Summary
Allogeneic bone marrow transplantation (BMT) may offer the only chance of cure for children with acute myeloid leukemia (AML) in second complete remission (CR2) or with relapsed disease, but the outcome of these patients has not been clearly defined. We conducted a retrospective study of 58 children, median age 7.4 years (range 0.8–17.3), who received matched related or unrelated BMT at our institution for AML in CR2 (n = 12), in untreated first relapse (n = 11) or with refractory disease (n = 35), to identify risk factors associated with disease-free survival (DFS). Life threatening to fatal regimen-related toxicity was observed in 22% of patients. Estimates of DFS at 5 years (95% confidence interval) for patients in CR2, with untreated first relapse and refractory disease were 58% (27–80%), 36% (11–63%) and 9% (2–21%), respectively. Non-relapse mortality estimates were 0%, 27% (0–54%) and 17% (5–30%), and relapse estimates were 42% (14–70%), 36% (8–65%) and 74% (60–89%), respectively. Advanced disease phase and cytogenetic abnormalities at the time of transplantation were each associated with decreased DFS and increased relapse in multivariable regression models. Survival for children transplanted in CR2 or untreated first relapse is higher than that previously reported, but relapse remains the major cause of treatment failure regardless of disease stage.
Keywords: acute myeloid leukemia, childhood leukemia
Allogeneic bone marrow transplantation (BMT) has been used for the past three decades as treatment for childhood acute myeloid leukemia (AML). Recent reports from cooperative group studies suggest that compared to chemotherapy alone, BMT from an HLA-identical sibling offers a survival advantage for children with AML in first complete remission (CR).1–4 However, less than one-third of children have a sibling donor, and nearly 50% of patients relapse after achieving a first CR with chemotherapy alone.5–7 About 50% of children who receive re-induction chemotherapy for a first relapse achieve a remission, and their probability of a second relapse is in excess of 75%.6–9 For children with AML beyond first CR, allogeneic BMT may offer the only chance for cure, but the clinical outcome of these patients has not been clearly defined.
Several prognostic factors have been associated with an increased risk of relapse and mortality after BMT for patients with AML, including white blood cell count and cytogenetic abnormalities at the time of diagnosis, duration of first CR, history of extramedullary disease (EMD), age and phase of disease at transplantation and cytomegalo-virus (CMV) seropositivity.9–14 These associations have not been thoroughly studied in children with advanced AML. Studies published to date on outcome of BMT for children with AML beyond first CR contain data from a relatively small number of these patients combined with other childhood diseases or with adult patients, making interpretation difficult.11–21 Survival for children transplanted for AML beyond first CR is reported to be less than 40%. Compared to patients with AML in first CR, patients with more advanced disease are reported to have increased transplant-related morbidity and mortality.14,22
The main objective of this study was to analyze the outcome of children who received an allogeneic BMT for AML beyond first CR, and to identify risk factors associated with failure of disease-free survival (DFS). We also report transplant-related morbidity including regimen-related toxicity (RRT) and graft-versus-host disease (GVHD).
Patients and methods
Patients
This retrospective study included pediatric patients consecutively admitted to the Fred Hutchinson Cancer Research Center (Seattle, WA, USA) for allogeneic BMT between January 1990 and December 1999. Patients included were younger than 18 years at the time of transplant, had AML beyond first medullary CR and received a first BMT from a related or unrelated donor. Recipients of peripheral blood stem cells or umbilical cord blood and children with Down’s syndrome were excluded from this study. Data were analyzed as of December 2003. A total of 19 patients had been previously reported as part of a study of unrelated donor BMT for children and adults with AML.13 Five patients had been reported on a previous study of AML in children younger than 2 years.14
The initial diagnosis of AML was made at the referring institution and was confirmed at our institution by review of diagnostic bone marrow examinations. Disease subtypes were classified using the French–American–British (FAB) system.23 Therapy at diagnosis and subsequent re-induction chemotherapy regimens varied among referring institutions. Phase of disease at the time of BMT was determined by histopathologic examination and cytogenetic analysis of bone marrow and cerebrospinal fluid performed no earlier than 2 weeks prior to transplantation. Patients were considered in second complete remission (CR2) if they had less than 5% blasts in a normocellular bone marrow and normal peripheral blood counts after re-induction chemotherapy. Patients who had greater than 5% marrow blasts after a first CR and had not received re-induction chemotherapy were considered as in untreated first relapse, and those with greater than 5% marrow blasts who failed to achieve remission after therapy were considered to have refractory disease. This latter group included patients with disease persisting from initial diagnosis to after at least 3 months of chemotherapy (primary refractory AML), and patients with ongoing relapse who had achieved a CR (persistent relapse). Results of marrow cytogenetic studies at initial diagnosis and at the time of transplantation were available for all patients, and classified as normal or abnormal. Cytogenetic abnormalities were classified as favorable if t(8;21), t(15;17) or inversion 16 was present, unfavorable if involving 11q23 abnormalities or monosomy 7, and indeterminate for all other abnormalities.14,24 EMD was considered present if there were active site(s) or previous history of nonmedullary involvement at the time of BMT.
