Summary
There is evidence that inositol isomers may help protect against formation of toxic fibrils of Aβ fragments in Alzheimer disease mouse models. Scyllo-inositol is one of the more promising inositol isomers for the potential treatment of Alzheimer's disease (AD) and can be detected using MRS in human subjects. In this manuscript we demonstrate using MRS, in two different mouse models of AD (APP×PS1 and APP×PS1×tau), that we could detect increased scyllo-inositol in the hippocampus and frontal cortex in mice fed water supplemented with 16.5mg/L of scyllo-inositol equivalent to about 3.3mg/kg/day. We used both brain extracts using solution MRS as well as intact brain tissue using high resolution magic angle spinning (HRMAS) to ensure that any membrane associated scyllo-inositol would be detected. By brain extracts we detected a 3.0 fold increase in scyllo-inositol in the scyllo-fed AD mice compared to normal diet (p<0.001). Using HRMAS we detected a 2.2-2.4-fold increase in scyllo-inositol (p<0.001). Scyllo-inositol treatment was associated with an increase in glutamine in hippocampus. The concentrations of scyllo-inositol were higher in the hippocampus than in the frontal cortex. Mice have a smaller concentration of scyllo-inositol than humans (ca. 100μM vs. 500μM in humans). Given the ease with which scyllo-inositol can be measured in human MRS data with high signal to noise ratios, these data suggest that MRS will prove useful for evaluation of inositol treatment trials in AD subjects.
Keywords: Alzheimer's disease, scyllo-inositol, transgenic mouse, MRS, magic angle spinning
Introduction
Soluble Aβ is believed to be as or more toxic than fibrillar Aβ in plaques (Dickson, 1997; Wang et al., 2002). Therefore, strategies that can minimize formation or oligomeriztiion of Aβ may be preventative or palliative for treatment of Alzheimer's disease (AD). Studies with cyclohexanols (inositols) have provided evidence that certain isomers, such as scyllo-inositol, may be useful for such a purpose by complexing with and stabilizing Aβ1-42 and preventing it from progressing to oligomers and fibrils that may be the toxic moieties (McLaurin et al., 2000). Complexes of scyllo-inositol with Aβ1-42 and Aβ1-40 rendered the Aβ much less toxic to cells (McLaurin et al., 2000). It turned out that scyllo-inositol was much more effective (especially with the more toxic Aβ1-42) than myo-inositol at the doses used and was able to provide multiple protective benefits in an AD mouse model even when treatment was started long after symptoms began (McLaurin et al., 2006).
Magnetic resonance spectroscopy (MRS) is useful for following neurochemical changes in a variety of neurodegenerative disorders, and particularly in mouse models of such diseases (Choi et al., 2007; Choi et al., 2009). MRS studies of AD indicate a loss of NAA and an increase in myo-inositol (Shonk et al., 1995). By combining the increase in myo-inositol with the decrease in NAA, Ross and colleagues were able to distinguish AD from other dementias (Shonk et al., 1995). Other studies have confirmed the increase in myo-inositol and decrease in NAA in AD (Klunk et al., 1996; Pettegrew et al., 1997). The significance of this increase is not clear at this time. Myo-inositol has a well established role in osmoregulation as proposed by Thurston (Thurston et al., 1989) and verified in subsequent studies (Isaacks et al., 1999). Myo-inositol has a much higher concentration in glial cells than in neurons (Brand et al., 1993), thus it may also serve as a good marker for gliosis. Given the evidence that myo-inositol may provide protective benefits in AD (McLaurin et al., 2000) it is tempting to suggest that the increase noted in AD may be a response to toxic processes in the development of the disease. Elevated scyllo-inositol has recently been detected in AD brains using in vivo MRS at 3T (Griffith et al., 2007). Scyllo-inositol is found in relatively high concentrations in human brain, although in most subjects it has a concentration about 12 times lower than myo-inositol. Some apparently healthy controls have greatly increased scyllo-inositol concentrations, although such individuals are rare (Seaquist and Gruetter, 1998).
