Abstract
Inhibition of mTOR by rapamycin has been shown to suppress seizures in TSC/PTEN genetic models. Rapamycin, when applied immediately before or after a neurological insult, also prevents the development of spontaneous recurrent seizures (epileptogenesis) in an acquired model. In the present study, we examined the mTOR pathway in rats that had already developed chronic spontaneous seizures in a pilocarpine model. We found that mTOR is aberrantly activated in brain tissues from rats with chronic seizures. Furthermore, inhibition of mTOR by rapamycin treatment significantly reduces seizure activity. Finally, mTOR inhibition also significantly suppresses mossy fiber sprouting. Our findings suggest the possibility for a much broader window for intervention for some acquired epilepsies by targeting the mTOR pathway.
Keywords: mTOR, rapamycin, chronic seizure, acquired epilepsy, mossy fiber sprouting
Introduction
Epilepsy is a chronic, common and sometimes devastating neurological disorder. Epilepsy is characterized by recurrent seizures that are unpredictable and sometimes progressively severe. It is also associated with significant mortality and morbidities (Rice and DeLorenzo, 1998; Sutula, 2004, 2005). Some forms of epilepsy are caused by an inherited vulnerability to seizures, while other forms are a consequence of neurological insults such as head trauma, stroke, and tumors (Manning et al., 2002; Chang and Lowenstein, 2003; Inoki et al., 2005; Holmes and Stafstrom, 2007).
In epilepsy animal models, an initial brain insult such as status epilepticus induced by electrical stimuli or convulsive agents such as pilocarpine or kainate (KA) triggers widespread neuronal loss followed by neurogenesis, gliosis, and mossy fiber sprouting, along with changes in synaptic transmission in the hippocampus (Gruenthal et al., 1986; Cavazos and Sutula, 1990; Sutula, 1991; Sutula et al., 1992; Sankar et al., 2000; Borges et al., 2003; Borges et al., 2006). The structural changes in these animal models are similar to those observed in human mesial temporal-lobe epilepsy (TLE) (Cavazos and Sutula, 1990; Chang and Lowenstein, 2003). Most animals will subsequently develop spontaneous recurrent seizure within 1-3 months (Williams et al., 2009).
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase and belongs to the phosphatidylinositol kinase-related kinase family (Heitman et al., 1991; Brown et al., 1994; Chiu et al., 1994; Sabatini et al., 1994). It regulates cell growth, proliferation and survival (Avruch et al., 2006; Reiling and Sabatini, 2006; Um et al., 2006; Wullschleger et al., 2006). In the CNS, the mTOR pathway is regulated by glutamate receptor activation (Lenz and Avruch, 2005; Huang et al., 2007) and is involved in neurite growth, synaptic plasticity and cell survival, presumably by influencing protein translation or Akt activity (Burnett et al., 1998; Tang et al., 2002; Cammalleri et al., 2003; Jaworski et al., 2005; Kumar et al., 2005; Tavazoie et al., 2005; Guertin et al., 2006). The mTOR pathway is closely associated with epilepsy. For example, mutations in the tuberous sclerosis complex (TSC) including TSC1 and TSC2 that act upstream of the mTOR not only lead to a wide spread development of benign tumors and mental retardation, but also a high incidence of epilepsy (Manning et al., 2002; Inoki et al., 2005; Holmes and Stafstrom, 2007). Furthermore, rapamycin treatment that inhibits the mTOR pathway attenuates structural abnormalities and reduces seizures in TSC and PTEN mouse models (Ehninger et al., 2008; Meikle et al., 2008; Zeng et al., 2008; Ljungberg et al., 2009; Zhou et al., 2009), suggesting that the aberrant mTOR activation interferes with normal brain development and leads to epilepsy. Most recent studies also indicate that pharmacological inhibition of the mTOR pathway, either before or immediately following neurological insults, can prevent pathological changes in animal brains and the development of spontaneous recurrent seizure in an acquired epilepsy model ( Zeng et al., 2009). Furthermore, chronic hippocampal infusion of the mTOR inhibitor rapamycin reduces mossy fiber sprouting in a pilocarpine model (Buckmaster et al., 2009). Therefore, it has been proposed that the mTOR pathway could be a target for preventing epilepsy following neurological insults (Crino, 2008; Ehninger et al., 2008; Meikle et al., 2008; Zeng et al., 2008; Zeng et al., 2009; Zhou et al., 2009).
We report here that mTOR is hyperactivated in rat brains with chronic spontaneous seizures. Inhibition of the mTOR pathway by rapamycin markedly reduces seizure activity, along with inhibition of mossy fiber sprouting. Our data suggest that inhibition of mTOR could be a new therapeutic strategy for managing acquired epilepsy.
Material and method
Animals
Adult (200-300g or 8-10 week old) male Sprague-Dawley rats were purchased from Taconic (Taconic, NY). Rats were housed in a room with ad libitum access to food and water under a 12-hr light/dark cycle (lights on 0700). All experiments were performed in accordance with National Institutes of Health guidelines for the care and use of laboratory animals and were approved by our Institutional Animal Care and Use Committees.
