Abstract
Although B cells are crucial antigen-presenting cells in the initiation of T cell autoimmunity to islet β cell autoantigens in type 1 diabetes (T1D), adhesion molecules that control migration of B cells into pancreatic lymph nodes (PanLN) in the nonobese diabetic (NOD) mouse model of human T1D have not been defined. In this study, we found that B cells from PanLN of 3-4-week-old female NOD mice expressed high levels of α4 integrin and LFA-1 and intermediate levels of β7 integrin; half of B cells were L-selectinhigh. In short-term in vivo lymphocyte migration assays, B cells migrated from the bloodstream into PanLN more efficiently than into peripheral LNs. Moreover, antibodies to mucosal addressin cell adhesion molecule 1 (MAdCAM-1) and α4β7 integrin inhibited >90% of B cell migration into PanLN. In contrast, antibodies to peripheral node addressin, L-selectin or LFA-1 partially inhibited B cell migration into PanLN. Furthermore, one intraperitoneal injection of anti-MAdCAM-1 antibody into 3-week-old NOD mice significantly inhibited entry of B cells into PanLN for at least 2 weeks. Taken together, these results indicate that the α4β7 integrin/MAdCAM-1 adhesion pathway plays a predominant role in migration of B cells into PanLN in NOD mice. Thus, specific blockage of α4β7 integrin/MAdCAM-1 adhesion pathway-mediated B cell migration may be a potential treatment for T1D.
Keywords: lymphocyte migration, B cells, adhesion molecules, pancreatic lymph nodes, type 1 diabetes, nonobese diabetic mouse
1. Introduction
Type 1 diabetes (T1D) is a T cell-mediated autoimmune disease characterized by the infiltration of β cell autoantigen-specific memory/effector T cells and other leukocytes into pancreatic islets and eventually destruction of insulin-producing islet β cells [1]. The β cell antigen-specific memory T cells are generated in pancreatic lymph nodes (PanLN) when naive autoreactive T cells migrate from the bloodstream into PanLN and encounter β cell autoantigen-bearing dendritic cells. In the nonobese diabetic (NOD) mouse model of human T1D, several studies in B cell-depleted or B cell-deficient mice have demonstrated that B cells are critical for the development of T1D [2-5]. B cells in PanLN are crucial antigen-presenting cells (APCs) not only to initiate and optimize priming of naive autoreactive CD4+ T cells by β cell antigens, but also to contribute to epitope spreading of T cell autoimmunity among β cell antigens [6-11]. B cells in inflamed islets also promote the survival of intraislet CD8+ T cells, leading to enhanced development of T1D [12].
Migration of lymphocytes from the bloodstream into secondary lymphoid tissues such as LNs and Peyer's patches (PP) is essential for the generation of organ-selective, antigen-specific adoptive immunity to pathogens and antigens, including autoantigens. This migration is in part controlled by adhesion molecules and chemokine receptors on lymphocytes and their ligands on blood vessel high endothelial venules (HEVs) in LNs and PP [13-15]. We and others have shown that L-selectin/peripheral node addressin (PNAd) and α4β7 integrin/mucosal addressin cell molecule 1 (MAdCAM-1) (lymphocyte/endothelia adhesion molecule pairs, referred to here as “adhesion pathway”) predominantly mediate migration of lymphocytes from the bloodstream into peripheral LNs (PLN) and PP, respectively, in adult NOD mice [16, 17]. Although T cell autoimmunity to β cell antigens is primarily initiated in PanLN of NOD mice during 3-4 weeks (wk) of age [18-21], the adhesion molecules that control the migration of lymphocytes into PanLN from the bloodstream have not been defined.
Given the crucial role of B cells as APCs in the initiation of β cell antigen-autoimmunity in PanLN, the aims of this study were to determine the expression profile of adhesion molecules on PanLN B cells and to define the major lymphocyte/endothelia adhesion pathways that mediate migration of B cells from the bloodstream into PanLN of 3-4-wk-old NOD mice. We found that the α4β7 integrin/MAdCAM-1 pathway is crucial for the migration of B cells from the bloodstream into PanLN and that the L-selectin/PNAd pathway and LFA-1 are partially involved. Moreover, treatment of 3-wk-old NOD mice with one dose of anti-MAdCAM-1 antibody efficiently prevented entry of B cells into PanLN for at least 2 wk. Thus, specific blockage of α4β7 integrin/MAdCAM-1 pathway-mediated B cell migration may be a potential therapy for T1D.
2. Materials and Methods
2.1. Mice
NOD mice were purchased from the Jackson Laboratory (Bar Harbor, ME), bred, and housed in the Stanford University Animal Facility. Three to four-wk-old female NOD mice were used for all experiments unless otherwise indicated. Stanford University's Administrative Panel of Laboratory Animal Care approved all studies.