Treatment
Transplant preparative regimens varied depending on the stage of disease, type of donor used and protocols active at the time of transplantation. Regimens included busulfan and cyclophosphamide, or cyclophosphamide with 12–15.75 Gy of total body irradiation (TBI). Oral busulfan was administered at a dose ranging from 16 to 28 mg/kg divided in 16 doses over 4 days, as previously described.25 Cyclophosphamide was administered at a total dose of 120 mg/kg intravenously over 2 days. Patients with previous history of central nervous system (CNS) disease received two pretransplant doses and four post transplant doses of intrathecal methotrexate. Patients with active EMD at the time of transplant received local radiation in addition to TBI in the preparative regimen, with total radiation doses ranging from 10 to 24 Gy for cranial and 4 to 20 Gy for other sites. The Institutional Review Board approved all transplant and research study protocols.
Testing for donor–recipient histocompatibility was performed at the FHCRC Clinical Immunogenetics Laboratory using methods previously described.26,27 Mismatched related donors were family members matched at HLA-A, -B and -DR/DRB1 in one haplotype, and not matched at one to three antigens on the second haplotype. Unrelated donors were either matched by serology at HLA-A, -B and -DR/DRB1, or mismatched at one single HLA locus either within the same crossreactive group for HLA-A and -B or within the same -DR specificity. All patients received unmanipulated bone marrow collected and infused using established methods.28
GVHD prophylaxis consisted of methotrexate with or without cyclosporine, depending on the treatment protocol used at the time. Methotrexate alone was given as 15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6, 11 and weekly until day 102 after BMT. The combination of cyclosporine and methotrexate was given as previously reported.29 A short course of four doses of methotrexate was given as 15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6 and 11. Cyclosporine was initiated the day prior to the marrow infusion, targeting serum levels between 150 and 450 ng/ml. In patients with no evidence of GVHD, cyclosporine was slowly tapered starting on day 50 until approximately day 180. All patients had central venous access catheters and received nutritional support with parenteral hyperalimentation. Infection prophylaxis varied according to standard practices in place at the time of BMT, and consisted of single conventional or laminar airflow rooms, broad-spectrum antibacterial prophylaxis during neutropenia and, from 1992, fluconazole for fungal prophylaxis and gancyclovir for CMV prophylaxis. Patients remained hospitalized from the time of initiation of conditioning until achievement of engraftment and resolution of acute transplant-related complications.
Statistical methods
Neutrophil engraftment was defined as the first of three consecutive days with an absolute neutrophil count greater than 500 cells/μl. Platelet engraftment was defined as the first of three consecutive days with a platelet count greater than 20 000/μl following seven consecutive days without a platelet transfusion for a platelet count less than 20 000/μl. Life threatening to fatal RRT was defined as organ toxicity not attributable to GVHD or infection, and of grade 4 or greater according to the National Cancer Institute Common Toxicity Criteria, version 2.0.30 Acute and chronic GVHD were diagnosed and graded using methods previously described.28,31,32
Estimates of overall survival and DFS were obtained using the method of Kaplan and Meier.33 The first to occur of relapse or death was considered as failure for the end point of DFS. Cumulative incidence estimates were used to summarize the probabilities of chronic GVHD, relapse and nonrelapse mortality (NRM).34 Death without GVHD and relapse were considered as competing risks for GVHD and relapse, respectively. Relapse was considered a competing risk for NRM. Simple proportions were used to estimate the probabilities of acute GVHD and RRT. Cox regression models were fit for the end points DFS, relapse and chronic GVHD, and logistic regression models were fit for RRT and acute GVHD. Linear regression was used to model engraftment among patients who engrafted. Explanatory variables examined for the regression models included patient age and gender, presence of cytogenetic abnormalities at diagnosis and at BMT, presence of EMD, time from diagnosis to CR, duration of first remission (ie time from diagnosis to first relapse), time from diagnosis to BMT, phase of disease at BMT, donor type, CMV serologic status of patient and donor, preparative regimen, GVHD prophylaxis and marrow cell dose. All reported two-sided P-values from regression models were derived from the Wald’s test. Model improvement with the addition of a variable was assessed using the likelihood ratio test. No adjustments were made for multiple comparisons.
Results
Patient characteristics
Characteristics of the 58 patients studied are summarized in Table 1. The majority of patients had refractory disease at the time of BMT; 18 patients had primary refractory AML and 17 had persistent relapse. Age at transplantation, sex, FAB classification, EMD, duration of first remission, donor type, CMV serologic status, preparative regimen, GVHD prophylaxis and marrow cell dose were distributed similarly among patients in CR2, untreated first relapse and refractory disease. The marrow source was a mismatched relative or unrelated donor in 67% of cases. Most patients received a TBI-containing regimen (84%) and GVHD prophylaxis with cyclosporine and methotrexate (78%). The TBI dose was 12 Gy in five patients, 14.4 Gy in 35 patients and 15.75 Gy in nine patients.
Table 1.
Patient characteristics
| Characteristics | Number of patients |
|---|---|
| Total number of patients | 58 |
| Age at transplantation (years)a | 7.4 (0.8–17.3) |
| Sex (females:males) | 23:35 |
| FAB classification at diagnosis | |
| M1/M2 | 14/7 |
| M3/M4/M5 | 5/13/5 |
| M6/M7 | 3/11 |
| Cytogenetics at transplantationb | |
| Normal | 27 |
| Favorable | 1 |
| Unfavorable | 9 |
| Indeterminate | 21 |
| EMDc | |
| Isolated CNS | 8 |
| Other sites±CNS | 14 |
| High disease burdend | |
| Presence of circulating blasts | 29 |
| ≥25% marrow blasts | 21 |
| Phase of disease at transplantation | |
| Second remission | 12 |
| Untreated first relapse | 11 |
| Refractory disease | 35 |
| Months from diagnosis to transplantationa | 7.9 (1.9–54.7) |
| Donor type | |
| HLA-identical related | 19 |
| Mismatched related | 17 |
| Unrelated | 22 |
| CMV serology | |
| (+) recipient/(±) donor | 29 |
| (−) recipient/(+) donor | 10 |
| (−) recipient/(−) donor | 19 |
| Preparative regimen | |
| Non-TBI containing | 9 |
| TBI-containing | 49 |
| GVHD prophylaxis | |
| Methotrexate alone | 13 |
| Cyclosporine and methotrexate | 45 |
| Marrow cell dose (cells × 108/kg)a | 4.4 (1.1–15.6) |
Data are median (range).
