Abstract
Estradiol increases mRNA and/or protein expression of the nitric oxide synthase (NOS) isoforms in a variety of tissues including kidney. In this study we determined the relationship between cyclical variations in estradiol levels and renal function and total NO production in the virgin female rat. In addition, we used an aromatase inhibitor (Anastrozole), to inhibit synthesis of estradiol from testosterone. Estradiol levels were higher in proestrus vs. diestrus, and were markedly suppressed by 7 days treatment with aromatase inhibitor. There was no difference in total NO production (from urinary and plasma nitrate + nitrite = NOX) between proestrus and diestrus but aromatase inhibition resulted in increases in total NO production. The renal cortical NOS activity and protein abundance also increased in aromatase-inhibited female rats. There were no differences in blood pressure (BP) in any group but the renal vascular resistance (RVR) was low in proestrus, increased in diestrus and did not change further after aromatase inhibition. In summary, the cyclical changes in renal function correlate with estradiol but not NO levels. Pharmacologic castration with aromatase inhibition leads to a marked increase in total and renal NOS. This contrasts to earlier work where surgical castration causes decreased NOS.
Keywords: Anastrozole, renal vascular resistance, sodium excretion, testosterone, nitric oxide synthase
Introduction
In addition to the well recognized protective effects of estradiol and female sex on the cardiovascular system [1, 2], the kidney is also protected. Women and female rats are protected from age-dependent kidney damage vs. males [2,3] and premenopausal women and cycling rats with non diabetic chronic renal disease have a slower rate of progression compared to males [1,4,5]. Estrogen has many cardiovascular / renal actions that could contribute to protection, some of which are mediated by nitric oxide (NO). Estrogens have both acute (nongenomic) effects to stimulate NO release, as well as long term, genomic actions to increase mRNA and protein expression of NO synthase (NOS) isoforms, and to increase NOS activity in a variety of tissues [1,2]. In the kidney, female rats have higher levels of NOS protein compared to male and ovariectomized rats [6, 7].
In this study we investigated the effect of physiological (cyclic) alterations in estradiol levels on systemic and renal hemodynamics and on total NO production in the virgin female rat. As an alternative to ovariectomy we also employed the aromatase inhibitor, Anastrozole (Arimidex), to inhibit synthesis of 17B-estradiol from testosterone. Anastrozole is a potent and highly selective inhibitor of aromatase with no intrinsic hormonal activity [8]. We investigated the renal hemodynamic response to aromatase inhibition as well as the impact on total NO production and renal NOS activity/abundance.
Experimental
Studies were performed on 21 female Sprague Dawley rats (Harlan Sprague Dawley Inc., Indianapolis, IN) aged 4–5 months as approved by the West Virginia University Animal Care and Use Committee and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Rats were given a low nitrate but complete diet (AIN 76C semi purified diet, ICN Pharmaceuticals, Costa Mesa, CA) and water ad libitum and maintained in a 12h light/dark cycle. Stages of the estrous cycle were confirmed by daily vaginal smears. Urine collections (24h) were made in Nalgene metabolic cages and aliquots were frozen for later analysis.
Eleven rats were placed on low nitrate diet for 3 days prior to beginning the study and then two 24 hour urines were collected, one in proestrus and one in the diestrus stage of the estrous cycle. Next, surgery was performed to implant chronic femoral arterial and venous catheters and a urinary bladder catheter, under general anesthesia and using full sterile technique, as described previously [9]. Two days after surgery, daily vaginal smears were begun to monitor the estrous cycle, while rats were trained to accept the laboratory environment. At least 7 days after surgery and after rats had resumed a normal cycle the first of two “baseline” renal function experiments were performed on each rat; once between 8AM and 1PM on the day of proestrus (maximal estradiol levels) and once between 8AM and 1PM on day 1 of diestrus (lowest estradiol levels) [10]. After these measurements, each rat was lightly anesthetized and an osmotic mini pump (0.5µL/hr; Alzet; Palo Alto, CA) was implanted under the skin of the back. Minipumps either contained vehicle (n=6) or the aromatase inhibitor Anastrozole (n=5; Zeneca Pharmaceuticals, Macclesfield, UK). Anastrozole was suspended in 25%EtOH: 25% Polyethylene glycol: 50%, 0.9% sodium chloride at a concentration based on the rats’ weight at the time of implantation to deliver a dose of 1 mg/kg/day. This dose inhibits ovulation and lowers plasma estradiol without any pharmacological side effects [8,11].
