Abstract
Lipopolysaccharide (LPS/endotoxin) is a potent immunologic stimulant. Many commercial-grade reagents used in research are not screened for LPS contamination. LPS induces a wide spectrum of proinflammatory responses in microglia, the immune cells of the brain. Recent studies have demonstrated that a broad range of endogenous factors including plasma-derived proteins and bioactive phospholipids can also activate microglia. However, few of these studies have reported either the LPS levels found in the preparations used or the effect of LPS inhibitors such as polymyxin B (PMX) on factor-induced responses. Here, we used the Limulus amoebocyte lysate assay to screen a broad range of commercial- and pharmaceutical-grade proteins, peptides, lipids, and inhibitors commonly used in microglia research for contamination with LPS. We then characterized the ability of PMX to alter a representative set of factor-induced microglial activation parameters including surface antigen expression, metabolic activity/proliferation, and NO/cytokine/chemokine release in both the N9 microglial cell line and primary microglia. Significant levels of LPS contamination were detected in a number of commercial-grade plasma/serum- and nonplasma/serum-derived proteins, phospholipids, and synthetic peptide preparations, but not in pharmaceutical-grade recombinant proteins or pharmacological inhibitors. PMX had a significant inhibitory effect on the microglia-activating potential of a number of commercial-, but not pharmaceutical-grade, protein preparations. Novel PMX-resistant responses to α2-macroglobulin and albumin were incidentally observed. Our results indicate that LPS is a frequent and significant contaminant in commercial-grade preparations of previously reported microglia-activating factors. Careful attention to LPS levels and appropriate controls are necessary for future studies in the neuroinflammation field.
Keywords: LPS, endotoxin, microglia, thrombin, albumin
INTRODUCTION
Lipopolysaccharide (LPS/endotoxin) is a heat-stable nonproteinacious bacterial cell wall component. It is found ubiquitously in the environment and can induce a wide spectrum of biologic activities (Dixon and Darveau, 2005; Janeway et al., 2005; Westphal et al., 1981). LPS initiates numerous metabolic and cellular changes in vitro and in vivo. LPS’s synonym, endotoxin, derives from its ability to induce secretion of overwhelming concentrations of proinflammatory cytokines in vivo leading to severe vascular, hemodynamic, and respiratory changes (Janeway et al., 2005). In addition, LPS triggers numerous and profound changes in the immune system. It is immunogenic in the absence of T-helper cells, possesses strong adjuvant properties and induces a polyclonal antibody response in B-cells (Dixon and Darveau, 2005; Jacobs and Morrison, 1977). In the CNS, LPS induces neuroinflammatory responses including microglial activation, astrogliosis, and focal necrosis (Cai et al., 2003; Herber et al., 2006; Pang et al., 2006; Szczepanik et al., 1996). LPS can also augment sensitization to brain antigens (Becker et al., 2005) and induce cross-tolerance (Rosenzweig et al., 2004) in stroke models. LPS is a pathogen-associated molecular pattern (PAMP) and many of its responses are mediated by Toll-like receptor 4 (TLR4) (Lee and Lee, 2002; Palsson-McDermott and O’Neill, 2004). The biologically active portion of the LPS macromolecule is its phospholipid anchor known as lipid A (Morrison and Jacobs, 1976a). The cationic polypeptide polymyxin B (PMX) binds to this region and potently abrogates many LPS-induced responses (Jacobs and Morrison, 1977; Morrison and Jacobs, 1976a,b).
Microglial cells are the tissue macrophages of the CNS. Under normal conditions they are characterized by a small cell body with fine, ramified processes, and low expression of surface antigens (Kreutzberg, 1996). Although sometimes referred to as “resting,” recent live imaging studies have demonstrated that microglial cellular processes are constantly in motion, monitoring their environment for signals from surrounding neural cells (Davalos et al., 2005; Melchior et al., 2006; Nimmerjahn et al., 2005). During brain injury, microglia rapidly activate by transforming their morphology, increasing surface antigen expression, proliferating and releasing large quantities of neurotoxic compounds including nitric oxide (NO) and TNF-α (Melchior et al., 2006; Streit et al., 1999). In selected settings, however, activated microglia can function in a neuroprotective manner and promote regeneration by releasing growth factors and modulating the immune response (Melchior et al., 2006; Schwartz et al., 2006; Streit, 2002). Microglia must respond rapidly and efficiently to a wide range of exogenous and endogenous signals that indicate a threat to the structural/functional integrity of the CNS. For this purpose, microglia are equipped with an array of sensitive receptors, including TLRs for PAMPs such as LPS (Olson and Miller, 2004). LPS induces a broad spectrum of microglial activation parameters including NO/cytokine release (Kim et al., 2004), antigen presentation (Gregerson et al., 2004), and upregulation of surface antigens (Kloss et al., 2001; Menendez Iglesias et al., 1997; Terrazzino et al., 2002). LPS also modulates microglial proliferation, enhancing it in some experimental paradigms (Lee et al., 1994) and inhibiting it in others (Bianco et al., 2006; Ganter et al., 1992; Gebicke-Haerter et al., 1989; Northoff et al., 1989).
A number of endogenous plasma/serum proteins (Hooper et al., 2005; Möller et al., 1997a,b, 2000; Pul et al., 2002) and disease-associated factors (D’Aversa et al., 2005; Jekabsone et al., 2006) as well as bioactive phospholipids (Möller et al., 2001; Nakajima et al., 2002; Wang et al., 1999) have also been reported to induce microglial activation. Studies in the immunology literature have suggested that the activation of peripheral macrophages/monocytes by commercially available preparations of heat-shock protein (Bausinger et al., 2002; Gao and Tsan, 2003a,b) and pokeweed mitogen (Yang et al., 2006) were due to LPS contamination. Recently, we reported that a number of microglial activation responses induced by commercial-grade preparations of thrombin were due to protein contaminants (Hanisch et al., 2004; Weinstein et al., 2005). LPS was also detected in several of the thrombin preparations (Weinstein et al., 2005) but its functional significance was not examined. The extent of LPS contamination in most commercial-grade proteins, peptides, phospholipids, and pharmacological inhibitors used commonly in the neuroinflammation field has not been examined or reported. The goals of this study were to (1) systematically quantify LPS content in a number of commercially available factors used commonly in microglia research and (2) determine the functional impact of LPS contamination on microglial cell activation.
MATERIALS AND METHODS
Solutions and Reagents
Control standard endotoxin (CSE) a highly purified form of LPS whose endotoxin activity has been determined by the manufacturer with respect to the reference United States Standard endotoxin, was purchased from Associates of Cape Cod, Falmouth, MA. Commercial-grade plasma-derived bovine α-thrombin (pb-thr) (T-4648, 40–165 U/mg) and PMX sulfate (P-4932) were purchased from Sigma (St. Louis, MO). The pharmaceutical-grade [current Good Manufacturing Practices (cGMP)-compliant (FDA, 2002)] recombinant human α-thrombin (rh-thr) (3,490 U/mg) was a kind gift of Zymo-Genetics (Seattle, WA). Recombinant mouse interferon-γ (rm-IFN-γ) was purchased from R&D systems, Minneapolis, MN. Commercial-grade serum-derived human α2-macroglobulin was purchased from Sigma (M-6159) and Calbiochem/EMD Biosciences, La Jolla, CA (441251) (sh-α2M-5 and sh-α2M-7, respectively). Commercial-grade essentially fatty acid free (A6414) and Fraction V Powder (A6272) serum-derived rat albumin was purchased from Sigma (sr-Alb-efaf and sr-Alb-FV, respectively). Commercial-grade 2,4,6-trinitrophenyl-conjugated hen egg white-derived ovalbumin (tnp-Oval) (T5051) was from Biosearch Technologies, Novato, CA. All solutions were freshly prepared from frozen stock solutions or lyophilized preparations. All materials were handled in a sterile manner using endotoxin-free microfuge tubes (Eppendorf/Fisher Scientific, Santa Clara, CA), polypropylene tubes (Becton Dickinson Labware, Franklin Lakes, NJ), polystyrene culture vessels (Becton Dickinson Labware), serological pipettes (Costar/Corning, Corning, NY), precision pipette tips (Rainin Instruments, LCC, Oakland, CA), water (Associates of Cape Cod), and PBS (Gibco/Invitrogen, Carlsbad, CA). Suppliers, enzyme specific activities, and catalog numbers of additional reagents used only for determination of endotoxin levels by Limulus assay are listed in Table 1.
TABLE 1.
