Abstract
The alcohol-preferring (P) rat is a valid animal model of alcoholism. However, the effect of alcohol on sleep in P or alcohol non-preferring (NP) rats is unknown. Since alcohol consumption has tremendous impact on sleep, the present study compared the effects of binge alcohol administration on sleep-wakefulness in P and NP rats.
Using standard surgical procedures, the P and NP rats were bilaterally implanted with sleep recording electrodes. Following post-operative recovery and habituation, pre-ethanol (baseline) sleep-wakefulness was electrographically recorded for 48 hr. Subsequently, ethanol was administered beginning with a priming dose of 5 g/Kg followed by two doses of 2 g/Kg every 8 hrs on the first day and three doses of 3 g/Kg/8 hr on the second day. On the following day (post-ethanol), undisturbed sleep-wakefulness was electrographically recorded for 24 hr.
Our initial results suggest that, during baseline conditions, the time spent in each of the three behavioral states: wakefulness, non-rapid eye movement (NREM) sleep and REM sleep, was comparable between P and NP rats. However, the P rats were more susceptible to changes in sleep-wakefulness following 2 days of binge ethanol treatment. As compared to NP rats, the P rats displayed insomnia like symptoms including a significant reduction in the amount of time spent in NREM sleep coupled with a significant increase in wakefulness on post-ethanol day. Subsequent analysis revealed that binge ethanol induced increased wakefulness and reduced NREM sleep in P rats occurred mainly in the dark period.
This is the first study that : 1) demonstrates spontaneous sleep-wake profile in P and NP rats, and 2) compares the effects of binge ethanol treatment on sleep in P and NP rats. Our results suggest that as compared to NP rats, the P rats were more susceptible to sleep disruptions after binge ethanol treatment. In addition, the P rats exhibited insomnia-like symptoms observed during abstinence from alcohol in human subjects.
Keywords: Alcohol preferring P rats, wakefulness, NREM sleep, ethanol, REM sleep
Ethanol intake has a profound impact on sleep. In healthy non-alcoholics, acute ethanol intake promotes NREM sleep, but suppresses REM sleep during first half of the sleep period. However, during the second half, increased wakefulness coupled with increased REM sleep occurs resulting in severe disturbance in sleep architecture (Roehrs and Roth, 2001).
Acute discontinuation of alcohol in alcoholics results in severe insomnia coupled with profound sleep disruptions (Roehrs and Roth, 2001; Brower, 2003; Brower, 2001). In addition, recent studies suggest that sleep disturbances are predictors of relapse in recovering alcoholics (reviewed in Brower, 2003).
Strong evidence suggests that the alcohol-preferring (P) rat is a viable model of alcoholism (McBride and Li, 1998; Murphy et al., 2002). The P and its counterpart, alcohol-non-preferring NP rat were selectively bred for high and low alcohol-drinking behavior at the Indiana University (Lumeng et al., 1977). In last 30 years, the P rat has been extensively studied and meets all the criteria put forth for a valid animal model of alcohol abuse and alcoholism (Cicero, 1979; Bell et al., 2006a).
The P and NP rats showed profound differences in behavioral responses to ethanol. For example, compared to NP rats, the P rats displayed 1) genetic predisposition for high ethanol consumption (Bell et al., 2006a), 2) early onset high alcohol-consuming behavior (McBride and Li, 1998; Murphy et al., 2002; Bell et al., 2006b), 3) binge- or bender-like excessive ethanol drinking in short time period (Rodd-Henricks et al., 2001; Rodd-Henricks et al., 2000), 4) reduced sensitivity to ataxic and hypothermic effects of ethanol (Bell et al., 2001; Stewart et al., 1992), 5) rapid tolerance to the ataxic and hypnotic [as evidenced by a longer latency to lose righting reflex] effects of ethanol (Kurtz et al., 1996; Bell et al., 2001), 6) increased locomotor activity after low doses of ethanol (Waller et al., 1986; Rodd et al., 2004; Lumeng et al., 1982), 7) greater behavioral activation in odor-enhanced novel environment (Nowak et al., 2000), 8) loss of control over drinking under relapse conditions (Bell et al., 2006a) and 9) reinstatement of ethanol consumption and the expression of an alcohol deprivation effects (Rodd-Henricks et al., 2000; Rodd-Henricks et al., 2002). As described above, the P and NP rats have been extensively evaluated for many behavioral responses to ethanol; however, sleep-wakefulness following ethanol has not been evaluated in these animals.