Two additional 24 hour urine collections were performed, before each of the renal function experiments conducted in the mornings of days 3 and 7 after minipump implantation. After the last renal function experiment, rats were sacrificed, blood collected and plasma stored frozen for later measurement of estradiol and plasma NOX and the kidneys were harvested onto dry ice, separated into cortex and medulla, frozen in liquid nitrogen and stored at −80°C for later analysis.
During renal function experiments, the arterial line was connected to a pressure transducer for measurement of mean arterial pressure (BP) and occasional blood sampling. A continual iv infusion was given of [3H] Inulin (5µCi/mL; New England Nuclear) and para-aminohippuric acid (PAH; 1g %) at 5 µl/min/100g body weight; this low infusion rate allowed rats to remain euvolemic. The bladder catheter was unplugged for urine collection. The clearances of [3H] Inulin and PAH were used to measure glomerular filtration rate (GFR) and renal plasma flow (RPF), respectively, in 2 × 20 min clearance periods.
The following analyses were performed: Urine and plasma NO3 and NO2 (=NOx) was measured by the Griess reaction as described by us previously [12]. Plasma concentrations of estradiol were measured by radioimmunoassay by the method of Rozell & Keisler [13]. Plasma estradiol levels were measured in samples from 5 rats treated for 7 days with Anastrozole as well as six samples from control rats, three each in diestrus and proestrus. Samples from the renal function experiments were analyzed for urine volume, and plasma and urine concentrations of [3H]Inulin, PAH and sodium. Blood hematocrit and plasma protein concentration was also measured. These measurements allowed for calculation of GFR, RPF, filtration fraction (FF), RVR and urinary sodium excretion (UNaV). Details of these analyses and calculations are given in previous publications [9]. In 10 separate female rats plasma testosterone was measured after 7 days of anastrozole (n=6) and 7 days sham treatment (n=4), using the Count-A-Count total testosterone, Diagnostic Products kit according to the manufacturers instructions.
NOS activity was measured from the conversion of 3H-arginine to 3H-citrulline in the soluble fraction of renal cortex and medulla, after removal of the endogenous L-arginine and additional of 5 µM L-Arginine, all essential cofactors and the arginase inhibitors, 10 mM valine and 10 mM proline. For each sample, two sets of triplicates were run, one at baseline and one in the presence of the non-selective NOS inhibitors, L-NMA (10 mM) and L-NAME (20 mM). Data are expressed as pmoles of [3H]L-Citrulline converted per minute mg protein (pmol Citrulline/min/mg protein) and corrected for background and for the L-NMA and L-NAME inhibitable fraction. Details of this method have been published by us previously [3,14]. The abundance of the NOS3 and NOS1 proteins were measured on homogenates of kidney cortex by Western blot analysis. The NOS3 was detected with mouse monoclonal antibody (Transduction Laboratories, 1:250 dilution, 1 hour; secondary antibody goat, anti-mouse IgG-HRP conjugate, Transduction Labs., 1:2000 dilution, 1 hour). Separate membranes were probed for detection of NOS1 using rabbit polyclonal antibody (1:5000 dilution, one hour incubation; secondary antibody, goat, anti-rabbit IgG-HRP, Biorad; 1:3000 dilution, one hour). Equal protein-loading and transfer was confirmed by Ponceau red staining. The NOS3 and NOS1 abundance was measured by image analysis (Optimas 6.2, Bothell, WA) of the integrated optical density, normalized for total protein using ponceau red. Details of these techniques have been published by us previously [3,14].
Results are expressed as mean ± SEM. Statistical analysis was by paired and unpaired t-test and 1 way ANOVA. Values of p<0.05 are considered to be significantly different.
RESULTS
As previously reported [10], estradiol levels were higher in rats in proestrus than in diestrus and treatment with the aromatase inhibitor, Anastrozole, for 7 days inhibited plasma estradiol further (Figure 1). In separate rats, 7 days of Anastrozole markedly increased the plasma testosterone compared to sham treated female rats (2,728±396 vs. 315±75 pg/ml; p<0.001).
Figure 1.
Plasma estradiol concentration, plasma NOx (nitrite + nitrate) concentration and 24h urinary NOx excretion in proestrus (PRO), diestrus (DI) and after 7 days of aromatase inhibition. * denotes p<0.05 difference compared to proestrus, # denotes p<0.05 difference between diestrus and 7 days of aromatase inhibition.