Drug treatment and seizure monitoring
Rapamycin (Tecoland) was first dissolved in DMSO and further diluted in a vehicle solution containing 5% tween-20 and 4% ethanol. Rats were pretreated with rapamycin at 5mg/kg/day i.p. for three consecutive days before the induction of seizures by administration of pilocarpine (300 mg/kg). Pilocarpine administration was performed as described (Huang et al., 2002). Briefly, rats were injected with methylscopolamine and terbutaline (2 mg/kg each i.p. in 0.9% NaCl) 15–30 min prior to pilocarpine (300 mg/kg, i.p.) to minimize peripheral side effects. Seizures were terminated with sodium pentobarbital (25 mg/kg, i.p.) 60 min after administration of pilocarpine. Seizure activity was recorded by a digital camera and graded according to Racine's standard classification: Stage 1, behavioral arrest with mouth and facial movement; stage 2: head nodding/ “wet dog shakes”; stage 3: forepaw clonus; stage 4: rearing and stage 5: rearing and falling. Spontaneous seizures were continuously monitored for 91 h/week (13 h/d, 7 d/week) by video camera from week 3 to 10 after pilocarpine injection. Seizure events were mainly identified and graded by reviewing video by observers blinded to treatment. To determine the effect of rapamycin on chronic spontaneous seizures, adult rats that had already developed chronic spontaneous seizures were paired based on seizure frequency and seizure scale and assigned into two groups (vehicle vs rapamycin treated). Rats in the rapamycin group were treated with rapamycin at 5 mg/kg/day i.p. for three consecutive days, followed by treatment on every other day. Spontaneous seizures were monitored via continuous video recording for 13h/d for three weeks. Total seizure events, total duration and percentage of rats that had at least one seizure during the monitoring period were quantified.
Western Blot
Rats were treated with rapamycin (5 mg/kg/day, i.p.) or vehicle for three days followed by pilocarpine (300 mg/kg, i.p.). Pentobarbital (25 mg/Kg, i.p) was given after one hour to terminate seizures. Rats were sacrificed 30, 60, and 120 minutes after pilocarpine. Brain tissues were removed immediately and then homogenized in lysis buffer consisting of 50 mM Tris, pH 7.4, 2 mM EDTA and proteinase inhibitor set (Roche). The lysates were then mixed with equal volumes of 2 XSDS sample buffer and heated at 95°C for 5 min. Insoluble cell debris was removed by centrifugation at 10,000 g for 10 min, the resulting protein samples were dissolved in 8% Bis-Tris gel and then transferred onto 0.45 μM nitrocellulose membrane. Membranes were first blocked in 5% nonfat dry milk in TBST (25 mM Tris-HCl, pH 7.4; 1.5 M NaCl; 0.05% Tween-20) for 1 hour at room temperature and then incubated with rabbit anti-S6 or anti-phospho-S6 antibodies with 1:1000 dilution (Cell signaling Technology) at 4°C overnight. After removal of primary antibodies by several washings in TBST, membranes were incubated with HRP-conjugated secondary antibody (1:5000 dilution) in 5% milk in TBST. The signals were visualized with ECL reagent (Pierce). To examine mTOR activity in the epileptic brains, rats with chronic spontaneous seizures were sacrificed and brain tissues were processed accordingly. Rats that were from the same batch, but failed to show chronic spontaneous seizures after status epilepticus induced by pilocarpine were used as controls.
Timm staining
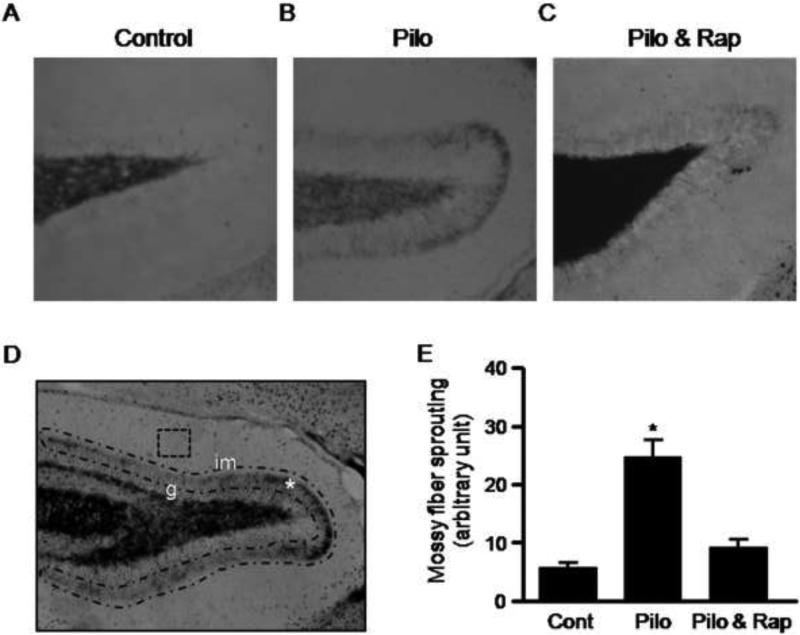
Rats that had already developed chronic spontaneous seizures were treated with rapamycin (5mg/kg) every other day for three weeks. Mossy fiber sprouting (MFS) was examined by Timm staining as described previously (Chen et al., 2007). Briefly, rats were anesthetized, transcardially perfused with sulfide solution containing 0.9% NaCl, 1.2% Na2S·9H2O and 1.0% NaH2PO4 and then fixed with 4% paraformaldehyde in 0.1M phosphate buffer, pH 7.4. After further postfixation in 4% paraformaldehyde overnight, brains were cryprotected in 30% sucrose at 4°C for 2-3 days. Brain sections were cut in the coronal plane at 40 μm and developed in the dark for 40-45 min in a solution of gum Arabic (50% w/v), hydroquinone (5.67% w/v, protect from light), citric acid/sodium citrate buffer (26% citric acid w/v, 24% sodium citrate w/v), and silver nitrate (17% w/v). After washing, sections were dehydrated in alcohol, cleared in xylene, and mounted on slides with DPX mounting medium. Images were acquired using a light microscope under a low power objective (10X). Supragranular layer (inner molecular layer) optical intensity of Timm staining was measured by drawing a contour along the midline of the granular layer and around the inner third of the molecular layer as indicated in Figure 6D. Optical intensity in a square box drawn in the middle and outer molecular layers served as background staining. At least three brain sections (1 in every 4 series) from each animal were quantified.
