Abstract
Postmenopausal osteoporosis and hyperparathyroidism are to two common forms of bone loss caused primarily by an expansion of the osteoclastic pool only partially compensated by a stimulation of bone formation. The intimate mechanisms by which estrogen deficiency and excessive production of PTH cause bone loss remain to be determined in part because in vitro studies do not provide the means to adequately reproduce the effects of ovx and PTH overproduction observed in vivo. This article examines the connection between T cells and bone in health and disease and reviews the evidence in favor of the hypothesis that T cells play an unexpected critical role in the mechanism of action of estrogen and PTH in bone.
INTRODUCTION
The association between inflammation and bone loss has long been recognized, leading to the assumption the cells of the immune system may participate in the control of bone remodeling. The discovery that the RANKL/RANK system play a pivotal role in adaptative immunity and osteoclastogenesis has provided the molecular evidence that has firmly established the link between the immune system and bone. A seminal work by Kong at al (1) demonstrating that T cell produced RANKL plays a pivotal role in the bone loss observed adjuvant arthritis has later provided the first incontrovertible evidence about the capacity of T cells to cause bone loss. It is now clear that T cells regulate bone homeostasis via direct interactions with bone marrow (BM) stromal cells (SCs) and osteoblasts (OBs) and by releasing osteoclastogenic cytokines and Wnt ligands. This article focuses on the role of T cells in the mechanism by which two essential calciotrophic hormones, estrogen and PTH, regulate bone homeostasis.
DEVELOPMENT, HOMING, AND FUNCTIONS OF BONE MARROW T CELLS
T cells are critical mediators of the adaptive immune response. These lymphocytes may be subdivided into major classes according to the subunits which form the T cell receptor (TCR). T cells express either an αβ or γδ TCR on the cell surface, and these receptors are responsible for recognizing a diverse range of antigens (2). Most T lymphocytes are αβ T cells, a lineage which express either the CD4 or CD8 marker. By contrast, the majority of γδ T cells lack expression of CD4 and CD8 and their function remains largely unknown (3). Another small subset of T cells (< 1 % total T cells in the mouse) is known as natural killer T (NKT) cells, which can be defined based on the expression of both the NK marker NK1.1 and the αβTCR (4). Although small in number, NKT cells can produce large amounts of cytokines, and have been implicated in a variety of immune responses including autoimmunity, graft rejection, and responses to pathogens (4). CD4+ T cells can also be subdivided based on the types of cytokines produced and broadly grouped into the Th1 (IFNγ), Th2 (IL-4), and Th17 (IL-17) subsets. Of these, Th17 T cells have been suggested to be the osteoclastogenic T cells. Th17 are produced when naïve T cells are activated by TGFβ and IL-6 in the mouse, or TGFβ and inflammatory stimuli in humans. The resulting clonal memory T-cell population will be instructed to produce the Th17 signature cytokines IL-17A, IL-17F, IL-22, and IL-26 (5). IL-17A, a cytokine which induces RANKL expression in SCs and OBs, is the only Th17 signature cytokine currently known to influence the biology of osteoclasts. However, Th17 produce additional non-signature cytokines relevant for bone, including RANKL and TNF (6). A subset of Th17 cells also produce small amounts of IFNγ, which in vitro moderate the osteoclastogenic activity of Th17 (7). The issue of whether IFNγ producing Th17 are more or less osteoclastogenic than non- IFNγ producing Th17 in vivo remains to be determined. The cytokine repertoires of specific Th17 subsets depend on master differentiation factors present in the microenvironment during initial antigen recognition (5)].
Another type of CD4 positive T cell can be defined based on expression of CD25 and the transcription factor FoxP3. These cells are known as regulatory T cells (Tregs), and are critical in the prevention of autoimmune disease. There are a number of mechanism by which Tregs dampen the immune response, including some requiring cell to cell contact.
Like all blood cells, T cells derive from hematopoietic stem cells (HSCs) that reside in the BM. HSCs can self-renew or differentiate into multipotent progenitors (MPPs), which in turn will give rise to lymphoid-primed multipotent progenitors (LMPPs), which then produce common lymphoid progenitors (CLPs). These progenitors mobilize from the BM into the blood, and from there into the thymus. Upon thymic entry, thymic settling progenitors differentiate into the earliest defined T-cell precursors in the thymus, tthe early T progenitors, which ultimately produce naïve T cells that go on to populate the periphery. BM T cells arrive from the blood route and, after homing to the BM, can move back to the blood and then migrate to other lymphoid organs. T cells can exit from the BM only through the blood route, as there are no lymphatic vessels that drain this organ (8). T-cell colonization of the BM is a competitive process between the incoming T cells and the resident T cells, which already inhabit the same saturable niche (9).
The BM serves as a site for the initiation of naïve T cells responses and as a reservoir of CD8 T cells (8). T cells represent ~5–8 % of the total mononuclear BM cells. The percentage of activated T cells is much higher in the BM than in other secondary lymphoid organs and this feature is both cytokine and antigen (Ag) driven (8). As a result, the BM is the lymphoid organ with the highest percentage and number of proliferating T cells, apart from the thymus. A distinctive feature of BM T cells is the high proportion of memory T cells. The BM is a crucial organ in mature T-cell traffic and contributes greatly to the homeostatic regulation of peripheral CD8 T cell number. The BM can prime naive T cells and recruit effector T cells, but also that it serves as a site of preferential proliferation for CD4 and CD8 T cells (8).
CROSS TALK BETWEEN T CELLS AND STROMAL CELLS
Within the BM, cells of hematopoietic origin are embedded in a heterogeneous mixture of SCs of mesenchymal origin, which includes fibroblasts, reticular cells and adipocytes. It is well recognized that BM SCs support hematopoiesis by establishing appropriate anatomical niches and secreting specific cytokines and growth factors (10). BM SCs support also the maintenance of mature B lymphocytes, long-lived plasma cells, and possibly naive and memory T cells (11).
Importantly, SCs exert an important suppressive effect on T cells (12). The involved mechanisms have not been completely elucidated. However, SCs have been found to inhibit antigen specific T cell proliferation, and to induce the generation of Tregs (12). Additional mechanisms involve the generation of nitric oxide, which is a potent suppressor of T cell responsiveness (13). Attesting to the potency of SCs as immunosuppressants, SCs have been successfully used to for the treatment of severe graft versus host disease (14) and to blunt skin graft rejection (12). Relevant for bone biology is the capacity of SCs to block T cell activation upon exposure to IFNγ or TNF (13), a finding which suggest that SCs may contribute to moderate T cell dependent bone loss.
The cross talk between SCs and T cells is bidirectional because while SCs suppress T cell function, T cells regulate SC activity. For example T cells produce IL-17, an inflammatory and osteoclastogenic cytokine that regulate SC proliferation, survival and function (15). Another important T cell/SC interaction relevant for bone is the capacity of activated T cells induce SC apoptosis via the Fas/Fas ligand pathway, thus blunting the compensatory increase in bone formation that limits bone loss in ovx mice (16)
Another mediator of T cell-SC crosstalk is the T cell costimulatory molecule CD40L (17). This surface molecule, also known as CD154, exerts its effects by binding to CD40 (18) and several integrins (19,20). CD40 is expressed on antigen presenting cells, hemopoietic progenitors and cells of the osteoblastic lineage (21). The CD40/CD40L system is crucial for many additional functions of the immune system and myelopoiesis. Recently, CD40L has been linked to post natal skeletal maturation because T cells, through the CD40L/CD40 system, promote production of the anti-osteoclastogenic factor OPG by B-cells (22). Consequently, CD40L deficient mice attain a reduced peak bone volume due to stimulated bone resorption (22). Low bone density has also been found in children affected by X-linked hyper-IgM syndrome, a condition in which CD40L production is impaired due to a mutation of the CD40L gene (23). However, mice lacking T cell expressed CD40L are protected against parathyroid hormone (PTH) induced bone loss (24), raising the possibility that CD40L may exert anti resorptive activities in unstimulated conditions, while promoting bone resorption under conditions of bone stress. .
T CELLS IN THE MAINTENANCE OF BASELINE BONE HOMEOSTASIS
It is well established that Infection and inflammation lead to T cell activation, and T cell production of osteoclastogenic cytokines such as RANKL and TNFα (TNF). Indeed activated T cells have been implicated in the bone loss in inflammation, autoimmune disorders (1), periodontitis (25), and in animal models of postmenopausal osteoporosis (26). However, under basal conditions T cells are not considered a significant source of RANKL, and T cell deficient nude mice do not show evidence of diminished RANKL mRNA in their BM (22). Moreover, resting T cells have been shown to blunt OC formation in vitro (27) and may contribute to dampen bone resorption in vivo (22). Indeed depletion of CD4+ and CD8+ T lymphocytes in mice in vivo enhances OC formation by a mechanism involving the complete suppression of osteoprotegerin production (28). Providing further support to this hypothesis others have found that 1,25 dihydroxyvitamin D3 was a more potent inducer of OC formation in cultures of BM from T cell depleted mice than from control mice (28). The bone protective role of resting T cells was however clearly demonstrated by the finding that T cell deficient mice have a significant increased in basal OC number and reduced bone density as compared to controls (29).
As discussed above the mechanisms by which T cells promote basal skeletal integrity appear to involve the regulation of OPG production by B cells by T cells through CD40L/CD40 interactions (22). In vivo, the dominant source of the ligand for CD40 (CD40L) is T cells. Consistent with this notion, animals deficient in CD40 and CD40L expression (CD40 and CD40L KO mice) recapitulate the same bone phenotype as T cell and B cell null mice, displaying diminished BMD and mass, elevated bone resorption and an elevated RANKL/OPG ratio, due to diminished B cell OPG production (22).