A number of indices of activity of the NO system were measured. There was no difference in 24h UNOXV or PNOX between proestrus and diestrus stages of the estrous cycle. Seven days of aromatase inhibition resulted in significant increases in both 24h UNOXV and PNOX (Figure 1). There was no change in Hct or plasma sodium concentration during the estrous cycle or with anastrozole (Table 1), suggesting that the increased plasma PNOX was not a result of alterations in plasma volume.
Table 1.
Effect of the estrus cycle and aromatase inhibition on hematocrit (hct), plasma sodium concentration (PNa), urinary sodium excretion (UNaV) and urine flow (V) in the conscious chronically catheterized female rat.
| Hct (%) |
PNa (mEq/l) |
UNaV µeq/min) |
V µl/min) |
|
|---|---|---|---|---|
| Proestrus | 42±1 | 137±1 | 0.9±0.2 | 10±1 |
| Diestrus | 43±1 | 139±1 | 1.1±0.3 | 12±3 |
| Sham (day 7) | 41±1 | 136±2 | 2.0±0.7 | 16±2 |
| AI (day 7) | 42±1 | 136±1 | 2.5±1.1 | 21±9 |
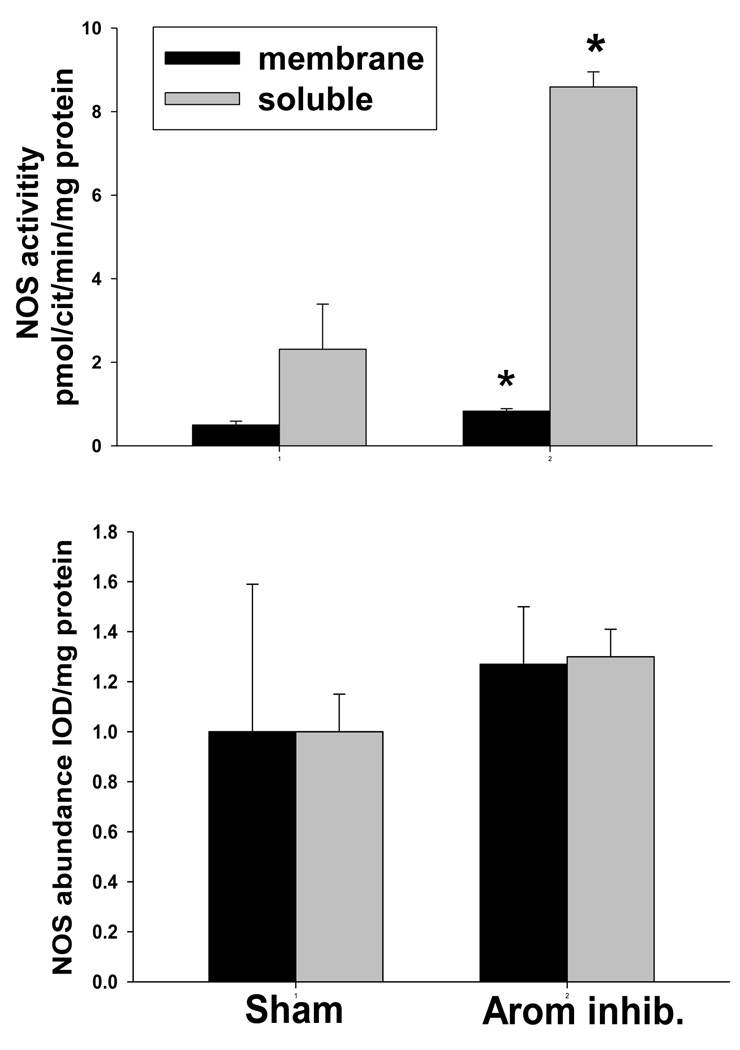
In vitro NOS activity in homogenates of renal cortex was markedly elevated in rats after 7d aromatase inhibition vs. shams in both the soluble and the membrane fractions (Figure 2). However, the protein abundance (by Western blot) in renal cortex of both NOS1 and NOS3 were similar in 7d aromatase inhibited rats and shams (Figure 2). In medulla, membrane fraction NOS activity was lower with aromatase inhibition (1.6±0.2 vs. 2.4±0.3 pmol Cit formed/min/mg protein, p<0.05) and was not different in the soluble fraction (1.2±0.1 vs. 1.3±0.3 pmol Cit formed/min/mg protein). In contrast to kidney cortex, the cerebellar NOS activity was similar in shams and after 7 days of aromatase inhibition (44±5 vs. 47±3 pmol Cit formed/min/mg protein).
Figure 2.