2.2. Antibodies and other reagents
Rat monoclonal antibodies (mAbs) against MAdCAM-1 (clone MECA367, ATCC, Manassas, VA), PNAd (MECA79, provided by Dr. Eugene C. Butcher, Stanford University, Stanford, CA), L-selectin (MEL-14, Dr. Butcher), α4β7 integrin (DATK32, ATCC), LFA-1 (FD441.8, Dr. Butcher), human CD44 (9B5, rat IgG negative control, Dr. Butcher), and granule cells in neonatal mouse cerebellum (OZ42, rat IgM negative control, Dr. Leslie Pickford, Menlo Park, CA) were used for in vivo blocking experiments. Fluorochrome-conjugated mAbs against B220 (RA3-6B2) (eBioscience, San Diego, CA), α4 integrin (SG31), L-selectin (MEL-14) and β7 integrin (M293) (BD Biosciences, San Diego, CA) were used for fluorescence-activated cell sorter (FACS) analysis. Tetramethylrhodamine-5(6)-isothiocyanate mixed isomers (TRITC) was purchased from Molecular Probes (Eugene, OR).
2.3. FACS analysis of adhesion molecules on B cells
Lymphocyte suspensions were prepared from PanLN, PLN, mesenteric LN (MLN) and PP of 3-4-wk-old female NOD mice. Cells from each tissue were pooled from 3-4 mice. The suspensions were stained with a FITC-anti-B220 mAb combined with either a PE-conjugated mAb to α4 integrin, L-selectin or β7 integrin, or a PE-conjugated negative control mAb on ice for 30 minutes (min), and analyzed on a BD FACSCalibur flow cytometer. The percentage of B cells that express each adhesion molecule and the mean fluorescence intensity of the expression were evaluated on at least 10,000 B220+ B cells in the lymphocyte forward scatter/side scatter gate.
2.4. Short-term in vivo lymphocyte migration assays
Short term in vivo lymphocyte migration assays were performed as previously described [17, 22]. Briefly, lymphocytes were prepared from LNs and spleens of 3-4-wk-old female donor NOD mice, labeled with TRITC, and injected i.v. into age-matched female host mice. Each host mouse received 5×107 donor cells. To block endothelia MAdCAM-1 or PNAd, each host mouse was given 500 μg of anti-MAdCAM-1, anti-PNAd or isotype-matched negative control mAb i.v. 30 min before the cell transfer. To block lymphocyte α4β7 integrin, L-selectin or LFA-1, donor lymphocytes were pretreated with 10 μg/ml anti-α4β7 integrin, L-selectin or LFA-1 mAb or with negative control mAb on ice for 15 min. To ensure saturating mAb levels in vivo, additional mAb (100 μg anti-endothelia adhesion molecule mAb or 200 μg anti-lymphocyte adhesion molecule mAb) was given to each host mouse along with the donor cells. In some experiments, 4-, 5- or 8-wk-old mice, which were treated i.p. with anti-MAdCAM-1 mAb or negative control mAb at 3 wk of age, were used as host mice. In all experiments, host mice were sacrificed 2 hours (h) after transfer. Lymphocyte suspensions were prepared from PanLN, PLN, MLN, PP, spleen and peripheral blood, and stained with a FITC- or Alexa Fluor 647-anti-B220 mAb. Donor B cells (TRITC+B220+ cells) in each tissue of each host mouse were identified using FACS analysis. Migration of donor B cells in the anti-adhesion molecule mAb-treated group is expressed as the percentage of the migration in the negative control mAb-treated group, in which the migration was set at 100%.
2.5. In vivo blockage of MAdCAM-1
Three-wk-old female NOD mice were injected i.p. with anti-MAdCAM-1 mAb or control mAb (30 μg mAb/g body weight) and sacrificed at 4, 5 or 8 wk of age. Lymphocyte suspensions were prepared from PanLN, PLN and MLN. The total number of cells in each suspension was determined using a hemacytometer, and the relative number of B cells was identified by staining with a FITC-anti-B220 mAb and FACS analysis. Absolute number of B cells in each tissue of each mouse was calculated by multiplying the percentage of B220+ cells by the total number of cells.
2.6. Statistical analysis
Data are presented as mean ± SD. One-way ANOVA test was used to evaluate the difference between groups. P<0.05 is considered to be statistically significant.
3. Results and Discussion
3.1. PanLN B cells has a unique expression profile of adhesion molecules
It was previously shown that the expression patterns of adhesion molecules on lymphocytes from PLN, MLN and PP of 6-10-wk-old NOD and BALB/c mice were similar, but NOD LNs contained more β7 integrinhigh lymphocytes than did BALB/c LNs [16]. Although PanLN B cells are important APCs during the development of autoimmunity to β cell antigens, the adhesion molecules that control migration of B cells into PanLN are not known. Here we determined the expression of adhesion molecules on PanLN B cells in NOD mice at 3-4 wk of age, which is the critical period for the initiation of the autoimmune response in NOD mice. We focused on α4 integrin, which is a subunit of α4β7 integrin (that mainly binds to MAdCAM-1) and of α4β1 integrin (that mainly binds to VCAM-1); β7 integrin, which is a subunit of α4β7 integrin and of αEβ7 integrin (that binds to epithelial E cadherin); L-selectin, which mainly binds to PNAd and, in some tissues, also to MAdCAM-1; and LFA-1, that binds to endothelia ICAM-1 and ICAM-2 [13, 23].