ROLE OF T CELLS IN THE MECHANISM OF ACTION OF PTH IN BONE
1. Effects of PTH on bone
PTH plays a critical regulatory role in calcium metabolism. Secreted in response to small decrements in serum ionized calcium, this hormone defends against hypocalcemia, in part by stimulating bone resorption and thereby the release of calcium from the skeleton. In addition to its role in regulating the level of serum calcium, sustained overproduction, or in vivo continuous infusion of PTH (cPTH), is a cause of bone disease. Secondary hyperparathyroidism has been implicated in the pathogenesis of senile osteoporosis (30), and primary hyperparathyroidism, is associated with accelerated bone loss and osteopenia (31). However, when injected daily in humans and animals at low dose, a regimen known as intermittent PTH (iPTH) treatment, the hormone stimulates trabecular and cortical bone formation, leading to marked increases in bone volume and strength. Attesting to potency, iPTH, has been shown to decrease the risk of fractures in humans, and is an FDA approved treatment modality for postmenopausal women and men with osteoporosis (32).
PTH exerts its anabolic activity by binding to the PPR receptor (also known as PTH-1R), a G protein coupled receptor expressed on OBs, osteocytes and SCs (33). Ligand binding to PPR activates the cyclic AMP–dependent protein kinase A, and calcium-dependent protein kinase C signaling pathways. Mice in which the PPR has been ablated by homologous recombination have decreased trabecular bone and increased thickness of cortical bone during fetal development (34). Conversely, osteoblastic expression of the constitutively active PPR increases osteoblastic function in the trabecular and endosteal compartments, and decreases OB activity in the periosteum (35). The net effect of these actions is a substantial increase in trabecular bone volume and a decrease in cortical bone thickness of the long bones (35). Transgenic mice expressing a constitutively active PTH receptor exclusively in osteocytes also exhibit increased bone mass and bone remodeling, as well as reduced expression of the osteocyte-derived Wnt antagonist sclerostin, increased Wnt signaling, increased OC and OB number, and decreased OB apoptosis (36). PTH receptor signaling in osteocytes has been shown to increase bone mass and the rate of bone remodeling through LRP5 dependent and independent mechanisms, respectively (36).
PTH promotes bone formation by increasing the number of OBs (37,38) through multiple effects, including activation of quiescent lining cells (39), increased OB proliferation (40) and differentiation (41), attenuation of pre-OB and OB apoptosis (42,43), and signaling in osteocytes (36). However, the specific contribution of each of these effects to the overall anabolic activity of PTH remains controversial.
PTH signaling has been observed to intersect Wnt pathways in OBs. Wnts are secreted signaling proteins which influence diverse developmental processes. Activation of Wnt signaling induces OB proliferation (44) and differentiation (45), prevents pre-OB and OB apoptosis (46), and augments OB production of OPG (47). Wnt proteins initiate a canonical signaling cascade by binding to receptors of the Frizzled family together with coreceptors, members of the low-density lipoprotein receptor-related protein (LRP) family, LRP5 and LRP6, which results in the stabilization of cytosolic β-catenin. Recently a new member of this family LRP4, has been discovered on OBs and implicated in the mechanism of action of PTH (48). Lrp4 is expressed in bone and cultured osteoblasts and binds Dkk1 and sclerostin in vitro (48). A nuclear complex of beta-catenin and the T-cell factor/lymphoid enhancer factor (TCF/LEF) family of transcription factors then interacts with DNA to regulate the transcription of Wnt target genes (49). Wnt proteins also signal through non-canonical pathways which involve the Src/ERK and Pi3K/Akt cascades (46).
PTH is a canonical Wnt signaling agonist which increases β catenin levels in osteoblastic cells (50). PTH, once bound to PPR, is also capable of forming a complex with LRP6 which results in LRP6 signaling and β catenin activation (51). Thus, PTH activates Wnt signaling in osteoblastic cells through both Wnt ligands dependent and Wnt ligands independent mechanisms. Moreover, PTH down regulates the production of sclerostin, an osteocyte derived Wnt antagonist which blocks Wnt signaling by binding to LRP5 and LRP6 (52,53). Recently, convincing evidence has emerged that PTH receptor signaling in osteocytes and the resulting direct regulation of sclerostin production play a particularly relevant role in the anabolic activity of PTH (36). PTH also regulates Dickkopf-1, a soluble LRP5 and LRP6 signaling inhibitor (50), and Sfrp-4, a factor which binds Wnt proteins thus antagonizing both canonical and non-canonical Wnt signaling (54). Uncertainty remains with regard to the identity and the source of Wnt ligands which activate Wnt signaling in response to PTH treatment are not completely understood.
2. T cells and PTH induced bone loss
T lymphocytes, expresses functional PPR (55), responds to PTH (56), and stimulates OB differentiation (57). Yet a role for T cells in the mechanism of action of PTH has remained undetected for a long period of time in part because transplantation of tumors producing PTH and/or PTHrP in nude mice is known to cause hypercalcemia and increased bone resorption in spite of the absence of T cells in the host (58). Hory et al (59), were the first to report that transplantation of human parathyroid gland fragments from patients with primary and secondary hyperparathyroidism into nude mice fails to stimulate OC formation and bone resorption. The apparent discrepancy between these reports is explained by the higher levels of circulating PTH/PTHrP attained by transplanting PTH/PTHrP producing tumors, as compared to those obtained by transplanting hyperplastic parathyroid tissues.
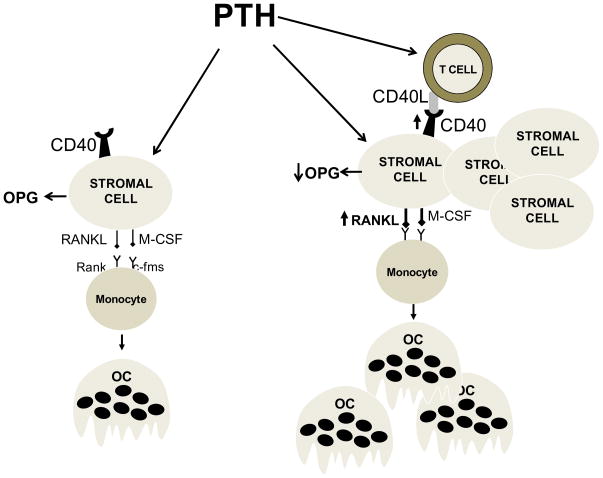
These observations prompted more in depth investigations on the role of T cells as mediators of the pro-resorptive effect of cPTH treatment. We found that an infusion of cPTH that mimics hyperparathyroidism fails to induce OC formation, bone resorption and cortical bone loss in mice lacking T cells (24). By contrast, cPTH equally stimulated bone formation in T cell replete and T cell deficient mice. These studies further revealed the existence of a cross-talk between T cells and SCs mediated by the CD40L/CD40 signaling system (Figure 1). T cells provide proliferative and survival cues to SCs and sensitize SCs to PTH through CD40L, a surface molecule of activated T cells that induces CD40 signaling in SCs. An important element of this regulatory loop is the capacity of PTH to upregulate the expression of CD40 in SC from T cell replete mice but not from T cell deficient mice (24). Thus T cells contribute to the CD40L/CD40 mediated exchange of information between T cells and SCs in two ways. Firstly by providing CD40L. Secondly, by upregulating the expression of CD40 on SCs. As a result, mice lacking T cells or T cell expressed CD40L have lower number of SCs. Furthermore, these SCs produce lower amount of RANKL and have an even smaller suppression of OPG secretion in response to PTH. Therefore, SCs from T cell deficient mice have a lower capacity to support OC formation in vivo and in vitro. The alteration in SC function is the ultimate reason why deletion of T cells or T cell expressed CD40L blunts the bone catabolic activity of PTH (24).
Figure 1.
Schematic representation of the role of T cells in the mechanism by which continuous PTH stimulates OC formation. In the absence of T cells (left panel) BM SCs are fewer in number (not shown), and exhibit a blunted capacity to support PTH induced OC formation due to diminished production of RANKL and continuous production of the RANKL decoy receptor OPG. In the presence of T cell expressed CD40L (right panel) the pool of SCs in the BM is 2-fold larger. Furthermore, CD40L/CD40 signaling increases the osteoclastogenic activity of SCs by augmenting the SC production of RANKL and blunting their secretion of OPG. The result is a potentiation of the capacity of PTH to stimulate the formation of OCs.
Preliminary studies have also shown that cPTH increases the T cell production of TNF. Attesting to the relevance of this cytokine, mice lacking the production of TNF by T cells are protected against the bone loss induced by cPTH treatment. If confirmed, these observation would suggest the existence of a second regulatory mechanism by which T cells potentiate the bone catabolic activity of cPTH.
In spite of a smaller SC pool and a blunted SC osteoclastogenic activity, T cell deficient mice possess a normal anabolic response to cPTH. This may suggest that the SCs which produce osteoclastogenic factors and support OC formation are not the same cells that differentiate into OBs, the cells that form osteoid and mineralize it, a hypothesis recently proposed by O’Brien et al (60). Indeed osteoclastogenic SCs express intercellular adhesion molecule-1 (ICAM-1) which is necessary for binding to OC precursors (61), while matrix producing osteoblastic cells are ICAM-1 negative (62). However, it is also possible that a defective stimulation of bone formation might emerge in T cell deficient mice in response to long-term cPTH treatment.
Antigen activated Th2 cells have been shown to produce PTH, which contributes to maintain the anabolic activity of OBs under inflammatory conditions (63). However mice lacking T cells are not protected against the catabolic activity of cPTH because of the absence of Th2 cell produced PTH. In fact, activated Th2 cells mitigate bone resorption by lowering the RANKL/OPG ratio largely through PTH independent mechanisms (63).
Cortical bone represents about 80 % of the entire skeletal mass and cortical volume and thickness are major predictors of bone strength and fracture risk (64). Thus, the T cell dependent bone effects of PTH are relevant for the risk of long bone fractures associated with primary and secondary hyperparathyroidism.