Upper panel: Nitric oxide synthase (NOS) activity measured from the conversion of 3H-L-arginine to 3H-L-citrulline) in membrane fraction (open column) and soluble fraction (closed column) in kidney cortex from sham treated rats and after 7 days of aromatase inhibition (Arom Inhib). Lower panel: Relative abundance of the NOS3 protein (open column) and NOS1 protein (closed column) in rat kidney cortex from sham and 7 d aromatase inhibited rats. * denotes p<0.05 between sham and 7 days of aromatase inhibition.
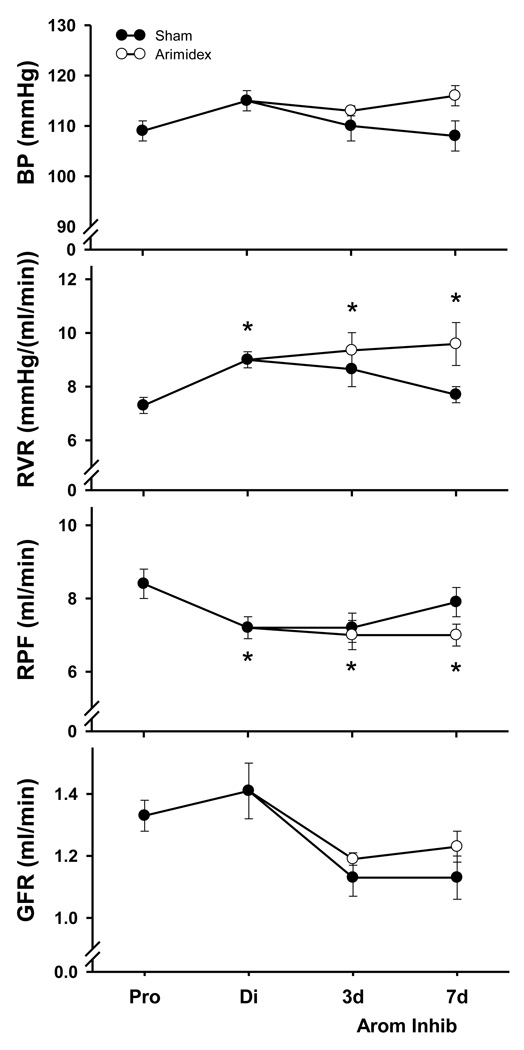
Data from the functional studies are presented in Figure 3; the data for normal proestrus and diestrus (prior to minipump implantation) are pooled for rats subsequently randomized as either shams or aromatase inhibited rats. There was no change in BP during the estrous cycle or in aromatase inhibited rats. At proestrus, RVR was low and increased by 25±8% at diestrus (p<0.01 by paired t-test). The RVR remained elevated at the diestrus value at days 3 and 7 of aromatase inhibition but showed cyclic fluctuations in the shams. There was no change in GFR during the estrous cycle, or after minipump implantation in either group. There was no difference in urine flow rate (V) or urinary excretion of sodium (UNaV) between the cycling rats and rats treated with aromatase inhibitor (Table 1).
Figure 3.
Summary of blood pressure (BP, renal vascular resistance (RVR), renal plasma flow (RPF) and glomerular filtration rate (GFR) in normal cycling rats and after 3 and 7 days of aromatase inhibition (Arom Inhib) shown by the solid circles and line. * denotes p<0.05 vs. the proestrus value.
DISCUSSION
The main, novel findings in the present study are that the renal vasculature in the cycling female rat is vasodilated at proestrus when estrogen levels are maximal but that the estrogen levels do not correlate with indices of total NO production. Also, reduction of circulating estradiol in the virgin female rat by aromatase inhibition, causes increased total production of NO and increased renal cortical NOS activity but the kidney is vasoconstricted relative to proestrus.
Virgin rats have a four to five day estrous cycle which consists of proestrus, estrous, and 2–3 days of diestrus. Peak and trough physiological estradiol levels occur in cycling rats on a standard light / dark cycle, with maximal and minimal plasma estradiol levels during proestrus and diestrus, respectively [10]. We have confirmed that circulating estradiol is >2X higher in proestrus vs diestrus. However, total systemic NO production (indicated from UNOXV and plasma NOX under conditions of controlled NOX intake) is not different, indicating that physiologic fluctuations in estradiol do not impact overall NO production. The clinical literature is variable, with reports of increased and unchanged plasma NOX, unchanged early morning UNOXV and increased, unchanged and decreased NO in expired air during the normal menstrual cycle [15 –21]. In one clinical study 24h urine NOx and cGMP (2nd messenger of NO) were unchanged throughout the normal menstrual cycle [22]. In a study in macaque monkeys an inverse correlation between plasma estradiol and plasma NOx was reported [23]. Since none of these experiments controlled for dietary NOX intake, they are difficult to evaluate [24]. While the present studies show no change in total NO production throughout the estrus cycle there is no question that estrogen stimulates NO locally in the circulation and elsewhere [2]. However, local variations in NO production cannot always be detected from plasma and urinary NOx measurements [24].