Do P rats exhibit sleep disturbances observed in humans during withdrawal? The main objective of this study was to evaluate sleep-wakefulness following ethanol administration in P and NP rats.
EXPERIMENTAL
Animals
Adult male P and NP rats, (age 5 weeks) were obtained from Indiana University. After 8 weeks of quarantine at the University of Missouri Animal facility, the animals were moved to the Harry S. Truman Memorial Veteran's Hospital Animal Facility and housed under constant temperature, 12:12 h light and dark cycle (light on =0200 to 1400 hr) and with ad libitum availability of food and water for one week before surgery. All animals were treated in accordance with the American Association for Accreditation of Laboratory Animal Care's policy on care and use of laboratory animals and every effort was made to minimize animal suffering and to reduce the number of animals used. All experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23; revised 1996) and approved by the Harry S. Truman Memorial Veterans Hospital Animal Committee.
Surgery
Surgical procedures were done under aseptic conditions and under inhalation anesthesia (1% isoflurane). Adult P and NP rats (N=6/group; 350–500 gm) were stereotaxically implanted with bilateral screw electrodes fixed onto the skull above the frontal area (4 mm anterior to the bregma and 3 mm lateral to the mid saggital suture for recording electroencephalogram (EEG) (Thakkar et al., 2003). Two flexible wires with stainless steel loops were fixed onto the superior nuchal muscles for recording electromyogram (EMG). These electrodes were connected to a multichannel electrode pedestal (MS363, Plastics One Inc, Roanoke, VA), and fixed on the skull by acrylic cement.
Post operative recovery and habituation
Experiments were conducted in a sound-attenuated chamber with the same light conditions, and with food and water available ad libitum. After 3 days of post-operative recovery, the rats were tethered to a light weight recording cable and habituated for 7 days before the experiment was begun.
Ethanol
The 200 proof ethyl alcohol (Acros Organics) was purchased from Fischer Scientific and diluted to 35% (v/v) in sterile water. Ethanol or sterile water was administered by intragastric intubation (Thakkar et al., 2010).
Experimental Protocol
Following habituation to the recording cable the experiment was begun with the initiation of sleep-wakefulness recording on experimental day 1 at light onset (ZT0). The electrographic recording of sleep-wakefulness with a 16 channel, bipolar Physiodata Amplifier System (Model 15LT; Grass Technologies, West Warwick, RI) and 4 Quad Neuroamplifiers (Model 15A54). The EEG signal was recorded differentially from two bilateral skull electrodes with high pass filter settings at 0.3 Hz and low pass filter settings at 100 Hz. Similarly, the EMG signal was recorded differentially from two bilateral implanted electrodes in the nuchul muscle and filtered between 30 to 300 Hz. In addition, both EEG and EMG signals were filtered at 60 Hz with a notch filter.
On experimental day 1 and 2, the animals were administered sterile water (10 ml/Kg; by intragastric intubation) with the first dose administered at ZT6 (6 hr after light onset) followed by a second dose at ZT14 (2 hr after dark onset) and the third dose at ZT22 (10 hr after dark onset). This experimental paradigm allowed maximal undisturbed sleep during the light period. Sleep-wakefulness recording that began at ZT0 on Experimental day 1 was continued until experiment day 3 (described below).
For next two days (Experimental Days 3 and 4), a dose of 9 g/kg of ethanol was administered each day in three divided doses. The priming dose of 5 g/kg of ethanol (35% v/v in sterile water) was administered on experimental day 3 at ZT6. This was followed by administration of two doses; each dose of 2 g/Kg administered at ZT 14 and ZT22. On the second day (Experimental Day 4), three doses of 3 g/Kg were administered every 8 hr beginning with the first dose administered at ZT6 and ending at ZT22 on experiment day 4 (Marutha Ravindran et al., 2007; Majchrowicz, 1975; Mendelson et al., 1978; Obernier et al., 2002). We had to stop recording on experimental day 3 and unhook the animals from the recording cables because the cables interfered with inadvertently assumed positions of intoxicated animals (especially the NP rats). Thus, we did not continue recording during alcohol treatment and the animals were left unhooked from the cable until the last dose of ethanol administration on experiment day 4.