As shown in Fig. 1, more than 90% of B cells from PanLN, PLN and MLN were α4 integrin+, β7 integrin+ or LFA-1+. All B cells from PP were LFA-1+ and β7 integrin+, whereas 75% of B cells were α4 integrin+. Approximately half of B cells in PanLN and MLN were L-selectinhigh as compared to 75% B cells in PLN and 25% of B cells in PP. Moreover, PanLN B cells expressed higher levels of α4 integrin and LFA-1 but intermediate levels of β7 integrin as compared to PLN and MLN. In contrast, PP B cells expressed lower levels of α4 integrin and β7 integrin but intermediate levels of LFA-1 as compared to LNs. These results indicate that the expression profile of adhesion molecules on PanLN B cells most closely resembles that on MLN B cells. Thus, high expression levels of α4 integrin and LFA-1, intermediate expression levels of β7 integrin, and half of L-selectinhigh are characteristic of PanLN B cells.
Figure 1. Expression of adhesion molecules on PanLN B cells.
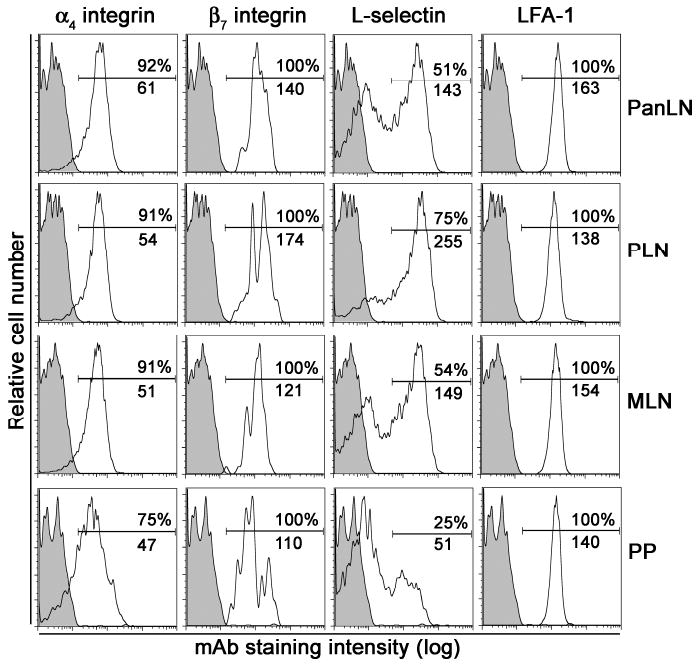
Lymphocytes were isolated from PanLN, PLN, MLN and PP of 3-4-wk-old female NOD mice, stained with a FITC-anti-B220 mAb combined with a PE-conjugated mAb to α4 integrin, β7 integrin, L-selectin or LFA-1, or a PE-conjugated negative control mAb, and evaluated by FACS analysis. The histograms in each panel show the staining with the anti-lymphocyte adhesion molecule mAb (unshaded) and the isotype control mAb (shaded) on B220+ B cells. The numbers in each panel indicate the percentage of B cells that express each adhesion molecule (top) and the mean fluorescence intensity of the B cell staining (bottom). Data are one representative of three experiments. In each experiment, lymphocytes were pooled from 3-4 mice.
3.2. B cells migrate into PanLN more efficiently than into PLN
We previously showed that B cells migrated equally well into MLN and PP, but significantly less well into PLN in old NOD mice, as compared to T cells [17]. It is not known whether B cells migrate into PanLN more efficiently than into other LNs and PP in young NOD mice. To evaluate the ability of NOD B cells to migrate into LNs and PP in young NOD mice, we performed short-term in vivo lymphocyte migration assays by i.v. injecting TRITC-labeled lymphocytes from 3-4-wk-old NOD mice into age-matched NOD mice. Two h after transfer, we found that the proportion of donor B cells (TRITC+B200+ cells) in the total lymphocyte population of PanLN of host mice was significantly higher than that in PLN, but was significantly lower than that in PP (Fig. 2). There was no significant difference in the proportion of donor B cells in PanLN and MLN of host mice. Similar trend for migration of B cells into different LNs and PP was also seen in nonautoimmune-prone mice (not shown). Thus, PanLN is distinct from PLN and PP but similar to MLN in terms of recruiting B cells from the bloodstream into tissues.
Figure 2. B cells migrate into PanLN more efficiently than into PLN.