PTH does not increase antigen presentation and T cell activation. However, since the expression of CD40L is a feature of activated T cells, it is likely that baseline Ag presentation, which leads to spontaneous activation of T cells in the BM, may be required for PTH to induce its catabolic effect. Indeed the BM contains a relative large number of memory T cells which have increased reactivity to self peptides and foreign Antigens. T cell activation takes place in the presence of several signals. The first is the presentation to the T cell receptor (TCR) of Ag derived peptides bound to MHC molecules which are expressed on the surface of Ag presenting cells (APCs). A second set of signals is provided by the interaction of the costimulatory molecules on APCs with the T cell expressed counter receptors such as CD28 and CD40L. A pharmacological approach to test the role of costimulation is provided by Abatacept, an agent approved for the treatment of Rheumatoid Arthritis which also blocks the bone loss induced by ovariectomy (65). We found that PTH induced bone loss is prevented using this inhibitor of costimulation. In the same study we found that arrest of Ag presentation through silencing of class I and class II MHC-TCR interactions prevents the capacity of cPTH to induce cortical bone loss. These findings provide further evidence of a novel regulatory link between the immune system and the mechanism of action of PTH.
3. Role of T cells in the anabolic activity of intermittent PTH treatment
The hypothesis that T cells may play a role in the anabolic response to iPTH was first proposed by Pettway et al (66), who investigated the effects of daily injections of PTH for up to 7 weeks on the growth of ectopic “ossicles” implanted in nude mice. This investigation disclosed that iPTH increased the bone content of the implanted WT ossicles, a structure which contains normal BM, but failed to induce vertebral bone growth in host nude mice, a strain devoid of T cells (66).
These observations were followed by our investigation on whether T cells contribute to the anabolic response to iPTH. Studies conducted in four strains of T cell deficient mice (TCRβ −/−, RAG2 −/−, class I and II MHC double KO mice, and nude mice) revealed that mice lacking T cells, exhibit a blunted increase in bone formation and trabecular bone volume in response to iPTH (55). Furthermore, adoptive transfer of T cells into T cell deficient mice restored a normal response to iPTH. T cells were found to augment the capacity of iPTH to improve architecture in trabecular but not in cortical bone. Although the reason of this selectivity is unknown, a lack of access of T cells to cortical surfaces is not a likely explanation, as T cells reach endosteal and periosteal bone surface through blood vessels and recirculate in and out of the BM (67).
The effects of iPTH on bone volume and the indices of bone strength measurable by μCT are blunted but not abolished in the absence of T cells. By contrast, direct measurements of bone strength by 4-point bending revealed that the capacity of iPTH to improve bone strength is completely dependent on the presence of T cells. Although the reason for this discrepancy is unknown, is possible that T cells might be required to improve the material property of bone. It should also be noted that while the stimulation of bone formation induced by iPTH was severely blunted in T cell deficient mice, T cells did not improve the capacity of cPTH to stimulate bone formation. The reason for this critical difference remains to be determined.
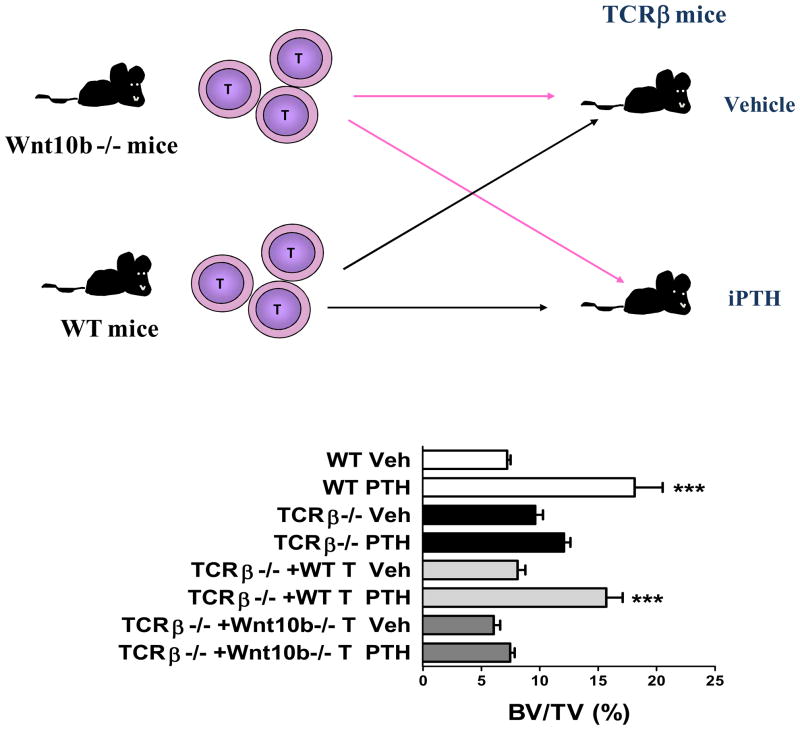
With regard of the mechanism by which T cells potentiate the bone anabolic activity of iPTH, studies have disclosed that in the absence of T cells iPTH is unable to increase the commitment of SCs to the osteoblastic lineage, induce OB proliferation and differentiation, and mitigate OB apoptosis. All of these actions of PTH were found to hinge on the capacity of T cells to activate Wnt signaling in osteoblastic cells (55). Although it is well established that Wnt activation is a key mechanism by which iPTH expands the osteoblastic pool, little information is available on the nature and the source of the Wnt ligand required to activate Wnt signaling in OBs. We have found that PTH stimulates BM CD8+ T cells to produce large amounts of Wnt10b (55), a Wnt protein which activates Wnt signaling in SCs and OBs, thus increasing OB proliferation, differentiation and life-span Treatment with iPTH also caused a small increase in the production of Wnt10b by BM CD4+ cells which was associated with a slightly diminished anabolic response in class II MHC −/− mice, suggesting that production of Wnt10b by CD4+ cells contributes, in small part, to the anabolic activity of iPTH. The relevance of CD8+ cells was demonstrated by the inability of iPTH to promote bone anabolism in class I MHC −/− mice, a strain that lacks CD8+ cells (55). Additional studies revealed that iPTH does not improve bone architecture in T cell deficient mice reconstituted with CD4+ cells, while it does so in mice adoptively transferred with CD8+ cells (55). The pivotal role of T cell produced Wnt10b was revealed by the hampered effect of iPTH on bone volume in TCRβ −/− mice reconstituted with T cells from Wnt10b −/− mice (Figure 2). It is likely that iPTH directly targets CD8+ T cells and stimulates their production of Wnt10b. This hypothesis is supported by the strong expression of PPR in CD8+ T cells, and the capacity of in vitro PTH treatment to promote cAMP production and Wnt10b expression in CD8+ murine and human lymphocytes.
Figure 2.
Analysis of the effects of iPTH treatment in WT mice, TCRβ −/− mice, and TCRβ −/− mice subjected to adoptive transfer of T cells derived from WT mice and Wnt10b −/− mice 1 week before initiation of iPTH. The upper panel shows a schematic representation of the experimental design. The bottom panel shows trabecular bone volume (BV/TV) as measured by μCT. *** = p<0.001 compared to the corresponding vehicle treated group.
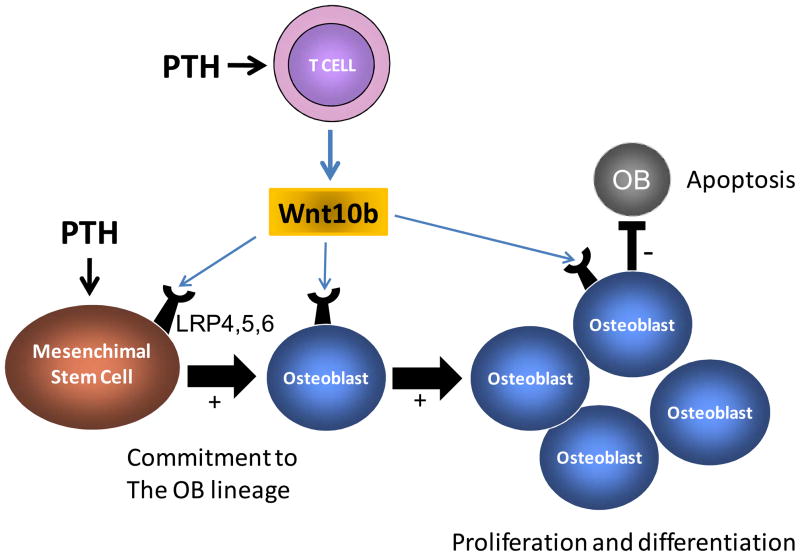
Together the data indicate that CD8+ T cells potentiates the anabolic activity of PTH by providing Wnt10b, which is a critical Wnt ligand required for activating Wnt signaling in osteoblastic cells Therefore in the absence of CD8+ cells, stimulation of osteoblastic cells by PTH is not sufficient to elicit maximal Wnt activation due to the lack of a critical Wnt ligand (Figure 3). The residual bone anabolic activity of PTH observed in T cell deficient mice is presumably due to ligand independent activation of LRP6 (51), and suppressed production of sclerostin (36,52,53).
Figure 3.
Schematic representation of the role of T cells in the mechanism by which intermittent PTH treatment stimulates bone formation. PTH stimulates T cells to secrete Wnt10b, a Wnt ligand required to activate Wnt signaling in SCs and OBs. In the presence of T cell produced Wnt10b, stimulation of osteoblastic cells by PTH result in the activation of the Wnt signaling pathway. This event leads to increased commitment of mesenchimal stem cells to the osteoblastic lineage, increased osteoblast proliferation and differentiation, and decreased osteoblast apoptosis.
While in vitro PTH treatment increased Wnt10b production by all T cells, iPTH upregulated Wnt10b production only by BM T cells. This diversity might be explained by the different dose and time of exposure to PTH. However, since adoptive transfer of spleen T cells into TCRβ −/− mice was followed by a restoration of a full responsiveness to iPTH, the data suggest that the capacity of T cells to upregulate their production of Wnt10b in response to iPTH is not an intrinsic feature of T cells, but rather is induced by environmental cues.