Cytogenetics was classified as favorable if t(8;21), t(15;17) or inversion 16 was present, unfavorable if involving 11q23 abnormalities or monosomy 7, and indeterminate for all other abnormalities.14,24
Refers to EMD at any time prior to transplantation.
Defined as presence of circulating blasts or ≥25% marrow blasts in patients with untreated first relapse or refractory disease (n = 34/46).
Marrow cytogenetic abnormalities at the time of transplantation were present more commonly in patients with refractory disease (69%) compared to patients in CR2 (25%), P = 0.008. Abnormal cytogenetics was of favorable subtype in 3% of cases, unfavorable in 29% and indeterminate in 68% of the cases. Unfavorable cytogenetics was not observed in patients in CR2, and was present in 9% of those in untreated first relapse and in 22% of those with refractory disease. High disease burden, as evidenced by ≥25% marrow blasts or presence of circulating blasts, was seen in 33% of patients in untreated first relapse and in 57% of the patients with refractory disease (P = 0.185). The median time from diagnosis to first relapse was 7 (2–32) months. Patients in untreated first relapse had a shorter time from diagnosis to first relapse than patients in CR2 or other refractory relapse (5±2 vs 10±7 months, P = 0.03). Of the 12 patients in CR2, 25% proceeded to BMT within 12 months after diagnosis compared to 74% of patients with refractory disease and all of the patients in untreated first relapse (P = 0.0003). Patients in untreated first relapse proceeded to transplant at a median of 5 months (range 2–10) compared to a median of 11 months (range 2–55) for those in refractory relapse, P = 0.06. Time from diagnosis to first relapse and time from diagnosis to BMT were highly correlated (R = 0.67, P<0.0001).
Engraftment and RRT
Of the 58 patients, 55 (95%) experienced neutrophil engraftment at a median of 20 days (range 12–30). The only variable significantly associated with time to engraftment was presence of EMD, where patients with EMD had an average time to engraftment 2.6 days longer than patients without EMD (P = 0.04). Two patients died with an absolute neutrophil count of 0 cells/μl, on days 18 and 29 from persistent disease and multiorgan failure, respectively. A third patient had primary graft failure and received a second BMT from an alternative donor, later dying from relapsed disease. A total of 42 patients (72%) experienced platelet engraftment at a median of 26 days (range 12–90). Eight patients died and eight relapsed without achieving platelet engraftment.
A total of 13 patients (22%) experienced RRT. The most common severe toxicities observed were mucositis, veno-occlusive disease of the liver and cystitis (Table 2). A total of 11 of the RRT events occurred among the 35 (31%) patients transplanted with refractory disease, while a single RRT occurred both among the 12 (8%) patients transplanted in CR2 and the 11 (9%) patients transplanted in untreated first relapse. Although no factors were significantly associated with the development of RRT, the analysis suggested that patients transplanted with refractory disease were more likely to experience RRT than patients in CR2 or with untreated first relapse (odds ratio (OR) = 4.8, 95% confidence interval (CI) 1.0–24.2, P = 0.06). In all, 12 of 49 (24%) patients who received TBI developed RRT compared to one of nine (11%) patients who did not receive TBI, but this difference was not statistically significant (OR = 2.6, 95% CI 0.3–22.9, P = 0.39). Only one of the seven patients who received additional cranial radiotherapy for active CNS disease at the time of BMT experienced RRT.
Table 2.
Regimen-related toxicities
| Toxicity | Gradeab |
|||
|---|---|---|---|---|
| Mild–moderate | Severe | Life-threatening | Fatalc | |
| Mucositis | 4 | 49 | 4 | — |
| Dermatitis | 11 | 9 | — | — |
| Enteritis | 34 | 10 | 1 | — |
| VODd | 11 | 35 | 4 | 1 |
| Pulmonary | 9 | 9 | 1 | 2 |
| Cardiac | 2 | 1 | — | — |
| Renal | 13 | 3 | 1 | 1 |
| Cystitis | 21 | 13 | 2 | — |
| Neurologic | — | 4 | 1 | — |
Grading was carried out using NCI-Common Toxicity Criteria, v2.0:27 1 = mild, 2 = moderate, 3 = severe, 4 = life-threatening, 5 = fatal.
Refers to number of events in 58 patients.
Two patients died from toxicity, one with multiorgan involvement.
VOD = veno-occlusive disease of the liver.