Just before light onset on experiment day 5, the animals were reconnected to the recording cable and the recording was begun at ZT0 on experimental day 5 (withdrawal day) and continued for 24 hr. There was no treatment and the animals were left undisturbed for the next 24 hr except for sleep-wakefulness recordings to ascertain the effects during withdrawal.
Analysis of Behavioral States
The sleep-wakefulness was visually scored in 10 sec epochs as (1) Wakefulness, which included both active and quiet waking; (2) NREM sleep; (3) REM sleep. Wakefulness was identified by the simultaneous presence of desynchronized EEG with high (active W) or reduced (quiet W) EMG activity, whereas NREM sleep was identified by the simultaneous presence of synchronized EEG without transient periods of desynchronization and reduced EMG activity. REM sleep was identified by the concomitant presence of desynchronized EEG with no muscle tone (Thakkar et al., 2008).
Data analysis
The percent time spent in each state of behavior, by P and NP rats, on baseline and post-ethanol days, was analyzed separately by t-test with α = 0.05.
RESULTS
Sleep-wakefulness in P and NP rats during pre-ethanol baseline
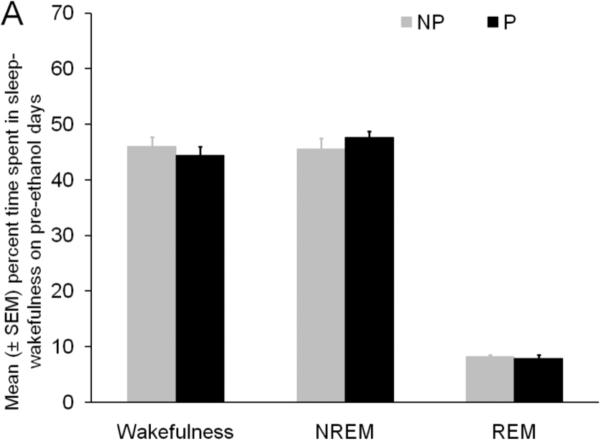
The percent time spent in wakefulness, NREM sleep and REM sleep during baseline days were comparable in P and NP rats (Figure 1A) suggesting that there are no inherent difference in sleep-wakefulness states between P and NP rats.
Figure 1.
Panel A: The percent time spent in sleep–wakefulness by P and NP rats during 24 hr of pre-ethanol baseline is described. The amount of time spent in each behavioral state by P and NP rats was comparable suggesting that there was no inherent difference in sleep-wakefulness in P and NP rats.
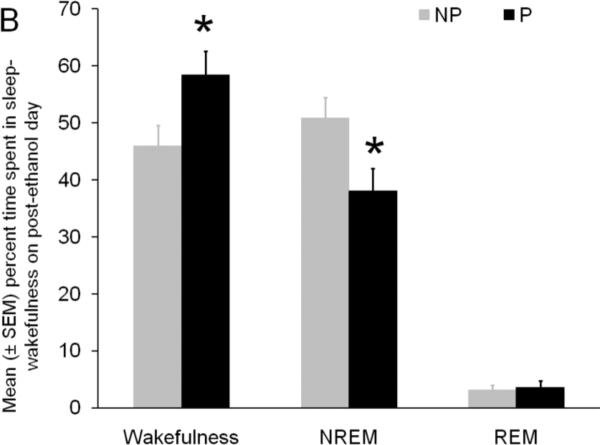
Panel B: The effect of chronic binge ethanol treatment on the percent time spent in sleep-wakefulness during the 24 hr period of post-ethanol day is described. As compared to NP rats, the P rats displayed a significant increase in wakefulness coupled with a significant decrease in NREM sleep on post ethanol day. REM sleep was comparable in P and NP rats. * = p<0.05
Sleep-wakefulness in P and NP rats on post-ethanol day
The effect of binge ethanol treatment on the percent time spent in sleep-wakefulness in P and NP rats is described in Figure 1B. The P rats spent significantly (t = 2.3; df = 10; p < 0.05) more time in wakefulness (Mean ± SEM = 58.4% ± 4.2%) and significantly (t = 2.7; df = 10; p <0.05) less time in NREM sleep (Mean ± SEM = 38.0% ± 3.2%) as compared to NP rats (Mean ± SEM; Wakefulness =45. 9% ± 3.7%; NREM = 50.9% ± 3.6%). Interestingly, the amount of time spent in REM sleep was comparable in P (Mean ± SEM = 3.6% ± 1.1%) and NP rats (Mean ± SEM = 3.1% ± 0.9%).