One attractive feature of MRS is the ability to measure both scyllo- and myo-inositol. Therefore one can readily measure the increases in the brain in a regionally selective manner. In this communication, we show that low, therapeutically useful doses of scyllo-inositol lead to increases in scyllo-inositol in the brain in two different brain regions and in two different mouse models. The ability to measure regionally specific drug increases in conjunction with the therapeutic response will be invaluable.
Materials and Methods
Mice and Scyllo-inositol Treatment
All animal studies were carried out with the consent and approval of the institutional subcommittee on research and care of small animals. We used two different AD mouse models. The first was a double transgene model overexpressing the human Aß with the Swedish mutation (Hsiao et al., 1996), APPTg2576 were crossbred with homozygous transgenic female mice expressing mutant human presenilin-1 (PS-1M146V) to generate double transgenic mice heterozygous for APP and PS-1 (APP×PS1). These mice were treated with water containing scyllo-inositol at a concentration of 16.5mg/L. No differences were noted in the ad libitum water consumption between the treated (n=5) and un-treated mice (n=5) with measured water consumption of 5ml/day. This consumption is equivalent to a dose of approximately 3.3mg/kg/day. Scyllo-inositol treatment was initiated starting at 5 months of age and continued for two months and then were sacrificed for MRS as previously described (Dedeoglu et al., 2004; Jenkins et al., 2000). Briefly, mice were euthanized under using CO2 under isoflurane anesthetic. Then the right hemisphere was dissected coronally at the bregma level and 1 mm of cortex from bregma going posterior to bregma was immediately placed into pre-weighed Eppendorf tubes sitting in dry ice and weighed again for tissue weight and stored at -80°C until chemical extraction (see Fig. 1). All tissue samples were sonicated in 250 μL of 0.1 M perchloric acid (PCA) and centrifuged for 10 min and supernatant containing brain chemicals was separated and pH was adjusted to 7.2 by adding NaOH in PBS.
Figure 1.
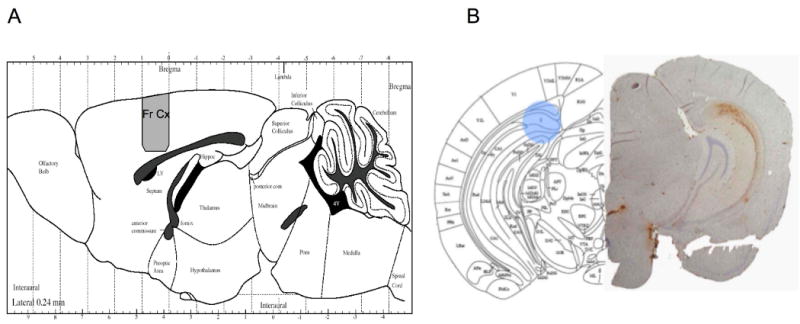
A) Figure showing the region from which tissue was taken for both frontal cortical studies using the APP×PS1 and triple trasngene mice. B) Picture from a mouse brain atlas at the levels of the hippocampus (subiculum) showing the approximate region (blue) from which the tissue punches were taken. Shown on the right is the corresponding slice from a six month old mouse showing immunohistochemical stains for amyloid ß protein (Aβ1-42) in the hippocampus (shown in the figure on the right). The region from which the punch was taken is a region showing intense Aβ accumulation.
A second AD mouse model was also studied. We used a triple transgenic mouse model developed by Frank LaFerla and his colleagues (Oddo et al., 2003) that harbors three mutant human genes: amyloid precursor protein (APPswe), presenilin-1 (PS1M146V) and tauP301L that are expressed at comparable levels. These mice develop Aβ deposits beginning at 6 months of age followed by tau pathology beginning at 9 months of age in a distribution that closely mimics human AD brain. They also show cognitive deficits that correlate with intraneuronal accumulation of Aβ and release of soluble tau, but not with Aβ alone (Oddo et al., 2006). For this group we chose to utilize high resolution magic angle spinning as this technique will retain membrane-associated chemicals as well as the water – soluble chemicals and is close to in vivo MRS with the exception of much narrower linewidths. In this case mice were fed scyllo-inositol ad libitum in the water at the same dose as the APP×PS1 mice (16.5mg/L) starting at 7.6 months of age for two months, with no difference being noted in the water consumption between the two groups. Then mice were sacrificed as described above. A coronal slice was dissected at the level of the hippocampus (subiculum) on dry ice (see Fig. 1B) and a tissue punch with a diameter of 1.2mm was taken and placed at -80°C until the MRS was run. A tissue punch from the cortex, from the same region as the APP×PS1 mice with a diameter of 1.2 mm was also taken for analysis.