Figure 6.
Rapamycin reduces mossy fiber sprouting. Rats with spontaneous recurrent seizures were treated with rapamycin or vehicle for three weeks. Photomicrographs of Timm staining were acquired from brain slides prepared from naïve rats (A), rats with spontaneous recurrent seizures (B) and rats with spontaneous seizure treated with rapamycin (C). The dense band of mossy fibers in the supragranular layer (marked by * in panel D) and measured by drawing a contour along the midline of the granular cell layer (g) and the inner third of the inner molecular layer (im). The optical intensity in the square box in panel D was used to measure background staining. (E) Data are presented as average intensity of Timm staining in the contour area (control: 5.4 ± 1.07; pilocarpine: 27.03 ± 3.51; Pilocarpine plus rapamycin: 9.01± 1.42) from 5 sets of animals. *p<0.05 by t-test (mean ± SE).
Statistical analysis was performed using GraphPad (GraphPad Software, Inc., La Jolla, CA USA). Groups were compared using student's t-test or Wilcoxon signed rank test.
Results
Activation of mTOR in rat brain by pilocarpine-induced seizures
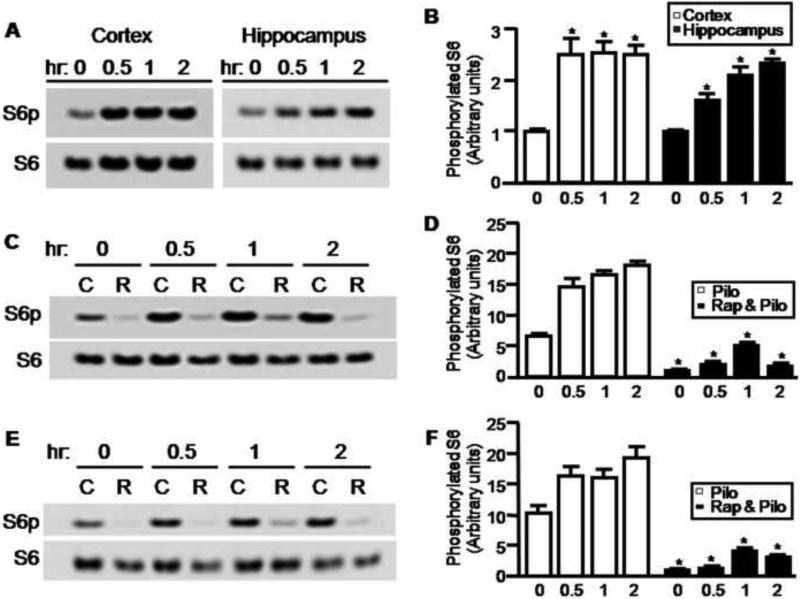
Administration of pilocarpine, a muscarinic receptor agonist, is known to cause sustained increases in extracellular glutamate levels in the hippocampus (Liu et al., 1997; Smolders et al., 1997). Previous studies revealed that pretreatment with the NMDA receptor blocker MK801 can antagonize pilocarpine-induced cell death and prevent spontaneous seizures (Rice and DeLorenzo, 1998), suggesting that NMDA receptors are critically involved in this epilepsy model. Our previous studies have shown that excessive activation of NMDA receptors inhibits mTOR activity (Huang et al., 2007), while others have shown that transient activation of NMDA receptors activates mTOR in primary cultured neurons (Lenz and Avruch, 2005). To determine whether pilocarpine treatment influences the mTOR signaling pathway in rat brain, we used western blots to monitor the phosphorylation of S6, a downstream target of mTOR in rat hippocampus and cortex. We observed that S6 phosphorylation was markedly increased within 30 min in the cortex and hippocampus after pilocarpine injection and peaked around 1 hr (Figure 1A and 1B). To confirm the involvement of the mTOR pathway in S6 phosphorylation in brains, we first treated rats with the mTOR inhibitor rapamycin (5mg/kg/day) for three days. We found that phospho-S6 elicited by pilocarpine was almost completely depleted by rapamycin treatment in cortex and hippocampus, suggesting that the levels of phospho-S6 reflect the in vivo mTOR activity (Figure 1C, 1D, 1E and 1F). It is worth noting that rapamycin pretreatment has no significant effect on the severity of acute seizure activity induced by pilocarpine in adult rats (data not shown).
Figure 1.