GVHD
A total of 46 (79%) patients developed grade II–IV acute GVHD, 25 (43%) had acute GVHD of grade II, 19 (33%) grade III and two (3%) grade IV. No factors were significantly associated with the probability of grade II–IV or III–IV acute GVHD. Recipients of alternate donor marrow had somewhat higher odds of grade III–IV acute GVHD compared to recipients of matched-sibling donor marrow, but the difference was not significant (OR = 1.9, 95% CI 0.6–6.4, P = 0.30). Similarly, the odds of grade III–IV acute GVHD were not significantly different in patients who received TBI compared to those who did not (OR = 2.4, 95% CI 0.4–12.7, P = 0.31), and in patients over 10 years of age compared to 10 years or younger (OR = 1.9, 95% CI 0.6–5.6, P = 0.27). A longer time from diagnosis to transplant was also not statistically significantly associated with an increased probability of grade III–IV acute GVHD (P = 0.12).
A total of 28 patients developed chronic GVHD for an estimated 2-year probability of 0.48. Table 3 summarizes a multivariable model for chronic GVHD. The only significant risk factor for the development of chronic GVHD was time from diagnosis to transplant. The risk of chronic GVHD increased 1.6-fold (95% CI 1.3–1.96) for every year passed from diagnosis to transplant. There was no correlation between time from diagnosis to transplant and time to first CR, duration of first remission, phase of disease or donor type. Recipients of TBI-containing regimens were 6.8 times (95% CI 0.9–51.3) more likely to develop chronic GVHD than recipients of non-TBI-containing regimens (P = 0.06). The presence of acute GVHD did not have a qualitative effect on this association.
Table 3.
Multivariable regression analyses for selected variables
| Variable | N | Chronic GVHD |
DFS |
Relapse |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | ||
| Phase of disease | ||||||||||
| CR2 | 12 | 0.3 | 0.1–0.7 | 0.008 | 0.3 | 0.1–0.8 | 0.02 | |||
| First untreated relapse | 11 | 0.5 | 0.2–1.2 | 0.14 | 0.4 | 0.1–1.0 | 0.06 | |||
| Refractory relapse | 35 | 1 | — | — | 1 | — | — | |||
| Marrow cytogenetics | ||||||||||
| Normal | 27 | 1 | — | — | 1 | — | — | |||
| Abnormal | 31 | 2.7 | 1.4–5.3 | 0.003 | 2.7 | 1.3–5.6 | 0.008 | |||
| Preparative regimen | ||||||||||
| Non-TBI containing | 9 | 1 | — | — | ||||||
| TBI containing | 47 | 6.8 | 0.9–51.3 | 0.06 | ||||||
| Time from diagnosis to BMTa | 58 | 1.6 | 1.3–1.96 | 0.02 | ||||||
GVHD = graft-versus-host disease; DFS = disease-free survival; CR2 = second remission; TBI = total body irradiation; BMT = bone marrow transplant; HR = hazard ratio; CI = confidence interval.
Modeled as a continuous linear variable. The HR is presented as an increase in 1 year.
DFS, relapse and nonrelapse death
In all, 15 patients are disease-free survivors, 4–13 years after BMT, with a median follow-up of 9 years. A total of 43 patients died by date of last contact, and 34 (79%) of relapse after transplant. One patient relapsed and survives in remission after a second BMT. The estimated DFS at 5 years for all patients was 24% (95% CI 14–36%). The estimates of DFS at 5 years for patients in CR2, untreated first relapse and refractory disease were 58% (95% CI 27–80%), 36% (95% CI 11–63%) and 9% (95% CI 2–21%), respectively (Figures 1–3). There was no difference in DFS between patients with primary refractory disease and those with persistent relapse (14 vs 6%, P = 0.316). Table 3 summarizes a multivariable regression model for failure for the end point DFS. Patients in CR2 had a superior DFS compared to those with refractory disease (P = 0.008). Patients with abnormal cytogenetics were 2.7 times more likely to die or relapse than those with normal cytogenetics (P = 0.003). Other variables such as type of donor (P = 0.32), type of GVHD prophylaxis (P = 0.10) or EMD (P = 0.15) did not significantly improve the regression model for DFS summarized in Table 3. Patients with untreated first relapse or refractory disease who also had high disease burden were 1.7 times more likely to die or relapse than those with untreated first relapse or refractory disease without high disease burden. Neither marrow cell dose nor CMV serologic status of patient and donor was associated with DFS.
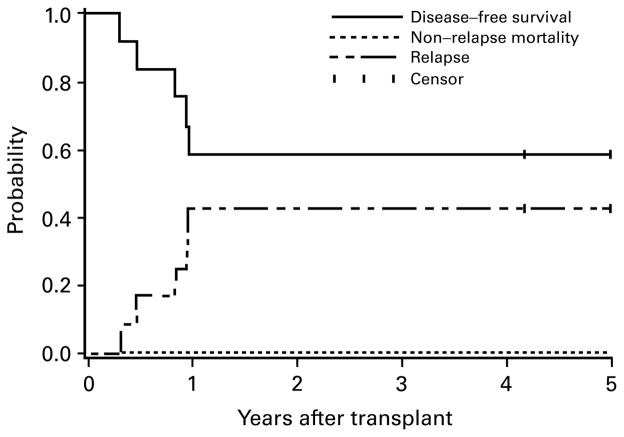
Figure 1.
Estimates of the probability of DFS, relapse and NRM for patients transplanted in second remission. Five patients alive without relapse beyond 5 years (5.5–11.9 years) are indicated as censored observations at 5 years. No events occurred beyond 5 years.
