Abstract
Coxiella burnetii is the bacterial agent of Q fever in humans. Acute Q fever generally manifests as a flu-like illness and is typically self-resolving. In contrast, chronic Q fever usually presents with endocarditis and is often life-threatening without appropriate antimicrobial therapy. Unfortunately, available options for the successful treatment of chronic Q fever are both limited and protracted (>18 months). Pentamidine, an RNA splice inhibitor used to treat fungal and protozoal infections, was shown to reduce intracellular growth of Coxiella by ca. 73% at a concentration of 1 μM (ca. 0.6 μg/mL) compared with untreated controls, with no detectable toxic effects on host cells. Bacterial targets of pentamidine include Cbu.L1917 and Cbu.L1951, two group I introns that disrupt the 23S rRNA gene of Coxiella, as demonstrated by the drug's ability to inhibit intron RNA splicing in vitro. Since both introns are highly conserved among all eight genotypes of the pathogen, pentamidine is predicted to be efficacious against numerous strains of C. burnetii. To our knowledge, this is the first report describing antibacterial activity for this antifungal/antiprotozoal agent.
Keywords: Coxiella, Pentamidine, Group I intron, RNA splicing
1. Introduction
Coxiella burnetii is an obligate intracellular γ-proteobacterium and is the agent of Q fever in humans. It is one of the most infectious pathogens known, with a 50% infective dose (ID50) of one to ten bacteria in the guinea pig model [1]. Human infections with C. burnetii are generally zoonoses acquired by inhalation of contaminated aerosols. Q fever typically presents as an acute, self-limiting flu-like illness accompanied by pneumonia or hepatitis. In roughly 1% of cases a severe chronic infection can occur in which endocarditis predominates [2]. These attributes and its past use as a biological weapon component [3] were grounds for classifying Coxiella as a `Health and Human Services (HHS) Select Agent'.
The recommended antimicrobial therapy for acute Q fever, when required, consists of a 14-day course of doxycycline (200 mg/day) and is highly effective [2]. The current recommended therapy for chronic Q fever in adults is doxycycline (100 mg by mouth twice daily) in combination with hydroxychloroquine (600 mg by mouth once daily) for at least 18 months [4,5]. Mortality rates of ca. ca. 5% have been reported [4,5] despite therapeutic intervention with this combinational therapy for chronic Q fever.
Previous research from our laboratory has shown that all eight genotypes of C. burnetii possess two highly conserved, self-splicing group I introns named Cbu.L1917 and Cbu.L1951 that disrupt the pathogen's single-copy 23S rRNA gene [6]. Because of this genetic arrangement, intron-mediated RNA splicing and exon re-ligation are essential to the formation of a mature 23S rRNA in Coxiella. Thus, the associated RNA splicing events constitute an `Achilles heel' that provides an antimicrobial target for C. burnetii.
In this study, we present data showing that pentamidine, a group I intron RNA splice inhibitor [7], significantly decreases intracellular growth of C. burnetii and inhibits the splicing efficiency of both group I intron RNAs in vitro. Pentamidine is normally used as a chemotherapeutic agent to treat Pneumocystis carinii infections and is also efficacious against other fungal and protozoal pathogens. Results of this study suggest that pentamidine may provide an alternative for antimicrobial therapy in chronic cases of Q fever, especially considering the limited number of available options for treating this potentially life-threatening infection.
2. Materials and methods
2.1. Bacterial strains
Synchronised cultures of C. burnetii Nine Mile, phase II, clone 4 (RSA439) were established in African green monkey kidney (Vero) epithelial cells (CCL-81) [American type Culture Collection (ATCC), Manassas, VA] as previously described [6,8]. RSA439 was chosen as a model for this study as it is the only attenuated Coxiella strain exempt from select agent status and it possesses the Cbu.L1917 and Cbu.L1951 group I introns common to all eight genotypes of the pathogen [6].
2.2. Pentamidine susceptibility and growth assays
To assess the effect of pentamidine on growth, Vero cells were seeded in tissue culture dishes (5 × 105 per well), cultured overnight and then inoculated with Coxiella small cell variants (SCVs) at a multiplicity of infection (MoI) of 664:1 and prepared as previously described [8]. MoIs were determined from C. burnetii genome equivalents from quantitative polymerase chain reaction (qPCR) using a primer set specific to the pathogen's rpoS gene as previously described [8]. A high MoI was intentionally used to allow for quantification of Coxiella at time zero. Pentamidine isethionate (Sigma-Aldrich, St Louis, MO) was added to a final concentration of 0–10 μM at 4 h after infecting Vero cells with bacteria. The minimum inhibitory concentration (MIC) was defined as the lowest concentration of pentamidine that significantly inhibited growth of Coxiella in infected Vero cells at 96 h compared with negative controls and at a concentration where increased dosage did not significantly increase the growth inhibition. The 96-h time point was chosen because: (i) it occurs in log-phase growth of Coxiella [8]; (ii) intron splicing from 23S rRNA precursors (i.e. pentamidine's target) would be maximal during ribosome formation in log-phase growth [6]; and (iii) the half-life of pentamidine is ca. 6.5 h [9].