Solution MRS
pH adjusted tissue extracts were lyophilized overnight and dissolved in 0.7 mL of 99.9% D2O (Sigma-Aldrich, Milwaukee, WI) containing 0.5 mM of DSS (Chenomx, Edmonton, Alberta) as an internal reference for quantification. All samples were run on Bruker 14T (Billerica, MA) at 25°C with a spinning rate of 20 Hz. Spectrum was acquired using a simple one pulse sequence with two hundred averages with a pulse width of 12 μs, 32k data points, 7100 Hz spectral width, and a repetition time of 12 s.
High Resolution Magic Angle Spinning
We collected high resolution magic angle spinning (HRMAS) spectra on Bruker 14T (Billerica, MA). We obtained tissue punches of freshly frozen hippocampus from mice. The dissected tissue sample was placed into a glass cylinder positioned in a 3 mm zirconium oxide MAS rotor (volume 50μL). HRMAS measurements were performed using a sample spinning rate, of 3.6 kHz selected to push the spinning side bands outside the frequency region of the metabolites. The experiments were performed at 4°C to minimize tissue degradation.
Data was acquired using a rotor synchronized, T2-filtered Carr–Purcell–Meiboom–Gill (CPMG) pulse sequence [90 – (τ – 180 – τ – Acq)n] with two different effective TEs (100ms/10ms). The longer TE serves to remove the lipid/macromolecular resonances and the short TE retains them. The interpulse delay, τ, is synchronized to the rotor frequency, and is 272μs. The n value for the relatively short T2 filter was 36 and for the long TE was 360. The short τ value removes all the T2* - like effects on the lineshapes. The long T2 filter yields approximately 95% of the total spectral intensity of all metabolites of interest compared to the short TE. Other acquisition parameters were a 90° pulse of 5-10 μs, a spectral width of 8 kHz, 16K complex points, 256 averages and a TR of 5s. Samples were placed in the rotor with a small amount of D2O (Sigma-Aldrich, Milwaukee, WI) for locking and shimming.
Data Analysis and Statistics
Analysis of the in vitro spectra, both solution and HRMAS, was performed using the Chenomx NMR Suite 4.6 (Edmonton, Alberta) fitting, and integrating, the full model spectra determined from simulated reference spectra provided by the Chenomx library with the exception of scyllo-inositol which was not in the existing libraries but was added to the library as a singlet at 3.36ppm with 6 equivalent protons. For the in vitro spectra concentrations were measured using DSS as an internal standard as described above. Each compound was fit to the full model spectrum for all protons. For the HRMAS spectra we measured all the values as molar ratios to the total creatine resonance at 3.03 ppm – a technique that is relatively commone in clinical MRS measurements. Statistical comparisons between the scyllo-fed and normal diet animals in both frontal cortex and hippocampus were made using a one-way ANOVA for all the metabolites with a Tukey HSD correction for multiple comparisons.