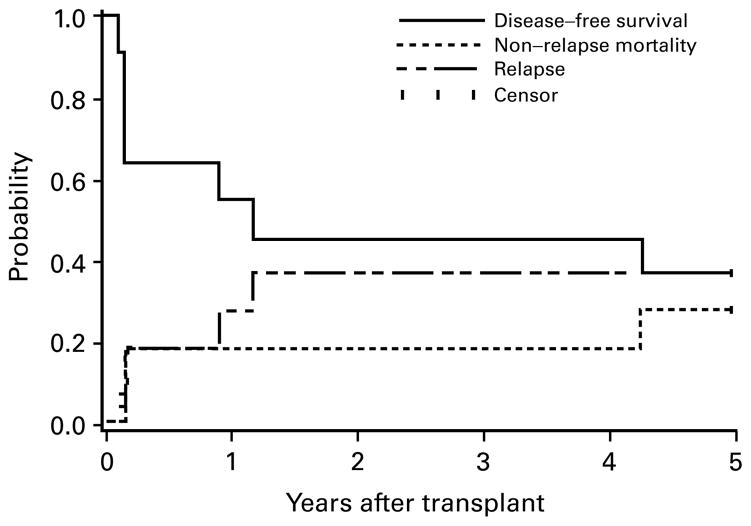
Figure 3.
Estimates of the probability of DFS, relapse and NRM for patients transplanted in refractory disease. Three patients alive without relapse beyond 5 years (11.3–13.6 years) are indicated as censored observations at 5 years. No events occurred beyond 5 years.
A total of 35 patients relapsed within 15 months after BMT. Estimates of the probability of relapse at 5 years are 42% (95% CI 14–70%), 36% (95% CI 8–65%) and 74% (95% CI 60–89%) for patients in CR2, untreated first relapse and refractory relapse, respectively. Because the majority of failures for the end point DFS were due to relapse (35 of 44), the results of regression modeling for relapse lead to the same qualitative conclusions as modeling for DFS (Table 3). Patients in CR2 (HR 0.3, 95% CI 0.1–0.8, P = 0.02) and patients in first untreated relapse (HR 0.4, 95% CI 0.1–1.0, P = 0.06) were less likely to relapse than were patients with refractory disease. The presence of cytogenetic abnormalities was also associated with a higher risk of relapse (HR 2.7, 95% CI 1.3–5.6, P = 0.008).
Estimates of overall mortality at day 100 and 1-year post transplantation were 27 and 55%, respectively. Nine (16%) patients died from nonrelapse causes (Table 4). Infection without GVHD was the most common cause of NRM. All the nonrelapse deaths occurred among patients transplanted with refractory disease (six of 35) or untreated first relapse (three of 11). NRM estimates were 0%, 27% (95% CI 0–54%) and 17% (95% CI 5–30%) for patients in CR2, untreated first relapse and refractory relapse, respectively. Comparing patients in CR2 vs all others, this difference is suggestive of an association between phase of disease and NRM (P = 0.06, log-rank test). No other variables were statistically significantly associated with the hazard of NRM, but the power to detect such differences is limited by the small number of events (n = 9).
Table 4.
Causes of death before and after 100 days post transplantation
| Cause of death | Total (43 patients) | <100 days (16 patients) | ≥100 days (27 patients) |
|---|---|---|---|
| Relapse | 34 (80) | 9 | 25 |
| Infection | 5 (11) | ||
| Disseminated Aspergillus | 1 | 0 | |
| CMV pneumonitisa | 3 | 1 | |
| GVHD | 2 (5) | ||
| Acute, multisystem | 1 | 0 | |
| Chronic, BOOP | 0 | 1 | |
| Toxicity | 2 (5) | ||
| Diffuse alveolar damage | 1 | 0 | |
| Multiorgan failureb | 1 | 0 |
Results shown as number of patients (%).
GVHD = graft-versus-host disease; CMV = cytomegalovirus; BOOP = bronchiolitis obliterans with organizing pneumonia
One patient with underlying chronic GVHD died from infection on day 185.
One patient died from veno-occlusive disease of the liver, acute renal failure and idiopathic pneumonia.
Discussion
Survival for children with advanced AML has been reported to be poor with either chemotherapy alone or BMT.8,11–13,17 Our study suggests that allogeneic marrow transplantation for children with AML in CR2 results in an excellent outcome, with an estimated 5-year DFS of 58%. Previously reported estimates of DFS for children with AML in CR2 range from 40 to 50%.11,12,15–21 As in our study, these reports included a modest number of patients in CR2. Survival for patients in untreated first relapse in our study is higher than that previously reported.14,35 While direct comparisons to previous studies cannot validly be made, we consider the current results to be encouraging. Although time from diagnosis to transplantation was not independently associated with DFS, patients in untreated first relapse proceeded to transplant much sooner than those in refractory relapse, suggesting the presence of more readily available allogeneic donors. This bias could explain in part the difference in DFS between these two groups.
Phase of disease at the time of transplantation and abnormal marrow cytogenetics at the time of BMT were each found to be risk factors for low DFS following transplantation in a multivariable regression model. These findings are similar to those reported by others.10,11,13,18,36 The presence of unfavorable cytogenetics at diagnosis has been associated with poor survival after chemotherapy or BMT for children with AML in first CR.24 The association between poor DFS and unfavorable cytogenetics at the time of BMT in children with more advanced disease found in our study has not been previously described, and should be considered when discussing prognosis and treatment options for these patients. Our analysis confirms the association between high disease burden and poor survival. This finding supports the current practice of attempting cytoreduction before proceeding with transplantation for patients with circulating blasts or extensive marrow disease.
