(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 11.Langenbach SY, Zheng L, McWilliams T, Levvey B, Orsida B, Bailey M, Williams TJ, Snell GI. Airway vascular changes after lung transplant: potential contribution to the pathophysiology of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2005;24:1550–1556. doi: 10.1016/j.healun.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Aris RM, Walsh S, Chalermskulrat W, Hathwar V, Neuringer IP. Growth factor upregulation during obliterative bronchiolitis in the mouse model. Am J Respir Crit Care Med. 2002;166:417–422. doi: 10.1164/rccm.2102106. [DOI] [PubMed] [Google Scholar]
- 13.Dragun D, Muller DN, Brasen JH, Fritsche L, Nieminen-Kelha M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, Mazak I, Plehm R, Schonemann C, Unger T, Budde K, Neumayer HH, Luft FC, Wallukat G. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 14.Dubel L, Farges O, Johanet C, Sebagh M, Bismuth H. High incidence of antitissue antibodies in patients experiencing chronic liver allograft rejection. Transplantation. 1998;65:1072–1075. doi: 10.1097/00007890-199804270-00011. [DOI] [PubMed] [Google Scholar]
- 15.Levitan I, Christian AE, Tulenko TN, Rothblat GH. Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells. J Gen Physiol. 2000;115:405–416. doi: 10.1085/jgp.115.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christian AE, Haynes MP, Phillips MC, Rothblat GH. Use of cyclodextrins for manipulating cellular cholesterol content. J Lipid Res. 1997;38:2264–2272. [PubMed] [Google Scholar]
- 17.Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768:1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci U S A. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 20.Levitan I, Gooch KJ. Lipid rafts in membrane-cytoskeleton interactions and control of cellular biomechanics: actions of oxLDL. Antioxid Redox Signal. 2007;9:1519–1534. doi: 10.1089/ars.2007.1686. [DOI] [PubMed] [Google Scholar]
- 21.Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–789. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- 22.del Pozo MA, Alderson NB, Kiosses WB, Chiang HH, Anderson RG, Schwartz MA. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- 23.Miceli MC, Moran M, Chung CD, Patel VP, Low T, Zinnanti W. Co-stimulation and counter-stimulation: lipid raft clustering controls TCR signaling and functional outcomes. Semin Immunol. 2001;13:115–128. doi: 10.1006/smim.2000.0303. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Mouton C, Abad JL, Mira E, Lacalle RA, Gallardo E, Jimenez-Baranda S, Illa I, Bernad A, Manes S, Martinez AC. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc Natl Acad Sci U S A. 2001;98:9642–9647. doi: 10.1073/pnas.171160298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao S, Aberg F, Nieves E, Band Horwitz S, Orr GA. Identification by mass spectrometry of a new alpha-tubulin isotype expressed in human breast and lung carcinoma cell lines. Biochemistry. 2001;40:2096–2103. doi: 10.1021/bi002323d. [DOI] [PubMed] [Google Scholar]
- 26.Dozier JH, Hiser L, Davis JA, Thomas NS, Tucci MA, Benghuzzi HA, Frankfurter A, Correia JJ, Lobert S. Beta class II tubulin predominates in normal and tumor breast tissues. Breast Cancer Res. 2003;5:R157–169. doi: 10.1186/bcr631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hein S, Scheffold T, Schaper J. Ischemia induces early changes to cytoskeletal and contractile proteins in diseased human myocardium. J Thorac Cardiovasc Surg. 1995;110:89–98. doi: 10.1016/S0022-5223(05)80013-3. [DOI] [PubMed] [Google Scholar]
- 28.Krebs R, Tikkanen JM, Nykanen AI, Wood J, Jeltsch M, Yla-Herttuala S, Koskinen PK, Lemstrom KB. Dual role of vascular endothelial growth factor in experimental obliterative bronchiolitis. Am J Respir Crit Care Med. 2005;171:1421–1429. doi: 10.1164/rccm.200408-1001OC. [DOI] [PubMed] [Google Scholar]
- 29.Dudez T, Borot F, Huang S, Kwak BR, Bacchetta M, Ollero M, Stanton BA, Chanson M. CFTR in a lipid raft-TNFR1 complex modulates gap junctional intercellular communication and IL-8 secretion. Biochim Biophys Acta. 2008;1783:779–788. doi: 10.1016/j.bbamcr.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest. 2002;110:597–603. doi: 10.1172/JCI16390. [DOI] [PMC free article] [PubMed] [Google Scholar]