Activation of mTOR by pilocarpine-induced seizures. (A) Rats received pilocarpine 300 mg/kg i.p. Brian cortical and hippocampal tissues were harvested either before or 0.5, 1, 2 hr after pilocarpine injection. Phosphorylated S6 was visualized by western blot using a phospho-specific antibody. Blots for total S6 were used as a loading control. Phosphorylated S6 was quantified by densitometry (1 ± 0.01; 2.45 ± 0.3; 2.52 ± 0.2; and 2.48 ± 0.2 in cortex; 1 ± 0.01; 1.61 ± 0.12; 2.08 ± 0.15; and 2.31 ± 0.15 in hippocampus for before or 0.5, 1, 2 hr after pilocarpine injection respectively) (B). Rats were pretreated with rapamycin 5 mg/kg for 3 days and then treated with pilocarpine for 0.5, 1 and 2 hr as indicated. Phosphorylated S6 and total S6 in rat cortex (C) and hippocampus (E) were visualized by western blots and quantified by densitometry (D & F) (pilocarpine: 6.5 ± 0.17; 14.54 ± 1.34; 16.57 ± 0.5; and 18.05 ± 0.78; rapamycin & pilocarpine: 1 ± 0.05; 2.04 ± 0.09; 4.98 ± 0.27; and 1.64 ± 0.21, in cortex for before and 0.5, 1, and 2hr after pilocarpine injection respectively) (pilocarpine: 10.21 ± 1.38; 16.27 ± 1.57; 16.1 ± 1.28; and 19.37 ± 1.71; rapamycin & pilocarpine: 1 ± 0.05; 1.32 ± 0.04; 4.13 ± 0.43; and 3.07 ± 0.17, in hippocampus for before and 0.5, 1, and 2hr after pilocarpine injection respectively)(*P < 0.05, t-test).
Hyperactivation of mTOR in rat brains with chronic spontaneous recurrent seizures
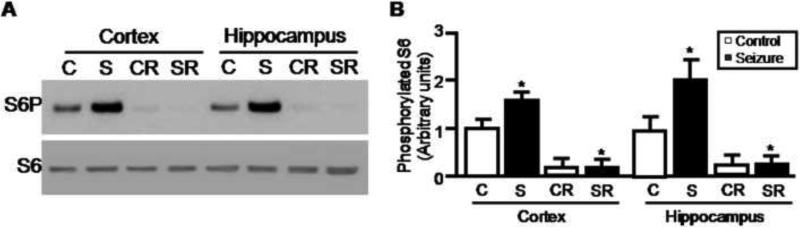
Seizures reflect hypersynchronization and hyperactivation of a large group of neurons. Accordingly, we investigated whether mTOR activity is altered in rat brains with chronic spontaneous seizures. Brain cortical and hippocampal tissues were isolated from seizure and age-matched control rats. We observed significantly higher levels of phosphorylated S6 in cortical and hippocampal tissues from rats with chronic seizures compared to age-matched controls (Figure 2A and 2B). Again, hyperphosphorylation of S6 was markedly inhibited by rapamycin treatment suggesting that mTOR signaling is aberrantly activated in epileptic brains.
Figure 2.
Hyperactivation of mTOR in rat brains with chronic spontaneous recurrent seizure. Brains tissues were harvested from rats with chronic spontaneous seizures and aged-matched controls. Rats were treated with rapamycin for 2 days. Phosphorylated S6 and total S6 in rat cortex and hippocampus were visualized by western blots (A) and quantified from three experiments (B) (control: 1.0 ± 0.17; seizure: 1.51 ± 0.12; control treated with rapamycin: 0.16 ± 0.04; seizure treated with rapamycin: 0.13 ± 0.04 in cortex; control: 1.0 ± 0.21; seizure: 1.97 ± 0.43; control treated with rapamycin: 0.16 ± 0.03; seizure treated with rapamycin: 0.20 ± 0.06 in hippocampus)(*P < 0.05, t-test).
Rapamycin suppresses seizure activity in rats with chronic spontaneous seizures
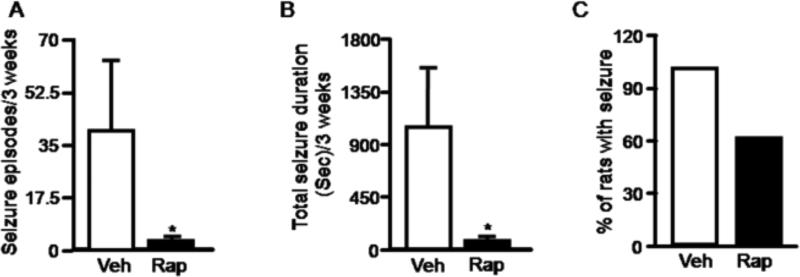
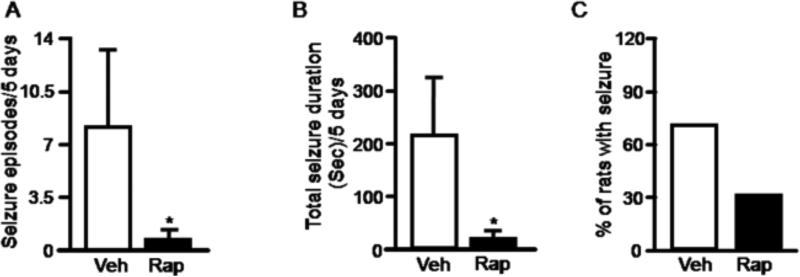
Previous studies have shown that the mTOR pathway regulates neuronal morphology and synaptic plasticity in primary neuronal cultures and brain slices (Tang et al., 2002; Cammalleri et al., 2003; Jaworski et al., 2005; Kumar et al., 2005; Tavazoie et al., 2005). Rapamycin also reverses morphological changes and suppresses chronic spontaneous seizure in the TSC and PTEN models that are associated with increased mTOR activity (Meikle et al., 2008; Zeng et al., 2008; Zhou et al., 2009). Since mTOR is hyperactivated in rat brains with chronic seizures in our acquired model, we wondered whether rapamycin could suppress chronic seizures. We began video recording (13 hr/day; 7days/week) from week 3 to 10 after pilocarpine injection to identify rats with spontaneous recurrent seizures. Behavioral seizure activities were quantified by frequency and duration. Rats with spontaneous seizures were then paired based on seizure frequency and duration and divided into two groups (rapamycin & vehicle control) (Supplemental Figure 1). Rats were