A previous study of marrow transplantation for AML in children younger than 2 years found that EMD at any time before transplantation was associated with poor survival.14 We did not find this variable to be predictive of outcome in children of all ages with advanced AML, although the power to detect a statistically significant difference was limited due to the relatively small number of events. Previous studies have reported associations between low DFS and marrow cell dose below 3.5 cells × 108/kg, high disease burden and CMV seropositivity.13,37 These studies limited analysis to recipients of unrelated donor marrow and included both children and adults. The median marrow cell dose in our study was 4.4 × 108/kg, and 60% of patients received a cell dose above 3.5 × 108/kg. This is likely related to the exclusion of adult patients from our analysis, and may explain in part why we did not find a significant association between this variable and DFS, although the limited power due to the low event rate could have also contributed to the lack of such a difference. We did not find a statistically significant association between CMV seropositivity and DFS. Previous studies that have identified this association included patients transplanted before the introduction of gancyclovir.13 The majority of patients who were CMV seropositive in our study received prophylactic gancyclovir during the transplant period, possibly contributing to reduced mortality in this patient group. However, the lack of power may have also contributed to the failure to observe an association between DFS and CMV serostatus.
NRM was not observed in the subset of 12 patients transplanted in CR2. The reported incidence of NRM for patients with AML beyond first CR in the current literature ranges from 10 to 50%.10–11,13–15 As in our study, the number of patients reported has been modest. However, the low incidence of NRM found in our study is encouraging and supports the use of allogeneic marrow transplantation in this patient group. The incidence of life threatening to fatal RRT in our study was 22%. There are no previous studies on this outcome specifically for children with advanced AML. The incidence of RRT reported here is comparable to that seen in children with acute leukemia in general, particularly when considering the large number of alternate donor recipients in the cohort.1,13,18,22 Previous studies have identified TBI, unrelated donor transplant, low marrow cell dose and transplant in relapse as risk factors for RRT.22,38 Our analysis failed to reveal any specific risk factors statistically significantly associated with the probability of RRT, perhaps due in part to the limited number of events.
The incidence of acute and chronic GVHD is similar to that previously reported for patients with AML.4,13,18 Recipients of alternative donor marrow, those older than 10 years or those receiving TBI conditioning had a tendency for a higher probability of grade III–IV acute GVHD. These associations have been previously described,39,40 and while the differences observed in our study were not statistically significant, the power to detect such associations was limited. Chronic GVHD was associated with time from diagnosis to transplantation and with TBI-containing preparative regimens. The association with TBI has been described before and thought to be related to increased inflammatory cytokine production.41 It is possible that the same mechanism explains the association between chronic GVHD and time to transplantation, since typically patients who wait longer for a transplant receive more cycles of chemotherapy than those who proceed to transplant earlier.
Our study supports allogeneic BMT as therapy for patients with recurrent AML, particularly in earlier disease phases. Despite all the advances in marrow transplantation, survival of children with AML transplanted in refractory disease has not changed significantly since the first recipients of marrow transplantation were reported over two decades ago.42,43 Relapse remains the main cause of treatment failure for patients with advanced AML, with mortality occurring mostly within the first 2 years after transplantation. These findings suggest the need to explore novel approaches to treat these patients such as targeted therapies and new preparative regimens. Risk factors for mortality and relapse identified in our study can serve to guide clinicians when providing recommendations for allogeneic BMT in children with AML beyond first CR.
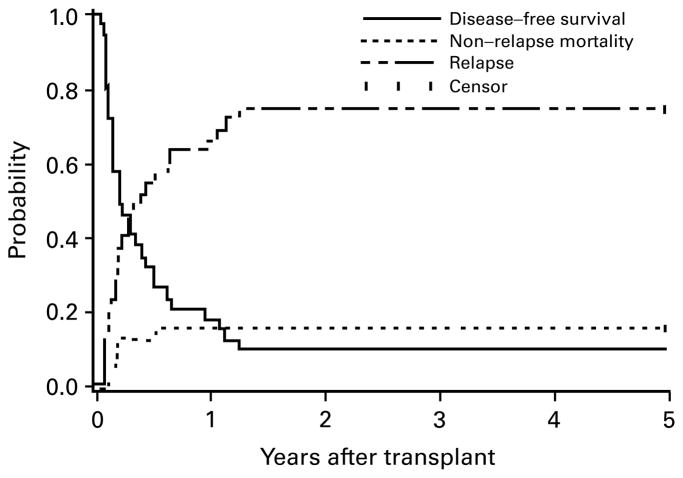
Figure 2.
Estimates of the probability of DFS, relapse and NRM for patients transplanted in untreated first relapse. Four patients alive without relapse beyond 5 years (7.3–13.7 years) are indicated as censored observations at 5 years. No events occurred beyond 5 years.
Acknowledgments
We thank Mr Paul Hoffmeister for his assistance in data collection. This work was supported in part by Grants CA100394 and CA18029 from the National Cancer Institute, National Institutes of Health.
