Abstract
The mesocorticolimbic dopamine system, which governs components of reward and goal-directed behaviors, undergoes final maturation during adolescence. Adolescent social stress contributes to adult behavioral dysfunction, and is linked to adult psychiatric and addiction disorders. Here, behavioral, corticosterone, and limbic dopamine responses to amphetamine were examined in adult male rats previously exposed to repeated social defeat stress during mid-adolescence. Amphetamine (2.5 mg/kg, ip) was administered after a novel environment test, with behavior observed in the same context for 90 min thereafter. Adult rats that had been defeated in adolescence showed increased locomotion in the novel environment, but reduced amphetamine-induced locomotion relative to non-defeated age matched controls. Monoamine and corticosterone responses to amphetamine were examined following a second amphetamine injection 3 days later. In previously defeated rats, corticosterone and medial prefrontal cortex dopamine responses to amphetamine were blunted while dopamine responses in the nucleus accumbens core were elevated. Our results suggest that experience of social defeat stress during adolescent development can contribute to altered behavioral and endocrine responses to amphetamine in adulthood. Furthermore, these effects are paralleled by changes in amphetamine-induced dopamine responses in corticolimbic systems implicated in addiction disorders.
Keywords: social stress, psychostimulant, adolescence, dopamine, corticosterone
1. Introduction
Bullying is a severe social stressor experienced by many teenagers that can result in both immediate and long-term negative psychological consequences (Grossman et al., 2003; Kaltiala-Heino et al., 2000; Nansel et al., 2001; Rigby and Slee, 1993). The vulnerability of adolescents to the detrimental effects of such stressful events is thought to result from disruption of the extensive re-organization of limbic monoamine systems occurring at this time (reviewed by Andersen, 2003; Andersen and Teicher, 2009; Spear, 2000). Based on the role of prior stress exposure in potentiating acquisition and maintenance of drug taking (Covington and Miczek, 2001,Covington and Miczek, 2005; Gordon, 2002; Koob and Le Moal, 2008; Kreek and Koob, 1998; Piazza and Le Moal, 1998), stress-induced disruptions of mesocorticoaccumbal dopamine (DA) system development and maturation during this critical phase are likely to result in alterations in behavioral responses to drugs of abuse later in life (Wise, 1996). With social activity at its peak during adolescence (Douglas et al., 2003; Primus and Kellogg, 1989; Varlinskaya and Spear, 2008), there is a high probability that negative social experiences will have similar long-lasting detrimental effects that persist into adulthood. Indeed, numerous clinical studies have shown that repeated adolescent experience of social stress, including bullying, is correlated with greater incidence of stress-related psychiatric and addictive disorders in adulthood (Gladstone et al., 2006; Hoffmann et al., 2000; Newman et al., 2005; Rossow and Lauritzen, 2001; Tharp-Taylor et al., 2009; Wals and Verhulst, 2005).
Bullying is characterized by an imbalance of power in which the victim is less powerful than the aggressor and is unable to defend themselves adequately. This power imbalance becomes even more noticeable over time, with the bullying increasing in severity (Björkqvist, 2001). The closest parallels of such hierarchical relationships in animal models arise from social defeat paradigms utilizing a resident-intruder paradigm (Björkqvist, 2001). In adult rats, repeated social defeat has been shown to cause increased emotive responses and drug-seeking behavior (Covington and Miczek, 2005; Miczek et al., 2004; Rygula et al., 2005) comparable to that reported for adult recipients of human bullying (Björkqvist, 2001). In particular, rats exposed to repeated defeat in adulthood exhibit increased psychostimulant-induced locomotion and more rapid acquisition of psychostimulant self-administration and greater overall intake (Covington and Miczek, 2001,Covington and Miczek, 2005; Haney et al., 1995; Tidey and Miczek, 1997). Repeated social defeat of adult rats is also associated with functional changes in limbic monoaminergic regions that mediate drug-seeking (Buwalda et al., 2005; Lucas et al., 2004; Miczek et al., 2004; Czeh et al., 2007). However, the effects of repeated social defeat experiences in adolescence on subsequent adult behavioral and neural responses to psychostimulants remain to be determined.
Recently, we demonstrated that male rats exposed to repeated social defeat during the peak of adolescent neural re-organization (postnatal days (P) 35–39, Andersen, 2003; Spear, 2000) display long-lasting alterations to adult monoaminergic function (Watt et al., 2009). In particular, previously defeated rats exhibited decreased baseline medial prefrontal cortex (mPFC) DA content as adults, in the absence of any behavioral or pharmacological manipulation prior to tissue collection (Watt et al., 2009). Previous work has shown that decreased mPFC DA content is associated with increased self administration and responsiveness to psychostimulants (Beyer and Steketee, 1999; Piazza et al., 1991b; Schenk et al., 1991; Ventura et al., 2004). This suggests that the changes to mPFC DA following repeated exposure to social defeat in adolescence may similarly alter responses to psychostimulants in adulthood.
In the current study, we investigated whether experience of adolescent social defeat stress would induce long-lasting effects to subsequently alter adult behavioral, corticosterone and neural responses to amphetamine. We chose to examine these variables in early adulthood (P59-62), as the emergence of symptoms characterizing stress-related addictive disorders often occurs at the equivalent stage of human maturation (Andersen, 2003; Park et al., 2006; Kessler et al., 2007). Since decreased mPFC DA content was found in adult rats defeated during adolescence (Watt et al., 2009), we hypothesized that these animals would exhibit a correspondingly diminished amphetamine-induced DA response in the mPFC. Based on reports that decreased mPFC DA activity potentiates phasic and drug-evoked DA release in the NAc following normal development (Del Arco and Mora, 2008; Mitchell and Gratton, 1992; Pascucci et al., 2007), we hypothesized that DA responses to amphetamine would be higher in the NAc of adult rats subjected to social defeat during adolescence. Furthermore, because greater DA release in the NAc is associated with increased locomotion responses (Johnson et al., 1996; Pijnenburg and van Rossum, 1973) and reinforcing properties of psychostimulants (Di Chiara and Imperato, 1988; Kalivas and O’Brien, 2008), we predicted that rats pretreated with social defeat in adolescence would show increased amphetamine-induced behaviors such as locomotion and stereotypy. We also investigated whether adult corticosterone responses to amphetamine were altered by adolescent social defeat, since psychostimulant-induced increases in plasma corticosterone may play a role in psychostimulant-related behaviors (Covington and Miczek, 2005; Goeders, 2002). We therefore predicted that adolescent defeat, in addition to increasing the expression of behaviors induced by amphetamine, would also result in heightened corticosterone responses to amphetamine in adulthood.