Quantification of LPS (Endotoxin) Levels in Various Commercial- and Pharmaceutical-Grade Preparations and Reagents
| Preparation/Reagent | Source | Species | Supplier | Catalog# | Specific activity of endotoxin contamination |
Endotoxin contamination level at typical working concentrations |
Potential functional significance in microgliab |
|---|---|---|---|---|---|---|---|
| Pharmaceutical-grade recombinant proteins | |||||||
| α-Thrombin (rh-thr) (3,495 U/mg)c | Recombinant | Human | 1 | N/A | <0.0003 EU/U | <0.03 EU/mL in 100 U/mL | − |
| Anti-thrombina,c | Recombinant | Human | 2 | N/A | <0.006 EU/U | <0.0006 EU/mL in 0.1 U/mL | − |
| Commercial-grade recombinant proteins | |||||||
| Trypsinc | Recombinant | Human | 3 | TRY001-10 | 0.0124 EU/U | 0.22 EU/mL in 100 nM | ++ |
| Interferon-γ (rm-IFNγ) | Recombinant | Mouse | 4 | 485-MI | <0.0001EU/U | <0.001 EU/mL in 10 U/mL | − |
| Commercial-grade, nonrecombinant, plasma/serum-derived proteins | |||||||
| α-Thrombin (pb-thr) (115 U/mg)c | Plasma | Bovine | 5 | T4648 | 0.21 EU/U | 21 EU/mL in 100 U/mL | ++++ |
| α-Thrombin (2,114 U/g)c | Plasma | Bovine | 6 | BT830a | 0.003 EU/U | 0.3 EU/mL in 100 U/mL | ++ |
| α-Thrombin (416 U/g)c | Plasma | Human | 5 | T7572 | 0.0036 EU/U | 0.36 EU/mL in 100 U/mL | ++ |
| α-Thrombin (3,074 U/g)a,c | Plasma | Human | 6 | HT2340PA | <0.001 EU/U | <0.1 EU/mL in 100 U/mL | − |
| α-Thrombin (1,560 U/g)c | Plasma | Mouse | 5 | T-8397 | 0.02 EU/U | 0.2 EU/mL in 10 U/mL | ++ |
| α2-Macroglobulin (sh-α2M-5) | Serum | Human | 5 | M6159 | 4.8 EU/nmol | 0.48 EU/mL in 100 nM | +++ |
| α2-Macroglobulin (sh-α2M-7) | Serum | Human | 7 | 441251 | 60 EU/nmol | 6 EU/mL in 100 nM | ++++ |
| Albumin-essentially fatty acid free (sr-Alb-efaf) | Serum | Rat | 5 | A6414 | 0.016 EU/nmol | 0.0024 EU/mL in 1 mg/mL | − |
| Albumin-fraction V (sr-Alb-FV) | Serum | Rat | 5 | A6272 | >2.78 EU/nmol | >0.43 EU/mL in 1 mg/mL | +++ |
| Albumin-delipidated | Serum | Bovine | 5 | A0281 | 0.065 EU/nmol | 0.01 EU/mL in 1 mg/mL | − |
| Complement C3a | Serum | Human | 7 | 204881 | 0.1 EU/nmol | 0.01 EU/mL in 100 nM | − |
| Complement C5a | Serum | Human | 5 | C5788 | 0.8 EU/nmol | 0.08 EU/mL in 100 nM | + |
| IgM | Serum | Bovine | 5 | I-8135 | 4,000 EU/nmol | 4,200 EU/mL in 1 mg/L | ++++ |
| Commercial-grade, nonrecombinant, nonplasma/serum-derived proteins | |||||||
| Trinitrophenol-ovalbumin (tnp-Oval) | Egg white | Chicken | 8 | T-5051 | 4.9 EU/nmol | 0.49 EU/mL in 100 nM | +++ |
| IgG2bk | Hybridoma | Rat | 9 | 553986 | 9.0 EU/nmol | 30 EU/mL in 50 μg/mL | ++++ |
| IgMk | Hybridoma | Mouse | 9 | 553472 | 45 EU/nmol | 0.18 EU/mL in 100 μg/mL | ++ |
| IgAk | Hybridoma | Mouse | 9 | 550924 | 8 EU/nmol | 2.3 EU/mL in 100 μg/mL | ++++ |
| IgA | Myeloma | Mouse | 10 | 02-6500 | >861 EU/nmol | >246 EU/mL in 100 μg/mL | ++++ |
| IgMkd | Hybridoma | Hamster | 9 | 553957 | 2 EU/nmol | 0.22 EU/mL in 100 μg/mL | ++ |
| Commercial-grade bioactive phospholipids | |||||||
| LPA | Synthetic | N/A | 5 | L7260 | <0.0001 EU/nmol | 0.001 EU/mL in 10 μM | − |
| LPA | Synthetic | N/A | 11 | 857119 | 0.04 EU/nmol | 0.4 EU/mL in 10 μM | +++ |
| S-1-P | Synthetic | N/A | 5 | S9666 (lot #1) | <0.0001 EU/nmol | 0.001 EU/mL in 10 μM | − |
| S-1-P | Synthetic | N/A | 5 | S9666 (lot #2) | 1.5 EU/nmol | 15 EU/mL in 10 μM | ++++ |
| Commercial-grade peptides | |||||||
| PAR1 activating peptide (SFLLRN) | Synthetic | Human | 12 | H-8365 | <0.000013 EU/nmol | <0.01 EU/mL in 770 μM | − |
| PAR1 activating peptide (SFFLRN) | Synthetic | Mouse | 12 | 4047522 | 0.00041 EU/nmol | 0.27 EU/mL in 660 μM | ++ |
| PAR4 activating peptide (GYPGKF) | Synthetic | Mouse | 12 | H-4404 | 0.000083 EU/nmol | 0.06 EU/mL in 720 μM | + |
| Amyloid β-protein (1–40) | Synthetic | Human | 12 | H-5568 | 0.016 EU/nmol | 0.31 EU/mL in 20 μM | ++ |
| Commercial-grade pharmacological inhibitors | |||||||
| SB 203580 (p38 MAP Kinase inhibitor) | Synthetic | N/A | 7 | 559389 | 0.000038 EU/nmol | 0.00019 EU/mL in 5 μM | − |
| U0126 (MEK1/2 MAP Kinase inhibitor) | Synthetic | N/A | 7 | 662005 | 0.000038 EU/nmol | 0.00019 EU/mL in 5 μM | − |
| SP 600125 (JNK MAP Kinase inhibitor) | Synthetic | N/A | 7 | 420119 | 0.00099 EU/nmol | 0.00099 EU/mL in 1 μM | − |
| SK&F 9635 (inhibitor of receptor-mediated Ca2+ release) |
Synthetic | N/A | 13 | CA-230 | 0.00020 EU/nmol | 0.0020 EU/mL in 10 μM | − |
| BAPTA | Synthetic | N/A | 7 | 196419 | <0.001 EU/nmol | <0.01 EU/mL in 10 μM | − |
| PPACK (thrombin inhibitor) | Synthetic | N/A | 7 | 520222 | 0.00013 EU/nmol | <0.0025 EU/mL in 10 μg/mL | − |
Supplier key: (1) Zymogenetics, Seattle, WA; (2) GTC Biotherapeutics, Framingham, MA; (3) Polymun Scientific, Vienna, Austria; (4) R&D Systems, Minneapolis, MN; (5) Sigma, St. Louis, MO; (6) Enzyme Research Laboratories, South Bend, IN; (7) Calbiochem/EMD Biosciences, La Jolla, CA; (8) Biosearch Technologies, Novato, CA; (9) BD PharMingen, San Diego, CA; (10) Zymed Laboratories, San Francisco, CA; (11) Avanti Polar Lipids, Alabaster, AL; (12) Bachem, King of Prussia, PA; and (13) Biomol Research Laboratories, Plymouth Meeting, PA.
LAL assay inhibition detected in unboiled samples.
Based on surface antigen response to control standard endotoxin (CSE) in N9 cells.
Boiled 2 min before testing.
Purchased as “No azide/Low Endotoxin” (NA/LE) product.