The anabolic activity of iPTH is not identical in all strains of T cell deficient mice. In fact, while some strains had no increase in bone volume in response to PTH, other exhibited a blunted but not a completely absent response. Osteoblastic cells produce several bone anabolic Wnt ligands including Wnt10b, Wnt7a and Wnt3b (68,69). These factors are likely to contribute to the T cell independent anabolic activity of iPTH, and quantitative differences in their production may account, in part, to the strain dependent variability in the response to iPTH observed herein. Furthermore, the magnitude of the anabolic response to iPTH in T cell null mice may be related to a strain and age dependent capacity of iPTH to inhibit the bone cells production of Wnt inhibitors such as sclerostin, (52,53), Dickkopf-1 (50) and Sfrp-4 (54). These factors have been shown to contribute to the anabolic activity of iPTH through T cell independent mechanisms. The enhancement of bone formation induced by iPTH is accompanied by a stimulation of bone resorption which is driven by increased production of RANKL and decreased release of OPG in the bone microenvironment. The direct effects of PTH on RANKL/OPG production are mitigated, in part, by the iPTH induced activation of β catenin in OBs, as this transcriptional regulator stimulates their production of OPG (47) and represses that of RANKL (70). The latter is one of the mechanisms which prevent bone resorption from offsetting the anabolic activity of iPTH.
Osteoblastic cells from WT mice treated with iPTH in vivo exhibited increased commitment to the osteoblastic lineage, proliferation, differentiation and life span in vitro, as compared to the corresponding cells from T cell deficient mice. Thus, T cells, like PTH, affect all aspects of OB life cycle. Remarkably, these differences were demonstrated in OBs purified from BM cultured for 7 days without the addition of PTH, suggesting that in vivo the hormone regulates early commitment steps of SCs and their osteoblastic progeny through T cell produced Wnt10b, and that these steps are not reversed by the absence of PTH and T cells in vitro. This model is consistent with the capacity of Wnt signaling to guide cell fate determination (71). A similar paradigm has been described in ovariectomized mice, a model where estrogen withdrawal in vivo leads to the formation of SCs which exhibit an increased osteoclastogenic activity which persists in vitro for 4 weeks (72).
T CELLS AND THE BONE LOSS INDUCED BY MENOPAUSE
1. Mechanisms by which menopause causes bone loss
The decline of ovarian function at menopause results in decreased production of estrogen and a parallel increase in FSH levels. The combined effects of estrogen deprivation and raising FSH production cause a marked stimulation of bone resorption (73) and a period of rapid bone loss which is central for the onset of postmenopausal osteoporosis. In mice the acute effects of estrogen deprivation and FSH elevation are modeled by ovariectomy (ovx) which, like natural menopause in humans, stimulates bone resorption by increasing osteoclast (OC) formation (26) and lifespan (74,75). The net bone loss caused by ovx is limited, in part, by an increase in bone formation resulting from stimulated osteoblastogenesis (76). This compensation is fueled by an expansion of the pool of BM SCs, increased commitment of such pluripotent precursors toward the osteoblastic lineage (76), and enhanced proliferation of early OB precursors (77). The capacity of estrogen deficiency to stimulate OB apoptosis (78,79) and to extend OC lifespan (74,75) is the likely reason why after ovx bone formation does not increase as much as resorption. The stimulatory effect of ovx on SCs is equally relevant for osteoclastogenesis as one of the consequences of estrogen deprivation is the formation of osteoblastic cells with an increased osteoclastogenic activity (72), that is the capacity to support OC formation.
It is well established that estrogen withdrawal leads to two distinct forms of bone loss (80). The first is a rapid loss of trabecular and cortical bone due to stimulation of bone resorption which occurs in the first few weeks after ovx, driven by increased osteoclastogenesis and decreased OC apoptosis (26,81). This phase is followed by a slower but more prolonged loss of mainly cortical bone, which is due to incomplete refilling of the resorption cavities, which is in turn secondary to insufficient OB activity and lifespan (81). An expansion of the osteoclastic pool is therefore the key mechanism responsible for the bone loss which occurs early after ovx. One cytokine responsible for augmented osteoclastogenesis during estrogen deficiency is TNF, and its relevance has been demonstrated in multiple animal models. For example, ovariectomy (ovx) fails to induce bone loss in TNF knockout mice and in mice lacking the p55 TNF receptor (82). Likewise, transgenic mice insensitive to TNF due to the overexpression of a soluble TNF receptor (83), and mice treated with the TNF inhibitor TNF binding protein (84) are protected from ovx induced bone loss. The presence of increased levels of TNF in the BM of ovx animals and in the conditioned media of peripheral blood cells of postmenopausal women is well documented (85–87).
Attesting to the relevance of T cells in estrogen deficiency induced bone loss in vivo, are reports from our laboratory that ovx fails to induce trabecular and cortical bone loss in nude mice (82,88–90), WT mice deleted of T cells by injection of anti T cells antibodies (91), and WT mice treated with Abatacept (65) an agent which blocks T cell costimulation and induces T cell anergy and apoptosis (92,93). An independent confirmation of the role of T cells in ovx induced bone loss was provided by Yamaza et al who reported that ovx fails to induce bone loss in nude mice, and in WT mice in which T cell activation was blocked by aspirin (16). Studies have also shown that menopause increases T cell activations and T cell production of TNF and RANKL in humans (94). By contrast, Lee et al (95) showed that nude mice are protected against the loss of cortical, but not trabecular bone induced by ovx. In the same study other strains of T cell, and T and B cell deficient mice were found to lose either trabecular or cortical bone after ovx (95). The discrepancy between our reports (65,82,88–91) and that of Lee at al (95) is likely explained by differences in the experimental design, the lack of B cells in some models, and compensation mechanisms.
T cells are key inducers of bone-wasting because ovx increases T cell TNF production to a level sufficient to augment RANKL-induced osteoclastogenesis (88). The specific relevance of T cell TNF production in vivo was demonstrated by the finding that while reconstitution of nude recipient mice with T cells from wild type (WT) mice restores the capacity of ovx to induce bone loss, reconstitution with T cells from TNF deficient mice does not (82). T cell produced TNF may further augment bone loss by stimulating T cell RANKL production.
2. Mechanisms of estrogen regulation of T cell TNF production
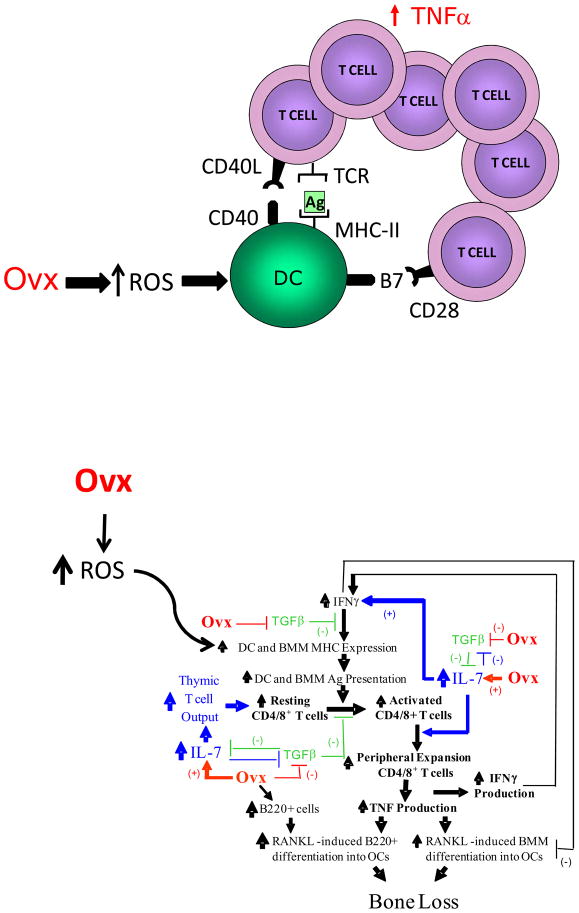
Ovx upregulates T cell TNF production primarily by increasing the number of TNF producing T cells (82). This is the result of a complex pathway which involves the thymus and the BM. The mechanisms by which estrogen deficiency expands the pool of TNF producing T cells are summarized in Figure 4. In the BM, ovx promotes T cell activation, resulting in increased T cell proliferation and life span through antigen presentation by macrophages and dendritic cells (DCs) (96,97). This process is due to the ability of estrogen deficiency to upregulate their expression of MHCII (96–98). The question thus arises as to the nature of the antigens. Estrogen deficiency is likely to increase T cell reactivity to a pool of self and foreign antigens physiologically present in healthy animals and humans. This is consistent with the fact that T cell clones expressing TCRs directed against self antigens not expressed in the thymus, survive negative selection during T cell maturation (99). Such clones (“autoreactive” or “self-reactive” T cells) reside in peripheral lymphatic organs of adult individuals. In addition, foreign antigens of bacterial origin are physiologically absorbed in the gut. As these peptides come into contact with immune cells locally and systemically, they induce a low grade T cell activation. Thus, a moderate immune response is constantly in place in healthy humans and rodents due to presentation by MHCII and MHCI molecules of both self and foreign peptides to CD4+ and CD8+ T cells (100). This autoreactive response is thought to be essential for immune cell survival and renewal (101).
Figure 4.
Schematic representation of the mechanism by which ovx increases antigen presentation through ROS. The upper diagram shows that ovx increases the activity of DCs through ROS generation, leading to increased T cell activation, T cell proliferation and secretion of TNF through a mechanism involving the T cell receptor and costimulatory molecules. The bottom diagram shows in detail the cells and the cytokines by which ovx leads to T cell production of TNF and bone loss.
The effects of ovx on antigen presentation and the resulting changes in T cell activation, proliferation and lifespan are explained by a stimulatory effect of ovx on the expression of the gene encoding Class II Transactivator (CIITA). The product of CIITA is a non-DNA binding factor induced by IFNγ that functions as a transcriptional coactivator at the MHC II promoter (102). Increased CIITA expression in macrophages results from ovx-mediated increases in both T cell IFNγ production and the responsiveness of CIITA to IFNγ (96). This factor was initially described as an anti-osteoclastogenic cytokine because is a potent inhibitor of osteoclastogenesis in vitro (103). The notion that IFNγ is an inhibitor of bone resorption was reinforced by the finding that silencing of IFNγR−/− signaling leads to a more rapid onset of collagen induced arthritis and bone resorption (104) as compared to WT controls, and by the report that IFNγ decreases serum calcium and osteoclastic bone resorption in nude mice (105). However, observations in humans and in experimental models of disease indicate that IFNγ promotes bone resorption and causes bone loss in a variety of conditions. Studies with IFN −/− and IFNR −/− mice have revealed that among these conditions are estrogen deficiency and endotoxin-induced bone disease (90,96). Mice lacking IFNγ production are also protected against infection induced alveolar bone loss (106), while in erosive tubercoloid leprosy and psoriatic arthritis IFNγ production correlates positively with tissue destruction (107). In addition, randomized controlled trials have shown that IFNγ does not prevent bone loss in patients with RA (108), nor the bone wasting effect of cyclosporin A (109). Furthermore, IFNγ has been reported to be efficacious in the treatment of osteopetrosis through restoration of bone resorption, both in humans (110) and rodents (111). These latter findings conclusively demonstrate that in some conditions, including estrogen deficiency, the net effect of IFNγ in vivo is that of stimulating osteoclastic bone resorption.