Most animal studies investigating the impact of estradiol on renal NO production have utilized comparisons between male and female, and/ or intact and ovariectomized females, with or without estradiol replacement. For example, in the kidney medulla, NOS2 and NOS3 protein levels were higher in female rats than in male or ovariectomized rats and estrogen replacement restored NOS levels after ovariectomy [6]. In the Dahl salt sensitive rat (on low salt diet) ovariectomy of 1 month duration had no impact on renal NOS but after 9 months medullary NOS2 and NOS3 was reduced vs. intact females and this was reversed by estradiol replacement. [25]. In the Sprague Dawley, although UNOXV were not different between the sexes, both NOS3 protein and mRNA levels were higher in kidney homogenates from female vs. male rats [7]. In young adult male and female Sprague Dawley rats, we reported that UNOXV, renal cortical eNOS, nNOS protein abundance and NOS activity were similar [3] although we subsequently observed that medullary NOS was higher in females (unpublished observations). Similar results have been reported in the SHR by Sullivan and colleagues with higher eNOS and nNOS seen only in the inner medulla of females [26].
In the present study we have used synthesis inhibition rather than ovariectomy to manipulate estradiol levels. Testosterone is converted to 17B-estradiol through the enzymatic action of aromatase, present predominantly in the ovary [27] and in various regions of the brain [28]. The early aromatase inhibitors exhibited other steroidal actions but Anastrozole was developed as an aromatase inhibitor that lacks any estrogenic, anti-estrogenic, androgenic, anti-androgenic, progestogenic, glucocorticoid, anti-glucocorticoid or mineralocorticoid activity [8]. Using selective aromatase inhibition to reduce estradiol production in female rats, we were surprised to find several indices of increased NOS activity.
Both 24h UNOXV and plasma NOX increased with aromatase inhibition, which clearly indicates increased total NO production since these rats were on a controlled low NOX diet. We have separate evidence from the increased kidney NOS activity that increased renal NO generation also occurs, selectively in cortex. Since the kidney contributes only a small fraction of the total NO [24], it is likely that aromatase inhibition also activates the NO system in other locations. It is interesting that we see no change in renal cortex eNOS and nNOS protein abundance but given the extensive post-translational regulation of the constitutive NOS, this is not surprising [29]. The present observations show that when estradiol levels are reduced by synthesis inhibition the impact on renal NOS is very different to that seen when estradiol is reduced by ovariectomy [6,25] and this is discussed further below.
We also investigated the renal functional effects of both the normal ovarian cycle and aromatase inhibition. The most striking difference was the lower value of RVR in proestrus compared to both diestrus and aromatase inhibition. The RPF was also higher in proestrus but GFR was not different, because of a lower filtration fraction. Our findings in the cycling rat contrast to studies in premenopausal women, where cyclic fluctuations in GFR were seen, but with maximum GFR in the mid and late luteal phase when estradiol levels were low, compared to the preovulatory phase when estradiol levels were high [22,30,31]. We do not know the mechanism of the proestrus renal vasodilation in the cycling rat but this may not be estrogen mediated since further reduction in estradiol (with aromatase inhibition) had no further effect on RVR in these females. Indeed, Chapman and colleagues suggest that the changes in renal hemodynamics in normal women during the menstrual cycle associate with changes in cAMP, not NOx or cGMP [22].We earlier reported that ovariectomy (low circulating estradiol) reduces RVR vs. the intact female value [32] whereas in the present study reduction in estradiol with aromatase inhibition was associated with increased RVR. Both interventions lower estradiol substantially, yet have opposite effects on the renal NOS activity and the RVR. There are many differences between the 2 methods of estradiol reduction: Ovariectomy removes all hormones that originate from the ovaries including the androgens and the renal vasodilator relaxin [33]. With aromatase inhibition the other ovarian hormones are intact and since androgens are no longer converted to estradiol, they increase. We observed marked increases in testosterone with anastrozole in this study as also reported by others in rats [34] and women [35].