2.3. Nucleic acid purification, manipulation and RNA splicing assays
DNA was extracted from 96-h infected cultures or Vero cell cultures using a DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Genome quantities for C. burnetii were determined using qPCR as above. qPCR on Vero cell DNA was done using a primer set specific to the Chlorocebus (green monkey) cytochrome oxidase gene (GenBank accession no. AY972804): Verocox_F, TTAGCAGGAAA CTTCTCCCATCC; and Verocox_R, AAGGTAG TGTTGAGGTTGCGGTC. Nucleic acids were quantified by spectrophotometry at 260 nm. The effect of pentamidine on group I intron RNA splicing was assayed by 35S-guanosine-5'-triphosphate (35S-GTP) incorporation into spliced intron RNAs in the presence of 0, 25 or 50 mM pentamidine in vitro as described previously [10].
2.4. Statistical analysis
At least three independent determinations were used to calculate the mean and standard deviation for all numerical data. Statistics and graphs were done using InStat3 (GraphPad, La Jolla, CA) and/or Excel 2003 (Microsoft, Redmond, WA) software. Statistical significance was determined using Student's t-test and a P-value of <0.05 was considered significant.
3. Results and discussion
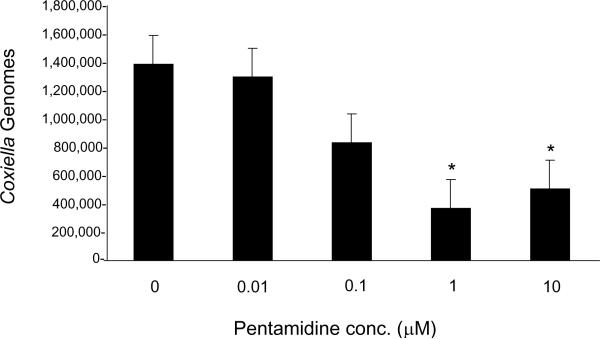
Results of the growth assays showed a dose-dependent inhibition of C. burnetii growth in response to increasing pentamidine concentrations, with a significant reduction (73.3% less than negative controls; P < 0.05) in Coxiella genome numbers at 1 μM (ca.. 0.6 μg/mL) pentamidine (Fig. 1). Increasing the dosage to 10 μM pentamidine did not significantly increase growth inhibition above that observed at the 1 μM MIC (Fig. 1). MIC results for pentamidine are comparable with the MIC obtained in vitro for doxycycline (ca. 1 μg/mL) and the C. burnetii Nine Mile phase I strain (RSA493) [11].
Fig. 1.
Effect of varying concentrations of pentamidine on Coxiella burnetii growth. Coxiella genomes were quantified using quantitative polymerase chain reaction (qPCR) [8] after 96 h of growth in infected Vero cells in the presence of 0–10 μM pentamidine. Values represent the mean ± standard deviation (n = 3). * Statistically significant difference from results of the untreated control (P < 0.05 by Student's t test).
A previous study examining maximal plasma concentrations of pentamidine during therapy for Trypanosoma gambiense infections showed a range of 0.42 μM to 13.42 μM [12]. These results suggest that concentrations above the in vitro MIC determined in this study could be attained in vivo during therapy.
To ensure that growth inhibition did not simply reflect a reduction in available host cells for C. burnetii to infect, Vero cells were cultured for 96 h in the presence of the same pentamidine concentrations as shown in Fig. 1. DNA was then extracted as above and genome quantities were determined by qPCR using a primer set specific for the Vero cell cytochrome oxidase gene. Results of the qPCR showed that regardless of pentamidine dosage, average cycle threshold (CT) values for the Vero cell cytochrome oxidase gene were not significantly different from untreated controls or from each other (Fig. 2). In addition, overt signs of cytotoxicity (e.g. altered morphology, loss of adherence, etc.) were not observed in Vero cells grown in the presence of pentamidine when cultures were examined by phase-contrast microscopy (data not shown). Live/dead staining using Trypan blue was also conducted and cytotoxicity was measured with a Cytotox96 Kit (Promega Corp., Madison, WI). However, no significant differences were found in the number of viable Vero cells that excluded Trypan blue per unit area or in levels of lactate dehydrogenase released to the culture supernatant (Cytotox96 assays) from Vero cells cultured for 96 h in the presence of 0–10 μM pentamidine (data not shown). Taken together, the results demonstrate that pentamidine does not affect host cell numbers at the concentrations employed in this study and suggest that pentamidine directly affects C. burnetii rather than the host cell in which the bacterium replicates (Figs 1 and 2).