Results
We first examined the effects of scyllo-inositol in hippocampus and frontal cortex of triple transgene mice using HRMAS. These mice were fed for two months with a concentration of 16.5mg/L in the drinking water. We examined small tissue punches from the frontal cortex (Fig. 1A) and from the subiculum area of the hippocampus where there is significant pathology (Fig 1B). Shown in Fig. 2A are spectra from hippocampus in four separate mice (two scyllo-fed and two normal diet) showing that the two scyllo-fed mice have large increases in the scyllo-inositol singlet at 3.36 ppm. The quantitative results are shown in Table 1 as molar ratios to creatine. There is an increase in scyllo-inositol in the scyllo-inositol fed animals by a factor of 2.38 in the hippocampus (p<0.001) and 2.18 in the frontal cortex (p<0.01). There were little changes in the spectra between the control and scyllo-fed animals for any of the metabolites except scyllo-inositol. Glutamate and NAA values were higher in cortex than in hippocampus for both the regular diet (p<0.0001) and the scyllo-treated animals (p<0.0001). The scyllo-inositol/Cr values were smaller in both the regular diet animals (p<0.04) and the scyllo-treated animals (p <0.003) in the frontal cortex compared to the hippocampus (see Table 1). There was little overlap of scyllo-inositol values between the groups as a function of diet.
Figure 2.
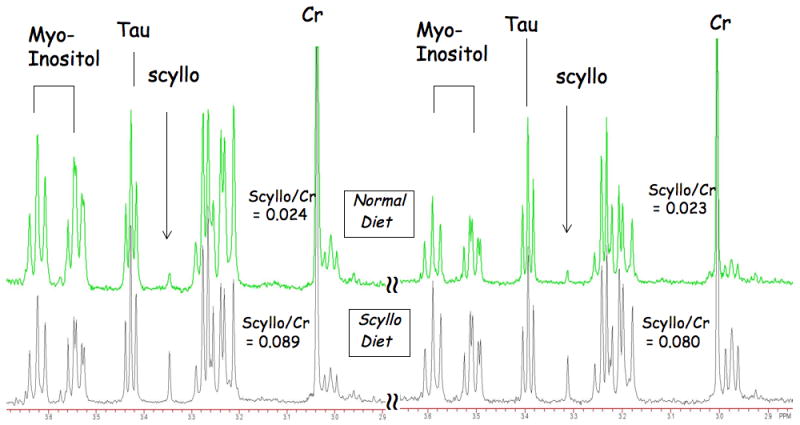
Four HRMAS spectra from the hippocampus of two triple transgene mice with and two without dietary scyllo-inositol supplementation. There was little overlap in the scyllo-inositol values between the two groups of mice (p<0.001).
Table 1. Increased scyllo-inositol in frontal cortex and hippocampus in triple transgene mice using HRMAS.
| Chemical/Cr | Frontal Cortex | Hippocampus | ||
|---|---|---|---|---|
| Scyllo (n=5) | Reg (n=3) | Scyllo (n=7) | Reg (n=7) | |
| GABA | 0.306±.041 | 0.362±.138 | 0.356±.157 | 0.315±.053 |
| Acetate | 0.033±.009 | 0.039±.006 | 0.049±.017 | 0.053±.022 |
| Alanine | 0.075±.008 | 0.084±.007 | 0.060±.019 | 0.066±.013 |
| Aspartate | 0.350±.037 | 0.317±.080 | 0.366±.082 | 0.334±.042 |
| Choline | 0.286±.034 | 0.258±.043 | 0.171±.049 | 0.196±.076 |
| Glutamate | 1.132±.135 | 1.106±.099 | 0.667±.114 | 0.679±.063 |
| Glutamine | 0.360±.060 | 0.394±.053 | 0.458±.107 | 0.368±.031 |
| Glycine | 0.116±.017 | 0.112±.004 | 0.131±.023 | 0.134±.025 |
| Lactate | 0.806±.081 | 0.689±.055 | 0.961±.183 | 0.819±.228 |
| NAA | 0.825±.069 | 0.796±.075 | 0.534±.067 | 0.485±.078 |
| Scyllo-Inositol | 0.035±.008* | 0.016±.003 | 0.074±.018* | 0.031±.011 |
| Taurine | 1.580±.215 | 1.553±.277 | 1.07±.230 | 0.986±.133 |
| myo-Inositol | 0.736±.112 | 0.759±.170 | 0.701±.110 | 0.748±.114 |
Concentrations reported as molar ratios to creatine.