Androgens have variable actions on NO production, with inhibition, stimulation and no effect of testosterone on NO-dependent vasodilation being reported for different parts of the circulation [36]. Perusquía and Stallone review convincing evidence that androgens elicit increases in NO release via non-genomic pathways and importantly, this effect is not abrogated by aromatase inhibition [37]. Androgens stimulate cultured endothelial cell NO production and this effect is not influenced by aromatase inhibition [38]. Of relevance to the present study, this androgen- induced increase in eNOS activity is evoked by phosphorylation of the serine 1177 site with no change in eNOS protein abundance. Thus, our finding of increased renal cortical NOS activity without change in protein abundance with aromatase inhibition may reflect the action of increased testosterone, although there is very little information on the direct actions of testosterone on renal NO production. Weiner et al report that 5 days of testosterone supplementation to intact female guinea pigs had no effect on renal NOS activity [37]. In contrast, Perez-Torres and colleagues reported that 4 weeks of testosterone supplementation in castrated male rats, led to a reduction in renal eNOS [38]. Whether testosterone plays a role in increased renal and/or systemic NOS activity seen with aromatase inhibition in the female rat remains to be determined. It is evident, however, that there are complex interactions between the sex steroids, their receptors as well as heterogeneity of action throughout the body [1]. This study clearly illustrates that we cannot look at estrogen levels in isolation when we consider the likely impact on NO production.
ACKNOWLEDGEMENTS
These studies were supported by NIH grant #R01 DK60421. We gratefully acknowledge the gifts of Anastrozole from Zeneca Pharmaceuticals, Macclesfield, UK, and the NOS1 antibody from Dr Kim Lau, UT Dallas. We thank Kevin Engels and Lennie Samsell for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dubey RK, Jackson EK. Protective effects of estrogen on the cardiovascular and renal systems: Potential cellular, biochemical and molecular mechanisms. Am J Physiol Renal Physiol. 2001;280:F365–F388. doi: 10.1152/ajprenal.2001.280.3.F365. [DOI] [PubMed] [Google Scholar]
- 2.Baylis C. Sexual dimorphism in the aging kidney: differences in the nitric oxide system. Nature Clinical Practice Nephrology. 2009;5:384–396. doi: 10.1038/nrneph.2009.90. [DOI] [PubMed] [Google Scholar]
- 3.Erdely A, Greenfeld Z, Wagner L, Baylis C. Sexual dimorphism in the aging kidney; inverse relationship between injury and nitric oxide system. Kidney Intl. 2003;63:1021–1026. doi: 10.1046/j.1523-1755.2003.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J Am Soc Nephrol. 2000;11:319–329. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 5.Mulroney SE, Woda C, Johnson M, Pesce C. Gender differences in renal growth and function after uninephrectomy in adult rats. Kidney Intl. 1999;56:944–953. doi: 10.1046/j.1523-1755.1999.00647.x. [DOI] [PubMed] [Google Scholar]
- 6.Neugarten J, Ding Q, Friedman A, Lei J, Silbiger S. Sex hormones and renal nitric oxide synthases. J Am Soc Nephrol. 1997;8:1240–1246. doi: 10.1681/ASN.V881240. [DOI] [PubMed] [Google Scholar]
- 7.Reckelhoff JF, Hennington BS, Moore AG, Blanchard EJ, Cameron J. Gender differences in the renal nitric oxide (NO) system: dissociation between expression of endothelial NO synthase and renal hemodynamic response to NO synthase inhibition. Am J Hypertens. 1998;11:97–104. doi: 10.1016/s0895-7061(97)00360-9. [DOI] [PubMed] [Google Scholar]
- 8.Dukes M, Edwards PN, Large M, Smith IK, Boyle T. The preclinical pharmacology of “Arimidex” (Anastrozole; ZD1033) – A potent, selective aromatase inhibitor. J Steroid Biochem Molec Biol. 1996;58:439–445. doi: 10.1016/0960-0760(96)00064-7. [DOI] [PubMed] [Google Scholar]
- 9.Baylis C, Collins RC. Angiotensin II inhibition on blood pressure and renal hemodynamics in pregnant rats. Am J Physiol Renal. 1986;250:F308–F314. doi: 10.1152/ajprenal.1986.250.2.F308. [DOI] [PubMed] [Google Scholar]