References
- 1.Woods WG, Neudorf S, Gold S, et al. A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood. 2001;97:56–62. doi: 10.1182/blood.v97.1.56. [DOI] [PubMed] [Google Scholar]
- 2.Amadori S, Testi AM, Arico M, et al. Prospective comparative study of bone marrow transplantation and postremission chemotherapy for childhood acute myelogenous leukemia. The Associazione Italiana Ematologia ed Oncologia Pediatrica Cooperative Group. J Clin Oncol. 1993;11:1046–1054. doi: 10.1200/JCO.1993.11.6.1046. [DOI] [PubMed] [Google Scholar]
- 3.Burnett AK, Wheatley K, Goldstone AH, et al. The value of allogeneic bone marrow transplant in patients with acute myeloid leukaemia at differing risk of relapse: results of the UK MRC AML 10 trial. Br J Haematol. 2002;118:385–400. doi: 10.1046/j.1365-2141.2002.03724.x. [DOI] [PubMed] [Google Scholar]
- 4.Feig SA, Lampkin B, Nesbit ME, et al. Outcome of BMT during first complete remission of AML: a comparison of two sequential studies by the Children’s Cancer Group. Bone Marrow Transplant. 1993;12:65–71. [PubMed] [Google Scholar]
- 5.Wells RJ, Woods WG, Buckley JD, et al. Treatment of newly diagnosed children and adolescents with acute myeloid leukemia: a Children’s Cancer Group study. J Clin Oncol. 1994;12:2367–2377. doi: 10.1200/JCO.1994.12.11.2367. [DOI] [PubMed] [Google Scholar]
- 6.Ravindranath Y, Yeager AM, Chang MN, et al. Autologous bone marrow transplantation versus intensive consolidation chemotherapy for acute myeloid leukemia in childhood. Pediatric Oncology Group. N Engl J Med. 1996;334:1428–1434. doi: 10.1056/NEJM199605303342203. [DOI] [PubMed] [Google Scholar]
- 7.Stevens RF, Hann IM, Wheatley K, Gray RG. Marked improvements in outcome with chemotherapy alone in paediatric acute myeloid leukemia: results of the United Kingdom Medical Research Council’s 10th AML trial. MRC Childhood Leukaemia Working Party. Br J Haematol. 1998;101:130–140. doi: 10.1046/j.1365-2141.1998.00677.x. [DOI] [PubMed] [Google Scholar]
- 8.Fleischhack G, Hasan C, Graf N, et al. IDA-FLAG (idarubicin, fludarabine, cytarabine, G-CSF), an effective remission-induction therapy for poor-prognosis AML of childhood prior to allogeneic or autologous bone marrow transplantation: experiences of a phase II trial. Br J Haematol. 1998;102:647–655. doi: 10.1046/j.1365-2141.1998.00836.x. [DOI] [PubMed] [Google Scholar]
- 9.Dini G, Boni L, Abla O, et al. Allogeneic bone marrow transplantation in children with acute myelogenous leukemia in first remission. Bone Marrow Transplant. 1994;13:771–776. [PubMed] [Google Scholar]
- 10.Schiller G, Feig SA, Territo M, et al. Treatment of advanced acute leukaemia with allogeneic bone marrow transplantation from unrelated donors. Br J Haematol. 1994;88:72–78. doi: 10.1111/j.1365-2141.1994.tb04979.x. [DOI] [PubMed] [Google Scholar]
- 11.Aladjidi N, Auvrignon A, Leblanc T, et al. Outcome in children with relapsed acute myeloid leukemia after initial treatment with the French Leucemie Aique Myeloide Enfant (LAME) 89/91 protocol of the French Society of Pediatric Hematology and Immunology. J Clin Oncol. 2003;21:4377–4385. doi: 10.1200/JCO.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 12.Webb DK, Wheatley K, Harrison G, et al. Outcome for children with relapsed acute myeloid leukaemia following initial therapy in the Medical Research Council (MRC) AML 10 trial. MRC Childhood Leukaemia Working Party. Leukemia. 1999;13:25–31. doi: 10.1038/sj.leu.2401254. [DOI] [PubMed] [Google Scholar]
- 13.Sierra J, Storer B, Hansen JA, et al. Unrelated donor marrow transplantation for acute myeloid leukemia: an update of the Seattle experience. Bone Marrow Transplant. 2000;26:397–404. doi: 10.1038/sj.bmt.1702519. [DOI] [PubMed] [Google Scholar]
- 14.Woolfrey AE, Gooley TA, Sievers EL, et al. Bone marrow transplantation for children less than 2 years of age with acute myelogenous leukemia or myelodysplastic syndrome. Blood. 1998;92:3546–3556. [PubMed] [Google Scholar]
- 15.Casper J, Camitta B, Truitt R, et al. Unrelated bone marrow donor transplants for children with leukemia or myelodysplasia. Blood. 1995;85:2354–2363. [PubMed] [Google Scholar]
- 16.Davies SM, Wagner JE, Shu XO, et al. Unrelated donor bone marrow transplantation for children with acute leukemia. J Clin Oncol. 1997;15:557–565. doi: 10.1200/JCO.1997.15.2.557. [DOI] [PubMed] [Google Scholar]
- 17.Balduzzi A, Gooley T, Anasetti C, et al. Unrelated donor marrow transplantation in children. Blood. 1995;86:3247–3256. [PubMed] [Google Scholar]
- 18.Dinndorf P, Bunin N. Bone marrow transplantation for children with acute myelogenous leukemia. J Pediatr Hematol Oncol. 1995;17:211–224. doi: 10.1097/00043426-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Hongeng S, Krance RA, Bowman LC, et al. Outcomes of transplantation with matched-sibling and unrelated-donor bone marrow in children with leukaemia. Lancet. 1997;350:767–771. doi: 10.1016/S0140-6736(97)03098-5. [DOI] [PubMed] [Google Scholar]