Errors reported are standard deviations.
p<0.001; t-test corrected for multiple comparisons
We next examined the effects of scyllo-inositol treatment in the frontal cortex of APP×PS1 double transgenic mice using brain extracts. Mice were fed the same dose as the triple transgenic animals, also for two months prior to the study. In Table 2, the results from all the mice are shown for the brain extract spectra. We observed an increase in scyllo-inositol slightly higher than what we observed in the triple transgenic animals (an increase of 2.97 (p<0.001) compared to 2.18 for the triple transgene animals in frontal cortex). There were no other changes noted in the spectra between the two groups, although there were a relatively small numbers of animals per group (n=5). There was no overlap in the scyllo-inositol values between the two groups. The lowest value in the scyllo-fed group was 0.198 (range from 0.198-0.317 μmol/mg/tissue) whereas the highest value in the regular diet group was 0.129 (range from 0.055-0.129 μmol/mg/tissue). A bar graph showing the scyllo-inositol/Cr values is shown in Fig. 3 comparing the two different techniques, mouse models and brain regions. We used the ratio to creatine for the extract data, as opposed to the absolute concentrations reported for these data in Table 2, since the HRMAS data does not yield absolute concentrations. These data show comparable increases in the scyllo-inositol concentrations for the two brain regions and techniques. The baseline values are larger in the hippocampus/HRMAS data than in either of the frontal cortex regions and the scyllo/Cr values in the frontal cortex of the regular diet animals is similar for the two mouse models and MRS techniques. There was no difference in scyllo-inositol values between the APP×PS1 mice in frontal cortex extracts and the triple transgene mice in frontal cortex HRMAS spectra for either the scyllo-fed (q = 1.09; p = 0.86) or the regular diet animals (q = 1.4 p = 0.88). In a separate study of wild-type animals in frontal cortex (n=10) with the same background as the APP×PS1 animals and with regular diet APP×PS1 animals (n=10) (Choi et al., 2009) we detected a concentration of scyllo-inositol very close to the values determined here of 1.08±0.037 μmol/g wet weight in the APP×PS1 animals and 0.86±0.03 in the WT animals - quite close to the value we determined here for the regular diet animals.
Table 2. Increased scyllo-inositol in frontal cortex in APP×PS1 Mice.
| Chemical | Scyllo-Fed (n=5) | Regular Diet (n=5) |
|---|---|---|
| GABA | 1.415±.601 | 1.292±.402 |
| Acetate | 0.282±.099 | 0.164±.222 |
| Alanine | 0.502±.084 | 0.483±.089 |
| Aspartate | 2.530±.518 | 2.543±.147 |
| Choline | 0.982±.250 | 0.997±.132 |
| Creatine | 7.149±1.598 | 7.290±1.009 |
| Glutamate | 8.927±.815 | 8.946±1.429 |
| Glutamine | 4.616±1.203 | 4.235±.800 |
| Glutathione | 0.846±.223 | 1.225±.466 |
| Glycine | 0.686±.128 | 0.672±.077 |
| Lactate | 6.058±1.155 | 5.567±.438 |
| NAA | 5.114±.750 | 5.034±.452 |
| NAAG | 0.214±.111 | 0.234±.049 |
| Scyllo-Inositol | 0.264±.043* | 0.089±.031 |
| Succinate | 0.341±.032 | 0.334±.065 |
| Taurine | 8.952±1.860 | 9.014±.715 |
| myo-Inositol | 3.391±.678 | 3.354±.639 |
Concentrations reported as μmol/g tissue wet weight.
Errors reported are standard deviations.
p<0.001; t-test corrected for multiple comparisons
Figure 3.