2. Results
2.1. Locomotion in a novel environment and amphetamine-induced behavior
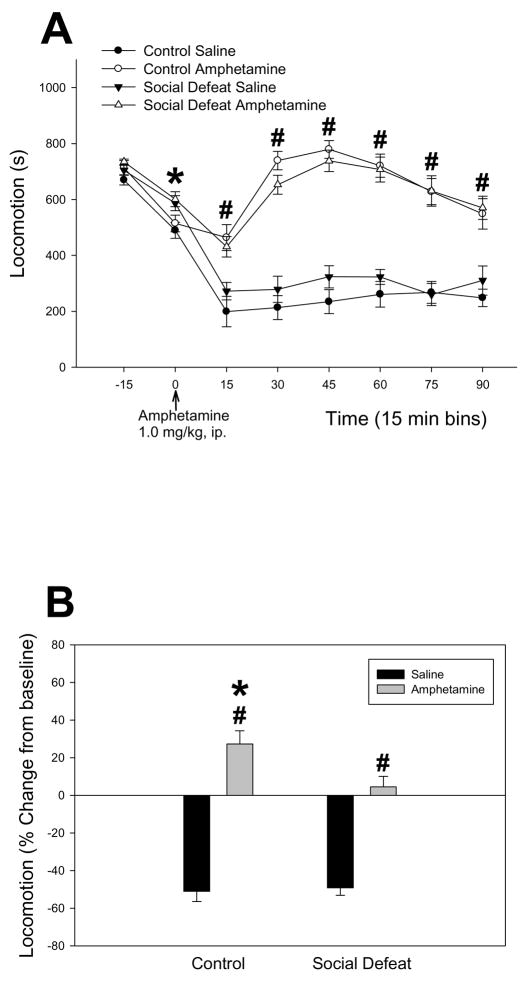
Two way ANOVA across each time point revealed that previously defeated rats that would subsequently receive either amphetamine or saline spent more time engaged in locomotion over the last 15 min of the 30 min testing period (zero time point) compared to all non-defeated rats, F(1,38) = 11.635; p = 0.002 (Figure 1A). Following amphetamine or saline injection, there was a significant effect of drug at all post-injection time points (p < 0.001), with all amphetamine-receiving subjects exhibiting higher locomotion than their saline-injected counterparts (Figure 1A). To control for the fact that locomotion was found to be significantly higher in previously defeated rats prior to injection, data were calculated as a percent change from baseline (respective saline control) to examine amphetamine-stimulated behavior. There was a significant effect of drug F(1,38) = 142.124; p < 0.001 and a significant interaction between adolescent stress pretreatment and drug, F(1,38) = 4.972; p = 0.032. While all rats receiving amphetamine exhibited increased percent change in locomotion compared to their saline controls over the subsequent 90 min, this was significantly lower for previously defeated rats (SNK, p = 0.007, Figure 1B). There were no differences in locomotion between previously defeated and control rats following saline injection (SNK, p = 0.806, Figure 1B)
Figure 1.
(A) Mean ± SEM number of seconds engaged in locomotion for controls and rats defeated in adolescence during acclimation to a novel testing environment and following amphetamine treatment in adulthood. (B) Percent change in from baseline locomotion (mean ± SEM) following amphetamine or saline treatment. *Significant difference according to adolescent stress pretreatment (P<0.05), #Significant difference according to drug (P<0.05).
To ensure that the decreased time engaged in locomotion observed in rats defeated in adolescence was not due to an increased expression of focused stereotyped behavior that excludes locomotion (Forster et al., 2002; Scholl et al., 2009), the intensity of stereotyped behavior was scored at each 15 min time point. Amphetamine-treated socially defeated rats exhibited a maximal stereotypy score of 2.22 ± 0.24, while amphetamine-treated controls showed a maximal stereotypy score of 2.30 ± 0.23 (see Table 1 for intensity of stereotyped ratings). Therefore, both groups of rats were engaged in comparable amounts of non-oral based stereotypy such as repetitive head movements, grooming with limbs and rearing during these observation times. Mann-Whitney tests at each 15 min time period of the testing session determined that intensity of stereotypy did not differ between controls and socially defeated rats that received amphetamine (p > 0.05 for all time periods). This indicates that the decreased locomotion exhibited by rats defeated in adolescence could not be accounted for by an increase in the intensity of stereotypy.
Table 1.
Scale used to score intensity of stereotyped behavior.
| Rating | Behavioral description of stereotyped behavior |
|---|---|
| 0 | Inactive/Locomotion only |
| 1 | Intermittent, non-oral-bases stereotypy such as repetitive (3x in a row) head bobbing, grooming with limbs and rearing (<1.5min) |
| 2 | Continuous, non-oral based stereotypy expressed over wide area of enclosure (>1.5 min) |
| 3 | Continuous, non-oral based stereotypy expressed in a restricted area of enclosure (e.g., in corner) |
| 4 | Pronounced, oral-bases stereotypy directed at self in a restricted area such as repetitive (3x in a row) licking, biting and grooming with mouth |
| 5 | Intermittent, oral-based stereotypy directed externally such as repetitive licking or biting of walls and floor of enclosure (<1.5 min) |
| 6 | Continuous, oral-based stereotypy directed externally (>1.5min) |
2.2. Amphetamine-induced changes in plasma corticosterone and limbic monoamine concentrations
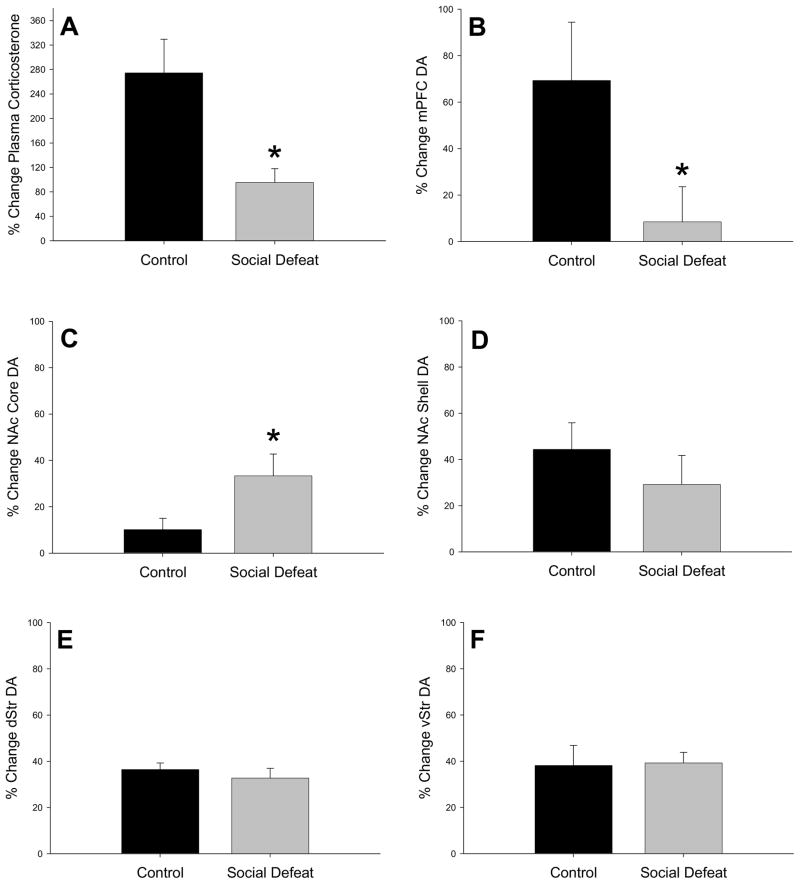
There was no difference in plasma amphetamine concentrations between previously defeated rats (81.6 ± 11.6 ng/ml) and control rats (88.3 ± 17.0 ng/ml) 30 min after injection, t = −0.326; df = 18; p = 0.748, indicating no difference in amphetamine pharmacokinetics as a result of adolescent pretreatment. However, experience of adolescent defeat altered corticosterone responses to amphetamine. Both previously defeated rats and controls treated with amphetamine showed increases in corticosterone 30 min after injection, but the percentage change in amphetamine-induced corticosterone levels from their corresponding saline controls was significantly lower in socially defeated rats compared to non-defeated controls (Figure 2A, F(1,9) = 12.789; p = 0.006).