The complex effects of IFNγ can be explained by the fact that IFNγ influences OC formation both via direct and indirect effects (90). IFNγ directly blocks OC formation through targeting of maturing OC (112). This effect is best observed in vitro (103). However, IFNγ is also a potent inducer of antigen presentation and thus of T cell activation. Therefore, when IFNγ levels are increased in vivo, activated T cells secrete pro-osteoclastogenic factors and this activity offsets the anti-osteoclastogenic effect of IFNγ.
One mechanism by which estrogen deficiency upregulates the production of IFNγ is through TGFβ. Estrogen has a direct stimulatory effect on the production of this factor which is mediated through direct binding of estrogen/ER complex to an ERE region in the TGFβ promoter (113). TGFβ is recognized a powerful repressor of T cell activation. Indeed, TGFβ exerts strong immunosuppressive effects by inhibiting the activation and the proliferation of T cells and their production of proinflammatory cytokines, including IFNγ. Studies in a transgenic mouse which expresses a dominant negative form of the TGFβ receptor exclusively in T cells have allowed the significance of the repressive effects of this cytokine on T cell function in the bone loss associated with estrogen deficiency to be established (89). This strain, known as CD4dnTGFβRII, is severely osteopenic due to increased bone resorption. More importantly, mice with T cell-specific blockade of TGFβ signaling are completely resistant to the bone sparing effects of estrogen (89). This phenotype results from a failure of estrogen to repress IFNγ production which, in turn, leads to increased T cell activation and T cell TNF production. Gain of function experiments confirmed that elevation of the systemic levels of TGFβ prevents ovx-induced bone loss and bone turnover (89).
Another mechanism by which estrogen regulates IFNγ and TNF production is by repressing the production of IL-7, a potent lymphopoietic cytokine and inducer of bone destruction in vivo (114). Importantly levels of IL-7 are significantly elevated following ovx (115–117) and in vivo IL- 7 blockade, using neutralizing antibodies, is effective in preventing ovx induced bone destruction (115) by suppressing T cell expansion and TNF and IFNγ production (116). The relevance of IL-7 in the mechanism of ovx induced bone loss has been confirmed, in part, by another investigation showing that ovx does not induce cortical bone loss in IL-7 KO mice (118). Indeed, the elevated BM levels of IL-7 contribute to the expansion of the T cell population in peripheral lymphoid organs through several mechanisms. Firstly, IL-7 directly stimulates T cell proliferation by lowering tolerance to weak self antigens. Secondly, IL-7 increases antigen presentation by upregulating the production of IFNγ. Thirdly, IL-7 and TGFβ inversely regulate each other’s production (119,120). The reduction in TGFβ signaling, characteristic of estrogen deficiency may serve to further stimulate IL-7 production, thus driving the cycle of osteoclastogenic cytokine production and bone wasting. In estrogen deficiency IL-7 compounds bone loss by suppressing bone formation thus uncoupling bone formation from resorption.
3. Estrogen, oxidative stress and T cell dependent bone loss
Reactive oxygen species (ROS) play an important role in postmenopausal bone loss by generating a more oxidized bone microenvironment (121,122). In vivo support of this hypothesis is found from experiments in which ovx induces oxidative stress and impairs the expression of antioxidants (65,81,123,124). Furthermore, administration of antioxidants prevent ovx induced bone loss (65,123,125), while depletion of glutathione by buthionine sulfoximine (BSO), which inhibits glutathione synthesis, enhances bone loss (123). Bone loss caused by BSO has significant similarities to bone loss induced by estrogen deficiency, as both processes are TNF-dependent (126). Moreover, soluble TNF receptors prevent both bone loss and the rise in thiol-based antioxidants characteristic of estrogen deficiency (126). The negative impact of oxidative stress is offset, in part, by oxidative defense mechanisms. Among them is the retention of FoxO transcription factors into the nucleus and the induction of FoxO mediate transcription of antioxidant enzymes, and genes involved in cell cycle, DNA repair and apoptosis (81). The bone wasting effects of ROS has indeed been linked to its capacity to activate the association of FoxO to β-catenin and promote FoxO mediate transcription at the expenses of β-catenin/T cell specific transcription factor mediated transcription (81,127). This matter has been addressed and reviewed extensively elsewhere (81,125,127–130) and will not be discussed herein.
An alternative explanation to link oxidative to ovx induced bone loss is through immune mediated mechanisms. ROS are indeed important stimulators of antigen presentation by DCs, and DC induced T cell activation. Conversely, anti-oxidants potently inhibit DC differentiation and their ability to activate T cells (131,132), in part by suppressing expression of MHC class II and costimulatory molecules in response to antigen (133). N-Acetyl-Cystein (NAC), which acts as an intracellular scavenger by restoring intracellular concentration of glutathione, can block DC maturation (134) and DC-mediated T cell activation (135). ROS are also generated upon DC interaction with T cells (136) and can reduce T cell lifespan by stimulating T cell apoptosis (137). Interestingly, NAC treatment has been shown to protect against ovx-induced bone loss (65,123,128). These data are consistent with studies demonstrating that NAC treatment blunts ovx-induced DC activation in the BM, decreases antigen presentation and expression of costimulatory molecules, and prevents T cell activation and TNF production (65).
We have found that ovx increases the production of ROS in the BM, which lead to expansion of mature DCs which express the costimulatory molecule CD80, and increased DC mediated Ag presentation (65). This effect is tissue specific because ovx does not increase oxidative stress outside the BM. Attesting to the relevance of oxidative stress we have found that treatment with antioxidants blocks the stimulatory effects of ovx on DC and T cell activation and, and prevents ovx induced bone loss (65). Multiple enzymatic pathways regulate the intracellular redox state through modulation of ROS levels (138). Ovx blunts the BM levels of GSH, a critical ROS scavenger, and those of APE1/Ref-1 and Prx-1, proteins which contributes to limit the production of intracellular ROS (139). Like the activation of DCs, the effects of ovx on the redox state are spatially limited to the BM a phenomenon previously noted by Lean et al (123).
The finding that ovx increases the expression of the costimulatory molecule CD80 on DCs, suggest that the process of costimulation plays an important role in ovx induced bone loss. This was confirmed by the finding that ovx induced bone loss is completely blocked by CTLA4-Ig (Abatacept) (65) an agent which blocks T cell costimulation and induces T cell anergy and apoptosis by binding to CD80 (and CD86) and blocking their interaction with CD28, (92,93). Abatacept also blocked the increase in T cell activation, proliferation and TNF production resulting from E withdrawal. Taken together, these data suggest a model for ovx-induced bone loss in which estrogen deficiency lowers antioxidant levels, thereby increasing ROS, which in turn induces TNF expression by OCs directly, as well as by stimulating the APC-induced expansion of TNF-producing T cells central to bone destruction.