In order to examine whether binge ethanol induced changes in sleep-wakefulness observed in P rats occurred during the inactive (light) or active (dark) period, sleep-wakefulness data for light and dark periods was separately analyzed.
Sleep-wakefulness in P and NP rats during the light (inactive) period on post-ethanol day
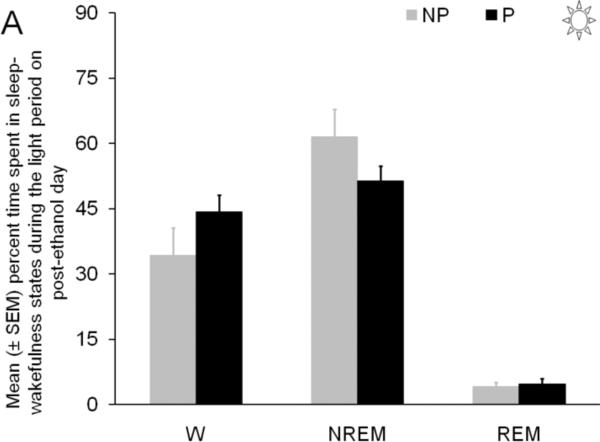
The percent time spent in wakefulness (t=1.4; df = 10; p = 0.2), NREM sleep (t=1.4; df = 10; p = 0.2), and REM sleep (t=0.7; df = 10; p = 0.9), during the light period on post-ethanol day were comparable in P and NP rats (Figure 2A).
Figure 2.
Panel A: The effect of chronic binge ethanol treatment on the percent time spent in sleep-wakefulness during the 12 hr light period of post-ethanol day is described. The P and NP rats spent comparable time in all three states of behavior during the light period on post-ethanol day.
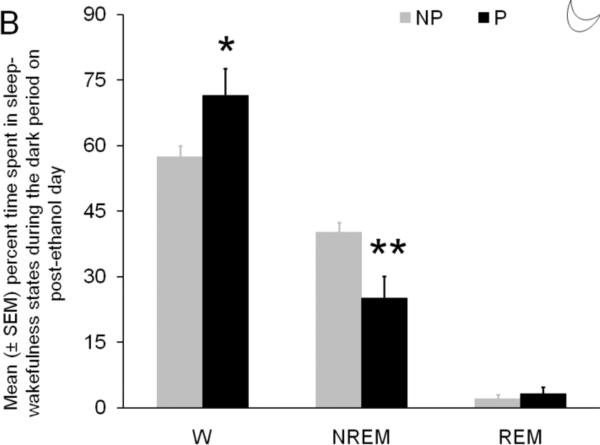
Panel B: The effect of chronic binge ethanol treatment on the percent time spent in sleep-wakefulness during the 12 hr dark period of post-ethanol day is described. As compared to NP rats, the P rats displayed a significant increase in wakefulness coupled with a significant decrease in NREM sleep. REM sleep was comparable in P and NP rats. * = p<0.05; ** =p<0.01
Sleep-wakefulness in P and NP rats during the dark (active) period on post-ethanol day
The effect of binge ethanol treatment on the percent time spent in three states of sleep-wakefulness in P and NP rats during the dark period is described in Figure 2B. As compared to NP rats(Mean ± SEM; 57.2% ± 2.5%), the P rats spent significantly (t = 2.5; df =10; p< 0.05) greater amount (> 25) of time in wakefulness (Mean ± SEM =72.5% ± 6.1%).
The P rats displayed a significant reduction in NREM sleep (t = 3.2; df =10; p <0.01) during the dark period as compared to NP rats. The mean (± SEM) percent time spent in NREM sleep by NP rats was 40.2% (± 2.1%). In contrast the P rats spent only 24.6% (± 4.4%) time in NREM sleep, a > 40% reduction in NREM sleep.
Surprisingly, both P (Mean ± SEM = 2.9% ± 1.3%) and NP rats (Mean ± SEM = 2.1% ± 0.9%) spent comparable time (t = 0.5; df =10; p = 0.6) in REM sleep during the dark period.