Figure 2.
Amphetamine-induced changes in plasma corticosterone and dopamine (DA) in adult rats that were socially defeated in adolescence and amphetamine treated controls. All values are represented as a percent change from saline injected controls 30 min after an amphetamine injection (2.5 mg/kg, ip) for both control and socially defeated rats. (A) plasma corticosterone (B) medial prefrontal cortex (mPFC) DA (C) nucleus accumbens (NAc) core DA (D) NAc shell DA (E) dorsal striatum (dStr) DA (F) ventral striatum (vStr) DA. (*P<0.05).
In all brain areas of both previously defeated and non-defeated rats, amphetamine treatment resulted in increased DA calculated as a percent change from their corresponding saline-injected controls. As expected, there was a negative percent change in DOPAC following amphetamine, indicating suppression of DA metabolism, but there was no difference between socially defeated and non-defeated control groups in amphetamine-induced DOPAC concentrations in any brain region (data not shown). When the percent increases in amphetamine-induced DA concentrations in the mPFC were compared, amphetamine-treated control rats had a significantly higher increase in mPFC DA than socially defeated rats that received amphetamine, F(1,18) = 4.772; p = 0.042 (Figure 2B). There were no differences in the mPFC between socially defeated and control rats in amphetamine-induced changes in 5-HT, 5-HIAA, or NE concentrations (data not shown).
In the NAc core, both socially defeated and control groups exhibited significant increases in percent changes in DA concentrations from saline-injected rats in response to amphetamine (Figure 2C), but this was significantly greater in defeated rats when compared to the change from saline values shown by amphetamine-treated controls, F(1,16) = 4.611; p = 0.047. In contrast, there was no effect of social defeat in adolescence on DA responses to amphetamine in the NAc shell (Figure 2D). Similarly, experience of adolescent defeat had no effect on DA responses to amphetamine in either the dStr or vStr (Figure 2E and 2F, respectively). Furthermore, percent change of 5-HT, 5-HIAA, and NE were not altered by experience of adolescent social defeat in any of these brain regions (data not shown).
3. Discussion
As hypothesized, amphetamine-induced DA increases in the mPFC were markedly attenuated in adult rats socially defeated in adolescence. Furthermore, the NAc core DA response to amphetamine was greater in previously defeated rats than in non-defeated controls. However, antithetical to our original hypothesis, previously defeated rats showed lower amphetamine-induced locomotion than amphetamine-treated controls when pre-existing differences in locomotion were accounted for. The absence of a significant difference in the expression of focused stereotyped behaviors suggests that the lower locomotion response in defeated subjects was not a function of increased time spent in drug-induced stereotypy. In addition, amphetamine-induced corticosterone release was attenuated in adult rats defeated in adolescence compared to amphetamine-treated controls. Together, these results suggest that social defeat in adolescence induces persistent effects to differentially alter neural, endocrine and locomotion responses to amphetamine in adulthood.
Male rats defeated in adolescence also exhibited more time engaged in locomotion when exposed to a novel open field as adults, with analyses across time showing that these subjects retained high levels of locomotion across the 30 min test in comparison to controls. This is in line with our earlier study in which previously defeated rats were shown to move greater distance both in a novel open field and in the open arms of an elevated plus maze as adults (Watt et al., 2009). Increased locomotion responses to a novel environment have been shown to predict increased behavioral sensitivity to psychostimulants (Hooks et al., 1991a, b; Kabbaj, 2006; Piazza et al., 1989). Both high novelty-responding rats and rats defeated in adolescence show reduced DA activity and lowered baseline DA concentrations in the mPFC, respectively (Piazza et al., 1991b; Watt et al., 2009). Rats that respond with high levels of locomotion to novel environments also show reduced cell surface DAT expression and DA clearance in the mPFC (Zhu et al., 2007), indicating altered mPFC DA activity in these animals. In addition, both excitotoxic mPFC lesions and experimental depletions of mPFC DA result in increased novelty-induced locomotion (Flores et al., 1996; Jaskiw et al., 1990). In the current study, adult rats defeated in adolescence exhibited an attenuated DA response to amphetamine in the mPFC, most likely a result of reductions in baseline mPFC DA concentrations following the defeat experience (Watt et al., 2009). It could be speculated that high novelty-responding rats with naturally reduced mPFC DA activity (Piazza et al., 1991b) would also exhibit a similarly blunted mPFC DA response to amphetamine. Combined with our earlier findings (Watt et al., 2009), this suggests that decreased adult mPFC DA content and activity following adolescent defeat experience may contribute to the increased locomotion response to a novel environment in adulthood.