CONCLUSIONS
Remarkable progress has been made in understanding how T cells participate in the regulation of bone remodeling in health and disease. In parallel with exciting discoveries about molecular and signaling pathways by which T cells and their products control OC and OB development and function, there has a been the recognition of specialized function of distinct T cell populations such as Tregs and Th17. Progress has been made in recognizing that T cells play an unexpected role in the function of major calciotrophic and reproductive hormones such as PTH and estrogen, and therefore in common and clinically relevant forms of bone loss such postmenopausal osteoporosis and hyperparathyroidism. Much remains to be done especially in translating observations accrued in experimental animals into studies in humans. Strategies to utilize T cells/bone cells interactions as the bases of new therapies also remain to be developed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402(6759):304–9. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 2.Mak TW, Ferrick DA. The gammadelta T-cell bridge: linking innate and acquired immunity. Nat Med. 1998;4(7):764–5. doi: 10.1038/nm0798-764. [DOI] [PubMed] [Google Scholar]
- 3.Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–85. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 4.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4(3):231–7. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 5.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9(6):650–7. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 6.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203(12):2673–82. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotake S, Nanke Y, Mogi M, Kawamoto M, Furuya T, Yago T, Kobashigawa T, Togari A, Kamatani N. IFN-gamma-producing human T cells directly induce osteoclastogenesis from human monocytes via the expression of RANKL. Eur J Immunol. 2005;35(11):3353–63. doi: 10.1002/eji.200526141. [DOI] [PubMed] [Google Scholar]
- 8.Di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 2005;26(7):360–366. doi: 10.1016/j.it.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Di Rosa F, Santoni A. Memory T-cell competition for bone marrow seeding. Immunology. 2003;108(3):296–304. doi: 10.1046/j.1365-2567.2003.01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6(2):93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 11.Tokoyoda K, Zehentmeier S, Chang HD, Radbruch A. Organization and maintenance of immunological memory by stroma niches. Eur J Immunol. 2009;39(8):2095–9. doi: 10.1002/eji.200939500. [DOI] [PubMed] [Google Scholar]
- 12.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–36. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 13.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141–50. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–86. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 15.Huang W, La Russa V, Alzoubi A, Schwarzenberger P. Interleukin-17A: a T-cell-derived growth factor for murine and human mesenchymal stem cells. Stem Cells. 2006;24 (6):1512–8. doi: 10.1634/stemcells.2005-0156. [DOI] [PubMed] [Google Scholar]
- 16.Yamaza T, Miura Y, Bi Y, Liu Y, Akiyama K, Sonoyama W, Patel V, Gutkind S, Young M, Gronthos S, Le A, Wang CY, Chen W, Shi S. Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS ONE. 2008;3 (7):e2615. doi: 10.1371/journal.pone.0002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–35. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 18.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–28. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 19.Andre P, Prasad KS, Denis CV, He M, Papalia JM, Hynes RO, Phillips DR, Wagner DD. CD40L stabilizes arterial thrombi by a beta3 integrin--dependent mechanism. Nat Med. 2002;8(3):247–52. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 20.Leveille C, Bouillon M, Guo W, Bolduc J, Sharif-Askari E, El-Fakhry Y, Reyes-Moreno C, Lapointe R, Merhi Y, Wilkins JA, Mourad W. CD40 ligand binds to alpha5beta1 integrin and triggers cell signaling. J Biol Chem. 2007;282(8):5143–51. doi: 10.1074/jbc.M608342200. [DOI] [PubMed] [Google Scholar]
- 21.Ahuja SS, Zhao S, Bellido T, Plotkin LI, Jimenez F, Bonewald LF. CD40 ligand blocks apoptosis induced by tumor necrosis factor alpha, glucocorticoids, and etoposide in osteoblasts and the osteocyte-like cell line murine long bone osteocyte-Y4. Endocrinology. 2003;144(5):1761–9. doi: 10.1210/en.2002-221136. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Toraldo G, Li A, Yang X, Zhang H, Qian WP, Weitzmann MN. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109(9):3839–48. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Granados E, Temmerman ST, Wu L, Reynolds JC, Follmann D, Liu S, Nelson DL, Rauch F, Jain A. Osteopenia in X-linked hyper-IgM syndrome reveals a regulatory role for CD40 ligand in osteoclastogenesis. Proc Natl Acad Sci U S A. 2007;104(12):5056–61. doi: 10.1073/pnas.0605715104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao Y, Wu X, Terauchi M, Li JY, Grassi F, Galley S, Yang X, Weitzmann MN, Pacifici R. T cells potentiate PTH-induced cortical bone loss through CD40L signaling. Cell Metab. 2008;8(2):132–45. doi: 10.1016/j.cmet.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng YT, Nguyen H, Gao X, Kong YY, Gorczynski RM, Singh B, Ellen RP, Penninger JM. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Invest. 2000;106(6):R59–67. doi: 10.1172/jci10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116(5):1186–94. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.John V, Hock JM, Short LL, Glasebrook AL, Galvin RJ. A role for CD8+ T lymphocytes in osteoclast differentiation in vitro. Endocrinology. 1996;137(6):2457–63. doi: 10.1210/endo.137.6.8641199. [DOI] [PubMed] [Google Scholar]
- 28.Grcevic D, Lee SK, Marusic A, Lorenzo JA. Depletion of CD4 and CD8 T lymphocytes in mice In vivo enhances 1, 25- dihydroxyvitamin D(3)-stimulated osteoclast-like cell formation In vitro by a mechanism that is dependent on prostaglandin synthesis. J Immunol. 2000;165(8):4231–8. doi: 10.4049/jimmunol.165.8.4231. [DOI] [PubMed] [Google Scholar]
- 29.Toraldo G, Roggia C, Qian W-P, Pacifici R, Weitzmann MN. IL-7 induces bone loss in vivo by induction of receptor activator of nuclear factor kappa B ligand and tumor necrosis factor alpha from T cells. 2003;100:125–130. doi: 10.1073/pnas.0136772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riggs BL, Melton LJ. Medical progress: involutional osteoporosis. N Eng J Med. 1986;314:1676–1684. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- 31.Potts J. Primary hyperparathyroidism. In: Krane LVAaS., editor. Metabolic Bone Diseases. Vol. 1. Academic Press; San Diego: 1998. pp. 411–442. #rd ed. [Google Scholar]
- 32.Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med. 2007;357(9):905–16. doi: 10.1056/NEJMra067395. [DOI] [PubMed] [Google Scholar]
- 33.Qin L, Raggatt LJ, Partridge NC. Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol Metab. 2004;15(2):60–5. doi: 10.1016/j.tem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Lanske B, Amling M, Neff L, Guiducci J, Baron R, Kronenberg HM. Ablation of the PTHrP gene or the PTH/PTHrP receptor gene leads to distinct abnormalities in bone development. J Clin Invest. 1999;104(4):399–407. doi: 10.1172/JCI6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, Kronenberg HM, Baron R, Schipani E. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest. 2001;107(3):277–86. doi: 10.1172/JCI11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Brien CA, Plotkin LI, Galli C, Goellner JJ, Gortazar AR, Allen MR, Robling AG, Bouxsein M, Schipani E, Turner CH, Jilka RL, Weinstein RS, Manolagas SC, Bellido T. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS ONE. 2008;3(8):e2942. doi: 10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindsay R, Cosman F, Zhou H, Bostrom MP, Shen VW, Cruz JD, Nieves JW, Dempster DW. A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. J Bone Miner Res. 2006;21(3):366–73. doi: 10.1359/JBMR.051109. [DOI] [PubMed] [Google Scholar]
- 38.Ma YL, Zeng Q, Donley DW, Ste-Marie LG, Gallagher JC, Dalsky GP, Marcus R, Eriksen EF. Teriparatide increases bone formation in modeling and remodeling osteons and enhances IGF-II immunoreactivity in postmenopausal women with osteoporosis. J Bone Miner Res. 2006;21(6):855–64. doi: 10.1359/jbmr.060314. [DOI] [PubMed] [Google Scholar]
- 39.Dobnig H, Turner RT. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology. 1995;136(8):3632–8. doi: 10.1210/endo.136.8.7628403. [DOI] [PubMed] [Google Scholar]
- 40.Pettway GJ, Meganck JA, Koh AJ, Keller ET, Goldstein SA, McCauley LK. Parathyroid hormone mediates bone growth through the regulation of osteoblast proliferation and differentiation. Bone. 2007 doi: 10.1016/j.bone.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng XW, Liang XG, Birchman R, Wu DD, Dempster DW, Lindsay R, Shen V. Temporal expression of the anabolic action of PTH in cancellous bone of ovariectomized rats. J Bone Miner Res. 1996;11(4):421–9. doi: 10.1002/jbmr.5650110402. [DOI] [PubMed] [Google Scholar]
- 42.Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104(4):439–46. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, Weinstein RS, O’Brien CA, Manolagas SC, Jilka RL. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003;278(50):50259–72. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- 44.Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157(2):303–14. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40(6):1434–46. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem. 2005;280(50):41342–51. doi: 10.1074/jbc.M502168200. [DOI] [PubMed] [Google Scholar]
- 47.Glass DA, 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8(5):751–64. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 48.Choi HY, Dieckmann M, Herz J, Niemeier A. Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS One. 2009;4(11):e7930. doi: 10.1371/journal.pone.0007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382(6592):638–42. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 50.Kulkarni NH, Halladay DL, Miles RR, Gilbert LM, Frolik CA, Galvin RJ, Martin TJ, Gillespie MT, Onyia JE. Effects of parathyroid hormone on Wnt signaling pathway in bone. J Cell Biochem. 2005;95(6):1178–90. doi: 10.1002/jcb.20506. [DOI] [PubMed] [Google Scholar]