treated for three weeks with rapamycin 5mg/kg or vehicle daily for the first three days, then every other day. Starting on day two, spontaneous seizures in all animals were monitored by video recording 13 hr/day for three weeks. Rapamycin treatment markedly reduced seizure activity including seizure frequency and duration, as well as the number of rats that had at least one seizure episode during the three week period (Figure 3A, 3B & 3C). A recent study found that nonconvulsive electrographic seizures usually occur during the first week after neurological insults, after which ictal EEG events are well correlated with behavioral seizures (Williams et al., 2009). It is however possible that we did not detect some behaviorally silent or subtle seizures. To determine when this inhibitory effect of rapamycin treatment on chronic spontaneous seizure began, we quantified seizure activity over a five day period beginning 24 hr after the first rapamycin injection. We observed a significant reduction in seizure activity in the rapamycin treated group (Figure 4A, 4B & 4C), suggesting that the inhibitory effect of rapamycin on chronic spontaneous seizures begins within days. Notably, rapamycin treatment markedly reduced seizures at stage 3-5 (Supplemental Table 1). However, stage 1-2 seizures, although very few, still occur in the rapamycin-treated group. It is conceivable that these behaviorally milder seizures represent attenuation of severe seizures (stage 3-5) by rapamycin.
Figure 3.
Rapamycin reduces spontaneous recurrent seizures. Rats with spontaneous recurrent seizures were treated with rapamycin (black bar) or vehicle (open bar) for three weeks. Seizure activity was quantified according to average number of seizures (A) (pilocarpine: 39.5 ± 22.96; pilocarpine plus rapamycin: 2.6 ± 0.68) (*P < 0.05, Wilcoxon signed rank test)(A), average total seizure duration (B) (pilocarpine: 1031.9 ± 493.8; pilocarpine plus rapamycin: 69.8 ± 18.37) (*P < 0.05, Wilcoxon signed rank test), and the percentage of rats with at least one seizure (C) (pilocarpine: 100%; pilocarpine plus rapamycin: 60%). (n= 10 per group).
Figure 4.
Rapamycin significantly reduces spontaneous recurrent seizures within five days. Rats with spontaneous recurrent seizures were treated with rapamycin (black bar) or vehicle (open bar) for five days. Seizure activity was quantified according to average number of seizures(A) (pilocarpine: 8 ± 5; pilocarpine plus rapamycin: 0.6 ± 0.38) (*P < 0.05, t- Wilcoxon signed rank test), average total seizure duration(B) (pilocarpine: 211.11 ± 103.31; pilocarpine plus rapamycin: 15.1 ± 9.47) (*P < 0.05, Wilcoxon signed rank test), and the percentage of rats with at least one seizure(C) (pilocarpine: 70%; pilocarpine plus rapamycin: 30%) (n= 10 per group).
The effect of rapamycin on chronic seizures is transient
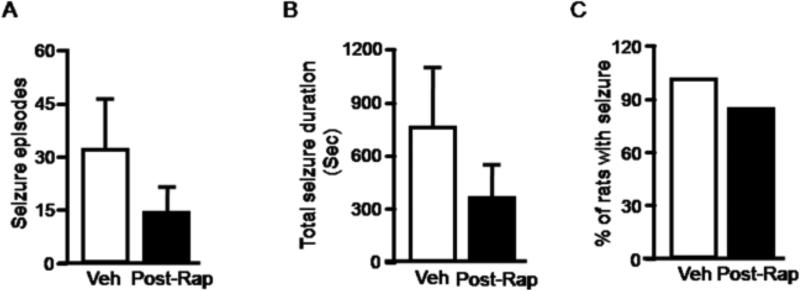
We monitored seizure activity for three weeks after termination of rapamycin treatment in order to assess the duration of the reduction in seizure activity. We found that seizures gradually increased after stopping rapamycin. Over this three week period, seizure activity in the rapamycin and vehicle groups did not differ statistically. These data suggests that the inhibitory effect of rapamycin on seizure activity is transient (Figure 5A, 5B & 5C).
Figure 5.
Seizures return after cessation of rapamycin administration. Rats with spontaneous recurrent seizures were first treated with rapamycin (black bar) or vehicle (open bar) for three weeks. Seizure activity was monitored for an additional three weeks after stopping rapamycin treatment and quantified according to average number of seizures(A) (pilocarpine: 31.5 ± 14.23; pilocarpine plus rapamycin: 13.67 ± 6.82), average total seizure duration(B) (pilocarpine: 751.67± 324.68; pilocarpine plus rapamycin: 341.5 ± 176.89) and the percentage of rats with at least one seizure(C) (pilocarpine: 100; pilocarpine plus rapamycin:83.3%). (n=6 per group).