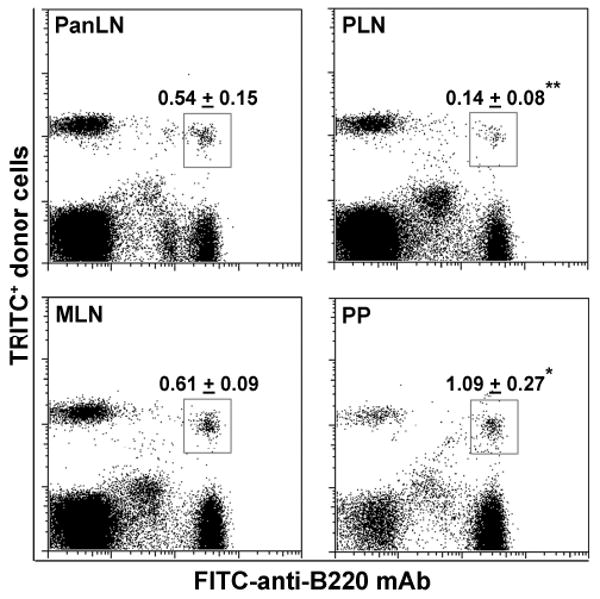
Fifty million TRITC-labeled lymphocytes from 3-4-wk-old female NOD mice were transferred i.v. into age-matched female NOD mice. Two h after cell transfer, donor B cells in PanLN, PLN, MLN and PP of host mice were identified by immunofluorescence staining with a FITC-anti-B220 mAb followed by FACS analysis. Representative FACS plots show donor B cells (TRITC+B220+ cells in box) in LNs and PP of host mice. Numbers in FACS plots are mean ± standard derivation of donor B cells as a percentage of total lymphocytes. One-way ANOVA test, *p<0.05 and **p<0.01 compared with PanLN, n=4 mice in each group.
3.3. α4β7 integrin/MAdCAM-1 pathway is critical for migration of B cells into PanLN
Phillips et al previously showed that HEVs in PanLN of neonatal NOD mice and 8-wk-old NOD.SCID mice expressed MAdCAM-1 [24]. In 6-10-wk-old NOD mice, Hanenien et al showed that anti-L-selectin mAb inhibited lymphocyte migration into PLN by 74% and into MLN and PP by 50-60% and that anti-MAdCAM-1 mAb inhibited the migration into PLN by 37% and into MLN and PP by 86-100% [16]. In >6-month-old NOD mice, we showed that mAbs to PNAd and L-selectin inhibited the migration into PLN by >90% and into MLN and PP by 50-60% [17]. In contrast, anti-α4β7 integrin mAb inhibited the migration into PP by >80% and into MLN by 60% but did not affect the migration into PLN [17]. However, both previous studies did not explore the role of adhesion molecules in migration of lymphocytes into PanLN.
In 3-4-wk-old NOD mice, MAdCAM-1 was expressed on approximately 95% of HEVs in PanLN, MLN and PP, but only on less than 5% of HEVs in PLN (Xu, Cook & Michie, manuscript in preparation). To determine which lymphocyte/endothelia adhesion pathways are important for B cell migration into PanLN during the critical period for the initiation of autoimmunity in T1D, we performed short-term in vivo lymphocyte migration assays in 3-4-wk-old NOD mice. In these experiments, host mice were treated with an anti-endothelial adhesion molecule mAb (MAdCAM-1 or PNAd) or a negative control mAb, or donor lymphocytes were treated with an anti-lymphocyte adhesion molecule mAb (α4β7 integrin, L-selectin or LFA-1) or a negative control mAb. We found that anti-MAdCAM-1 mAb inhibited 90% of migration of donor B cells into PanLN, MLN and PP and 60% of donor B cell migration into PLN (Fig. 3A). Similar results were observed when donor lymphocytes were treated with anti-α4β7 integrin mAb (Fig. 3B). In contrast, anti-PNAd mAb inhibited 50% of migration of donor B cells into PanLN and MLN, but did not significantly affect the migration into PP (Fig. 3C). Anti-L-selectin mAb inhibited 30-40% of migration of donor B cell into PanLN, MLN and PP (Fig. 3D). As in nonautoimmune prone mice [13], mAbs against PNAd and L-selectin significantly inhibited migration of donor B cells into NOD PLN by >80% (Figs. 3C and 3D). Anti-LFA-1 mAb inhibited migration of donor B cells into PLN by >70% and into PanLN, MLN and PP by 40-45% (Fig. 3E). Additionally, as in NOD mice, anti-MAdCAM-1 and PNAd mAbs blocked migration of B cells into PanLN by 84% and 43%, respectively, in nonautoimmune-prone mice (not shown).
Figure 3. MAbs to MAdCAM-1, α4β7 integrin, PNAd, L-selectin and LFA-1 inhibit migration of B cells into PanLN.