- 51.Wan M, Yang C, Li J, Wu X, Yuan H, Ma H, He X, Nie S, Chang C, Cao X. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev. 2008;22(21):2968–79. doi: 10.1101/gad.1702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC, Jilka RL. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146(11):4577–83. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 53.Keller H, Kneissel M. SOST is a target gene for PTH in bone. Bone. 2005;37(2):148–58. doi: 10.1016/j.bone.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 54.Qin L, Qiu P, Wang L, Li X, Swarthout JT, Soteropoulos P, Tolias P, Partridge NC. Gene expression profiles and transcription factors involved in parathyroid hormone signaling in osteoblasts revealed by microarray and bioinformatics. J Biol Chem. 2003;278(22):19723–31. doi: 10.1074/jbc.M212226200. [DOI] [PubMed] [Google Scholar]
- 55.Terauchi M, Li JY, Bedi B, Baek KH, Tawfeek H, Galley S, Gilbert L, Nanes MS, Zayzafoon M, Guldberg R, Lamar DL, Singer MA, Lane TF, Kronenberg HM, Weitzmann MN, Pacifici R. T lymphocytes amplify the anabolic activity of parathyroid hormone through Wnt10b signaling. Cell Metab. 2009;10(3):229–40. doi: 10.1016/j.cmet.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stojceva-Taneva O, Fadda GZ, Smogorzewski M, Massry SG. Parathyroid hormone increases cytosolic calcium of thymocytes. Nephron. 1993;64(4):592–9. doi: 10.1159/000187406. [DOI] [PubMed] [Google Scholar]
- 57.Rifas L. T-cell cytokine induction of BMP-2 regulates human mesenchymal stromal cell differentiation and mineralization. J Cell Biochem. 2006;98(4):706–14. doi: 10.1002/jcb.20933. [DOI] [PubMed] [Google Scholar]
- 58.Guise TA, Chirgwin JM, Favarato G, Boyce BF, Mundy GR. Chinese hamster ovarian cells transfected with human parathyroid hormone-related protein cDNA cause hypercalcemia in nude mice. Lab Invest. 1992;67(4):477–85. [PubMed] [Google Scholar]
- 59.Hory BG, Roussanne MC, Rostand S, Bourdeau A, Drueke TB, Gogusev J. Absence of response to human parathyroid hormone in athymic mice grafted with human parathyroid adenoma, hyperplasia or parathyroid cells maintained in culture. J Endocrinol Invest. 2000;23(5):273–9. doi: 10.1007/BF03343723. [DOI] [PubMed] [Google Scholar]
- 60.O’Brien C, Fu Q, Mommsen L, Dusevich V, Bonewald L, Manolagas SC, Jilka RL. Osteoblasts are not the source of RANKL and OPG in bone but are required for maintenance of osteoclast function. Journal Bone Mineral Research. 2006;22(Supplement 1) abstract. [Google Scholar]
- 61.Tanaka Y, Maruo A, Fujii K, Nomi M, Nakamura T, Eto S, Minami Y. Intercellular adhesion molecule 1 discriminates functionally different populations of human osteoblasts: characteristic involvement of cell cycle regulators. J Bone Miner Res. 2000;15 (10):1912–23. doi: 10.1359/jbmr.2000.15.10.1912. [DOI] [PubMed] [Google Scholar]
- 62.Everts V, Delaisse JM, Korper W, Jansen DC, Tigchelaar-Gutter W, Saftig P, Beertsen W. The bone lining cell: its role in cleaning Howship’s lacunae and initiating bone formation. J Bone Miner Res. 2002;17(1):77–90. doi: 10.1359/jbmr.2002.17.1.77. [DOI] [PubMed] [Google Scholar]
- 63.Young N, Mikhalkevich N, Yan Y, Chen D, Zheng WP. Differential regulation of osteoblast activity by Th cell subsets mediated by parathyroid hormone and IFN-gamma. J Immunol. 2005;175(12):8287–95. doi: 10.4049/jimmunol.175.12.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng X, Li J, Lu Y, Keyak J, Lang T. Proximal femoral density and geometry measurements by quantitative computed tomography: Association with hip fracture. Bone. 2006 doi: 10.1016/j.bone.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 65.Grassi F, Tell G, Robbie-Ryan M, Gao Y, Terauchi M, Yang X, Romanello M, Jones DP, Weitzmann MN, Pacifici R. Oxidative stress causes bone loss in estrogen-deficient mice through enhanced bone marrow dendritic cell activation. Proc Natl Acad Sci U S A 1. 2007;04(38):15087–92. doi: 10.1073/pnas.0703610104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pettway GJ, Schneider A, Koh AJ, Widjaja E, Morris MD, Meganck JA, Goldstein SA, McCauley LK. Anabolic actions of PTH (1–34): use of a novel tissue engineering model to investigate temporal effects on bone. Bone. 2005;36(6):959–70. doi: 10.1016/j.bone.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 67.Di Rosa F. T-lymphocyte interaction with stromal, bone and hematopoietic cells in the bone marrow. Immunol Cell Biol. 2008;87:20–29. doi: 10.1038/icb.2008.84. [DOI] [PubMed] [Google Scholar]
- 68.Rawadi G, Vayssiere B, Dunn F, Baron R, Roman-Roman S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res. 2003;18(10):1842–53. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- 69.Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y, Li X, Luu HH, Luo J, Montag AG, Haydon RC, He TC. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279(53):55958–68. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- 70.Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG. Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci. 2006;119(Pt 7):1283–96. doi: 10.1242/jcs.02883. [DOI] [PubMed] [Google Scholar]
- 71.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296(5573):1644–6. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 72.Kimble RB, Srivastava S, Ross FP, Matayoshi A, Pacifici R. Estrogen deficiency increases the ability of stromal cells to support murine osteoclastogenesis via an interleukin-1and tumor necrosis factor- mediated stimulation of macrophage colony-stimulating factor production. J Biol Chem. 1996;271(46):28890–7. doi: 10.1074/jbc.271.46.28890. [DOI] [PubMed] [Google Scholar]
- 73.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13(7):791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130(5):811–23. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 75.Krum SA, Miranda-Carboni GA, Hauschka PV, Carroll JS, Lane TF, Freedman LP, Brown M. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. Embo J. 2008;27(3):535–45. doi: 10.1038/sj.emboj.7601984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jilka RL, Takahashi K, Munshi M, Williams DC, Roberson PK, Manolagas SC. Loss of estrogen upregulates osteoblastogenesis in the murine bone marrow. Evidence for autonomy from factors released during bone resorption. J Clin Invest. 1998;101(9):1942–50. doi: 10.1172/JCI1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Di Gregorio GB, Yamamoto M, Ali AA, Abe E, Roberson P, Manolagas SC, Jilka RL. Attenuation of the self-renewal of transit-amplifying osteoblast progenitors in the murine bone marrow by 17beta-estradiol. J Clin Invest. 2001;107(7):803–812. doi: 10.1172/JCI11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104(5):719–30. [PubMed] [Google Scholar]
- 79.Kousteni S, Han L, Chen JR, Almeida M, Plotkin LI, Bellido T, Manolagas SC. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest. 2003;111(11):1651–64. doi: 10.1172/JCI17261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parfitt AM. Bone remodeling: relationship to the amount and structure of bone, and the pathogenesis and prevention of fractures. In: Riggs BL, editor. Osteoporosis. 1. Raven Press; New York: 1988. pp. 45–93. [Google Scholar]
- 81.Manolagas SC. From Estrogen-Centric to Aging and Oxidative Stress: A Revised Perspective of the Pathogenesis of Osteoporosis. Endocr Rev. 2010 doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R. Up-regulation of TNF-producing T cells in the bone marrow: A key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci U S A. 2001;98(24):13960–5. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ammann P, Rizzoli R, Bonjour JP, Bourrin S, Meyer JM, Vassalli P, Garcia I. Transgenic mice expressing soluble tumor necrosis factor-receptor are protected against bone loss caused by estrogen deficiency. J Clin Invest. 1997;99:1699–1703. doi: 10.1172/JCI119333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kimble R, Bain S, Pacifici R. The functional block of TNF but not of IL-6 prevents bone loss in ovariectomized mice. J Bone Min Res. 1997;12:935–941. doi: 10.1359/jbmr.1997.12.6.935. [DOI] [PubMed] [Google Scholar]
- 85.Pacifici R, Brown C, Puscheck E, Friedrich E, Slatopolsky E, Maggio D, McCracken R, Avioli LV. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci. 1991;88:5134–5138. doi: 10.1073/pnas.88.12.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ralston SH, Russell RGG, Gowen M. Estrogen inhibits release of tumor necrosis factor from peripheral blood mononuclear cells in postmenopausal women. J Bone Miner Res. 1990;5:983–988. doi: 10.1002/jbmr.5650050912. [DOI] [PubMed] [Google Scholar]
- 87.Shanker G, Sorci-Thomas M, Adams MR. Estrogen modulates the expression of tumor necrosis factor alpha mRNA in phorbol ester-stimulated human monocytic THP-1 cells. Lymphokine Cytokine Res. 1994;13:377–382. [PubMed] [Google Scholar]
- 88.Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, Pacifici R. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106(10):1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gao Y, Qian W-P, Dark K, Toraldo G, Lin ASP, Guldberg RE, Flavell RA, Weitzmann MN, Pacifici R. Estrogen prevents bone loss through transforming growth factor {beta} signaling in T cells. 2004;101:16618–16623. doi: 10.1073/pnas.0404888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao Y, Grassi F, Ryan MR, Terauchi M, Page K, Yang X, Weitzmann MN, Pacifici R. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117(1):122–32. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Robbie-Ryan M, Grassi F, Gao Y, Page K, Yang X, Weitzmann MN, Pacifici R. Silencing of T cell function in wild-type mice by immunodepletion or treatment with abatacept prevents the loss of cortical and trabecular bone and the increase in bone resorption induced by ovariectomy. J Bone Miner Res. 2006;21(Supplement 1) Abs. 1095. [Google Scholar]
- 92.Moreland L, Bate G, Kirkpatrick P. Abatacept. Nat Rev Drug Discov. 2006;5(3):185–6. doi: 10.1038/nrd1989. [DOI] [PubMed] [Google Scholar]
- 93.Ruderman EM, Pope RM. The evolving clinical profile of abatacept (CTLA4-Ig): a novel co-stimulatory modulator for the treatment of rheumatoid arthritis. Arthritis Res Ther. 2005;7(Suppl 2):S21–5. doi: 10.1186/ar1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.D’Amelio P, Grimaldi A, Di Bella S, Brianza SZ, Cristofaro MA, Tamone C, Giribaldi G, Ulliers D, Pescarmona GP, Isaia G. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone. 2008;43(1):92–100. doi: 10.1016/j.bone.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 95.Lee SK, Kadono Y, Okada F, Jacquin C, Koczon-Jaremko B, Gronowicz G, Adams DJ, Aguila HL, Choi Y, Lorenzo JA. T lymphocyte-deficient mice lose trabecular bone mass with ovariectomy. J Bone Miner Res. 2006;21(11):1704–12. doi: 10.1359/jbmr.060726. [DOI] [PubMed] [Google Scholar]