Rapamycin treatment reduces mossy fiber sprouting
Hippocampal mossy fiber sprouting is seen in human temporal lobe epilepsy and in various animal models. To determine whether rapamycin treatment has any influence on mossy fiber sprouting, we performed Timm staining on brain slices prepared from control or rapamycin-treated rats which had shown reduced seizure activity (Figure 3 and 4). We observed a moderate inhibition of mossy fiber sprouting in the supragranular dentate gyrus in the rapamycin-treated group (Figure 6A & 6B). This is consistent with a recent report that mossy fiber sprouting was attenuated by prolonged infusion of rapamycin (Buckmaster et al., 2009).
Discussion
We examined the effect of pharmacological inhibition of the mTOR pathway by rapamycin on chronic spontaneous seizures. The principal findings are that inhibition of the mTOR pathway by rapamycin markedly reduces seizure activity, and modestly reduces mossy fiber sprouting in adult rats that had already developed spontaneous recurrent seizures. The striking reduction in chronic spontaneous seizures in adult rats by rapamycin suggests that the mTOR pathway could be a therapeutic target for acquired epilepsy.
The mTOR pathway and epilepsy
There is an increasing body of evidence suggesting a role of the mTOR pathway in epilepsy. Genetic deficiencies of several cellular components in the mTOR signaling pathway including TSC and PTEN are associated with the development of epilepsy (Manning et al., 2002; Meikle et al., 2008; Zhou et al., 2009). Pharmacological inhibition of the mTOR pathway prevents the development of seizure in TSC and PTEN mouse genetic models (Manning et al., 2002; Meikle et al., 2008; Zeng et al., 2008; Zhou et al., 2009). Furthermore, genetic studies reveal that BDNF and its receptor TrkB, which are markedly induced in epilepsy models (Binder et al., 1999; Huang et al., 2002), are involved in epileptogenesis (He et al., 2004). Interestingly, BDNF signaling is known to regulate the mTOR pathway (Schratt et al., 2004). Finally, the ketogenic diet, high in fatty acids and low in carbohydrates, has been used clinically in managing epilepsy (Bough et al., 2006; Hartman et al., 2007). Low carbohydrate diets could influence AMP-dependent kinase and insulin signaling, which are known to crosstalk with the mTOR pathway (Hardie, 2007). Whether the mTOR pathway is involved in the anti-epileptic effect of ketogenic diets remains to be determined. Thus, several lines of evidence suggest that mTOR could be a therapeutic target in epilepsy. Consistent with this notion, recent studies show that inhibition of mTOR by rapamycin inhibits epileptogenesis and mossy fiber sprouting in acquired epilepsy models (Buckmaster et al., 2009; Zeng et al., 2009). However, the detailed molecular mechanism remains to be determined. The mTOR pathway is known to regulate synaptic plasticity and influences the expression of neuronal genes such as those coding for potassium channels (Raab-Graham et al., 2006). It has therefore been long suspected that inhibition of mTOR activity by rapamycin could influence brain excitability. However, several previous studies have not been able to confirm this notion. For example, rapamycin does not affect the bioelectrical activity and fEPSP in hippocampal tissues (Daoud et al., 2007). Furthermore, Ruegg et al reported that rapamycin neither changes the baseline of spontaneous neuronal activity, nor has any effect on voltage-dependent currents in cultured neurons (Ruegg et al., 2007). In the kainate model, rapamycin does not significantly change the acute manifestations of status epilepticus (Zeng et al., 2009).
The mTOR pathway and spontaneous recurrent seizures
We found that rapamycin reduces seizure activity in the pilocarpine model, even when given after the development of spontaneous recurrent seizures. This antiepileptic effect could have clinical implications for acquired epilepsy. The mechanism underlying the effect of rapamycin remains unclear, but our results suggest involvement of the mTOR pathway. The mTOR pathway is known to be regulated by glutamate receptor activation, providing a potential link between hyperexcitation of neurons, aberrant activation of the mTOR, and the development of spontaneous seizures (Lenz and Avruch, 2005; Zeng et al., 2009). Conceivably, rapamycin inhibits the aberrant activation of mTOR, thereby suppressing chronic spontaneous seizures. Consistent with this notion, rapamycin suppresses spontaneous recurrent seizures in several genetic models such as TSC and PTEN that are associated with excessive mTOR activity (Meikle et al., 2008; Zeng et al., 2009; Zhou et al., 2009). In our studies, we observed that the effect of rapamycin on chronic spontaneous seizure appears to be rapid and dramatic. We saw a marked reduction in seizure activity within 4-5 days after rapamycin treatment, in line with previous studies in TSC/PTEN mouse models (Zhou et al., 2009). This effect was persistent over a three week period during which rats were continuously treated with rapamycin. Rapamycin given before or immediately after status epilepticus induced by kainate reduces epileptogenesis (Zeng et al., 2009). Our findings suggest that inhibition of the mTOR pathway by rapamycin can suppress seizures in rats that have already developed chronic spontaneous seizures, raising the possibility for a much broader window for intervention in some acquired epilepsies.
Rapamycin treatment reduces seizures in TSC and PTEN mouse genetic models (Manning et al., 2002; Meikle et al., 2008; Zeng et al., 2008; Zhou et al., 2009). However, seizure relapse was observed after stopping rapamycin treatment (Meikle et al., 2008; Zeng et al., 2008; Zhou et al., 2009), presumably because of persistent activation of mTOR as a result of genetic mutations in TSC/PTEN. However, in our acquired epilepsy model, the antiepileptic effect of rapamycin is also not long lasting as seizures gradually return after cessation of rapamycin. This suggests that rapamycin prevents the hyperactivation of mTOR, thereby suppressing seizures, but does not reverse the hyperexcitability of neurons in epileptic brains.