In addition to an attenuated DA response to amphetamine in the mPFC, adult rats defeated in adolescence also showed enhanced DA responses to amphetamine in the NAc core. In contrast, no differences in amphetamine-induced DA responses between defeated and control rats were detected in the NAc shell and striatal regions. There also was no difference in amphetamine effects on DOPAC, indicating that the ability of amphetamine to inhibit monoamine oxidase metabolism of DA was not altered by adolescent pretreatment stress. Furthermore, following amphetamine treatment, there were no differences observed between defeated and control rats in NE, 5-HT or 5-HIAA in any brain region studied. This result suggests that the effects of adolescent social defeat on later amphetamine-induced monoamine responses in the brain regions studied are specific to corticoaccumbal DA systems. Previous studies have demonstrated an inverse relationship between mPFC DA activity and NAc DA release, such that phasic and drug-evoked NAc DA release is attenuated by increased mPFC DA activity, and potentiated when mPFC DA activity is reduced (Del Arco and Mora, 2008; Doherty and Gratton, 1996; Mitchell and Gratton, 1992; Pascucci et al., 2007; Ventura et al., 2004). Similarly, we propose that the attenuated amphetamine-induced DA response in the mPFC of previously defeated rats may have resulted in heightened DA responsiveness to amphetamine in the NAc core. It should be noted that these measures were taken following a second administration of amphetamine or saline 2 days after the locomotion test. A study by Vanderschuren et al. (1999) demonstrated that 3 days after a single injection of amphetamine (5 mg/kg), rats had potentiated electrically-evoked DA release in the NAc. Thus, the possibility that previously defeated rats in our study showed a greater NAc DA response because of sensitization to amphetamine following 2 injections cannot be discounted. Further experiments would be required to clarify this issue.
The NAc is a strong candidate for mediating amphetamine-induced locomotion, which appears to be a function of heightened extracellular DA release/activity in this region (Johnson et al., 1996; Pijnenburg and van Rossum, 1973; Sellings and Clarke, 2003, 2006). Our finding that rats defeated in adolescence showed reduced amphetamine-induced locomotion when pre-existing differences in locomotion were accounted for is incongruent with the observed increased NAc core DA response to amphetamine. One possibility is that rats defeated in adolescence may have sensitized in their NAc core DA response to amphetamine from the first injection (where locomotion was measured) to the second injection (where DA was measured) t. As mentioned earlier, Vanderschuren et al. (1999) demonstrated that a single injection of amphetamine can sensitize electrically-evoked DA release in the mPFC, NAc and caudate putamen. However, the current study did not show any difference between defeat and control rats in DA responses to amphetamine in the striatum. Moreover, DA responses to amphetamine in the mPFC were actually reduced in previously-defeated rats. Experimental depletion of DA in the mPFC by 6-hydroxydopamine lesions has been shown to decrease amphetamine (1.5 mg/kg, ip)-induced locomotion, but amphetamine-induced DA release in the NAc core was also attenuated (King et al., 1997). Therefore, it is possible that the lowered amphetamine-induced locomotion in previously defeated rats observed here may be more a function of reduced amphetamine-induced DA responses in the mPFC, and not entirely dependent on changes in NAc core DA responses to the drug. Further testing with lower doses of amphetamine in our model would be useful, since the ability of high novelty responses to predict high amphetamine-induced locomotion is based on lower (0.5–1.0 mg/kg) doses of amphetamine (Hooks et al., 1992; Kabbaj, 2006; Klebaur and Bardo, 1999). Furthermore, caution should be taken when interpreting ex vivo tissue concentrations as a measure of DA responses to amphetamine and extrapolating these to amphetamine-induced behavior. The advantage of this method was that the effects of adolescent social defeat on adult neural amphetamine responses could be assessed across many monoaminergic systems simultaneously. However, to better elucidate the connection between altered DA and behavioral responses to amphetamine following adolescent defeat stress, our neurochemical results should be confirmed using in vivo measures of extracellular DA release during amphetamine-induced locomotion.
In the current study, adult rats socially defeated in adolescence exhibited a reduced corticosterone response to amphetamine when compared to controls. While opposite to our original prediction, this result corresponded with the lowered amphetamine-evoked locomotion shown by previously defeated rats. The role of corticosterone in potentiating behavioral responses to psychostimulants is not entirely clear. While stress-evoked or exogenous corticosterone delivery increases both drug self-administration and locomotion responses to amphetamine (Deroche et al., 1992; Piazza, et al., 1991a; Piazza and Le Moal, 1998), changes in these behavioral parameters do not appear to be dependent on amphetamine-induced increases in plasma corticosterone (Marinelli and Piazza, 2002). However, adrenalectomized mice show reduced amphetamine-induced locomotion in response to a relatively high dose of amphetamine (5.0 mg/kg), but only after the first injection, suggesting that corticosterone may be required for unconditioned amphetamine-induced locomotion increases (Pauly et al., 1993). Thus, it is possible that the reduced amphetamine-induced corticosterone responses seen in previously defeated rats, combined with the dampened mPFC DA response, may have contributed to the lowered amphetamine-induced locomotion levels in these animals.
This is the first report that we are aware of investigating the effects of repeated social defeat during mid-adolescence on drug-induced locomotion and dopaminergic responses in adulthood. Experience of repeated heterotypic social stressors (comprising isolation, novel environment, crowding, litter-shifting and a single defeat episode) beginning in adolescence and continuing to early adulthood (P28-56) has been reported to reduce locomotion responses to acute and repeated administration of amphetamine (Kabbaj et al., 2002), similar to the lowered amphetamine-induced locomotion shown by rats defeated in adolescence. However, other studies indicate that brief daily social isolation and re-housing as a social stressor during mid-adolescence (P30-45) increases acute and sensitized locomotion responses to amphetamine in adulthood (P70), with this effect being most apparent in previously stressed females (Mathews et al., 2008; McCormick et al., 2005). In contrast, adult rats exposed to the same stressor showed no increase in amphetamine-induced locomotion when tested 3 weeks later (McCormick et al., 2005), suggesting that social stress comprising isolation and re-housing only has enduring effects on drug sensitivity if experienced during adolescent development. This differs from the effects of repeated social defeat in adulthood, which has been reported to cause increased locomotion responses to acute amphetamine that can persist up to 60 days following the final defeat session (Covington and Miczek, 2001; Nikulina et al., 2004). The increased behavioral sensitivity following adult defeat is accompanied by increased Fos-like immunoreactive labeling in the ventral tegmental area and in various dopaminergic terminal fields (Nikulina et al., 2004), suggesting enhanced amphetamine-induced functional activation in the mesolimbic DA system as a consequence of prior defeat. Combined with the current results, this suggests that social defeat can evoke long-lasting changes in behavioral and neural amphetamine sensitivity regardless of whether the defeat is experienced in adolescence or adulthood. However, the directionality of behavioral sensitivity to amphetamine appears to differ, with adolescent defeat reducing rather than increasing drug-evoked locomotion. At present, changes in amphetamine-induced DA content/release following adult defeat have not been assessed. Further experiments are needed to determine whether the altered mPFC and NAc core DA responses to amphetamine shown by previously defeated rats in the current study are specific to social defeat being experienced in adolescence.