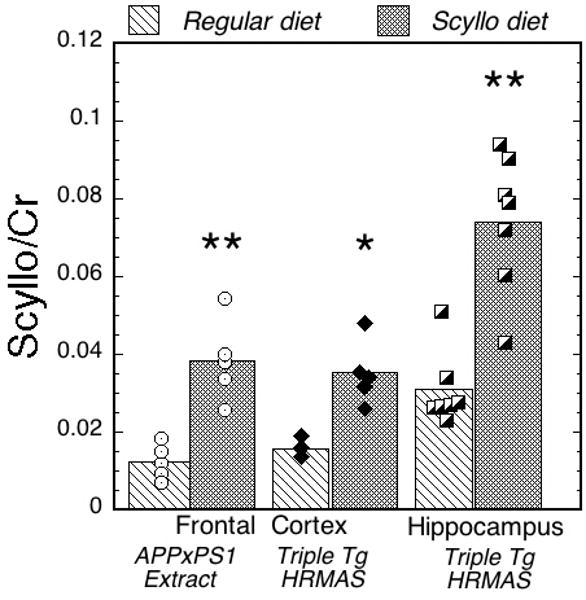
Bar graph showing the increase in scyllo-inositol in the scyllo-fed versus regular diet animals normalized to creatine. Values shown are molar ratios. The data come from brain extracts of frontal cortex in APP×PS1 mice and frontal cortex and hippocampus using HRMAS in APP×PS1×tau mice. The scyllo-inositol increases by about a factor of 2-3 in the two groups.
Discussion
These data demonstrate that dietary supplementation with scyllo-inositol can increase brain concentrations by a factor of 2-3. A prior study by McLaurin et al found that dietary supplementation with scyllo-inositol by oral gavage, at the same dose used in the present study, lowered both soluble and insoluble Aβ levels in an AD mouse model as well as ameliorated cognitive deficits and plaque burdens (McLaurin et al., 2006). In another study they found that these effects even occurred in aged AD mice with well advanced symptoms (Fenili et al., 2007). Whether or not such an increase will have therapeutic effects in humans remains to be seen, however there were some promising signs in our data that other compounds elevated in AD mouse models could be modulated with scyllo-inositol treatment. For instance, prior studies of APP, APP×PS1 and APP×PS2 mouse models showed that there were elevations in taurine, myoinositol and glutamine while there were decreases in glutamate and NAA (Choi et al., 2007; Choi et al., 2009; Dedeoglu et al., 2004; Marjanska et al., 2005; von Kienlin et al., 2005). Our data showed, albeit with small sample sizes and younger mice than those studied using MRS previously, trends towards modulation of glutamine, taurine and NAA with scyllo-inositol feeding (see Tables 1 and 2). Thus, MRS will likely prove valuable for following such therapeutic trials in mouse models – especially when combined with the behavioral measurements and pathology in the same animals. The ability to relate the direct increase in brain scyllo-inositol levels to the quantitative effects on other molecules in the same MR spectrum will be useful.
Detection of scyllo-inositol is quite a bit more difficult in rodents than in humans. This is because rodents have much more taurine than do humans. The taurine triplet at 3.43 ppm interferes with the scyllo-inositol singlet peak at 3.36 ppm. Nonetheless, using complete simulation of the metabolite spectra, where the intensity of the taurine triplet at 3.43 ppm will be constrained by the taurine multiplet at 3.26 ppm, with a program such as LCModel, it should be possible to quantify the scyllo-inositol peak at 3.36 ppm in vivo. Since the scyllo-inositol peak is a singlet with a relatively long T2 (ca. 170ms at 4T in humans (Seaquist and Gruetter, 1998)) it could potentially be detected at an echo time where taurine is nulled, at approximately 70ms. Further, in humans, there is approximately 5-10 times less taurine in the brain than in rodents. In rodents there appears to be considerably less scyllo-inositol than in humans. According to table 1, and the data we presented for wild-type animals the scyllo-inositol concentration is about 100 μM compared to about 500 μM in humans (Michaelis et al., 1993). Thus, it should be quite easy to identify in humans – especially at 3 or 4T since there are six magnetically equivalent protons for scyllo-inositol.