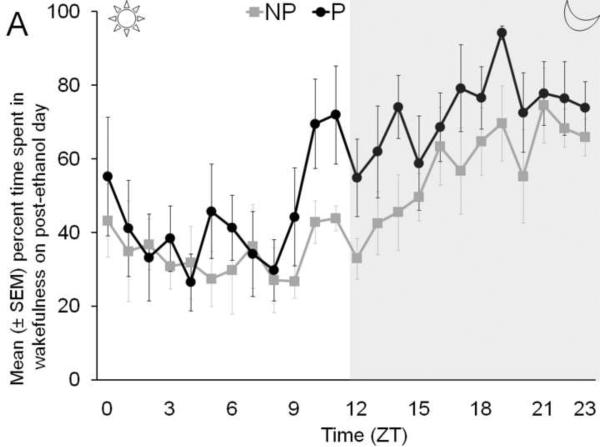
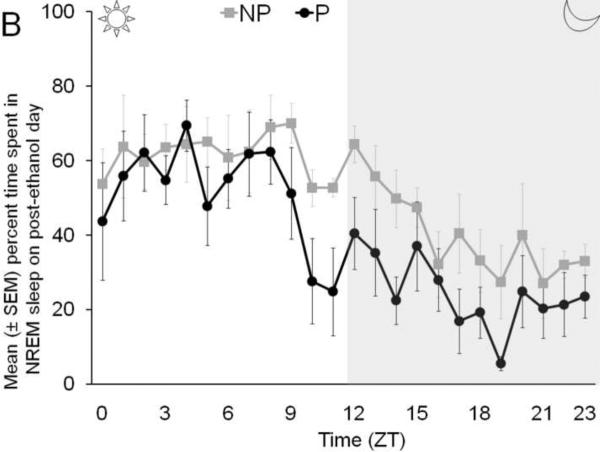
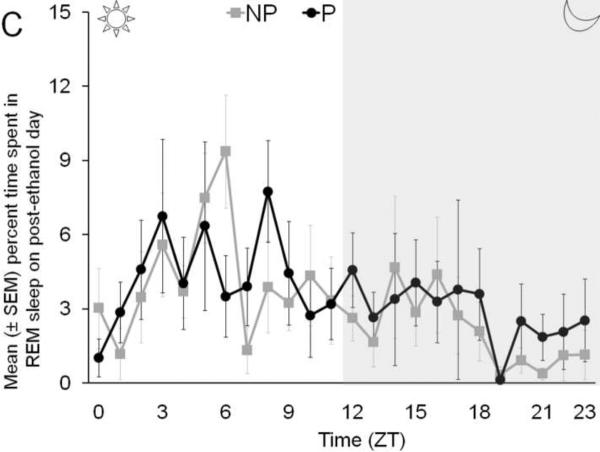
The temporal distribution of percent time spent in wakefulness (Panel A), NREM sleep (Panel B), and REM sleep (Panel C), by P and NP rats, across 24 hr following binge ethanol treatment is described in Figure 3.
Figure 3.
The temporal distribution of the percent time spent in wakefulness (Panel A), NREM sleep (Panel B) and REM sleep (Panel C) by P rats and NP rats across 24 hr on post ethanol day is described.
DISCUSSION
This is the first study that demonstrates 1) sleep-wakefulness profile in P and NP animals during normal conditions, and 2) the effects of binge ethanol treatment on sleep-wakefulness in P and NP rats. The results of our study suggest that both P and NP rats spent comparable time in each of the three states of behavior during baseline conditions. However, binge ethanol treatment affected sleep-wakefulness states differently in P and NP rats. The P rats showed a significant increase in wakefulness and a significant reduction in NREM sleep on post-ethanol day as compared to NP rats. The amount of time spent in REM sleep was comparable in P and NP rats.
We used the binge ethanol paradigm to evaluate the effects of ethanol on sleep-wakefulness in P and NP rats. The binge ethanol method has been extensively and successfully used [>300 papers citation of the original article in last 20 years (Faingold, 2008)] as a model to understand the effects of ethanol withdrawal in experimental animals. This method offers several advantages including 1) modeling binge drinking in experimental animals, 2) mimicking heavy ethanol consumption in alcoholics, 3) rapid induction of alcohol dependency with relatively short period of ethanol administration, and 4) relatively easy to use (Crews and Nixon, 2008; Faingold, 2008; Feng and Faingold, 2000; Mhatre et al., 1988; Crews et al., 2004).