Cell Culture
The mouse microglial cell line N9 was a kind gift of Dr. M. Righi, International School for Advanced Studies, Trieste, Italy, and was cultured in accordance with the original publication (Righi et al., 1995). Briefly, cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Mediatech, Herndon, VA), supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT) and penicillin/streptomycin (P/S, 50 I.U./50 μg/mL; Media-tech). Cells were passaged weekly with 0.05% trypsin (Gibco/Invitrogen) and serum starved in macrophage serum-free medium (MSFM; Gibco/Invitrogen) for at least 24 h before each experiment as detailed below. Primary microglia (pMG) were prepared from the cortex of new-born (p4) C57BL/6J mice as previously described (Giulian and Baker, 1986; Möller et al., 2000). In brief, cortical tissue was carefully freed from blood vessels and meninges, digested with 50-ng/mL DNase, triturated, and washed. Cortical cells were cultured in DMEM/10% FBS with P/S for 11–28 d (media change every 3–4 d). Microglia were separated from underlying astrocytic monolayer by gentle agitation, spun down (100g for 10 min). Cell pellet was resuspended in DMEM/10% FBS with P/S plus 20% L929 cell-conditioned medium and used for experiments as detailed below.
Assays for Microglial Metabolic Activity/Proliferation, NO Production, and Cytokine Release
For enzyme-linked immunosorbent assay (ELISA), metabolic activity/proliferation and NO measurements, microglial cells were plated in 96-well plates in DMEM/10% FBS. After 3 h, cells were washed once with PBS and cultured in DMEM/10% FBS. After 24 h of plating, medium was changed to 250 μL MSFM. After 24 h cells were stimulated with the various preparations. Metabolic activity/proliferation, NO production, and cytokine release were assessed in the supernatants 24-h post stimulation. Metabolic activity/proliferation was determined with the WST-1 reagent (Boehringer Mannheim, Indianapolis, IN) (Möller et al., 2000). NO production was assessed using the Griess reagent (Möller et al., 2000). Analyses of tumor necrosis factor-α (TNF-α) content in N9 cell-conditioned medium was carried out using mouse-specific antibody pairs and mouse protein standards designed for ELISA application in accordance with the manufacturer’s instructions (R&D Systems; Möller et al., 2000). For the measurement of cytokine (TNF-α) and chemokine (MIP-1α and KC) release from mouse pMG cultures, we used the luminex 100 IS system and cytokine/chemokine detection kits (Invitrogen) according to manufacturer’s instructions. CSE (100 EU/mL) stimulation served as a positive control. All cytokine/chemokine levels from both N9 and pMG cultures were normalized to the total cell protein measured using the bicinchoninic acid (BCA) protein assay (Sigma).
Flow Cytometry
N9 cells were plated onto polyornithine (Gibco/Invitrogen)-coated 24-well plates at density of 2 × 104 cells/well and grown in MSFM for 48 h. Cells were stimulated with the various preparations for 24 h and then dislodged in PBS containing 2-mM EDTA, pH 7.3 at room temperature, pelleted (200g for 6 min), and resuspended in ice-cold blocking solution [DMEM containing 1:100 dilutions of mouse, rat, and/or hamster sera (Sigma)]. Cells were transferred to a 96-well plate, washed, and stained for 1 h on ice with a 1:100 dilution of R-phycoerythrin (PE) -conjugated hamster anti-mouse CD95 (Fas) or biotin-conjugated rat anti-mouse CD40 antibodies (BD PharMingen, San Diego, CA). Isotype-matched control IgGs (BD Pharmingen) with corresponding detection systems were run in parallel. Strep-tavidin-PE-Cy7 (1:100, 30 min; BD Pharmingen) was used as secondary detection agent for CD40 and its iso-type control. Cells were then washed in ice-cold DMEM and resuspended in PBS. Analysis was performed on a FACScan flow cytometer (BD Biosciences) using Flojo™ software (Treestar, Ashland, OR). Specific binding was determined for each treatment group by subtracting the median fluorescent intensity of each isotype control from that of its corresponding specific antibody. Numerical data are given as the mean ± S.E.M. of the normalized specific binding median values from each independent experiment. All experiments were carried out in triplicate. Mouse pMG were processed for flow cytometry in a similar manner as earlier, except for the following: (i) cells were seeded into 24-well plates at a density of 0.5–1.0 × 105 cells/well, (ii) 2-ng/mL recombinant mouse macrophage colony stimulating factor (R&D Systems) was added during serum starvation, and (iii) pMG were dislodged from wells with 0.25% trypsin/PBS/2-mM EDTA (30 min at 37°C).
LPS (Endotoxin) Level Determination
LPS levels in all preparations were quantified using the pyrochrome® kit (Associates of Cape Cod), an endpoint chromogenic variant of the Limulus amoebocyte lysate (LAL) assay, as per manufacturer’s instructions. The LAL test is specific and highly sensitive with a detection limit (in our hands) of 0.005–0.01 EU/mL. It is approved by the FDA for end-product testing of human injectable drugs including biological products. A standard curve consisting of measured optical density plotted against known amounts of CSE was used to determine concentrations in the specimens. Indicated samples (Table 1) were denatured by boiling before LPS level determination to exclude false positive (because of serine protease activity) or false negative (because of protease inhibitor activity) results. LAL assays were also performed using Glucashield™ buffer (Associates of Cape Cod) as per manufacturer’s instructions to minimize the potential inhibiting/enhancing effects of β-d-glucans. A 0.4-EU/mL CSE “spike” positive control was included for each preparation to determine the presence or absence of LAL inhibitors in the sample. LPS levels in samples are expressed as endotoxin units (EU) per either unit (U) of enzyme activity or nanomole (nmol) of sample.
Statistics
Statistical evaluation was carried out using PRISM software (GraphPad, San Diego, CA). Comparisons were made using two-way ANOVA test with Bonferroni’s post-test. P < 0.05 was considered to be significant. Data are given as mean ± S.E.M.
RESULTS
Screening and Quantification of LPS Levels in Preparations Used in Microglia Research
We used an endpoint chromogenic version of the LAL assay to systematically screen and quantify LPS levels in a broad range of pharmaceutical- and commercial-grade proteins, peptides, lipids, and pharmacological inhibitors commonly used in microglia research (Table 1). We found that two pharmaceutical-grade (cGMP-compliant) human recombinant proteins, α-thrombin (rh-thr) and antithrombin had undetectable levels of LPS. As predicted, both samples required boiling before LAL assay to obtain accurate readings, because the serine protease activity in unboiled α-thrombin and the protease inhibitor activity in unboiled antithrombin induced artificially elevated readings and CSE “spike” control inhibition, respectively. The commercial-grade recombinant proteins we tested included one protease, human trypsin, which had a low but detectable level of LPS and one cytokine, rm-IFN-γ, which had an undetectable LPS level. Among commercial-grade, nonrecombinant, plasma/serum-derived proteins tested, significant (and in some cases strikingly high) LPS levels were found in multiple α-thrombin, α2-macroglobulin, and albumin preparations as well as in bovine immunoglobulin M (IgM). Among commercial-grade, nonrecombinant, nonplasma/serum-derived proteins, high levels of LPS were found in a trinitrophenyl conjugate of hen egg white-derived ovalbumin (tnp-Oval)—a derivative frequently used for immune complex studies. Hybridoma- and myeloma-derived Ig preparations were also found to have significant LPS levels (including one “no azide/low endotoxin” designated IgM product). We found significant LPS contamination levels in some commercial-grade synthetic bioactive phospholipids including one lysophosphatidic acid-18:1-oleoyl preparation and one (of two tested lots) of a sphingosine-1-phosphate preparation. Similarly, among commercial-grade synthetic peptides, two mouse proteinase-activated receptor (PAR) activating peptides and one human amyloid β-protein (1–40) preparation had functionally significant LPS levels. In contrast, none of the synthetic pharmacological inhibitors tested had significant LPS levels.