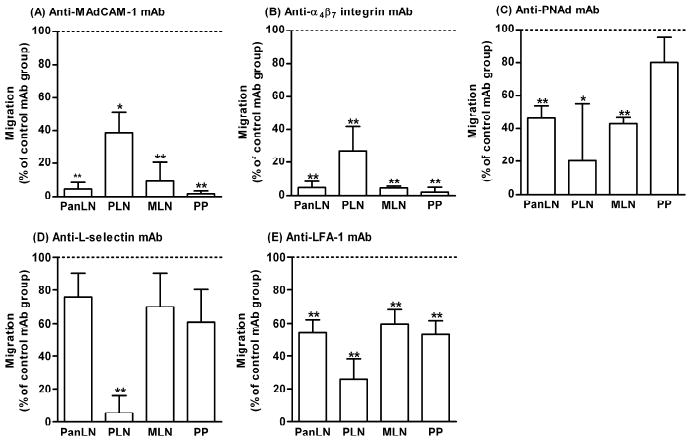
Short-term in vivo lymphocyte migration assays were used to determine the role of each adhesion molecule in migration of B cells into PanLN in 3-4-wk-old female NOD mice. (A, C): Fifty million TRITC-labeled lymphocytes from 3-4-wk-old female NOD mice were transferred i.v. into age-matched female NOD mice treated with a mAb to MAdCAM-1 (A) or PNAd (C), or with a negative control mAb (A, C). (B, D, E): Fifty million TRITC-labeled lymphocytes from 3-4-wk-old female NOD mice were pretreated with a mAb to α4β7 integrin (B), L-selectin (D) or LFA-1 (E), or with a negative control mAb (B, D, E) and were transferred i.v. into age-matched female NOD mice. In all experiments, host mice were sacrificed 2 h after cell transfer. Donor B cells in PanLN, PLN, MLN and PP of host mice were identified by suspension staining with a FITC-anti-B220 mAb and FACS analysis. Migration of donor B cells (TRITC+B220+ cells) into the anti-adhesion molecule mAb-treated group is expressed as the percentage of the migration into the negative control mAb-treated group, in which migration is set at 100% (horizontal dotted line). One-way ANOVA test, *p<0.05 and **p<0.01 compared with control mAb-treated group, n=3-4 mice in each group.
These results clearly demonstrate that the α4β7 integrin/MAdCAM-1 adhesion pathway is crucial for recruiting B cells from the bloodstream into PanLN in 3-4-wk-old NOD mice. Thus, B cell/endothelia adhesion pathway in PanLN differs from the PNAd/L-selectin-dominated pathway in PLN, but resembles the α4β7 integrin/MAdCAM-1-dominated pathway in PP and MLN. We and others previously showed that the α4β7 integrin/MAdCAM-1 pathway had minimal to no role (0-37%) in lymphocyte migration into PLN of young (6-10-wk-old) and adult (>6 months) NOD mice [16, 17]. In contrast, the α4β7 integrin/MAdCAM-1 pathway contributed remarkably to migration of B cells into PLN (60-70%) of 3-4-wk-old NOD mice. A few MAdCAM-1+ HEVs in PLN of 3-4-wk-old NOD mice (Xu, Cook & Michie, manuscript in preparation) as in the LNs of nonautoimmune-prone mice [25] may in part explain the difference between this study and previous studies. Taken together, migration of B cells into PanLN in 3-4-wk-old NOD mice is mediated predominantly by an α4β7 integrin/MAdCAM-1 pathway and partially by L-selectin/PNAd pathway and LFA-1.
3.4. α4β7 integrin/MAdCAM-1 pathway is crucial for B cell homeostasis in PanLN
The critical role of an α4β7 integrin/MAdCAM-1 pathway in migration of B cells into PanLN led us to examine whether this pathway contributes to B cell homeostasis in PanLN of young NOD mice. In a study parallel to this work (Xu, Cook & Michie, manuscript in preparation), we found that anti-MAdCAM-1 mAb bound to HEVs in PanLN and MLN of NOD mice at 4 and 5 wk of age that were injected with an anti-MAdCAM-1 mAb at 3 wk of age. Thus, we injected 3-wk-old NOD mice i.p. with an anti-MAdCAM-1 mAb or a control mAb, sacrificed them at different ages and evaluated B cell numbers in PanLN, MLN and PLN. As shown in Fig. 4A, anti-MAdCAM-1 mAb treatment reduced B cell numbers in PanLN and MLN by >80% and 40-50%, respectively, at 4 and 5 wk of age, but not at 8 wk of age, as compared to control mAb treatment (Fig. 4A). Anti-MAdCAM-1 mAb treatment did not reduce B cell numbers in PLN at any age. Moreover, short-term migration assays revealed that anti-MAdCAM-1 mAb treatment at 3 wk of age inhibited 80% of donor B cell migration into PanLN and MLN at 4 and 5 wk of age but not at 8 wk of age (Fig. 4B). Additionally, anti-MAdCAM-1 mAb treatment at 3 wk of age inhibited 30% of donor B cell migration into PLN at 4 wk of age. Thus, α4β7 integrin/MAdCAM-1 pathway is critically involved in maintenance of B cell homeostasis in PanLN by recruiting B cells from the bloodstream.
Figure 4. Blockage of MAdCAM-1 prevents homeostatic migration of B cells into PanLN.