In a study in a different mutant APP mouse model of AD (TgCRND8) McLaurin and colleagues showed, using gas chromatography and mass spectroscopy, that 10mg/ml of scyllo-inositol in the drinking water increased the scyllo-inositol concentration in whole brain hemisphere samples by a factor of about 7.5 (Fenili et al., 2007). This is about a 600-fold larger concentration of scyllo-inositol than we used and yet they were only able to elevate scyllo-inositol approximately 2.5 fold higher than we did using the much lower concentration. This suggests that the concentration of scyllo-inositol in the brain is saturated in the Fenili et al. study (Fenili et al., 2007) – perhaps by limited transport capacity. The brain has approximately a 10 fold higher concentration of scyllo-inositol than plasma, and it appears as if most of the scyllo-inositol comes from the blood into the brain via active transport rather than being synthesized in situ (Spector, 1978). Fenili et al. found that the scyllo-inositol was not incorporated into phosphatidylinositol lipids showing it could not substitute for myo-inositol in those pathways and consequently would have less chance of disrupting such metabolism (Fenili et al., 2007). These data, combined with the lack of side effects at the high doses, suggests that scyllo-inositol supplementation may be quite safe. Our data further suggest that a dose quite a bit lower than that used in the Fenili et al paper (Fenili et al., 2007) will likely increase brain scyllo-inositol by the same amount. These data also have important implications for continuing human trials since the high dose arms of ELND005 (scyllo-inositol) using 1000-2000 mg 2× per day were stopped due to an unusual number of deaths. Our data suggest that such a high dose will likely not lead to much higher brain levels of scyllo-inositol than can be attained using lower doses such as 250mg/day. MRS studies will prove invaluable in preliminary dose ranging studies for the promising effects of scyllo-inositol treatment in humans.
Acknowledgments
This research is supported by grants from the Department of Veteran Affairs (Merit Award) to AD, NIH (P30 AG13846 Boston University ADC). There is no conflict of interest. The authors thank Lokman Hussain for animal husbandary.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15:289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- Choi JK, Dedeoglu A, Jenkins BG. Application of MRS to mouse models of neurodegenerative illness. NMR Biomed. 2007;20:216–237. doi: 10.1002/nbm.1145. [DOI] [PubMed] [Google Scholar]
- Choi JK, Jenkins BG, Carreras I, Kaymakcalan S, Cormier K, Kowall NW, Dedeoglu A. Anti-inflammatory treatment in AD mice protects against neuronal pathology. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedeoglu A, Choi JK, Cormier K, Kowall NW, Jenkins BG. Magnetic resonance spectroscopic analysis of Alzheimer's disease mouse brain that express mutant human APP shows altered neurochemical profile. Brain Res. 2004;1012:60–65. doi: 10.1016/j.brainres.2004.02.079. [DOI] [PubMed] [Google Scholar]
- Dickson DW. The pathogenesis of senile plaques. J Neuropathol Exp Neurol. 1997;56:321–339. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- Fenili D, Brown M, Rappaport R, McLaurin J. Properties of scyllo-inositol as a therapeutic treatment of AD-like pathology. J Mol Med. 2007;85:603–611. doi: 10.1007/s00109-007-0156-7. [DOI] [PubMed] [Google Scholar]
- Griffith HR, den Hollander JA, Stewart CC, Evanochko WT, Buchthal SD, Harrell LE, Zamrini EY, Brockington JC, Marson DC. Elevated brain scyllo-inositol concentrations in patients with Alzheimer's disease. NMR Biomed. 2007;20:709–716. doi: 10.1002/nbm.1132. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Isaacks RE, Bender AS, Kim CY, Shi YF, Norenberg MD. Effect of ammonia and methionine sulfoximine on myo-inositol transport in cultured astrocytes. Neurochem Res. 1999;24:51–59. doi: 10.1023/a:1020928029845. [DOI] [PubMed] [Google Scholar]