Fig. 2.
Effect of pentamidine on Vero host cell numbers during 96 h of growth. Quantitative polymerase chain reaction (qPCR) was used to compare Vero cell genome numbers using a primer set specific to its cytochrome oxidase (GenBank accession no. AY972804). Values represent mean cycle threshold (CT) ± standard deviation (n = 6) following 96 h growth in media containing the indicated concentrations of pentamidine. CT values did not show a statistically significant difference from the untreated control (P > 0.05 by Student's t-test).
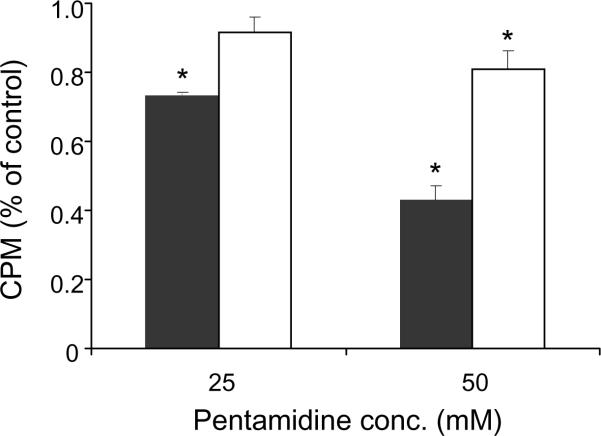
To address the hypothesis that pentamidine targets and inhibits splicing by Coxiella's group I intron RNAs, 35S-GTP incorporation into spliced intron RNAs was assayed in vitro in the presence of 0, 25 or 50 mM pentamidine. Results of the experiment showed that 25 mM and 50 mM pentamidine significantly (P < 0.05) reduced the splicing of Cbu.L1917 RNA by ca. 27% and 57%, respectively, compared with controls without the drug (Fig. 3). Cbu.L1951 was comparatively more resistant to splice inhibition and required 50 mM pentamidine to significantly (P < 0.05) reduce its splicing activity by ca. 19% (Fig. 3). The disparity in Cbu.L1917 and Cbu.L1951 sensitivities to pentamidine may reflect differences in intrinsic splicing rates, where Cbu.L1917 is much more active in vitro possibly owing to the larger size of Cbu.L1951 (719 bases vs. 287 bases, respectively) and partial reliance on potential maturase function of its encoded homing endonuclease. Previous work has shown that group I introns display differential sensitivity to pentamidine [13], possibly owing to specific RNA site interactions that subsequently affect folding and splicing rates [14]. Regardless, since both introns must splice and re-ligate the shared precursor RNA to form a mature 23S rRNA, inhibition of either intron would impair growth by deceasing the translational efficiency of Coxiella. Thus, although the introns display differential sensitivity to inhibition by pentamidine, they constitute two potential targets for the drug in every 23S rRNA precursor of C. burnetii. It was also observed that considerably greater quantities of pentamidine were needed to inhibit intron RNA splicing activities in vitro (25–50 mM) in contrast to inhibition of Coxiella growth within Vero cells (>1.0 μM). These observations may be due to the tendency of pentamidine to accumulate in cellular lysosomes [15], a characteristic that would, over time, increase the effective concentration of the drug in Coxiella's phagolysosomal niche.
Fig. 3.
Effect of pentamidine on RNA splicing by Coxiella burnetii group I intron RNAs in vitro. Values represent mean 35S-guanosine-5'-triphosphate (35S-GTP) incorporation into spliced RNAs [counts per min (CPMs) as a percent of the untreated control ± standard error of the mean]. Black and white bars denote Cbu.L1917 (n = 3) and Cbu.L1951 (n = 4), respectively. * Statistically significant difference from the untreated control (P < 0.05 by Student's t-test).