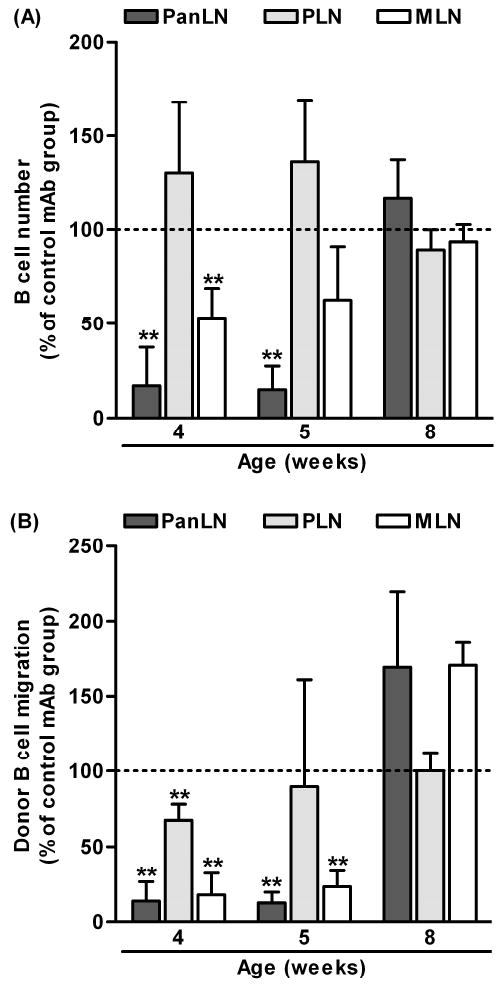
(A, B) Three-week-old female NOD mice were injected i.p. with an anti-MAdCAM-1 mAb or a negative control mAb (30 μg/g body weight). (A): Mice were sacrificed at 4, 5 or 8 wk of age. The absolute numbers of B cells in PanLN, PLN and MLN were determined from hemacytometer counts and FACS analysis. Absolute number of B cells in each LN of the anti-MAdCAM-1 mAb-treated group is given as the percentage of that in the negative control-mAb-treated group, in which the absolute number of B cells is set at 100% (horizontal dotted line). One-way ANOVA test, **p<0.01 anti-MAdCAM-1 mAb group compared to the control mAb group. (B): Mice at 4, 5 or 8 wk of age were injected i.v. with 5×107 TRITC-labeled lymphocytes from 3-4-wk-old female NOD mice. Two h after cell transfer, donor B cells in LNs of host mice were evaluated using FACS analysis. Migration of donor B cells (TRITC+B220+ cells) into the anti-MAdCAM-1 mAb-treated group is expressed as the percentage of the migration into the negative control mAb-treated group, in which migration is set at 100% (horizontal dotted line). One-way ANOVA test, **p<0.01 anti-MAdCAM-1 mAb group compared with the negative control mAb group (horizontal line, migration=100%), n=3-4 mice in each group.
3.5. Significance of present findings in T1D
It is well established that the α4β7 integrin/MAdCAM-1 pathway is critical for infiltration of lymphocytes into pancreatic islets and for the development of T1D in NOD mice [26-30]. Here, we found that this pathway is also critical for migration of B cells into PanLN in 3-4-wk-old NOD mice. Similarly, it is true for migration of naive T cells into PanLN in these mice (Xu, Cook & Michie, manuscript in preparation). Taken together, α4β7 integrin/MAdCAM-1 pathway directs not only migration of autoreactive naive lymphocytes into PanLN to initiate autoimmunity to β cell antigens, but also migration of autoreactive memory/effector lymphocytes into pancreatic islets to cause insulitis and to destroy insulin-producing β cells. Thus, the suppression of T1D in NOD mice by anti-MAdCAM-1 or anti-α4β7 integrin mAb treatment, as reported in early studies, was probably achieved by attenuating the entry of naive B and T lymphocytes into PanLN and of memory/effector lymphocytes into pancreatic islets [26-29].
B cells are becoming an attractive therapeutic target for prevention and/or reversal of T1D. In a transgenic NOD mouse line that expressed human CD20 on mouse B cells [31] or wild type NOD mice [32], depletion of the B cells with anti-CD20 mAb prevented or delayed the onset of diabetes, and even reversed established diabetes. Similar therapeutic efficacy has also been reported in NOD mice when B cells were depleted with anti-CD22 mAb conjugated to calicheamicin [33]. Both treatment strategies increased the frequency of regulatory T cells, regenerated a population of B cells with regulatory functions, and reduced the ability of APCs to stimulate antigen-specific proliferation and effector cytokine production (IFN-γ and IL-17) of autoreactive CD4+ T cells [31, 33]. Despite high therapeutic efficacy, these approaches may have side-effects. For example, depletion of naive B cells by an anti-B cell mAb may dampen B cell immunity in other organs such as lung and skin. The relative increases in regulatory T cells and B cells could cause immunosuppression, thus rendering the host susceptible to infection.