The mTOR pathway and mossy fiber sprouting
Previous study from Buckmaster's group (Buckmaster et al., 2009) reported that only early infusion of rapamycin into rat hippocampus immediately after pilocarpine-induced status epilepticus attenuates mossy fiber sprouting while late administration (2 months after status epilepticus) of rapamycin does not effectively reverse mossy fiber sprouting that had already developed. However, we observed that late treatment with rapamycin still moderately inhibits mossy fiber sprouting in rats with spontaneous recurrent seizure. The difference could be attributed to different experimental procedures. In contrast to local hippocampal infusion which presumably has less impact on chronic spontaneous seizure, our systemic treatment of rapamycin markedly reduces chronic seizure activity. It is conceivable that rapamycin could inhibit mossy fiber sprouting indirectly via inhibition of spontaneous recurrent seizures besides the direct inhibition of axonal growth reported in TSC models as well as in primary neuronal cultures (Jaworski et al., 2005; Kumar et al., 2005; Tavazoie et al., 2005, Huang et al., 2007).
In summary, we found that delayed administration of rapamycin reduces chronic spontaneous seizure in an acquired epilepsy model, and that this effect may be associated with inhibition of the mTOR pathway. Although the detailed mechanism of this antiepileptic action of rapamycin remains to be determined, this finding suggests an opportunity for clinical intervention in some acquired epilepsies by targeting this signaling pathway.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. doi: 10.1038/sj.onc.1209882. [DOI] [PubMed] [Google Scholar]
- Binder DK, Routbort MJ, McNamara JO. Immunohistochemical evidence of seizure-induced activation of trk receptors in the mossy fiber pathway of adult rat hippocampus. J Neurosci. 1999;19:4616–4626. doi: 10.1523/JNEUROSCI.19-11-04616.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges K, McDermott D, Irier H, Smith Y, Dingledine R. Degeneration and proliferation of astrocytes in the mouse dentate gyrus after pilocarpine-induced status epilepticus. Experimental neurology. 2006;201:416–427. doi: 10.1016/j.expneurol.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Experimental neurology. 2003;182:21–34. doi: 10.1016/s0014-4886(03)00086-4. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD, Dingledine RJ. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci. 2009;29:8259–8269. doi: 10.1523/JNEUROSCI.4179-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammalleri M, Lutjens R, Berton F, King AR, Simpson C, Francesconi W, Sanna PP. Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14368–14373. doi: 10.1073/pnas.2336098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazos JE, Sutula TP. Progressive neuronal loss induced by kindling: a possible mechanism for mossy fiber synaptic reorganization and hippocampal sclerosis. Brain Res. 1990;527:1–6. doi: 10.1016/0006-8993(90)91054-k. [DOI] [PubMed] [Google Scholar]
- Chang BS, Lowenstein DH. Epilepsy. The New England journal of medicine. 2003;349:1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- Chen Q, He S, Hu XL, Yu J, Zhou Y, Zheng J, Zhang S, Zhang C, Duan WH, Xiong ZQ. Differential roles of NR2A- and NR2B-containing NMDA receptors in activity-dependent brain-derived neurotrophic factor gene regulation and limbic epileptogenesis. J Neurosci. 2007;27:542–552. doi: 10.1523/JNEUROSCI.3607-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu MI, Katz H, Berlin V. RAPT1, a mammalian homolog of yeast Tor, interacts with the FKBP12/rapamycin complex. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:12574–12578. doi: 10.1073/pnas.91.26.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudomel O, Herman H, Nair K, Moshe SL, Galanopoulou AS. Age- and gender-related differences in GABAA receptor-mediated postsynaptic currents in GABAergic neurons of the substantia nigra reticulata in the rat. Neuroscience. 2009;163:155–167. doi: 10.1016/j.neuroscience.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino PB. Rapamycin and tuberous sclerosis complex: from Easter Island to epilepsy. Ann Neurol. 2008;63:415–417. doi: 10.1002/ana.21369. [DOI] [PubMed] [Google Scholar]
- Daoud D, Scheld HH, Speckmann EJ, Gorji A. Rapamycin: brain excitability studied in vitro. Epilepsia. 2007;48:834–836. doi: 10.1111/j.1528-1167.2006.00976.x. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/- mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenthal M, Armstrong DR, Ault B, Nadler JV. Comparison of seizures and brain lesions produced by intracerebroventricular kainic acid and bicuculline methiodide. Experimental neurology. 1986;93:621–630. doi: 10.1016/0014-4886(86)90181-0. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Developmental cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nature reviews. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Hartman AL, Gasior M, Vining EP, Rogawski MA. The neuropharmacology of the ketogenic diet. Pediatric neurology. 2007;36:281–292. doi: 10.1016/j.pediatrneurol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XP, Kotloski R, Nef S, Luikart BW, Parada LF, McNamara JO. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron. 2004;43:31–42. doi: 10.1016/j.neuron.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Stafstrom CE. Tuberous sclerosis complex and epilepsy: recent developments and future challenges. Epilepsia. 2007;48:617–630. doi: 10.1111/j.1528-1167.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- Huang Y, Doherty JJ, Dingledine R. Altered histone acetylation at glutamate receptor 2 and brain-derived neurotrophic factor genes is an early event triggered by status epilepticus. J Neurosci. 2002;22:8422–8428. doi: 10.1523/JNEUROSCI.22-19-08422.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Kang BN, Tian J, Liu Y, Luo HR, Hester L, Snyder SH. The cationic amino acid transporters CAT1 and CAT3 mediate NMDA receptor activation-dependent changes in elaboration of neuronal processes via the mammalian target of rapamycin mTOR pathway. J Neurosci. 2007;27:449–458. doi: 10.1523/JNEUROSCI.4489-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung DT, Schreiber SL. cDNA cloning of a human 25 kDa FK506 and rapamycin binding protein. Biochem Biophys Res Commun. 1992;184(2):733–8. doi: 10.1016/0006-291x(92)90651-z. [DOI] [PubMed] [Google Scholar]
- Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nature genetics. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M. Control of dendritic arborization by the phosphoinositide-3'-kinase-Akt-mammalian target of rapamycin pathway. J Neurosci. 2005;25:11300–11312. doi: 10.1523/JNEUROSCI.2270-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci. 2005;25:11288–11299. doi: 10.1523/JNEUROSCI.2284-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz G, Avruch J. Glutamatergic regulation of the p70S6 kinase in primary mouse neurons. The Journal of biological chemistry. 2005;280:38121–38124. doi: 10.1074/jbc.C500363200. [DOI] [PubMed] [Google Scholar]
- Liu Z, Gatt A, Werner SJ, Mikati MA, Holmes GL. Long-term behavioral deficits following pilocarpine seizures in immature rats. Epilepsy research. 1994;19:191–204. doi: 10.1016/0920-1211(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Liu Z, Stafstrom CE, Sarkisian MR, Yang Y, Hori A, Tandon P, Holmes GL. Seizure-induced glutamate release in mature and immature animals: an in vivo microdialysis study. Neuroreport. 1997;8:2019–2023. doi: 10.1097/00001756-199705260-00043. [DOI] [PubMed] [Google Scholar]
- Ljungberg MC, Sunnen CN, Lugo JN, Anderson AE, D'Arcangelo G. Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. Dis Model Mech. 2009;2:389–398. doi: 10.1242/dmm.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Molecular cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski DJ. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin., mTOR. inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab-Graham KF, Haddick PC, Jan YN, Jan LY. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science., New York, NY. 2006;314:144–148. doi: 10.1126/science.1131693. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. I. After-discharge threshold. Electroencephalography and clinical neurophysiology. 1972;32:269–279. doi: 10.1016/0013-4694(72)90176-9. [DOI] [PubMed] [Google Scholar]
- Rakhade SN, Jensen FE. Epileptogenesis in the immature brain: emerging mechanisms. Nat Rev Neurol. 2009;5:380–391. doi: 10.1038/nrneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene. 2006;25:6373–6383. doi: 10.1038/sj.onc.1209889. [DOI] [PubMed] [Google Scholar]
- Rice AC, DeLorenzo RJ. NMDA receptor activation during status epilepticus is required for the development of epilepsy. Brain Res. 1998;782:240–247. doi: 10.1016/s0006-8993(97)01285-7. [DOI] [PubMed] [Google Scholar]
- Ruegg S, Baybis M, Juul H, Dichter M, Crino PB. Effects of rapamycin on gene expression, morphology, and electrophysiological properties of rat hippocampal neurons. Epilepsy research. 2007;77:85–92. doi: 10.1016/j.eplepsyres.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Sankar R, Shin D, Liu H, Katsumori H, Wasterlain CG. Granule cell neurogenesis after status epilepticus in the immature rat brain. Epilepsia. 2000;41(Suppl 6):S53–56. doi: 10.1111/j.1528-1157.2000.tb01557.x. [DOI] [PubMed] [Google Scholar]
- Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci. 2004;24:7366–7377. doi: 10.1523/JNEUROSCI.1739-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders I, Khan GM, Manil J, Ebinger G, Michotte Y. NMDA receptor-mediated pilocarpine-induced seizures: characterization in freely moving rats by microdialysis. Br J Pharmacol. 1997;121:1171–1179. doi: 10.1038/sj.bjp.0701231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula T, Cavazos J, Golarai G. Alteration of long-lasting structural and functional effects of kainic acid in the hippocampus by brief treatment with phenobarbital. J Neurosci. 1992;12:4173–4187. doi: 10.1523/JNEUROSCI.12-11-04173.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula TP. Reactive changes in epilepsy: cell death and axon sprouting induced by kindling. Epilepsy research. 1991;10:62–70. doi: 10.1016/0920-1211(91)90096-x. [DOI] [PubMed] [Google Scholar]
- Sutula TP. Mechanisms of epilepsy progression: current theories and perspectives from neuroplasticity in adulthood and development. Epilepsy research. 2004;60:161–171. doi: 10.1016/j.eplepsyres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Sutula TP. Epilepsy after the Decade of the Brain: misunderstandings, challenges, and opportunities. Epilepsy Behav. 2005;6:296–302. doi: 10.1016/j.yebeh.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie SF, Alvarez VA, Ridenour DA, Kwiatkowski DJ, Sabatini BL. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nature neuroscience. 2005;8:1727–1734. doi: 10.1038/nn1566. [DOI] [PubMed] [Google Scholar]
- Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell metabolism. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, Dudek FE. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci. 2009;29:2103–2112. doi: 10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.