- Jenkins BG, Klivenyi P, Kustermann E, Andreassen OA, Ferrante RJ, Rosen BR, Beal MF. Nonlinear decrease over time in N-acetyl aspartate levels in the absence of neuronal loss and increases in glutamine and glucose in transgenic Huntington's disease mice. J Neurochem. 2000;74:2108–2119. doi: 10.1046/j.1471-4159.2000.0742108.x. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Xu C, Panchalingam K, McClure RJ, Pettegrew JW. Quantitative 1H and 31P MRS of PCA extracts of postmortem Alzheimer's disease brain. Neurobiol Aging. 1996;17:349–357. doi: 10.1016/0197-4580(96)00035-8. [DOI] [PubMed] [Google Scholar]
- Marjanska M, Curran GL, Wengenack TM, Henry PG, Bliss RL, Poduslo JF, Jack CR, Jr, Ugurbil K, Garwood M. Monitoring disease progression in transgenic mouse models of Alzheimer's disease with proton magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 2005;102:11906–11910. doi: 10.1073/pnas.0505513102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin J, Golomb R, Jurewicz A, Antel JP, Fraser PE. Inositol stereoisomers stabilize an oligomeric aggregate of Alzheimer amyloid beta peptide and inhibit abeta -induced toxicity. J Biol Chem. 2000;275:18495–18502. doi: 10.1074/jbc.M906994199. [DOI] [PubMed] [Google Scholar]
- McLaurin J, Kierstead ME, Brown ME, Hawkes CA, Lambermon MH, Phinney AL, Darabie AA, Cousins JE, French JE, Lan MF, Chen F, Wong SS, Mount HT, Fraser PE, Westaway D, St George-Hyslop P. Cyclohexanehexol inhibitors of Abeta aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat Med. 2006;12:801–808. doi: 10.1038/nm1423. [DOI] [PubMed] [Google Scholar]
- Michaelis T, Helms G, Merboldt KD, Hanicke W, Bruhn H, Frahm J. Identification of Scyllo-inositol in proton NMR spectra of human brain in vivo. NMR Biomed. 1993;6:105–109. doi: 10.1002/nbm.1940060116. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, Laferla FM. Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem. 2006 doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- Pettegrew JW, Klunk WE, Panchalingam K, McClure RJ, Stanley JA. Magnetic resonance spectroscopic changes in Alzheimer's disease. Ann N Y Acad Sci. 1997;826:282–306. doi: 10.1111/j.1749-6632.1997.tb48480.x. [DOI] [PubMed] [Google Scholar]
- Seaquist ER, Gruetter R. Identification of a high concentration of scyllo-inositol in the brain of a healthy human subject using 1H- and 13C-NMR. Magn Reson Med. 1998;39:313–316. doi: 10.1002/mrm.1910390220. [DOI] [PubMed] [Google Scholar]
- Shonk TK, Moats RA, Gifford P, Michaelis T, Mandigo JC, Izumi J, Ross BD. Probable Alzheimer disease: diagnosis with proton MR spectroscopy. Radiology. 1995;195:65–72. doi: 10.1148/radiology.195.1.7892497. see comments. [DOI] [PubMed] [Google Scholar]
- Spector R. The transport and metabolism of scyllo-inositol in the central nervous system. J Neurochem. 1978;31:1113–1115. doi: 10.1111/j.1471-4159.1978.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Thurston JH, Sherman WR, Hauhart RE, Kloepper RF. myo-inositol: a newly identified nonnitrogenous osmoregulatory molecule in mammalian brain. Pediatr Res. 1989;26:482–485. doi: 10.1203/00006450-198911000-00024. [DOI] [PubMed] [Google Scholar]
- von Kienlin M, Kunnecke B, Metzger F, Steiner G, Richards JG, Ozmen L, Jacobsen H, Loetscher H. Altered metabolic profile in the frontal cortex of PS2APP transgenic mice, monitored throughout their life span. Neurobiol Dis. 2005;18:32–39. doi: 10.1016/j.nbd.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Wang HW, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B, Viola KL, Klein WL, Stine WB, Krafft GA, Trommer BL. Soluble oligomers of beta amyloid (1-42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002;924:133–140. doi: 10.1016/s0006-8993(01)03058-x. [DOI] [PubMed] [Google Scholar]