Targeting α4β7 integrin/MAdCAM-1 pathway-mediated B cell migration could be an alternative to systemic depletion of B cells. For example, specific blockage of α4β7 integrin on B cells would reduce B cell numbers in PanLN but not PLN and mediastinal LN, thus inhibiting development of β cell antigen-autoimmunity without affecting cutaneous and bronchopulmonary immunity. As shown in this study, blockage of α4β7 integrin/MAdCAM-1 pathway reduces B cells in MLN, PP and probably intestines, thus harming intestinal immunity. However, intestinal immunity and inflammation have been shown to significantly influence the development of autoimmunity and T1D [34, 35]. MLN may also be involved in initiating autoimmunity to β cell antigens [36]. Thus, the reduction of B cells in MLN and PP by blocking the α4β7 integrin/MAdCAM-1 pathway could also be beneficial for T1D therapy. To consider α4β7 integrin/MAdCAM-1 pathway-mediated B cell migration as a therapeutic target for T1D, further studies are required to address its association with the progress of diabetes by specifically (or conditionally) inactivating β7 integrin on B cells using either Cre/LoxP recombination or small interfering RNA technology [37, 38].
In conclusion, we found that migration of B cells into PanLN in 3-4-wk-old NOD mice is mediated predominantly by an α4β7 integrin/MAdCAM-1 pathway and partially by L-selectin/PNAd pathway and LFA-1. Thus, specific blockage of α4β7 integrin/MAdCAM-1 pathway-mediated B cell migration may be a potential therapy for T1D.
Acknowledgments
We thank Dr. Eugene C. Butcher for providing mAbs and Evelyn Resurreccion for her technical assistance. This work was supported by National Institutes of Health grant DK67592 and JDF grant 1-2001-56 to SAM, and National Institutes of Health Digestive Disease Center grant DK-56339 to Stanford University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–85. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 2.Serreze DV, Chapman HD, Varnum DS, Hanson MS, Reifsnyder PC, Richard SD, Fleming SA, Leiter EH, Shultz LD. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu null mice. J Exp Med. 1996;184:2049–53. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silveira PA, Grey ST. B cells in the spotlight: innocent bystanders or major players in the pathogenesis of type 1 diabetes. Trends Endocrinol Metab. 2006;17:128–35. doi: 10.1016/j.tem.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Yang M, Charlton B, Gautam AM. Development of insulitis and diabetes in B cell-deficient NOD mice. J Autoimmun. 1997;10:257–60. doi: 10.1006/jaut.1997.0128. [DOI] [PubMed] [Google Scholar]
- 5.Noorchashm H, Noorchashm N, Kern J, Rostami SY, Barker CF, Naji A. B-cells are required for the initiation of insulitis and sialitis in nonobese diabetic mice. Diabetes. 1997;46:941–6. doi: 10.2337/diab.46.6.941. [DOI] [PubMed] [Google Scholar]
- 6.Falcone M, Lee J, Patstone G, Yeung B, Sarvetnick N. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J Immunol. 1998;161:1163–8. [PubMed] [Google Scholar]
- 7.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 1998;161:3912–8. [PubMed] [Google Scholar]
- 8.Tian J, Zekzer D, Lu Y, Dang H, Kaufman DL. B cells are crucial for determinant spreading of T cell autoimmunity among beta cell antigens in diabetes-prone nonobese diabetic mice. J Immunol. 2006;176:2654–61. doi: 10.4049/jimmunol.176.4.2654. [DOI] [PubMed] [Google Scholar]
- 9.Greeley SA, Moore DJ, Noorchashm H, Noto LE, Rostami SY, Schlachterman A, Song HK, Koeberlein B, Barker CF, Naji A. Impaired activation of islet-reactive CD4 T cells in pancreatic lymph nodes of B cell-deficient nonobese diabetic mice. J Immunol. 2001;167:4351–7. doi: 10.4049/jimmunol.167.8.4351. [DOI] [PubMed] [Google Scholar]
- 10.Wong FS, Wen L, Tang M, Ramanathan M, Visintin I, Daugherty J, Hannum LG, Janeway CA, Jr, Shlomchik MJ. Investigation of the role of B-cells in type 1 diabetes in the NOD mouse. Diabetes. 2004;53:2581–7. doi: 10.2337/diabetes.53.10.2581. [DOI] [PubMed] [Google Scholar]
- 11.Silveira PA, Johnson E, Chapman HD, Bui T, Tisch RM, Serreze DV. The preferential ability of B lymphocytes to act as diabetogenic APC in NOD mice depends on expression of self-antigen-specific immunoglobulin receptors. Eur J Immunol. 2002;32:3657–66. doi: 10.1002/1521-4141(200212)32:12<3657::AID-IMMU3657>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 12.Brodie GM, Wallberg M, Santamaria P, Wong FS, Green EA. B-cells promote intra-islet CD8+ cytotoxic T-cell survival to enhance type 1 diabetes. Diabetes. 2008;57:909–17. doi: 10.2337/db07-1256. [DOI] [PubMed] [Google Scholar]