Effect of PMX on Factor-Induced Surface Antigen Regulation in Microglia
To determine the functional significance of the LPS contamination found in many of the factors in the above screen, we selected eight proteins [one pharmaceutical-grade recombinant and seven commercial-grade (one recombinant, one plasma-, four serum-, and one egg white-derived)] to test for their ability to induce changes in N9 microglial cell surface antigen expression in the absence or presence of the LPS inhibitor PMX at 10 μg/mL. Dosages were selected to be within the range used in previously reported results (Badie et al., 2000; Hooper et al., 2005; Jarvis et al., 1999; Ryu et al., 2000; Suo et al., 2002; Weinstein et al., 2005). CD95 (Fas) a member of the TNF receptor family is a key microglial surface antigen and is upregulated in a variety of neuroinflammatory conditions (Choi and Benveniste, 2004; Lee et al., 2000). We found that all eight factors tested induced significant increases in CD95 (Fas) expression in the absence of PMX, but that PMX reduced these responses significantly in four of the six commercial-grade plasma/serum/egg white-derived (pb-thr, sh-α2M-5, sh-α2M-7, tnp-Oval) but neither of the two recombinant (rh-thr and rm-IFNγ) preparations (Fig. 1A). The magnitude of these reductions varied, but was quite substantial: 75% for pb-thr, 76% for sh-α2M-5, 74% for sh-α2M-7, and 92% for tnp-Oval. In all four of these cases, the presence of PMX inhibited the factor-induced CD95(Fas) upregulation enough to render these values no longer significantly different (statistically) from the baseline PMX-alone control, although pb-thr, sh-α2M-7, and tnp-Oval all appeared to have some residual PMX-resistant response. The remaining two commercial-grade serum-derived preparations (both rat albumins) also appeared to be inhibited by PMX (16% for sr-Alb-efaf and 36% for sr-Alb-FV); however, these reductions did not reach statistical significance and residual PMX-resistant responses were substantial. As predicted, PMX eliminated >98% of the CD95(Fas) upregulation induced by 100 EU/mL of CSE. Flow cytometric analysis of surface antigen expression in pMG is limited, because of strong cell adherence, and results in low yield. However, as a proof of principle, we examined the effects of two specific factors (sh-α2M-7 and sr-Alb-FV) on CD95(Fas) expression in mouse pMG in the absence or presence of PMX (Fig. 2A). Similar to the results in the N9 cell line, both factors induced robust increases in CD95(Fas) expression in pMG. PMX completely eliminated this effect in the case of sh-α2M-7 and (nonsignificantly) reduced the increase induced by sr-Alb-FV. As predicted, PMX also completely eliminated the CD95(Fas) expression increase induced by CSE in pMG.
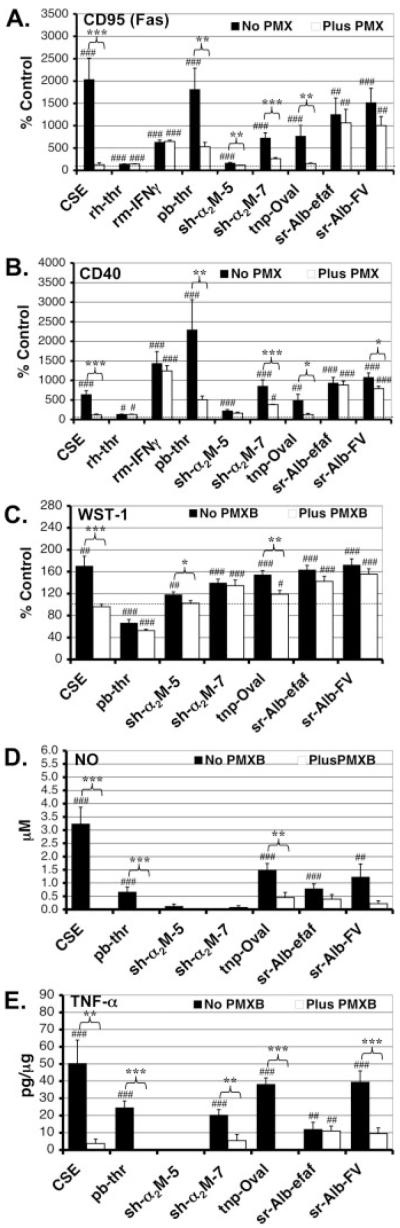
Fig. 1.
Comparison between effects of different commercial- and pharmaceutical-grade preparations in the absence or presence of PMX on a number of microglial activation parameters in the N9 cell line. N9 cells were either unstimulated or stimulated with 100 EU/mL of control standard endotoxin (CSE), 100-U/mL recombinant human α-thrombin (rh-thr 100), 10-U/mL recombinant mouse interferon-γ (rm-IFNγ), 100-U/mL plasma-derived bovine α-thrombin (pb-thr), 100-nM serum-derived human α2-macroglobulin from supplier #5 (sh-α2M-5), 100-nM serum-derived human α2-macroglobulin from supplier #7 (sh-α2M-7), 1-μM 2,4,6-trinitrophenol-conjugated egg-white-derived chicken ovalbumin (tnp-Oval), 15.4-μM (1 mg/mL) serum-derived rat serum albumin-essentially fatty acid free (sr-Alb-efaf), and/or 15.4-μM (1 mg/mL) serum-derived rat albumin-fraction V powder (sr-Alb-FV) as indicated in the absence or presence of PMX (10 μg/mL). Cell surface expression of CD95 (Fas) (A) and CD40 (B) was assessed 24 h later by flow cytometry. Values for the treatment groups that did and did not include PMX were normalized to their respective controls. Means ± S.E.M. for these values from at least three separate experiments are presented. *P < 0.05, **P < 0.01, ***P < 0.001 vs. corresponding preparation treatment without PMX. #P < 0.05, ##P < 0.01, ###P < 0.001 vs. unstimulated control or PMX alone control (dotted line). Prenormalization mean absolute fluorescent intensity units ± S.E.M. for unstimulated cells were 15.9 ± 2.5 and 15.9 ± 2.8 in the absence or presence of PMX, respectively, for CD95(Fas) and 7.4 ± 1.2 and 5.5 ± 1.1 for CD40. Metabolic activity/proliferation (C), NO production (D), and TNF-α release (E) were also assessed 24 h following stimulation using the WST-1 assay, Griess reagent and cytokine ELISA, respectively. Values for the treatment groups that did and did not include PMX were normalized to their respective controls. Data are expressed as mean ± S.E.M. n ≥ 9, exp ≥ 3. Prenormalization mean absolute values for unstimulated cells were 0.32 ± 0.015 and 0.34 ± 0.032 absorbance units (C), 0.0 and 0.0 μM NO (D), and 0.0 and 1.33 ± 1.33 pg TNF-α/μg total protein (E) in the absence or presence of PMX, respectively.
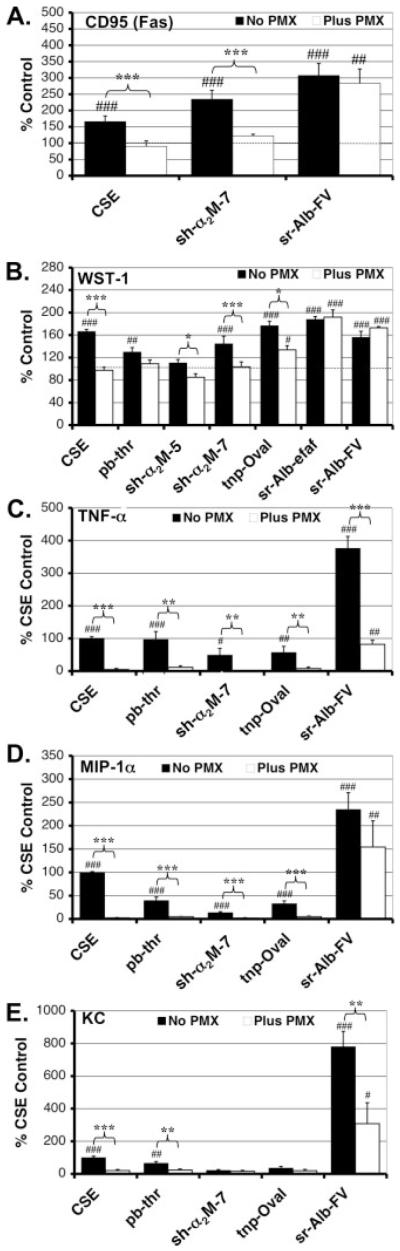
Fig. 2.