In our study, behavioral and neural responses to amphetamine in previously defeated subjects were examined after acute administration of the drug. In studies where adult rats are exposed to social defeat, subsequent repeated administration of psychostimulants results in increased behavioral sensitivity (Covington and Miczek, 2001,Covington and Miczek, 2005; Haney et al., 1995; Tidey and Miczek, 1997). Based on these findings, and the demonstration that reduced mPFC DA activity and increased NAc DA predict increased reinforcing effects of psychostimulants (Piazza et al., 1991b; Schenk et al., 1991), it is tempting to speculate that adult rats exhibiting a similar corticoaccumbal DA profile following adolescent social defeat may be more sensitive to repeated administration of amphetamine and to its rewarding effects.
In conclusion, our results suggest that while adolescent social defeat results in enhanced adult locomotion responses to novel environments, these same subjects exhibit lower locomotion than non-defeated controls following acute amphetamine treatment. Previously defeated rats also exhibited dampened corticosterone release, decreased mPFC DA and increases in NAc core DA in response to amphetamine when compared to non-defeated controls. Thus, it appears that the experience of repeated social defeat during adolescent development can have persistent effects to influence corticoaccumbal DA function and associated novelty and amphetamine-related behaviors in adulthood. Future experiments should test the effects of social defeat in adolescence on adult reward related behaviors to further our understanding of the consequences of social stress on the developing brain and later addictive disorders.
4. Experimental Procedures
4.1. Animals
Forty juvenile male Sprague-Dawley rats were obtained from the University of South Dakota Laboratory Animal Services at P21 (day of weaning) and pair-housed according to adolescent stress treatment (social defeat or control) under a reverse light cycle (lights off from 9:00–21:00 hrs). Food and water were available ad libitum. All testing procedures occurred between 10:00 and 15:00 hrs under red lighting. All procedures were approved by the Institutional Animal Care and Use Committee of the University of South Dakota, and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Every effort was made to minimize the number of animals used and their suffering.
4.2. Social defeat procedure
The social defeat procedures used in the current study have recently been shown to induce conditioned behavioral and endocrine defeat responses in adolescent male Sprague-Dawley rats (Watt et al., 2009). Mid-adolescent (P35, Spear, 2000; Andersen, 2003) male rats (n = 20, mass at P35 = 168.75 ± 2.99) were exposed to repeated social defeat in the home cage of a larger aggressive adult male Sprague-Dawley rat (mass = 360.00 g ± 10.00), using a modified version of the resident-intruder paradigm (Koolhaas et al., 1997; Martinez et al., 2002; Miczek, 1979). To encourage territoriality, each resident male (n = 10) was housed individually in a large plastic cage (40 × 25 × 17.5 cm) for one week prior to and throughout the course of the social defeat procedure, and was given daily access to a sexually receptive female. Females had been anesthetized with ketamine/xylazine (50/10 mg/kg, ip.) and ovariectomized earlier. Female sexual receptivity was induced via administration of 17β estradiol benzoate (5 μg/0.1 ml oil, sc, Sigma-Aldrich USA) followed 24 hrs later by progesterone (0.5 mg/0.1 ml oil, sc, Sigma-Aldrich USA) (Farmer et al., 1996). Several different females were used to ensure that each resident was paired with a receptive female every day. Females were removed from resident cages at the start of each defeat session and replaced afterwards. Age-matched controls (n = 20, mass at P35 = 157.85 g ± 4.38) experienced no social defeat or interaction, and were placed into a novel empty cage for a time period corresponding to the duration of each defeat trial to control for handling and novel environment stress (Watt et al., 2009).
For experimental defeat trials, each adolescent experimental subject was transferred from its home cage to that of a resident, with the interaction video-recorded for later measurement of latency to defeat. Adolescent intruders were considered defeated after exhibiting 3 consecutive submissive postures in response to resident attacks (Watt et al., 2009). Adolescents were then confined behind a wire mesh barrier for 35 min, which prevented further physical contact from the resident but allowed continued auditory, olfactory and visual intimidation. Following this, adolescent rats were returned to their home cages. Adolescent subjects were exposed daily to social defeat over a 5 day period, and were confronted with a different resident male each time using a completely randomized design to control for individual variance in defeat intensity. The latency to defeat across the 5 trials was 109 ± 14.9 sec (mean ± SEM). Following social defeat conditioning, subjects and controls were left in their original pairs in their home cages and allowed to mature undisturbed to early adulthood (P58).
4.3. Adult behavioral and amphetamine testing
At P59, previously defeated and control rats were assessed for locomotion responses after being placed in a novel opaque plastic enclosure (52 × 33.5 × 30 cm) for 30 min. Immediately following this, previously defeated and control rats were administered either amphetamine (2.5 mg/kg, ip) or saline of equivalent volume (n = 10 per group) and returned to the testing environment for 90 min. All behavior was video recorded for subsequent analysis using Ethovision v3.1 (Noldus Information Technology, Inc., Leesburg, VA, USA). The Noldus-defined behavior “movement” was used to score locomotion in this experiment. Locomotion duration was scored by Ethovision when the rat’s velocity of movement was greater than 2 cm/s, with a stop velocity of 1.75 cm/s and an averaging interval of 5 (user defined thresholds that exclude non-locomotion movements, Noldus Information Technology, Inc.). To further examine amphetamine-induced behavior, we scored intensity of stereotyped behavior expression excluding locomotion (Table 1). Using a previously described method (Forster et al., 2002; Scholl et al., 2009) two experimenters blind to treatment recorded stereotypy intensity over the last 2 min of every 15 min time interval.
Three days following the behavioral tests (P62), a second amphetamine (2.5 mg/kg, ip) or saline injection was administered 30 min prior to decapitation and trunk blood collection, with subjects remaining in their home cages between injection and sampling. Subjects received the same injection as during locomotion testing, i.e., those that had been given amphetamine received a second amphetamine injection prior to sample collection. Brains were rapidly removed, frozen on dry ice, and stored at −80° C. Trunk blood was centrifuged (5000 × g) and the plasma was drawn off and stored at −80° C.