- 96.Cenci S, Toraldo G, Weitzmann MN, Roggia C, Gao Y, Qian WP, Sierra O, Pacifici R. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc Natl Acad Sci U S A. 2003;100(18):10405–10. doi: 10.1073/pnas.1533207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grassi F, Pacifici R. Ovariectomy Increases The Formation Of T Cell Niches At The Resorption Surfaces. J Bone Min Res. 2005;20(Suppl 1) Abs. [Google Scholar]
- 98.Adamski J, Ma Z, Nozell S, Benveniste EN. 17beta-Estradiol inhibits class II major histocompatibility complex (MHC) expression: influence on histone modifications and cbp recruitment to the class II MHC promoter. Mol Endocrinol. 2004;18(8):1963–74. doi: 10.1210/me.2004-0098. [DOI] [PubMed] [Google Scholar]
- 99.Robey EA, Ramsdell F, Gordon JW, Mamalaki C, Kioussis D, Youn HJ, Gottlieb PD, Axel R, Fowlkes BJ. A self-reactive T cell population that is not subject to negative selection. Int Immunol. 1992;4(9):969–74. doi: 10.1093/intimm/4.9.969. [DOI] [PubMed] [Google Scholar]
- 100.Grossman Z, Paul WE. Self-tolerance: context dependent tuning of T cell antigen recognition. Semin Immunol. 2000;12(3):197–203. doi: 10.1006/smim.2000.0232. discussion 257–344. [DOI] [PubMed] [Google Scholar]
- 101.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276(5321):2057–62. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 102.Boss JM, Jensen PE. Transcriptional regulation of the MHC class II antigen presentation pathway. Curr Opin Immunol. 2003;15(1):105–11. doi: 10.1016/s0952-7915(02)00015-8. [DOI] [PubMed] [Google Scholar]
- 103.Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, Nakamura K, Taniguchi T. T-cell-mediated regulation of osteoclastogenesis by signalling cross- talk between RANKL and IFN-gamma. Nature. 2000;408(6812):600–5. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 104.Vermeire K, Heremans H, Vandeputte M, Huang S, Billiau A, Matthys P. Accelerated collagen-induced arthritis in IFN-gamma receptor-deficient mice. J Immunol. 1997;158(11):5507–13. [PubMed] [Google Scholar]
- 105.Sato K, Satoh T, Shizume K, Yamakawa Y, Ono Y, Demura H, Akatsu T, Takahashi N, Suda T. Prolonged decrease of serum calcium concentration by murine gamma-interferon in hypercalcemic, human tumor (EC-GI)-bearing nude mice. Cancer Res. 1992;52 (2):444–9. [PubMed] [Google Scholar]
- 106.Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999;67(6):2804–9. doi: 10.1128/iai.67.6.2804-2809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arnoldi J, Gerdes J, Flad HD. Immunohistologic assessment of cytokine production of infiltrating cells in various forms of leprosy. Am J Pathol. 1990;137(4):749–53. [PMC free article] [PubMed] [Google Scholar]
- 108.Cannon GW, Pincus SH, Emkey RD, Denes A, Cohen SA, Wolfe F, Saway PA, Jaffer AM, Weaver AL, Cogen L, et al. Double-blind trial of recombinant gamma-interferon versus placebo in the treatment of rheumatoid arthritis. Arthritis Rheum. 1989;32(8):964–73. doi: 10.1002/anr.1780320805. [DOI] [PubMed] [Google Scholar]
- 109.Mann GN, Jacobs TW, Buchinsky FJ, Armstrong EC, Li M, Ke HZ, Ma YF, Jee WS, Epstein S. Interferon-gamma causes loss of bone volume in vivo and fails to ameliorate cyclosporin A-induced osteopenia. Endocrinology. 1994;135:1077–1083. doi: 10.1210/endo.135.3.8070349. [DOI] [PubMed] [Google Scholar]
- 110.Key LL, Jr, Rodriguiz RM, Willi SM, Wright NM, Hatcher HC, Eyre DR, Cure JK, Griffin PP, Ries WL. Long-term treatment of osteopetrosis with recombinant human interferon gamma. N Engl J Med. 1995;332(24):1594–9. doi: 10.1056/NEJM199506153322402. [DOI] [PubMed] [Google Scholar]
- 111.Rodriguiz RM, Key LL, Jr, Ries WL. Combination macrophage-colony stimulating factor and interferon-gamma administration ameliorates the osteopetrotic condition in microphthalmic (mi/mi) mice. Pediatr Res. 1993;33(4 Pt 1):384–9. doi: 10.1203/00006450-199304000-00014. [DOI] [PubMed] [Google Scholar]
- 112.Takayanagi H, Kim S, Taniguchi T. Signaling crosstalk between RANKL and interferons in osteoclast differentiation. Arthritis Res. 2002;4(Suppl 3):S227–32. doi: 10.1186/ar581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang NN, Venugopalan M, Hardikar S, Glasebrook A. Identification of an estrogen response element activated by metabolites of 17β-estradiol and raloxifene. Science. 1996;273:1222–1225. doi: 10.1126/science.273.5279.1222. [DOI] [PubMed] [Google Scholar]
- 114.Miyaura C, Onoe Y, Inada M, Maki K, Ikuta K, Ito M, Suda T. Increased B-lymphopoiesis by interleukin 7 induces bone loss in mice with intact ovarian function: similarity to estrogen deficiency. Proc Natl Acad Sci USA. 1997;19:9360–9365. doi: 10.1073/pnas.94.17.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weitzmann MN, Roggia C, Toraldo G, Weitzmann L, Pacifici R. Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J Clin Invest. 2002;110(11):1643–50. doi: 10.1172/JCI15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ryan MR, Shepherd R, Leavey JK, Gao Y, Grassi F, Schnell FJ, Qian WP, Kersh GJ, Weitzmann MN, Pacifici R. An IL-7-dependent rebound in thymic T cell output contributes to the bone loss induced by estrogen deficiency. Proc Natl Acad Sci U S A. 2005;102(46):16735–40. doi: 10.1073/pnas.0505168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lindberg MK, Svensson J, Venken K, Chavoshi T, Andersson N, Moverare Skrtic S, Isaksson O, Vanderschueren D, Carlsten H, Ohlsson C. Liver-derived IGF-I is permissive for ovariectomy-induced trabecular bone loss. Bone. 2006;38(1):85–92. doi: 10.1016/j.bone.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 118.Lee SK, Kalinowski JF, Jacquin C, Adams DJ, Gronowicz G, Lorenzo JA. Interleukin-7 influences osteoclast function in vivo but is not a critical factor in ovariectomy-induced bone loss. J Bone Miner Res. 2006 doi: 10.1359/JBMR.060117. (In Press) [DOI] [PubMed] [Google Scholar]
- 119.Huang M, Sharma S, Zhu LX, Keane MP, Luo J, Zhang L, Burdick MD, Lin YQ, Dohadwala M, Gardner B, Batra RK, Strieter RM, Dubinett SM. IL-7 inhibits fibroblast TGF-beta production and signaling in pulmonary fibrosis. J Clin Invest. 2002;109(7):931–7. doi: 10.1172/JCI14685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dubinett SM, Huang M, Dhanani S, Economou JS, Wang J, Lee P, Sharma S, Dougherty GJ, McBride WH. Down-regulation of murine fibrosarcoma transforming growth factor-beta 1 expression by interleukin 7. J Natl Cancer Inst. 1995;87(8):593–7. doi: 10.1093/jnci/87.8.593. [DOI] [PubMed] [Google Scholar]
- 121.Basu S, Michaelsson K, Olofsson H, Johansson S, Melhus H. Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun. 2001;288(1):275–9. doi: 10.1006/bbrc.2001.5747. [DOI] [PubMed] [Google Scholar]
- 122.Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, Mecocci P, Senin U, Pacifici R, Cherubini A. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88 (4):1523–7. doi: 10.1210/jc.2002-021496. [DOI] [PubMed] [Google Scholar]
- 123.Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, Urry ZL, Chambers TJ. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest. 2003;112(6):915–23. doi: 10.1172/JCI18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Muthusami S, Ramachandran I, Muthusamy B, Vasudevan G, Prabhu V, Subramaniam V, Jagadeesan A, Narasimhan S. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin Chim Acta. 2005;360(1–2):81–6. doi: 10.1016/j.cccn.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 125.Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O’Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282(37):27285–97. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jagger CJ, Lean JM, Davies JT, Chambers TJ. Tumor necrosis factor-alpha mediates osteopenia caused by depletion of antioxidants. Endocrinology. 2005;146(1):113–8. doi: 10.1210/en.2004-1058. [DOI] [PubMed] [Google Scholar]
- 127.Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem. 2009;284(40):27438–48. doi: 10.1074/jbc.M109.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Almeida M, Martin-Millan M, Ambrogini E, Bradsher R, Han L, Chen XD, Roberson PK, Weinstein RS, O’Brien CA, Jilka RL, Manolagas SC. Estrogens Attenuate Oxidative Stress and the Differentiation and Apoptosis of Osteoblasts by DNA Binding-Independent Actions of the ERalpha. J Bone Miner Res. 2009 doi: 10.1359/jbmr.091017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, Jilka RL, O’Brien CA, Manolagas SC. The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol. 2010;24 (2):323–34. doi: 10.1210/me.2009-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ambrogini E, Almeida M, Martin-Millan M, Paik JH, Depinho RA, Han L, Goellner J, Weinstein RS, Jilka RL, O’Brien CA, Manolagas SC. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 11(2):136–46. doi: 10.1016/j.cmet.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mizuashi M, Ohtani T, Nakagawa S, Aiba S. Redox imbalance induced by contact sensitizers triggers the maturation of dendritic cells. J Invest Dermatol. 2005;124(3):579–86. doi: 10.1111/j.0022-202X.2005.23624.x. [DOI] [PubMed] [Google Scholar]
- 132.Rutault K, Alderman C, Chain BM, Katz DR. Reactive oxygen species activate human peripheral blood dendritic cells. Free Radic Biol Med. 1999;26(1–2):232–8. doi: 10.1016/s0891-5849(98)00194-4. [DOI] [PubMed] [Google Scholar]
- 133.Maemura K, Zheng Q, Wada T, Ozaki M, Takao S, Aikou T, Bulkley GB, Klein AS, Sun Z. Reactive oxygen species are essential mediators in antigen presentation by Kupffer cells. Immunol Cell Biol. 2005;83(4):336–43. doi: 10.1111/j.1440-1711.2005.01323.x. [DOI] [PubMed] [Google Scholar]
- 134.Vosters O, Neve J, De Wit D, Willems F, Goldman M, Verhasselt V. Dendritic cells exposed to nacystelyn are refractory to maturation and promote the emergence of alloreactive regulatory t cells. Transplantation. 2003;75(3):383–9. doi: 10.1097/01.TP.0000043924.09647.61. [DOI] [PubMed] [Google Scholar]
- 135.Verhasselt V, Vanden Berghe W, Vanderheyde N, Willems F, Haegeman G, Goldman M. N-acetyl-L-cysteine inhibits primary human T cell responses at the dendritic cell level: association with NF-kappaB inhibition. J Immunol. 1999;162(5):2569–74. [PubMed] [Google Scholar]
- 136.Matsue H, Edelbaum D, Shalhevet D, Mizumoto N, Yang C, Mummert ME, Oeda J, Masayasu H, Takashima A. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J Immunol. 2003;171(6):3010–8. doi: 10.4049/jimmunol.171.6.3010. [DOI] [PubMed] [Google Scholar]
- 137.Hildeman DA, Mitchell T, Teague TK, Henson P, Day BJ, Kappler J, Marrack PC. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10 (6):735–44. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- 138.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15(2):247–54. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 139.Ozaki M, Suzuki S, Irani K. Redox factor-1/APE suppresses oxidative stress by inhibiting the rac1 GTPase. Faseb J. 2002;16(8):889–90. doi: 10.1096/fj.01-0664fje. [DOI] [PubMed] [Google Scholar]