Comparison between effects of different commercial-grade preparations in the absence or presence of PMX on a number of microglial activation parameters in mouse primary microglia (pMG). Mouse pMG were either unstimulated or stimulated with 100 EU/mL of control standard endotoxin (CSE), 100-U/mL plasma-derived bovine α-thrombin (pb-thr), 100-nM serum-derived human α2macroglobulin from supplier #5 (sh-α2M-5), 100-nM serum-derived human α2macroglobulin from supplier #7 (sh-α2M-7), 1-μM 2,4,6-trinitrophenol-conjugated egg-white-derived chicken ovalbumin (tnp-Oval), 15.4-μM (1 mg/mL) serum-derived rat serum albumin-essentially fatty acid free (sr-Alb-efaf) and/or 15.4-μM (1 mg/mL) serum-derived rat albumin-fraction V powder (sr-Alb-FV) as indicated in the absence or presence of PMX (10 μg/mL). Cell surface expression of CD95 (Fas) (A) was assessed 24 h later by flow cytometry. Values for the treatment groups that did and did not include PMX were normalized to their respective controls. Means ± S.E.M. for these values from at least three separate experiments are presented. Statistical significance indicators are as in Figure 1. Prenormalization mean absolute fluorescent intensity units ± S.E.M. for unstimulated cells were 9.1 ± 1.8 and 8.8 ± 1.2 in the absence or presence of PMX, respectively. Metabolic activity/proliferation (B), TNF-α (C), MIP-1α (D), and KC (E) release were also assessed 24 h following stimulation using the WST-1 assay (B) and luminex system (C–E). Values for the treatment groups that did and did not include PMX were normalized to either their respective unstimulated (B) or CSE (C–E) controls. Data are expressed as mean ± S.E.M. n ≥ 4, exp ≥ 2. Prenormalization mean absolute values for unstimulated cells were 0.23 ± 0.008 and 0.22 ± 0.012 absorbance units (B), 5.27 ± 3.13 and 5.68 ± 3.38 pg TNF-α/μg total protein (C), 1.71 ± 0.50 and 2.02 ± 0.49 pg MIP-1α/μg total protein (D), 13.4 ± 7.19 and 20.7 ± 7.01 pg KC/μg total protein (E) in the absence or presence of PMX, respectively.
A similar pattern emerged for the CD40 regulation studies in the N9 cell line (Fig. 1B). All eight proteins tested induced significant increases in CD40 expression in the absence of PMX. PMX significantly reduced the effect for four of the six commercial-grade, plasma/serum/egg white-derived proteins (82% for pb-thr, 62% for sh-α2M-7, 93% for tnp-Oval, and 30% for sr-Alb-FV). In the case of pb-thr and tnp-Oval, PMX reduced the responses to levels not statistically different from the baseline PMX-alone control although, as seen with CD95(Fas), these preparations did appear to have some residual PMX-resistant CD40-inducing response. PMX also appeared to weakly inhibit the effects of rm-IFNγ, sh-α2M-5 and sr-Alb-efaf, but none of these reductions reached statistical significance. PMX had no impact on the small, but statistically significant, CD40 upregulation induced by pharmaceutical-grade rh-thr. Similar to the results seen for CD95(Fas), PMX eliminated > 96% of the CD40 upregulation induced by 100 EU/mL of CSE.
Effect of PMX on Factor-Induced Microglial Metabolic Activity/Proliferation, NO Production, and Cytokine/Chemokine Release
To characterize a larger spectrum of functional effects that could be attributed to LPS contamination we investigated several other commonly reported parameters of microglial activation including metabolic activity/proliferation, NO production, and cytokine/chemokine release. We treated N9 microglial cells with the same six commercial-grade plasma/serum/egg white-derived protein factors (pb-thr, sh-α2M-5, sh-α2M-7, sr-Alb-efaf, sr-Alb-FV, and tnp-Oval) used in the cell surface antigen regulation experiments above in the absence or presence of PMX. In the N9 cell line, all of the factors except pb-thr induced a significant increase in metabolic activity/proliferation in the absence of PMX (Fig. 1C). In contrast, pb-thr induced a significant decrease as has been reported previously (Weinstein et al., 2005). PMX significantly inhibited the effect in the case of sh-α2M-5 (83%) and tnp-Oval (65%). In the presence of PMX, metabolic activity/proliferation levels induced by sh-α2M-5 did not significantly differ from baseline. PMX partially blunted the effect of sh-α2M-7 (15%), sr-Alb-efaf (33%), and sr-Alb-FV (22%) as well, but these decreases were not statistically significant. In the case of pb-thr, PMX nonsignificantly enhanced (by 38%) the reduction in metabolic activity/proliferation levels seen in the absence of inhibitor. Similar to the results for surface antigen expression, PMX completely eliminated the large CSE-induced increase in metabolic activity/proliferation. We next examined the profile of effects induced by the panel of factors (±PMX) on metabolic activity/proliferation in pMG (Fig. 2B). Overall, results were similar to those seen in N9 cells. Five of the six factors (all except sh-α2M-5) induced significant increases in metabolic activity/proliferation in the absence of PMX. PMX completely eliminated and significantly reduced the increases mediated by sh-α2M-7 and tnp-Oval, respectively. PMX also completely blocked the CSE induced response in pMG.
Four of the factors (pb-thr, tnp-Oval, sr-Alb-efaf, and sr-Alb-FV) induced a significant increase in NO release in the N9 cell line in the absence of PMX, whereas neither of the two sh-α2M preparations did (Fig. 1D). PMX significantly inhibited this effect in the case of pb-thr (100%) and tnp-Oval (70%). It also appeared to strongly inhibit the responses to sr-Alb-FV (by 82%) and sr-Alb-efaf (by 50%), although these decreases did not reach statistical significance because of the high variability in the size of the responses elicited. In the presence of PMX, NO levels induced by these four factors no longer significantly differed from baseline. The substantial CSE-induced NO response was completely eliminated by PMX. In pMG, none of the factors induced significant NO responses in the absence or presence of PMX (data not shown).
TNF-α release was also induced by five of the six factors tested in the absence of PMX (only sh-α2M-5 did not induce its release) in the N9 cell line (Fig. 1E). Addition of PMX reduced TNF-α release induced by four of the five proteins: pb-thr (100%), sh-α2M-7 (73%), tnp-Oval (100%), and sr-Alb-FV (76%). For these four protein preparations, the residual TNF-α release induced in the presence of PMX no longer differed significantly from baseline. The small but statistically significant TNF-α release response to sr-Alb-efaf was unaffected by PMX. Again, as expected, PMX dramatically reduced (by 92%) the CSE-induced TNF-α release. In pMG, all factors tested (and positive control CSE) induced robust increases in TNF-α release in the absence of PMX (Fig. 2C). PMX either eliminated (in the case of CSE, pb-thr, sh-α2M-7, and tnp-Oval) or significantly reduced (for sr-Alb-FV) these effects. Also in pMG, the profile of effects induced by the various factor preparations on chemokine MIP-1α (Fig. 2D) and KC (Fig. 2E) release in the absence and presence of PMX was strikingly similar to that of TNF-α (although in the case of KC, neither sh-α2M-7 nor tnp-Oval induced any baseline increases). Notably, for both chemokines, PMX either eliminated or markedly reduced all factor-induced increases.
DISCUSSION
This study is the first systematic investigation of the extent and functional significance of LPS/endotoxin levels in major classes of research reagents relevant to the field of neuroinflammation. The results presented earlier demonstrate that LPS is a frequent and functionally significant contaminant in many commercial-grade preparations of proteins, peptides, and bioactive phospholipids used commonly in research on microglial activation, neural cell biology, and neurodegeneration. Recently, we have suggested that high molecular weight proteins contaminating some commercial-grade, plasma-derived thrombin preparations could potentially confound interpretation of studies characterizing thrombin-induced microglial responses (Hanisch et al., 2004; Weinstein et al., 2005). We also noted that some of these thrombin preparations contained detectable levels of LPS, which could further complicate interpretation of results (Möller et al., 2006; Weinstein et al., 2005). We initiated the current study as a follow-up to the latter observation. Interestingly, the findings not only confirmed the functionally significant levels of LPS contamination in the plasma-derived thrombin preparations, but also demonstrated the scope and severity of the LPS contamination problem for a host of other factors and compounds relevant to CNS pathophysiology. The results are also consistent with several isolated findings from the immunology literature describing the effects of LPS contamination on macrophage/monocyte activation by a few selected commercial-grade protein preparations including heat-shock protein-60 (Gao and Tsan, 2003b), −70 (Gao and Tsan, 2003) and the lectin pokeweed mitogen (Yang et al., 2006).