4.4. Plasma corticosterone assay
Measurement of plasma corticosterone was accomplished by previously described methods (Forster et al., 2008), using a corticosterone enzyme-linked immunosorbant assay kit (R&D Systems Minneapolis, MN, USA). Steroid displacement reagent (0.5 μl) was added to each 10 μl plasma sample, which was then diluted (1:100) with assay buffer and run in duplicate. Sample plasma corticosterone levels were detected by absorbance at 405 nm with wave length correction set at 595 nm using automated plate reader software, and were corrected for recovery using KinetiCalc Jr. software (Bio-Tek Instruments Winooski, VT, USA). Maximum binding percent ranged between 21.5 and 22 % and the percent of non-specific binding was 1.6 %, both within the manufacturer’s range. The detection limit sensitivity of this assay was 27.0 pg/ml.
4.5. Plasma amphetamine assay
To ensure that there was no difference in amphetamine pharmacokinetics between social defeat and control subjects, plasma amphetamine concentrations of all rats receiving amphetamine were measured 30 min post injection from trunk blood collected upon decapitation. Amphetamine was measured from 15 μl of plasma using an Amphetamine Ultra enzyme-linked assay kit (Neogen Corp., Lexington, KY, USA) according to manufacturer instructions. Samples were diluted (1:30), run in duplicate, and read at an absorbance of 450 nm.
4.6. Brain tissue dissection and monoamine analysis
To measure tissue monoamine concentrations in discrete amphetamine-responsive limbic regions, frozen brains were cut in 300 μm sections at −10° C using a Leica-Jung cryostat (North Central Instruments, MN, USA), thaw-mounted on glass slides and stored at −80° C. The mPFC, NAc shell, NAc core, dorsal striatum (dStr) and ventral striatum (vStr) were identified using Paxinos and Watson’s (1998) stereotaxic atlas. All dissections were performed bilaterally with a 430 μm (NAc) or 580 μm (Str and mPFC) inside diameter cannula on a freezing stage (Physitemp Clifton, NJ, USA). Dissected tissue was expelled into 60 μlL of sodium acetate buffer (pH 5.0) containing 9.42 pg/μl of the internal standard (dihydroxybenzylamine, DHBA). Cells were lyzed by freeze-thawing samples. Since the effects of social defeat appear to be specific to mesocortical systems (Watt et al., 2009), differences in amphetamine responses between defeated and control rats were not expected in striatal regions. Therefore, analysis of striatal tissue monoamine concentrations served as a control region.
Samples were analyzed for levels of norepinephrine (NE), dopamine (DA), the DA metabolite 3,4-dihydroxyphenylacetic acid (DOPAC), and serotonin (5-HT) and the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA) using high-pressure liquid chromatography (HPLC) with electrochemical detection. Details of this assay have been published elsewhere (Renner and Luine, 1984; Watt et al., 2007; Watt et al., 2009). Briefly, 2 μl of an ascorbate oxidase solution (1 mg/ml, Sigma Chemical Co., St. Louis, MO, USA) was added to each sample. Samples were centrifuged (17000 x g for 3 min), the supernatant was removed, and 45 μl was injected into an HPLC system (Waters Associates, Inc., Milford, MA, USA). These samples were analyzed electrochemically by a LC-4B potentiostat/amperometric detector (BioAnalytical Systems, West Lafayette, IN, USA), with the electrode potential set at +0.6 V with respect to an Ag/AgCl reference electrode. The mobile phase was composed of 14 g citric acid, 8.6 g sodium acetate, 110 mg 1-octanesulfonic acid, 150 mg EDTA disodium salt, and 100 ml of methanol in 1L of deionized H2O (Sigma Chemical Co.). Flow rate was maintained at 1.4 ml/min. Tissue pellets were dissolved in 0.4 N NaOH and protein concentrations were measured using a Bradford assay (Bradford, 1976). Monoamine concentrations were calculated with respect to peak height values obtained from standard runs set in the internal standard mode using the CSW32 data program (DataApex Ltd., Prague, Czech Republic). Neurotransmitter and metabolite levels were expressed as pg amine/μg protein.
4.7. Data analysis
Duration of locomotion in the novel testing enclosure during the 30 min habituation period and post amphetamine/saline treatment was collapsed into 15 min time intervals, and then compared across each time point between socially-defeated and control rats using separate 2-way ANOVA with main factors of adolescent stress pretreatment (defeat or control) and drug (amphetamine or saline). Significant main effects were further analyzed using 1-way ANOVA followed by Student-Newman-Keuls (SNK) posthoc tests.
Locomotion in the testing chamber was found to be significantly different between socially-defeated and control rats at the zero time point, i.e. in the 15 min interval prior to amphetamine injection. Therefore, post-amphetamine locomotion for each subject was expressed as a percent change from pre-drug for each group to control for differences present before injection. These percent change data for each subject were then averaged across the 6 post-injection time points of the 90 min test. A 2 way ANOVA (main factors of adolescent stress and drug) with SNK posthoc tests was then used to determine the effect of adolescent social defeat on amphetamine-induced locomotion. . Intensity of stereotyped behavior was analyzed between socially defeated rats and controls that received amphetamine at each time point using Mann-Whitney sum ranks tests because intensity ratings were not normally distributed.
Our previous study (Watt et al., 2009) had demonstrated that experience of social defeat in adolescence resulted in alterations to baseline monoamine concentrations in various regions of the adult brain. In particular, mPFC DA concentrations were reduced in previously defeated rats (Watt et al., 2009). Therefore, to control for any pre-existing group differences in baseline corticosterone and monoamine concentrations in socially defeated versus control rats prior to amphetamine administration, these variables were calculated as the percent change from corresponding saline injected rats, comparable to other studies using similar measures (Caccia et al, 1992; Chen et al, 1997; Slikker et al., 1988). . Separate 1-way ANOVA were then used to compare percent changes in corticosterone, DA, DOPAC, NE, 5-HIAA and 5-HT concentrations (percent change from mean of saline-injected rats in corresponding stress pretreatment groups) of socially-defeated rats with non-defeated control rats at 30 min post-amphetamine injection.
All analyses were performed using SigmaStat for Windows v3.0.1a (Systat Software Inc., San Jose, California, USA). The alpha level for all tests was set at 0.05.