- 10.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with the rescue of the corpus luteum of pseudo pregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 11.Plourde PV, Dyroff M, Dukes M. Arimidex: a potent and selective fourth-generation aromatase inhibitor. Breast Cancer Res Treat. 1994;30:103–111. doi: 10.1007/BF00682745. [DOI] [PubMed] [Google Scholar]
- 12.Suto T, Losonczy G, Qiu C, Hill C, Samsell L, Ruby J, Charon N, Venuto R, Baylis C. Acute changes in urinary excretion of nitrite + nitrate (UNOXV) do not predict renal vascular NO production. Kidney Int. 1995;48:1272–1277. doi: 10.1038/ki.1995.411. [DOI] [PubMed] [Google Scholar]
- 13.Rozell TG, Keisler DH. Effects of estradiol on LH, FSH and prolactin in ovariectomized ewes. J Reprod Fert. 1990;88:645–653. doi: 10.1530/jrf.0.0880645. [DOI] [PubMed] [Google Scholar]
- 14.Xiao S, Erdely A, Wagner L, Baylis C. Uremic levels of BUN do not cause nitric oxide deficiency in rats with normal renal function. Am J Physiol Renal. 2001;49:F996–F1000. doi: 10.1152/ajprenal.2001.280.6.F996. [DOI] [PubMed] [Google Scholar]
- 15.Jilma B, Kastner J, Mensik C, Vondrovec B, Hildebrandt J, Krejcy K, Wagner OF, Eichler HG. Sex differences in concentrations of exhaled nitric oxide and plasma nitrate. Life Sci. 1996;58:469–476. doi: 10.1016/0024-3205(95)02311-9. [DOI] [PubMed] [Google Scholar]
- 16.Rosselli M, Imthurm B, Macas E, Keller PJ, Dubey RK. Circulating nitrite/nitrate levels increase with follicular development: indirect evidence for estradiol mediated NO release. Biochem Bio Phys Res Commun. 1994;202:1543–1552. doi: 10.1006/bbrc.1994.2107. [DOI] [PubMed] [Google Scholar]
- 17.Kharitonov SA, Logan-Sinclair RB, Busset CM, Shinebourne EA. Peak expiratory nitric oxide differences in men and women: relation to the menstrual cycle. Br Heart J. 1994;72:243–245. doi: 10.1136/hrt.72.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cicinelli E, Ignarro LJ, Lograno M, Galantino P, Balzano G, Schonauer LM. Circulating levels of nitric oxide in fertile women in relation to the menstrual cycle. Fertil Steril. 1996;66:1036–1038. doi: 10.1016/s0015-0282(16)58706-8. [DOI] [PubMed] [Google Scholar]
- 19.Merki-Feld GS, Imthurn B, Keller PJ. The effect of the menstrual cycle and of ethinylestradiol on nitric oxide, endothelin-1 and homocysteine plasma levels. Horm Metab Res. 2000;32:288–293. doi: 10.1055/s-2007-978638. [DOI] [PubMed] [Google Scholar]
- 20.Ekerhovd E, Enskog A, Caidahl K, Klintland N, Nilsson L, Brännström M, Norström A. Plasma concentrations of nitrate during the menstrual cycle, ovarian stimulation and ovarian hyperstimulation syndrome. Hum Reprod. 2001;16:1334–1339. doi: 10.1093/humrep/16.7.1334. [DOI] [PubMed] [Google Scholar]
- 21.Mandhane PJ, Hanna SE, Inman MD, Duncan JM, Greene JM, Wang HY, Sears MR. Changes in exhaled nitric oxide related to estrogen and progesterone during the menstrual cycle. Chest. 2009;136:1301–1307. doi: 10.1378/chest.09-0604. [DOI] [PubMed] [Google Scholar]
- 22.Chapman AB, Zamudio S, Woodmansee W, Merouani A, Osorio F, Johnson A, Moore LG, Dahms T, Coffin C, Abraham WT, Schrier RW. Systemic and renal hemodynamic changes in the luteal phase of the menstrual cycle mimic early pregnancy. Am J Physiol. 1997;273:F777–F782. doi: 10.1152/ajprenal.1997.273.5.F777. [DOI] [PubMed] [Google Scholar]
- 23.Khorram O, Colman RJ, Kemnitz JW, Magness RR, Zhang J, Yao Z, Keller ET. The influence of sex hormones on circulating nitric oxide (NOx) levels in rhesus monkeys (Macaca Mulatta) Med Sci Monit. 2002;8:BR489–BR495. [PubMed] [Google Scholar]