To highlight the functional significance of the LPS contamination levels we found, it is important to point out that microglial cell surface antigen expression was significantly regulated by as little as 0.04 EU/mL of CSE, while other parameters of microglial activation including proliferation, NO production, and cytokine release were less sensitive to LPS (>0.4 EU/mL) (data not shown). Taking into account typical working concentrations for each particular reagent tested, the endotoxin contamination levels in a number of factors listed exceed one or more of these threshold values (see far right column of Table 1). However, potential costimulatory effects on microglia (by LPS in combination with factor of interest), variation in LPS chemical structure/composition, and differences in bacterial serotype from which a particular LPS molecule is derived make it difficult to accurately predict the impact of contaminating LPS on cellular responses by quantifying endotoxin levels alone. For this reason, we chose to demonstrate the functional significance of the LPS contamination levels we found in selected factors by directly measuring a broad spectrum of factor-induced microglia-activation parameters in the absence or presence of the potent LPS inhibitor PMX (Figs. 1 and 2). Use of the N9 microglial cell line allowed us to efficiently screen a number of factors for functional LPS contamination (see Fig. 1). However, to ensure that our findings were not an artifactual response of this particular cell line, we confirmed our results in mouse pMG using a more limited subset of factors (see Fig. 2). Overall, responses to the LPS-contaminated factors and the impact of PMX were just as striking (if not more so) in pMG as in the N9 cell line. Although PMX alone has been reported to influence some cellular responses in the absence of LPS (Valentinis et al., 2005), we found that the inhibitor alone at the concentration used had no significant impact on the parameters measured here (see legends for Figs. 1 and 2). LPS is classically described as a heat-resistant molecule and boiling has been used in the literature to sort out the effects of contaminating LPS on cellular responsiveness (Morrison and Ryan, 1979; Rietschel et al., 1993). However, recent studies (Gao and Tsan, 2003a; Gao et al., 2006) have cast significant doubt on the validity of this method and we chose not to pursue this experimental route.
A review of Table 1 demonstrates that the highest levels of LPS were found in commercial-grade, nonrecombinant protein preparations including α-thrombin, α2-macroglobulin, albumin, and immunoglobulin. Interestingly, PMX markedly reduced, but in some cases did not eliminate, cellular responses to these preparations. This finding is consistent with what has been seen with several heat-shock protein preparations on peripheral immune cells of myeloid origin (Tsan and Gao, 2004). The remaining PMX-resistant responses reflect either true receptor-mediated factor-induced effects, the presence of non-LPS cell-activating contaminants or some combination of both. In the case of pb-thr, there is strong evidence for the combination (Hanisch et al., 2004; Weinstein et al., 2005). Microglia are known to express PARs (Balcaitis et al., 2003; Suo et al., 2002, 2003) and as seen here (see Fig. 1) and previously (Weinstein et al., 2005), pharmaceutical-grade rh-thr induces small increases in CD95(Fas) and CD40 expression in the N9 cell line. However, other microglia-activating properties of pb-thr including cytokine release are mediated by nonthrombin high molecular weight protein contaminants (Hanisch et al., 2004). The Limulus assay detection of significant quantities of LPS contamination in the pb-thr and the effect of PMX on pb-thr-induced microglial responses indicate that endotoxin is an additional functionally important contaminant in this preparation.
Microglial cell responses to the proteinase inhibitor α2-macroglobulin have not been previously reported, although microglia are known to express low density lipoprotein receptor related protein, a scavenger receptor with high affinity for α2-macroglobulin–proteinase complexes (Marzolo et al., 2000). Such complexes have been shown to induce phospholipase A2 activity in macrophages (Misra and Pizzo, 2000). In the present study, sh-α2M-7 had considerably more LPS contamination than sh-α2M-5 and, as expected, tended to induce larger responses that were more sensitive to PMX. As seen for pb-thr, PMX substantially reduced many α2-macroglobulin-induced responses but left a significant PMX-resistant component remaining in some cases. The PMX-resistant effects could be due to either receptor-mediated actions of α2-macroglobulin (either alone or in complex with secreted proteinase) and/or non-LPS contaminants in the commercial-grade preparations.
Both sr-Alb-efaf and sr-Alb-FV have been reported to induce calcium transients and proliferation in both N9 cells and pMG, but not in macrophages (Hooper et al., 2005). However, LPS levels in the preparations were not quantified and the potential contribution of LPS to the albumin-induced responses was not reported (Hooper et al., 2005). The present data show that the sr-Alb-FV preparation contains significant levels of LPS, whereas the sr-Alb-efaf preparation does not. The present data also indicate that the albumin preparation-induced proliferation findings were largely resistant to PMX and therefore not due (at least primarily) to LPS, corroborating the interpretation of the results in the prior report (Hooper et al., 2005). At the same time, our data also indicate that a component of the sr-Alb-FV-induced microglial responses (including CD40 regulation, NO production, and TNF-α/KC release) are due to LPS contamination. Nevertheless, both rat albumin preparations exhibited a substantial PMX-resistant component. To our knowledge, neither albumin-induced microglial surface antigen regulation nor cytokine/chemokine release has been previously reported. In contrast, superoxide release from cultured microglia by serum albumin preparations has been previously reported (Nakamura et al., 2000; Si et al., 1997). Similar to our results, the former study (Si et al., 1997) showed that a component of the superoxide response was both resistant to PMX and independent of fatty acid content in the preparation. These findings exclude LPS or protein bound fatty acids as the sole mediator of the response. The molecular mechanisms underlying the PMX-resistant microglial responses to serum-derived albumins remain to be elucidated.
Experiments in the present study were done in the absence of serum; however, serum is often used in studies characterizing microglial cell activation. LPS is known to bind to LPS-binding protein in serum and the complex has greatly enhanced biological activity mediated through CD14 and the TLR4/MD-2 receptor complex (Palsson-McDermott and O’Neill, 2004). Thus, the biological effects of LPS contamination in preparations added to microglia cultured in the presence of serum could be even greater than seen here.
The results presented earlier demonstrate that LPS is a frequent and functionally significant contaminant in many commercial-grade preparations of proteins, peptides, and bioactive phospholipids of interest in microglia research and more broadly in the neuroinflammation field. In light of the findings presented here for microglia, and consistent with what has been previously suggested in the macrophage/monocyte literature (Gao and Tsan, 2003b), we propose that the following considerations be taken into account for future studies involving microglia. First, whenever possible, pharmaceutical-grade, endotoxin-free reagents be used. Second, the endotoxin levels be quantified for all cell-stimulating reagents, particularly plasma/serum-derived proteins. Third, the effect of PMX on factor-stimulated (and control/unstimulated) microglial cell responses be examined. Finally, the previous reports detailing microglial activation by commercial-grade factors (particularly, plasma/serum-derived proteins), which have not taken possible LPS contamination into account be cautiously reinterpreted.
ACKNOWLEDGMENTS
The authors thank Matthew Zhang for technical support. They also thank ZymoGenetics, Seattle, WA, USA and GTC Biotherapeutics, Framingham, MA, USA for the generous gifts of pharmaceutical-grade recombinant human α-thrombin and antithrombin, respectively.
Grant sponsor: NIH/NINDS; Grant numbers: NS44337, NS047309; Grant sponsor: National Multiple Sclerosis Society; Grant number: PP1134.