We performed two days of binge ethanol treatment because previous studies have shown that two days of binge ethanol treatment is sufficient to produce significant neuronal damage in rat brain (Obernier et al., 2002). We did not measure blood ethanol concentration because drawing of blood required restraining of the animals and causing pain which may affect sleep-wakefulness and confound our findings. However, previous studies suggest that the blood alcohol concentration is comparable in P and NP rats after binge ethanol treatment (Crews and Braun, 2003).
Our results suggest that, as compared to the NP rats, the P rats were more susceptible to changes in sleep-wakefulness following binge ethanol treatment. The P rats displayed insomnia like symptoms including a significant reduction in the amount of time spent in NREM sleep and a significant increase in wakefulness. Interestingly, there was no change in REM sleep. Further analysis revealed that the binge ethanol induced changes in sleep-wakefulness in P rats occurred during the dark period on post-ethanol day. There was no significant difference in either wakefulness or sleep (both NREM and REM phases) during the light period, most likely due to different cerebral metabolism rates in P and NP rats (Smith et al., 2001; Strother et al., 2008). Our data is supported by previous animal studies. Insomnia-like symptoms (increased wakefulness and reduced sleep) have also been observed in rodents, during withdrawal, after chronic ethanol exposure (Ehlers and Slawecki, 2000; Kubota et al., 2002; Rouhani et al., 1998; Mendelson et al., 1978). Clinical studies also suggest that insomnia is highly prevalent during acute and chronic withdrawal in recovering alcohol (Brower et al., 2001; Brower, 2001; Brower, 2003). Thus, our results suggest that the P rats exhibit sleep disruptions observed in human alcoholics following discontinuation of alcohol.
Our data suggesting insomnia like symptoms in P rats as compared to NP is not surprising because the P rats displayed greater region-specific brain damage after binge ethanol treatment than NP rat (Crews and Braun, 2003). Clinical evidence exists to suggest that ethanol induced neurotoxicity may be a likely cause of insomnia and sleep reduction in recovering alcoholics (Brower, 2003).
Although the mechanism of binge ethanol induced reduction in NREM sleep is unknown, our recent studies suggest that adenosinergic mechanisms in the wake-promoting basal forebrain have a critical role in mediating sleep promoting effects of ethanol (Sharma et al., 2010; Thakkar et al., 2010). Are basal forebrain adenosinergic mechanisms impaired in P rats after chronic binge alcohol treatment? How long does the effect of binge ethanol treatment on sleep-wakefulness last? Further studies are required to answer these questions. In addition, studies performed in recovering alcoholics suggest that sleep disruptions predict relapse (Brower, 2001; Brower et al., 2001; Foster and Peters, 1999; Drummond et al., 1998). Since ethanol intake promotes sleep, it has been suggested that alcoholics use ethanol to self-medicate sleep disturbances which then results in return to drinking. While P rats display “alcohol deprivation effect” (ADE) and are vulnerable to relapse-like drinking (Bell et al., 2006a), it is yet unknown whether alcohol dependent P rats consume ethanol to improve their sleep quality.
CONCLUSION
This is the first study to demonstrate sleep-wakefulness profile in P and NP rats, and to evaluate the effect of binge ethanol treatment on sleep in P and NP rats. Our data suggest that as compared to NP rats, the P rats are more susceptible to changes in sleep-wakefulness after binge ethanol treatment. Thus, the P rats may provide an appropriate animal model for future studies on the neurobiological regulation of sleep disturbances induced by ethanol.
ACKNOWLEDGEMENTS
This study was supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital, Columbia, Missouri. This research received grant support from the NIH [NS059831 and RAA017472A (MMT) RO1EY17294 (RRM)]. The Alcohol Research Resource Award (R24AA015512) from NIAAA to Indiana University provided support for the selective breeding of P and NP rats. We would like to thank Drs. L. Lumeng and R. Bell for providing us the animals, Timothy Onderbeke and Carrie Harris for animal care and Jenifer Krieger for sleep analysis.