- 20.Horowitz MM, Bortin MM. Results of bone marrow transplants from human leukocyte antigen-identical sibling donors for treatment of childhood leukemias. A report from the International Bone Marrow Transplant Registry. Am J Pediatr Hematol Oncol. 1993;15:56–64. doi: 10.1097/00043426-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Brochstein JA, Kernan NA, Groshen S, et al. Allogeneic bone marrow transplantation after hyperfractionated total-body irradiation and cyclophosphamide in children with acute leukemia. N Engl J Med. 1987;317:1618–1624. doi: 10.1056/NEJM198712243172602. [DOI] [PubMed] [Google Scholar]
- 22.Balduzzi A, Valsecchi MG, Silvestri D, et al. Transplant-related toxicity and mortality: an AIEOP prospective study in 636 pediatric patients transplanted for acute leukemia. Bone Marrow Transplant. 2002;29:93–100. doi: 10.1038/sj.bmt.1703337. [DOI] [PubMed] [Google Scholar]
- 23.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the acute leukaemias. French–American–British (FAB) co-operative group. Br J Haematol. 1976;33:451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 24.Webb DK, Harrison G, Stevens RF, et al. Relationships between age at diagnosis, clinical features, and outcome of therapy in children treated in the Medical Research Council AML 10 and 12 trials for acute myeloid leukemia. Blood. 2001;98 :1714–1720. doi: 10.1182/blood.v98.6.1714. [DOI] [PubMed] [Google Scholar]
- 25.Slattery JT, Sanders JE, Buckner CD, et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant. 1995;16:31–42. [PubMed] [Google Scholar]
- 26.Hansen JA, Mickelson EM, Choo SY, et al. Clinical bone marrow transplantation: donor selection and recipient monitoring. In: Rose NR, De Macario EC, Fahey JL, Friedman H, Penn GM, editors. Manual of Clinical Laboratory Immunology. American Society for Microbiology; Washington, DC: 1992. pp. 850–866. [Google Scholar]
- 27.Dupont B, Yang SY. Histocompatibility. In: Forman SJ, Blume KG, Thomas ED, editors. Bone Marrow Transplantation. Blackwell Scientific; Boston, MA: 1994. pp. 22–40. [Google Scholar]
- 28.Thomas ED, Storb R, Clift RA, et al. Bone-marrow transplantation. N Engl J Med. 1975;292:895–902. doi: 10.1056/NEJM197504242921706. [DOI] [PubMed] [Google Scholar]
- 29.Storb R, Deeg HJ, Whitehead J, et al. Marrow transplantation for leukemia and aplastic anemia: two controlled trials of a combination of methotrexate and cyclosporine v cyclosporine alone or methotrexate alone for prophylaxis of acute graft-v-host disease. Transplant Proc. 1987;19:2608–2613. [PubMed] [Google Scholar]
- 30.Cancer Therapy Evaluation Program. National Institutes of Health, National Cancer Institute; 1999. Common Toxicity Criteria Manual Version 2. [Google Scholar]
- 31.Doney KC, Weiden PL, Storb R, Thomas ED. Treatment of graft-versus-host disease in human allogeneic marrow graft recipients: a randomized trial comparing antithymocyte globulin and corticosteroids. Am J Hematol. 1981;11:1–8. doi: 10.1002/ajh.2830110102. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan KM, Shulman HM, Storb R, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57:267–276. [PubMed] [Google Scholar]
- 33.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 34.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Wiley; New York, NY: 1980. [Google Scholar]
- 35.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation during untreated first relapse of acute myeloid leukemia. J Clin Oncol. 1992;10:1723–1729. doi: 10.1200/JCO.1992.10.11.1723. [DOI] [PubMed] [Google Scholar]
- 36.Fung HC, Stein A, Slovak M, et al. A long-term follow-up report on allogeneic stem cell transplantation for patients with primary refractory acute myelogenous leukemia: impact of cytogenetic characteristics on transplantation outcome. Biol Blood Marrow Transplant. 2003;9:766–771. doi: 10.1016/j.bbmt.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Sierra J, Storer B, Hansen JA, et al. Transplantation of marrow cells from unrelated donors for treatment of high-risk acute leukemia: the effect of leukemic burden, donor HLA-matching, and marrow cell dose. Blood. 1997;89:4226–4235. [PubMed] [Google Scholar]
- 38.Bearman SI, Appelbaum FR, Buckner CD, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988;6:1562–1568. doi: 10.1200/JCO.1988.6.10.1562. [DOI] [PubMed] [Google Scholar]
- 39.Svennilson J, Remberger M, Ringden O. Risk factors for moderate-to-severe acute graft-vs-host disease after allogeneic stem cell transplantation in children. Pediatr Transplant. 2003;7:130–136. doi: 10.1034/j.1399-3046.2003.00030.x. [DOI] [PubMed] [Google Scholar]
- 40.Locatelli F, Uderzo C, Dini G, et al. Graft-versus-host disease in children: the AIEOP-BMT Group experience with cyclosporin A. Bone Marrow Transplant. 1993;12:627–633. [PubMed] [Google Scholar]
- 41.Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991:324. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 42.Thomas ED, Buckner CD, Banaji M, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. 1977;49:511–533. [PubMed] [Google Scholar]
- 43.Jonson FL, Hartmann JR, Thomas ED, et al. Marrow transplantation in treatment of children with aplastic anaemia or acute leukaemia. Arch Dis Child. 1976;51:403–410. doi: 10.1136/adc.51.6.403. [DOI] [PMC free article] [PubMed] [Google Scholar]