Whilst several studies have documented the utility of pentamidine for treatment of fungal (P. carinii and Candida albicans) and protozoal (Leishmania and Trypanosoma) infections, this is the first report describing the antibacterial activity of the drug in vitro. In addition, we have identified two potential targets for the drug's antibacterial activity. From these data, we predict that any molecule that blocks the activity of Coxiella's group I introns (e.g. other RNA splice inhibitors) constitutes a potential therapeutic agent for Q fever. Further, since both L1917 and L1951 group I introns are highly conserved elements that disrupt the single 23S rRNA gene of all eight genotypes of C. burnetii [6], we predict that pentamidine would be an effective antimicrobial against multiple Coxiella strains. Although the efficacy of pentamidine against C. burnetii in vivo remains to be determined, intron RNA splicing and formation of a mature 23S rRNA is a process that would also undoubtedly be required for replication in vivo. Thus, the drug has potential as an antimicrobial agent against C. burnetii, especially during chronic Q fever where available therapeutic options are limited.
Acknowledgments
Funding This work was supported by NIH grant R21 AI078125 to MFM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests None declared.
Ethical approval Not required.
References
- [1].Moos A, Hackstadt T. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect Immun. 1987;55:1144–50. doi: 10.1128/iai.55.5.1144-1150.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–53. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Regis E. The biology of doom: the history of America's secret germ warfare project. Henry Holt & Co.; New York, NY: 1999. [Google Scholar]
- [4].Karakousis PC, Trucksis M, Dumler JS. Chronic Q fever in the United States. J Clin Microbiol. 2006;44:2283–7. doi: 10.1128/JCM.02365-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Raoult D, Houpikian P, Dupont HT, Riss JM, Arditi-Djiane J, Brouqui P. Treatment of Q fever endocarditis: comparison of 2 regimens containing doxycycline and ofloxacin or hydroxychloroquine. Arch Intern Med. 1999;159:167–73. doi: 10.1001/archinte.159.2.167. [DOI] [PubMed] [Google Scholar]
- [6].Raghavan R, Miller SR, Hicks LD, Minnick MF. The unusual 23S rRNA gene of Coxiella burnetii: two self-splicing group I introns flank a 34-base-pair exon, and one element lacks the canonical omegaG. J Bacteriol. 2007;189:6572–9. doi: 10.1128/JB.00812-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Miletti KE, Leibowitz MJ. Pentamidine inhibition of group I intron splicing in Candida albicans correlates with growth inhibition. Antimicrob Agents Chemother. 2000;44:958–66. doi: 10.1128/aac.44.4.958-966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Coleman SA, Fischer ER, Howe D, Mead DJ, Heinzen RA. Temporal analysis of Coxiella burnetii morphological differentiation. J Bacteriol. 2004;186:7344–52. doi: 10.1128/JB.186.21.7344-7352.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Conte JE, Jr, Upton RA, Lin ET. Pentamidine pharmacokinetics in patients with AIDS with impaired renal function. J Infect Dis. 1987;156:885–90. doi: 10.1093/infdis/156.6.885. [DOI] [PubMed] [Google Scholar]
- [10].Raghavan R, Hicks LD, Minnick MF. A unique group I intron in Coxiella burnetii is a natural splice mutant. J Bacteriol. 2009;191:4044–6. doi: 10.1128/JB.00359-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rolain J-M, Maurin M, Raoult D. Bacteriostatic and bactericidal activities of moxifloxacin against Coxiella burnetii. Antimicrob Agents Chemother. 2001;45:301–2. doi: 10.1128/AAC.45.1.301-302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bronner U, Doua F, Ericsson Ö, Gustafsson LL, Miézan TW, Rais M, et al. Pentamidine concentrations in plasma, whole blood and cerebrospinal fluid during treatment of Trypanosoma gambiense infection in Côte d'lvoire. Trans R Soc Trop Med Hyg. 1991;85:608–11. doi: 10.1016/0035-9203(91)90364-5. [DOI] [PubMed] [Google Scholar]
- [13].Zhang Y, Bell A, Perlman PS, Leibowitz MJ. Pentamidine inhibits mitochondrial intron splicing and translation in Saccharomyces cerevisiae. RNA. 2000;6:937–51. doi: 10.1017/s1355838200991726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang Y, Li Z, Pilch DS, Leibowitz MJ. Pentamidine inhibits catalytic activity of group I intron Ca.LSU by altering RNA folding. Nucleic Acids Res. 2002;30:2961–71. doi: 10.1093/nar/gkf394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Glaumann H, Bronner U, Ericsson Ö, Gustafsson LL, Rombo L. Pentamidine accumulates in rat liver lysosomes and inhibits phospholipid degradation. Pharmacol Toxicol. 1994;74:17–22. doi: 10.1111/j.1600-0773.1994.tb01067.x. [DOI] [PubMed] [Google Scholar]