ABBRIVIATIONS
- ANOVA
Analysis of variance
- EEG
Electroencephalogram
- EMG
Electromyogram
- NP rats
Alcohol non-preferring NP rats
- NREM
Non-rapid eye movement
- P rats
Alcohol preferring P rats
- REM
Rapid eye movement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006a;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2006b;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Bell RL, Stewart RB, Woods JE, Lumeng L, Li TK, Murphy JM, McBride WJ. Responsivity and development of tolerance to the motor impairing effects of moderate doses of ethanol in alcohol-preferring (P) and -nonpreferring (NP) rat lines. Alcohol Clin Exp Res. 2001;25:644–650. [PubMed] [Google Scholar]
- Brower KJ. Alcohol's effects on sleep in alcoholics. Alcohol Res Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7:523–539. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EA, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry. 2001;158:399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ. A critique of animal analogues of alcoholism. In: Majchrowicz E, Noble EP, editors. Biochemistry and Pharmacology of Ethanol. Plenum Press; New York: 1979. pp. 533–560. [Google Scholar]
- Crews FT, Braun CJ. Binge ethanol treatment causes greater brain damage in alcohol-preferring P rats than in alcohol-nonpreferring NP rats. Alcohol Clin Exp Res. 2003;27:1075–1082. doi: 10.1097/01.ALC.0000075826.35688.0D. [DOI] [PubMed] [Google Scholar]
- Crews FT, Collins MA, Dlugos C, Littleton J, Wilkins L, Neafsey EJ, Pentney R, Snell LD, Tabakoff B, Zou J, Noronha A. Alcohol-induced neurodegeneration: when, where and why? Alcohol Clin Exp Res. 2004;28:350–364. doi: 10.1097/01.alc.0000113416.65546.01. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of Neurodegeneration and Regeneration in Alcoholism. Alcohol Alcohol. 2009;44(2):115–27. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, Smith TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol Clin Exp Res. 1998;22:1796–1802. [PubMed] [Google Scholar]
- Ehlers CL, Slawecki CJ. Effects of chronic ethanol exposure on sleep in rats. Alcohol. 2000;20:173–179. doi: 10.1016/s0741-8329(99)00077-4. [DOI] [PubMed] [Google Scholar]
- Faingold CL. The Majchrowicz binge alcohol protocol: an intubation technique to study alcohol dependence in rats. Curr Protoc Neurosci. 2008 doi: 10.1002/0471142301.ns0928s44. Chapter 9:Unit 9.28.:Unit. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Faingold CL. Modulation of audiogenic seizures by histamine and adenosine receptors in the inferior colliculus. Exp Neurol. 2000;163:264–270. doi: 10.1006/exnr.2000.7382. [DOI] [PubMed] [Google Scholar]
- Foster JH, Peters TJ. Impaired sleep in alcohol misusers and dependent alcoholics and the impact upon outcome. Alcohol Clin Exp Res. 1999;23:1044–1051. [PubMed] [Google Scholar]
- Kubota T, De A, Brown RA, Simasko SM, Krueger JM. Diurnal effects of acute and chronic administration of ethanol on sleep in rats. Alcohol Clin Exp Res. 2002;26:1153–1161. doi: 10.1097/01.ALC.0000024292.05785.03. [DOI] [PubMed] [Google Scholar]
- Kurtz DL, Stewart RB, Zweifel M, Li TK, Froehlich JC. Genetic differences in tolerance and sensitization to the sedative/hypnotic effects of alcohol. Pharmacol Biochem Behav. 1996;53:585–591. doi: 10.1016/0091-3057(95)02055-1. [DOI] [PubMed] [Google Scholar]
- Lumeng L, Hawkins TD, Li TK. New strains of rats with alcohol preference and non-preference. In: Thurman RG, Williamson JR, Drott H, Chance B, editors. Alcohol and Aldehyde Metabolizing Systems. Academic Press; New York: 1977. pp. 537–544. [Google Scholar]
- Lumeng L, Waller MB, McBride WJ, Li TK. Different sensitivities to ethanol in alcohol-preferring and -nonpreferring rats. Pharmacol Biochem Behav. 1982;16:125–130. doi: 10.1016/0091-3057(82)90023-5. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Marutha Ravindran CR, Mehta AK, Ticku MK. Effect of chronic administration of ethanol on the regulation of the delta-subunit of GABA(A) receptors in the rat brain. Brain Res. 2007;1174:47–52. doi: 10.1016/j.brainres.2007.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Majchrowicz E, Mirmirani N, Dawson S, Gillin JC, Wyatt RJ. Sleep during chronic ethanol administration and withdrawal in rats. J Stud Alcohol. 1978;39:1213–1223. doi: 10.15288/jsa.1978.39.1213. [DOI] [PubMed] [Google Scholar]