Abbreviations
- BAPTA
1,2-bis-(o-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid tetra-(acetoxymethyl) ester
- BCA
bicinchoninic acid
- cGMP
FDA’s current Good Manufacturing Practice
- CNS
central nervous system
- CSE
control standard endotoxin
- DMEM
Dulbecco’s modified Eagle’s medium
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- FDA
United States Food and Drug Administration
- FITC
fluorescein isothiocyanate
- Ig
immunoglobulin
- LAL
Limulus amoebocyte lysate
- LBP
lipopolysaccharide binding protein
- LPA
lysophosphatidic acid-18:1-oleoyl
- LPS
lipopolysaccharide/endotoxin
- MAP
mitogen activated kinase
- MSFM
macrophage serum-free medium
- NO
nitric oxide
- P/S
penicillin/streptomycin
- PAMP
pathogen-associated molecular pattern
- PAR
proteinase-activated receptor
- PBS
phosphate buffered saline
- pb-thr
plasma-derived bovine α-thrombin
- PE
R-phycoerythrin
- pMG
primary microglia
- PMX
polymyxin B
- PPACK
d-phenylalanyl-l-prolyl-l-arginine chloromethyl ketone dihydrochloride
- rh-thr
recombinant human α-thrombin
- rm-IFN-γ
recombinant mouse interferon-γ
- S-1-P
sphingosine-1-phosphate
- sh-α2M
serum-derived human α2-macroglobulin
- sr-Alb-efaf
serum-derived rat albumin-essentially fatty acid free
- sr-Alb-FV
serum-derived rat albumin-fraction V
- TLR4
Toll-like receptor-4
- TNF-α
tumor necrosis factor-α
- tnp-Oval
2,4,6-trinitrophenyl-conjugated egg-white-derived chicken ovalbumin
REFERENCES
- Badie B, Schartner J, Vorpahl J, Preston K. Interferon-gamma induces apoptosis and augments the expression of Fas and Fas ligand by microglia in vitro. Exp Neurol. 2000;162:290–296. doi: 10.1006/exnr.1999.7345. [DOI] [PubMed] [Google Scholar]
- Balcaitis S, Xie Y, Weinstein JR, Andersen H, Hanisch UK, Ransom BR, Möller T. Expression of proteinase-activated receptors in mouse microglial cells. Neuroreport. 2003;14:2373–2377. doi: 10.1097/00001756-200312190-00017. [DOI] [PubMed] [Google Scholar]
- Bausinger H, Lipsker D, Ziylan U, Manie S, Briand JP, Cazenave JP, Muller S, Haeuw JF, Ravanat C, de la Salle H, Hanau D. Endotoxin-free heat-shock protein 70 fails to induce APC activation. Eur J Immunol. 2002;32:3708–3713. doi: 10.1002/1521-4141(200212)32:12<3708::AID-IMMU3708>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25:1634–1644. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco F, Ceruti S, Colombo A, Fumagalli M, Ferrari D, Pizzirani C, Matteoli M, Di Virgilio F, Abbracchio MP, Verderio C. A role for P2x in microglia proliferation. J Neurochem. 2006;99:745–758. doi: 10.1111/j.1471-4159.2006.04101.x. [DOI] [PubMed] [Google Scholar]
- Cai Z, Pang Y, Lin S, Rhodes PG. Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res. 2003;975:37–47. doi: 10.1016/s0006-8993(03)02545-9. [DOI] [PubMed] [Google Scholar]
- Choi C, Benveniste EN. Fas ligand/Fas system in the brain: Regulator of immune and apoptotic responses. Brain Res Brain Res Rev. 2004;44:65–81. doi: 10.1016/j.brainresrev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- D’Aversa TG, Eugenin EA, Berman JW. NeuroAIDS: Contributions of the human immunodeficiency virus-1 proteins Tat and gp120 as well as CD40 to microglial activation. J Neurosci Res. 2005;81:436–446. doi: 10.1002/jnr.20486. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Dixon DR, Darveau RP. Lipopolysaccharide heterogeneity: Innate host responses to bacterial modification of lipid a structure. J Dent Res. 2005;84:584–595. doi: 10.1177/154405910508400702. [DOI] [PubMed] [Google Scholar]
- FDA . Good Manufacturing Practice. Rockville, MD: 2002. http://www.fda.gov/cdrh/comp/gmp.html [Google Scholar]
- Ganter S, Northoff H, Mannel D, Gebicke-Harter PJ. Growth control of cultured microglia. J Neurosci Res. 1992;33:218–230. doi: 10.1002/jnr.490330205. [DOI] [PubMed] [Google Scholar]
- Gao B, Tsan MF. Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumor necrosis factor alpha release by murine macrophages. J Biol Chem. 2003a;278:174–179. doi: 10.1074/jbc.M208742200. [DOI] [PubMed] [Google Scholar]
- Gao B, Tsan MF. Recombinant human heat shock protein 60 does not induce the release of tumor necrosis factor alpha from murine macrophages. J Biol Chem. 2003b;278:22523–22529. doi: 10.1074/jbc.M303161200. [DOI] [PubMed] [Google Scholar]
- Gao B, Wang Y, Tsan MF. The heat sensitivity of cytokine-inducing effect of lipopolysaccharide. J Leukoc Biol. 2006;80:359–366. doi: 10.1189/jlb.1205738. [DOI] [PubMed] [Google Scholar]
- Gebicke-Haerter PJ, Bauer J, Schobert A, Northoff H. Lipopolysaccharide-free conditions in primary astrocyte cultures allow growth and isolation of microglial cells. J Neurosci. 1989;9:183–194. doi: 10.1523/JNEUROSCI.09-01-00183.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregerson DS, Sam TN, McPherson SW. The antigen-presenting activity of fresh, adult parenchymal microglia and perivascular cells from retina. J Immunol. 2004;172:6587–6597. doi: 10.4049/jimmunol.172.11.6587. [DOI] [PubMed] [Google Scholar]
- Hanisch U-K, van Rossum D, Xie Y, Gast K, Misselwitz R, Ariola S, Goldsteins G, Koistinaho J, Kettenmann H, Möller T. The microglia-activating potential of thrombin: The protease is not involved in the induction of proinflammatory cytokines and chemokines. J Biol Chem. 2004;279:51880–51887. doi: 10.1074/jbc.M408318200. [DOI] [PubMed] [Google Scholar]
- Herber DL, Maloney JL, Roth LM, Freeman MJ, Morgan D, Gordon MN. Diverse microglial responses after intrahippocampal administration of lipopolysaccharide. Glia. 2006;53:382–391. doi: 10.1002/glia.20272. [DOI] [PubMed] [Google Scholar]
- Hooper C, Taylor DL, Pocock JM. Pure albumin is a potent trigger of calcium signalling and proliferation in microglia but not macrophages or astrocytes. J Neurochem. 2005;92:1363–1376. doi: 10.1111/j.1471-4159.2005.02982.x. [DOI] [PubMed] [Google Scholar]
- Jacobs DM, Morrison DC. Inhibition of the mitogenic response to lipopolysaccharide (LPS) in mouse spleen cells by polymyxin B. J Immunol. 1977;118:21–27. [PubMed] [Google Scholar]
- Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology: The immune system in health and disease. Garland Science/Taylor & Francis; New York/London: 2005. [Google Scholar]
- Jarvis JN, Xu C, Wang W, Petty HR, Gonzalez M, Morssy N, Waxman F, del Rio A Quintero. Immune complex size and complement regulate cytokine production by peripheral blood mononuclear cells. Clin Immunol. 1999;93:274–282. doi: 10.1006/clim.1999.4792. [DOI] [PubMed] [Google Scholar]
- Jekabsone A, Mander PK, Tickler A, Sharpe M, Brown GC. Fibrillar beta-amyloid peptide Abeta1-40 activates microglial proliferation via stimulating TNF-alpha release and H2O2 derived from NADPH oxidase: A cell culture study. J Neuroinflammation. 2006;3:24. doi: 10.1186/1742-2094-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Whang SY, Woo MS, Park JS, Kim WK, Han IO. Sodium butyrate suppresses interferon-gamma-, but not lipopolysaccharide-mediated induction of nitric oxide and tumor necrosis factor-alpha in microglia. J Neuroimmunol. 2004;151:85–93. doi: 10.1016/j.jneuroim.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Kloss CU, Bohatschek M, Kreutzberg GW, Raivich G. Effect of lipopolysaccharide on the morphology and integrin immunoreactivity of ramified microglia in the mouse brain and in cell culture. Exp Neurol. 2001;168:32–46. doi: 10.1006/exnr.2000.7575. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lee S. Toll-like receptors and inflammation in the CNS. Curr Drug Targets Inflamm Allergy. 2002;1:181–191. doi: 10.2174/1568010023344698. [DOI] [PubMed] [Google Scholar]
- Lee SC, Liu W, Brosnan CF, Dickson DW. GM-CSF promotes proliferation of human fetal and adult microglia in primary cultures. Glia. 1994;12:309–318. doi: 10.1002/glia.440120407. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Zhou T, Choi C, Wang Z, Benveniste EN. Differential regulation and function of Fas expression on glial cells. J Immunol. 2000;164:1277–1285. doi: 10.4049/jimmunol.164.3.1277. [DOI] [PubMed] [Google Scholar]