Acknowledgments
This work was supported by NIDA RO1 DA019921 (GLF), NIH R03 MH068364 (MJW), and NIH NCRR P20 RRO15567-10, which is designated a Center of Biomedical Research Excellence (COBRE). We thank Jamie Scholl for valuable technical assistance with these experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Desperately driven and no brakes: Developmental stress exposure and subsequent risk for substance abuse. Neurosci Biobehav Rev. 2009;33:516–24. doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer CE, Steketee JD. Dopamine depletion in the medial prefrontal cortex induces sensitized-like behavioral and neurochemical responses to cocaine. Brain Res. 1999;833:133–41. doi: 10.1016/s0006-8993(99)01485-7. [DOI] [PubMed] [Google Scholar]
- Björkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:435–42. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Kole MH, Veenema AH, Huininga M, de Boer SF, Korte SM, Koolhaas JM. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev. 2005;29:83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Caccia S, Fracasso C, Garattini S, Guiso G, Sarati S. Effects of short- and long-term administration of fluoxetine on the monoamine content of rat brain. Neuropharmacology. 1992;31:343–7. doi: 10.1016/0028-3908(92)90066-x. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Maier SE, West JR. Prenatal alcohol treatment attenuated postnatal cocaine-induced elevation of dopamine concentration in nucleus accumbens: a preliminary study. Neurotoxicol Teratol. 1997;19:39–46. doi: 10.1016/s0892-0362(96)00188-2. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–98. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl) 2005;183:331–40. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, Fuchs E. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol Biochem Behav. 2008;90:226–35. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Casolini P, Maccari S, Le Moal M, Simon H. Stress-induced sensitization to amphetamine and morphine psychomotor effects depend on stress-induced corticosterone secretion. Brain Res. 1992;598:343–8. doi: 10.1016/0006-8993(92)90205-n. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty MD, Gratton A. Medial prefrontal cortical D1 receptor modulation of the meso-accumbens dopamine response to stress: an electrochemical study in freely-behaving rats. Brain Res. 1996;715:86–97. doi: 10.1016/0006-8993(95)01557-4. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiol Behav. 2003;80:317–25. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Farmer CJ, Isakson TR, Coy DJ, Renner KJ. In vivo evidence for progesterone dependent decreases in serotonin release in the hypothalamus and midbrain central grey: relation to the induction of lordosis. Brain Res. 1996;711:84–92. doi: 10.1016/0006-8993(95)01403-9. [DOI] [PubMed] [Google Scholar]
- Flores G, Wood GK, Liang JJ, Quirion R, Srivastava LK. Enhanced amphetamine sensitivity and increased expression of dopamine D2 receptors in postpubertal rats after neonatal excitotoxic lesions of the medial prefrontal cortex. J Neurosci. 1996;16:7366–75. doi: 10.1523/JNEUROSCI.16-22-07366.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster GL, Falcon AJ, Miller AD, Heruc GA, Blaha CD. Effects of laterodorsal tegmentum excitotoxic lesions on behavioral and dopamine responses evoked by morphine and d-amphetamine. Neuroscience. 2002;114:817–23. doi: 10.1016/s0306-4522(02)00365-2. [DOI] [PubMed] [Google Scholar]
- Forster GL, Pringle RB, Mouw NJ, Vuong SM, Watt MJ, Burke AR, Lowry CA, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. European Journal of Neuroscience. 2008;28:299–310. doi: 10.1111/j.1460-9568.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone GL, Parker GB, Malhi GS. Do bullied children become anxious and depressed adults?: A cross-sectional investigation of the correlates of bullying and anxious depression. J Nerv Ment Dis. 2006;194:201–8. doi: 10.1097/01.nmd.0000202491.99719.c3. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Gordon HW. Early environmental stress and biological vulnerability to drug abuse. Psychoneuroendocrinology. 2002;27:115–26. doi: 10.1016/s0306-4530(01)00039-7. [DOI] [PubMed] [Google Scholar]
- Grossman AW, Churchill JD, McKinney BC, Kodish IM, Otte SL, Greenough WT. Experience effects on brain development: possible contributions to psychopathology. J Child Psychol Psychiatry. 2003;44:33–63. doi: 10.1111/1469-7610.t01-1-00102. [DOI] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Hoffmann JP, Cerbone FG, Su SS. A growth curve analysis of stress and adolescent drug use. Subst Use Misuse. 2000;35:687–716. doi: 10.3109/10826080009148417. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 1991a;9:121–8. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Individual differences in locomotor activity and sensitization. Pharmacol Biochem Behav. 1991b;38:467–70. doi: 10.1016/0091-3057(91)90308-o. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Neill DB, Justice JB., Jr Individual differences in amphetamine sensitization: dose-dependent effects. Pharmacol Biochem Behav. 1992;41:203–10. doi: 10.1016/0091-3057(92)90083-r. [DOI] [PubMed] [Google Scholar]
- Jaskiw GE, Karoum F, Freed WJ, Phillips I, Kleinman JE, Weinberger DR. Effect of ibotenic acid lesions of the medial prefrontal cortex on amphetamine-induced locomotion and regional brain catecholamine concentrations in the rat. Brain Res. 1990;534:263–72. doi: 10.1016/0006-8993(90)90138-2. [DOI] [PubMed] [Google Scholar]
- Johnson K, Churchill L, Klitenick MA, Hooks MS, Kalivas PW. Involvement of the ventral tegmental area in locomotion elicited from the nucleus accumbens or ventral pallidum. J Pharmacol Exp Ther. 1996;277:1122–31. [PubMed] [Google Scholar]
- Kabbaj M, Isgor C, Watson SJ, Akil H. Stress during adolescence alters behavioral sensitization to amphetamine. Neuroscience. 2002;113:395–400. doi: 10.1016/s0306-4522(02)00188-4. [DOI] [PubMed] [Google Scholar]
- Kabbaj M. Individual differences in vulnerability to drug abuse: the high responders/low responders model. CNS Neurol Disord Drug Targets. 2006;5:513–20. doi: 10.2174/187152706778559318. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kaltiala-Heino R, Rimpela M, Rantanen P, Rimpela A. Bullying at school--an indicator of adolescents at risk for mental disorders. J Adolesc. 2000;23:661–74. doi: 10.1006/jado.2000.0351. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB. Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry. 2007;20:359–364. doi: 10.1097/YCO.0b013e32816ebc8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D, Zigmond MJ, Finlay JM. Effects of dopamine depletion in the medial prefrontal cortex on the stress-induced increase in extracellular dopamine in the nucleus accumbens core and shell. Neuroscience. 1997;77:141–53. doi: 10.1016/s0306-4522(96)00421-6. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Bardo MT. Individual differences in novelty seeking on the playground maze predict amphetamine conditioned place preference. Pharmacol Biochem Behav. 1999;63:131–136. doi: 10.1016/s0091-3057(98)00258-5. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiol Scand Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Celen Z, Tamashiro KL, Blanchard RJ, Blanchard DC, Markham C, Sakai RR, McEwen BS. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124:449–457. doi: 10.1016/j.neuroscience.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with c-fos expression: a review. Stress. 2002;5:3–13. doi: 10.1080/102538902900012369. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, Mills RG, McCormick CM. Chronic social stress in adolescence influenced both amphetamine conditioned place preference and locomotor sensitization. Dev Psychobiol. 2008;50:451–9. doi: 10.1002/dev.20299. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Kopeikina K, Kelsey JE. Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Horm Behav. 2005;48:64–74. doi: 10.1016/j.yhbeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60:253–9. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Covington HE, 3rd, Nikulina EM, Jr, Hammer RP. Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev. 2004;27:787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Gratton A. Partial dopamine depletion of the prefrontal cortex leads to enhanced mesolimbic dopamine release elicited by repeated exposure to naturally reinforcing stimuli. J Neurosci. 1992;12:3609–18. doi: 10.1523/JNEUROSCI.12-09-03609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nansel TR, Overpeck M, Pilla RS, Ruan WJ, Simons-Morton B, Scheidt P. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. Jama. 2001;285:2094–100. doi: 10.1001/jama.285.16.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman ML, Holden GW, Delville Y. Isolation and the stress of being bullied. J Adolesc. 2005;28:343–57. doi: 10.1016/j.adolescence.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, 3rd, Ganschow L, Hammer RP, Jr, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–65. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Park MJ, Paul Mulye T, Adams SH, Brindis CD, Irwin CE., Jr The health status of young adults in the United States. J Adolesc Health. 2006;39:305–17. doi: 10.1016/j.jadohealth.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Pascucci T, Ventura R, Latagliata EC, Cabib S, Puglisi-Allegra S. The medial prefrontal cortex determines the accumbens dopamine response to stress through the opposing influences of norepinephrine and dopamine. Cereb Cortex. 2007;17:2796–804. doi: 10.1093/cercor/bhm008. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Robinson SF, Collins AC. Chronic corticosterone administration enhances behavioral sensitization to amphetamine in mice. Brain Res. 1993;620:195–202. doi: 10.1016/0006-8993(93)90156-h. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminiere JM, Le Moal M, Mormede P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci U S A. 1991a;88:2088–92. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deminiere JM, Kharoubi M, Le Moal M, Simon H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res. 1991b;567:169–74. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Pijnenburg AJ, van Rossum JM. Letter: Stimulation of locomotor activity following injection of dopamine into the nucleus accumbens. J Pharm Pharmacol. 1973;25:1003–5. doi: 10.1111/j.2042-7158.1973.tb09995.x. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22:633–43. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Renner KJ, Luine VN. Determination of monoamines in brain nuclei by high performance liquid chromatography with electrochemical detection: young vs middle aged rats. Life Sci. 1984;34:2193–9. doi: 10.1016/0024-3205(84)90320-5. [DOI] [PubMed] [Google Scholar]
- Rigby K, Slee PT. Dimensions of interpersonal relation among Australian children and implications for psychological well-being. J Soc Psychol. 1993;133:33–42. doi: 10.1080/00224545.1993.9712116. [DOI] [PubMed] [Google Scholar]
- Rossow I, Lauritzen G. Shattered childhood: a key issue in suicidal behavior among drug addicts? Addiction. 2001;96:227–40. doi: 10.1046/j.1360-0443.2001.9622275.x. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–34. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Schenk S, Horger BA, Peltier R, Shelton K. Supersensitivity to the reinforcing effects of cocaine following 6-hydroxydopamine lesions to the medial prefrontal cortex in rats. Brain Res. 1991;543:227–35. doi: 10.1016/0006-8993(91)90032-q. [DOI] [PubMed] [Google Scholar]
- Scholl JL, Feng N, Watt MJ, Renner KJ, Forster GL. Individual differences in amphetamine sensitization, behavior and central monoamines. Physiol Behav. 2009;96:493–504. doi: 10.1016/j.physbeh.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci. 2003;23:6295–303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellings LH, Clarke PB. 6-Hydroxydopamine lesions of nucleus accumbens core abolish amphetamine-induced conditioned activity. Synapse. 2006;59:374–7. doi: 10.1002/syn.20247. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Ali SF, Scallet AC, Frith CH, Newport GD, Bailey JR. Neurochemical and neurohistological alterations in the rat and monkey produced by orally administered methylenedioxymethamphetamine (MDMA) Toxicol Appl Pharmacol. 1988;94:448–57. doi: 10.1016/0041-008x(88)90285-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Tharp-Taylor S, Haviland A, D’Amico EJ. Victimization from mental and physical bullying and substance use in early adolescence. Addict Behav. 2009 doi: 10.1016/j.addbeh.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology (Berl) 1997;130:203–12. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Schmidt ED, De Vries TJ, Van Moorsel CA, Tilders FJ, Schoffelmeer AN. A single exposure to amphetamine is sufficient to induce long-term behavioral, neuroendocrine, and neurochemical sensitization in rats. J Neurosci. 1999;19:9579–9586. doi: 10.1523/JNEUROSCI.19-21-09579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res. 2008;188:398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Alcaro A, Cabib S, Conversi D, Mandolesi L, Puglisi-Allegra S. Dopamine in the medial prefrontal cortex controls genotype-dependent effects of amphetamine on mesoaccumbens dopamine release and locomotion. Neuropsychopharmacology. 2004;29:72–80. doi: 10.1038/sj.npp.1300300. [DOI] [PubMed] [Google Scholar]
- Wals M, Verhulst F. Child and adolescent antecedents of adult mood disorders. Curr Opin Psychiatry. 2005;18:15–9. [PubMed] [Google Scholar]
- Watt MJ, Forster GL, Korzan WJ, Renner KJ, Summers CH. Rapid neuroendocrine responses evoked at the onset of social challenge. Physiol Behav. 2007;90:567–75. doi: 10.1016/j.physbeh.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Burke AR, Renner KJ, Forster GL. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav Neurosci. 2009;123:564–76. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Bardo MT, Bruntz RC, Stairs DJ, Dwoskin LP. Individual differences in response to novelty predict prefrontal cortex dopamine transporter function and cell surface expression. Eur J Neurosci. 2007;26:717–28. doi: 10.1111/j.1460-9568.2007.05690.x. [DOI] [PubMed] [Google Scholar]