- 24.Baylis C, Vallance P. Editorial Review: Measurement of nitrite and nitrate (NOx) levels in plasma and urine; what does this measure tell us about the activity of the endogenous nitric oxide. (Ed.) Brenner BM. Current Opinion. Nephrol Hypert. 1998;7:1–4. doi: 10.1097/00041552-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Maric C, Xu Q, Sandberg K, Hinojosa-Laborde C. Age-related renal disease in female Dahl salt-sensitive rats is attenuated with 17 beta-estradiol supplementation by modulating nitric oxide synthase expression. Gend Med. 2008;5:147–159. doi: 10.1016/j.genm.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan JC, Pardieck JL, Hyndman KA, Pollock JS. Renal NOS activity, expression, and localization in male and female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R61–R69. doi: 10.1152/ajpregu.00526.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hickey GJ, Chen SA, Besman MJ, Shively JE, Hall PF, Gaddy-Kurten D, Richards JS. Hormonal regulation, tissue distribution, and content of aromatase cytochrome P450 messenger ribonucleic acid and enzyme in rat ovarian follicles and corpora lutea: relationship to estradiol biosynthesis. Endocrinology. 1998;122:1426–1436. doi: 10.1210/endo-122-4-1426. [DOI] [PubMed] [Google Scholar]
- 28.Shinoda K, Mori S, Ohtsuki T, Osawa Y. An aromatase-associated cytoplasmic inclusion, the “stigmoid body,” in the rat brain: I. Distribution in the forebrain. J Comp Neurol. 1992;322:360–376. doi: 10.1002/cne.903220306. [DOI] [PubMed] [Google Scholar]
- 29.Musicki B, Ross AE, Champion HC, Burnett AL, Bivalacqua TJ. Posttranslational modification of constitutive nitric oxide synthase in the penis. J Androl. 2009;30:352–362. doi: 10.2164/jandrol.108.006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davison JM, Noble MCB. Serial changes in 24 hour creatinine clearance during normal menstrual cycles and the first trimester of pregnancy. Br J Obstet Gynecol. 1981;88:10–17. doi: 10.1111/j.1471-0528.1981.tb00930.x. [DOI] [PubMed] [Google Scholar]
- 31.Phipps WR, Duncan AM, Merz BE, Kurzer MS. Effect of menstrual cycle of creatinine clearance in normally cycling women. Obstet Gynecol. 1998;92:585–588. doi: 10.1016/s0029-7844(98)00241-5. [DOI] [PubMed] [Google Scholar]
- 32.Munger K, Baylis C. Sex differences in renal hemodynamics in rats. Am J Physiol. 1988;254:F223–F231. doi: 10.1152/ajprenal.1988.254.2.F223. [DOI] [PubMed] [Google Scholar]
- 33.Jeyabalan A, Shroff SG, Novak J, Conrad KP. The vascular actions of relaxin. Adv Med Biol. 2007;612:65–87. doi: 10.1007/978-0-387-74672-2_6. [DOI] [PubMed] [Google Scholar]
- 34.Iino Y, Sugamata N, Owada S, Tago T, Sato H, Yokoe T, Maemura M, Morishita Y, Horiuchi R. Antitumor effects of a nonsteroidal aromatase inhibitor (CGS 16949A) on 7, 12 dimethylbenz[alpha]anthracene-induced mammary tumors in rats. Jpn J Clin Oncol. 1991;21:153–159. [PubMed] [Google Scholar]
- 35.Dunaif A, Longcope C, Canick J, Badger T, Crowley WF., Jr The effects of the aromatase inhibitor delta 1-testolactone on gonadotropin release and steroid metabolism in polycystic ovarian disease. J Clin Endocrinol Metab. 1985;60:773–780. doi: 10.1210/jcem-60-4-773. [DOI] [PubMed] [Google Scholar]
- 36.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–R249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 37.Perusquía M, Stallone JN. Do androgens play a beneficial role in the regulation of vascular tone? Nongenomic vascular effects of testosterone metabolites. Am J Physiol Heart Circ Physiol. 2010;298:H1301–H1307. doi: 10.1152/ajpheart.00753.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu J, Akishita M, Eto M, Ogawa S, Son BK, Kato S, Ouchi Y, Okabe T. Androgen receptor-dependent activation of endothelial nitric oxide synthase in vascular endothelial cells: role of phosphatidylinositol 3-kinase/akt pathway. Endocrinology. 2010;151:1822–1828. doi: 10.1210/en.2009-1048. [DOI] [PubMed] [Google Scholar]
- 39.Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of calcium-dependent nitric oxide synthases by sex hormones. PNAS. 1994;91:5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pérez-Torres I, Roque P, El Hafidi M, Diaz-Diaz E, Baños G. Association of renal damage and oxidative stress in a rat model of metabolic syndrome. Influence of gender. Free Radic Res. 2009;43:761–771. doi: 10.1080/10715760903045296. [DOI] [PubMed] [Google Scholar]