- Marzolo MP, von Bernhardi R, Bu G, Inestrosa NC. Expression of alpha(2)-macroglobulin receptor/low density lipoprotein receptor-related protein (LRP) in rat microglial cells. J Neurosci Res. 2000;60:401–411. doi: 10.1002/(SICI)1097-4547(20000501)60:3<401::AID-JNR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Melchior B, Puntambekar SS, Carson MJ. Microglia and the control of autoreactive T cell responses. Neurochem Int. 2006;49:145–153. doi: 10.1016/j.neuint.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez Iglesias B, Cerase J, Ceracchini C, Levi G, Aloisi F. Analysis of B7-1 and B7-2 costimulatory ligands in cultured mouse microglia: Upregulation by interferon-gamma and lipopolysaccharide and downregulation by interleukin-10, prostaglandin E2 and cyclic AMP-elevating agents. J Neuroimmunol. 1997;72:83–93. doi: 10.1016/s0165-5728(96)00155-5. [DOI] [PubMed] [Google Scholar]
- Misra UK, Pizzo SV. Cytosolic phospholipase A(2) activity associated with nuclei is not inhibited by arachidonyl trifluoromethyl ketone in macrophages stimulated with receptor-recognized forms of alpha(2)-macroglobulin. Arch Biochem Biophys. 2000;379:153–160. doi: 10.1006/abbi.2000.1878. [DOI] [PubMed] [Google Scholar]
- Morrison DC, Jacobs DM. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976a;13:813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Morrison DC, Jacobs DM. Inhibition of lipopolysaccharide-initiated activation of serum complement by polymyxin B. Infect Immun. 1976b;13:298–301. doi: 10.1128/iai.13.1.298-301.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison DC, Ryan JL. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Möller T, Contos JJ, Musante DB, Chun J, Ransom BR. Expression and function of lysophosphatidic acid receptors in cultured rodent microglial cells. J Biol Chem. 2001;276:25946–25952. doi: 10.1074/jbc.M102691200. [DOI] [PubMed] [Google Scholar]
- Möller T, Hanisch UK, Ransom BR. Thrombin-induced activation of cultured rodent microglia. J Neurochem. 2000;75:1539–1547. doi: 10.1046/j.1471-4159.2000.0751539.x. [DOI] [PubMed] [Google Scholar]
- Möller T, Kann O, Prinz M, Kirchhoff F, Verkhratsky A, Kettenmann H. Endothelin-induced calcium signaling in cultured mouse microglial cells is mediated through ETB receptors. Neuroreport. 1997a;8:2127–2131. doi: 10.1097/00001756-199707070-00008. [DOI] [PubMed] [Google Scholar]
- Möller T, Nolte C, Burger R, Verkhratsky A, Kettenmann H. Mechanisms of C5a and C3a complement fragment-induced [Ca2+]i signaling in mouse microglia. J Neurosci. 1997b;17:615–624. doi: 10.1523/JNEUROSCI.17-02-00615.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller T, Weinstein JR, Hanisch UK. Activation of microglial cells by thrombin: Past, present, and future. Semin Thromb Hemost. 2006;32(Suppl 1):69–76. doi: 10.1055/s-2006-939556. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Tohyama Y, Kohsaka S, Kurihara T. Ceramide activates microglia to enhance the production/secretion of brain-derived neurotrophic factor (BDNF) without induction of deleterious factors in vitro. J Neurochem. 2002;80:697–705. doi: 10.1046/j.0022-3042.2001.00752.x. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Si QS, Takaku T, Kataoka K. Identification of a peptide sequence in albumin that potentiates superoxide production by microglia. J Neurochem. 2000;75:2309–2315. doi: 10.1046/j.1471-4159.2000.0752309.x. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Northoff H, Bauer J, Schobert A, Flegel WA, Gebicke-Haerter PJ. Lipopolysaccharide (LPS)-free conditions allow growth and purification of postnatal brain macrophages (microglia) J Immunol Methods. 1989;116:147. doi: 10.1016/0022-1759(89)90324-4. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Palsson-McDermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Fan LW, Zheng B, Cai Z, Rhodes PG. Role of interleukin-6 in lipopolysaccharide-induced brain injury and behavioral dysfunction in neonatal rats. Neuroscience. 2006;141:745–755. doi: 10.1016/j.neuroscience.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Pul R, Nguyen D, Schmitz U, Marx P, Stangel M. Comparison of intravenous immunoglobulin preparations on microglial function in vitro: More potent immunomodulatory capacity of an IgM/IgA-enriched preparation. Clin Neuropharmacol. 2002;25:254–259. doi: 10.1097/00002826-200209000-00005. [DOI] [PubMed] [Google Scholar]
- Rietschel ET, Kirikae T, Schade FU, Ulmer AJ, Holst O, Brade H, Schmidt G, Mamat U, Grimmecke HD, Kusumoto S, Cahringer U. The chemical structure of bacterial endotoxin in relation to bio-activity. Immunobiology. 1993;187:169–190. doi: 10.1016/S0171-2985(11)80338-4. [DOI] [PubMed] [Google Scholar]
- Righi M, Letari O, Sacerdote P, Marangoni F, Miozzo A, Nicosia S. Myc-immortalized microglial cells express a functional platelet-activating factor receptor. J Neurochem. 1995;64:121–129. doi: 10.1046/j.1471-4159.1995.64010121.x. [DOI] [PubMed] [Google Scholar]
- Rosenzweig HL, Lessov NS, Henshall DC, Minami M, Simon RP, Stenzel-Poore MP. Endotoxin preconditioning prevents cellular inflammatory response during ischemic neuroprotection in mice. Stroke. 2004;35:2576–2581. doi: 10.1161/01.STR.0000143450.04438.ae. [DOI] [PubMed] [Google Scholar]
- Ryu J, Pyo H, Jou I, Joe E. Thrombin induces NO release from cultured rat microglia via protein kinase C, mitogen-activated protein kinase, and NF-kappa B. J Biol Chem. 2000;275:29955–29959. doi: 10.1074/jbc.M001220200. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Butovsky O, Bruck W, Hanisch UK. Microglial phenotype: Is the commitment reversible? Trends Neurosci. 2006;29:68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Si QS, Nakamura Y, Kataoka K. Albumin enhances superoxide production in cultured microglia. Glia. 1997;21:413–418. doi: 10.1002/(sici)1098-1136(199712)21:4<413::aid-glia9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Suo Z, Wu M, Ameenuddin S, Anderson HE, Zoloty JE, Citron BA, Andrade-Gordon P, Festoff BW. Participation of protease-activated receptor-1 in thrombin-induced microglial activation. J Neurochem. 2002;80:655–666. doi: 10.1046/j.0022-3042.2001.00745.x. [DOI] [PubMed] [Google Scholar]
- Suo Z, Wu M, Citron BA, Gao C, Festoff BW. Persistent protease-activated receptor 4 signaling mediates thrombin-induced microglial activation. J Biol Chem. 2003;278:31177–31183. doi: 10.1074/jbc.M302137200. [DOI] [PubMed] [Google Scholar]
- Szczepanik AM, Fishkin RJ, Rush DK, Wilmot CA. Effects of chronic intrahippocampal infusion of lipopolysaccharide in the rat. Neuroscience. 1996;70:57–65. doi: 10.1016/0306-4522(95)00296-u. [DOI] [PubMed] [Google Scholar]
- Terrazzino S, Bauleo A, Baldan A, Leon A. Peripheral LPS administrations up-regulate Fas and FasL on brain microglial cells: A brain protective or pathogenic event? J Neuroimmunol. 2002;124:45–53. doi: 10.1016/s0165-5728(02)00013-9. [DOI] [PubMed] [Google Scholar]
- Tsan MF, Gao B. Cytokine function of heat shock proteins. Am J Physiol Cell Physiol. 2004;286:C739–C744. doi: 10.1152/ajpcell.00364.2003. [DOI] [PubMed] [Google Scholar]
- Valentinis B, Bianchi A, Zhou D, Cipponi A, Catalanotti F, Russo V, Traversari C. Direct effects of polymyxin B on human dendritic cells maturation. The role of IkappaB-alpha/NF-kappaB and ERK1/2 pathways and adhesion. J Biol Chem. 2005;280:14264–14271. doi: 10.1074/jbc.M410791200. [DOI] [PubMed] [Google Scholar]
- Wang X, Bae JH, Kim SU, McLarnon JG. Platelet-activating factor induced Ca(21) signaling in human microglia. Brain Res. 1999;842:159–165. doi: 10.1016/s0006-8993(99)01849-1. [DOI] [PubMed] [Google Scholar]
- Weinstein JR, Hong S, Kulman JD, Bishop C, Kuniyoshi J, Andersen H, Ransom BR, Hanisch UK, Möller T. Unraveling thrombin’s true microglia-activating potential: Markedly disparate profiles of pharmaceutical-grade and commercial-grade thrombin preparations. J Neurochem. 2005;95:1177–1187. doi: 10.1111/j.1471-4159.2005.03499.x. [DOI] [PubMed] [Google Scholar]
- Westphal O, Luderitz O, Rietschel ET, Galanos C. Bacterial lipopolysaccharide and its lipid A component: Some historical and some current aspects. Biochem Soc Trans. 1981;9:191–195. doi: 10.1042/bst0090191. [DOI] [PubMed] [Google Scholar]
- Yang JS, Kim HJ, Ryu YH, Yun CH, Chung DK, Han SH. Endotoxin contamination in commercially available pokeweed mitogen contributes to the activation of murine macrophages and human dendritic cell maturation. Clin Vaccine Immunol. 2006;13:309–313. doi: 10.1128/CVI.13.3.309-313.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]