- Mhatre M, Mehta AK, Ticku MK. Chronic ethanol administration increases the binding of the benzodiazepine inverse agonist and alcohol antagonist [3H]RO15-4513 in rat brain. Eur J Pharmacol. 1988;153:141–145. doi: 10.1016/0014-2999(88)90599-7. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Nowak KL, Ingraham CM, McKinzie DL, McBride WJ, Lumeng L, Li TK, Murphy JM. An assessment of novelty-seeking behavior in alcohol-preferring and nonpreferring rats. Pharmacol Biochem Behav. 2000;66:113–121. doi: 10.1016/s0091-3057(00)00206-9. [DOI] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res. 2002;26:547–557. [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McKinzie DL, Webster AA, Murphy JM, Lumeng L, Li TK, McBride WJ. Low-dose stimulatory effects of ethanol during adolescence in rat lines selectively bred for high alcohol intake. Alcohol Clin Exp Res. 2004;28:535–543. doi: 10.1097/01.alc.0000122107.08417.d0. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring rats. Alcohol Clin Exp Res. 2001;25:1140–1150. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: II. Adult exposure. Alcohol Clin Exp Res. 2002;26:1642–1652. doi: 10.1097/01.ALC.0000036302.73712.9D. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Shaikh SR, Murphy JM, McBride WJ, Lumeng L, Li TK. Alcohol deprivation effect is prolonged in the alcohol preferring (P) rat after repeated deprivations. Alcohol Clin Exp Res. 2000;24:8–16. [PubMed] [Google Scholar]
- Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev. 2001;5:287–297. doi: 10.1053/smrv.2001.0162. [DOI] [PubMed] [Google Scholar]
- Rouhani S, Dall'Ava-Santucci J, Bajenaru O, Emmanouilidis E, Tran G, Manicom R, Dinh-Xuan AT, Poenaru S. Effects of muscimol or homotaurine on sleep-wake states in alcohol-dependent rats during withdrawal. Pharmacol Biochem Behav. 1998;59:955–960. doi: 10.1016/s0091-3057(97)00521-2. [DOI] [PubMed] [Google Scholar]
- Smith DG, Learn JE, McBride WJ, Lumeng L, Li TK, Murphy JM. Alcohol-naive alcohol-preferring (P) rats exhibit higher local cerebral glucose utilization than alcohol-nonpreferring (NP) and Wistar rats. Alcohol Clin Exp Res. 2001;25:1309–1316. [PubMed] [Google Scholar]
- Stewart RB, Kurtz DL, Zweifel M, Li TK, Froehlich JC. Differences in the hypothermic response to ethanol in rats selectively bred for oral ethanol preference and nonpreference. Psychopharmacology (Berl) 1992;106:169–174. doi: 10.1007/BF02801968. [DOI] [PubMed] [Google Scholar]
- Strother WN, Lumeng L, McBride WJ. Acute ethanol effects on local cerebral glucose utilization in select central nervous system regions of adolescent alcohol-preferring (P) and alcohol-nonpreferring (NP) rats. Alcohol Clin Exp Res. 2008;32:1875–1883. doi: 10.1111/j.1530-0277.2008.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Engemann SC, Sharma R, Sahota P. Role of wake-promoting basal forebrain and adenosinergic mechanisms in sleep promoting effects of ethanol. Alcohol Clin Exp Res. 2010 doi: 10.1111/j.1530-0277.2010.01174.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Engemann SC, Walsh KM, Sahota PK. Adenosine and the homeostatic control of sleep: Effects of A1 receptor blockade in the perifornical lateral hypothalamus on sleep-wakefulness. Neuroscience. 2008;153:875–880. doi: 10.1016/j.neuroscience.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic Basal forebrain. J Neurosci. 2003;23:4278–4287. doi: 10.1523/JNEUROSCI.23-10-04278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller MB, Murphy JM, McBride WJ, Lumeng L, Li TK. Effect of low dose ethanol on spontaneous motor activity in alcohol-preferring and -nonpreferring lines of rats. Pharmacol Biochem Behav. 1986;24:617–623. doi: 10.1016/0091-3057(86)90567-8. [DOI] [PubMed] [Google Scholar]