- 13.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 14.Campbell DJ, Kim CH, Butcher EC. Chemokines in the systemic organization of immunity. Immunol Rev. 2003;195:58–71. doi: 10.1034/j.1600-065x.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 15.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–78. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 16.Hanninen A, Salmi M, Simell O, Andrew D, Jalkanen S. Recirculation and homing of lymphocyte subsets: dual homing specificity of beta 7-integrin(high)-lymphocytes in nonobese diabetic mice. Blood. 1996;88:934–44. [PubMed] [Google Scholar]
- 17.Xu B, Wagner N, Pham LN, Magno V, Shan Z, Butcher EC, Michie SA. Lymphocyte homing to bronchus-associated lymphoid tissue (BALT) is mediated by L-selectin/PNAd, alpha4beta1 integrin/VCAM-1, and LFA-1 adhesion pathways. J Exp Med. 2003;197:1255–67. doi: 10.1084/jem.20010685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gagnerault MC, Luan JJ, Lotton C, Lepault F. Pancreatic lymph nodes are required for priming of beta cell reactive T cells in NOD mice. J Exp Med. 2002;196:369–77. doi: 10.1084/jem.20011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med. 1999;189:331–9. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turley S, Poirot L, Hattori M, Benoist C, Mathis D. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med. 2003;198:1527–37. doi: 10.1084/jem.20030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 22.Mikulowska-Mennis A, Xu B, Berberian JM, Michie SA. Lymphocyte migration to inflamed lacrimal glands is mediated by vascular cell adhesion molecule-1/alpha(4)beta(1) integrin, peripheral node addressin/l-selectin, and lymphocyte function-associated antigen-1 adhesion pathways. Am J Pathol. 2001;159:671–81. doi: 10.1016/s0002-9440(10)61738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–72. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 24.Phillips JM, Haskins K, Cooke A. MAdCAM-1 is needed for diabetes development mediated by the T cell clone, BDC-2.5. Immunology. 2005;116:525–31. doi: 10.1111/j.1365-2567.2005.02254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mebius RE, Streeter PR, Michie S, Butcher EC, Weissman IL. A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+ CD3- cells to colonize lymph nodes. Proc Natl Acad Sci U S A. 1996;93:11019–24. doi: 10.1073/pnas.93.20.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang XD, Sytwu HK, McDevitt HO, Michie SA. Involvement of beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in the development of diabetes in obese diabetic mice. Diabetes. 1997;46:1542–7. doi: 10.2337/diacare.46.10.1542. [DOI] [PubMed] [Google Scholar]
- 27.Hanninen A, Jaakkola I, Jalkanen S. Mucosal addressin is required for the development of diabetes in nonobese diabetic mice. J Immunol. 1998;160:6018–25. [PubMed] [Google Scholar]
- 28.Hanninen A, Salmi M, Simell O, Jalkanen S. Mucosa-associated (beta 7-integrinhigh) lymphocytes accumulate early in the pancreas of NOD mice and show aberrant recirculation behavior. Diabetes. 1996;45:1173–80. doi: 10.2337/diab.45.9.1173. [DOI] [PubMed] [Google Scholar]
- 29.Hanninen A, Taylor C, Streeter PR, Stark LS, Sarte JM, Shizuru JA, Simell O, Michie SA. Vascular addressins are induced on islet vessels during insulitis in nonobese diabetic mice and are involved in lymphoid cell binding to islet endothelium. J Clin Invest. 1993;92:2509–15. doi: 10.1172/JCI116859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faveeuw C, Gagnerault MC, Lepault F. Expression of homing and adhesion molecules in infiltrated islets of Langerhans and salivary glands of nonobese diabetic mice. J Immunol. 1994;152:5969–78. [PubMed] [Google Scholar]
- 31.Hu CY, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, Shlomchik MJ, Wen L. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117:3857–67. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiu Y, Wong CP, Bouaziz JD, Hamaguchi Y, Wang Y, Pop SM, Tisch RM, Tedder TF. B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions. J Immunol. 2008;180:2863–75. doi: 10.4049/jimmunol.180.5.2863. [DOI] [PubMed] [Google Scholar]
- 33.Fiorina P, Vergani A, Dada S, Jurewicz M, Wong M, Law K, Wu E, Tian Z, Abdi R, Guleria I, et al. Targeting CD22 reprograms B-cells and reverses autoimmune diabetes. Diabetes. 2008;57:3013–24. doi: 10.2337/db08-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turley SJ, Lee JW, Dutton-Swain N, Mathis D, Benoist C. Endocrine self and gut non-self intersect in the pancreatic lymph nodes. Proc Natl Acad Sci U S A. 2005;102:17729–33. doi: 10.1073/pnas.0509006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–13. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaakkola I, Jalkanen S, Hanninen A. Diabetogenic T cells are primed both in pancreatic and gut-associated lymph nodes in NOD mice. Eur J Immunol. 2003;33:3255–64. doi: 10.1002/eji.200324405. [DOI] [PubMed] [Google Scholar]
- 37.Kuhn R, Torres RM. Cre/loxP recombination system and gene targeting. Methods Mol Biol. 2002;180:175–204. doi: 10.1385/1-59259-178-7:175. [DOI] [PubMed] [Google Scholar]
- 38.Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319:627–30. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]


