Abstract
Mitochondrial bioenergetic studies mostly rely on isolated mitochondria thus excluding the regulatory role of other cellular compartments important for the overall mitochondrial function. In intact cardiomyocytes, we followed the dynamics of electron fluxes along specific sites of the electron transport chain (ETC) by simultaneous detection of NAD(H)P and flavoprotein (FP) fluorescence intensities using a laser-scanning confocal microscope. This method was used to delineate the effects of isoflurane, a volatile anesthetic and cardioprotective agent, on the ETC. Comparison to the effects of well-characterized ETC inhibitors and uncoupling agent revealed two distinct effects of isoflurane: uncoupling-induced mitochondrial depolarization and inhibition of ETC at the level of complex I. In correlation, oxygen consumption measurements in cardiomyocytes confirmed a dose-dependent, dual effect of isoflurane, and in isolated mitochondria an obstruction of the ETC primarily at the level of complex I. These effects are likely responsible for the reported mild stimulation of mitochondrial reactive oxygen species (ROS) production required for the cardioprotective effects of isoflurane. In conclusion, isoflurane exhibits complex effects on the ETC in intact cardiomyocytes, altering its electron fluxes, and thereby enhancing ROS production. The NAD(P)H-FP fluorometry is a useful method for exploring the effect of drugs on mitochondria and identifying their specific sites of action within the ETC of intact cardiomyocytes.
Keywords: NAD(P)H fluorometry, flavoprotein fluorometry, electron transport chain, cardiomyocytes, reactive oxygen species, isoflurane
1. Introduction
Mitochondria play a crucial role under physiological and pathological conditions, and as such were recognized as important target for therapeutic interventions [1]. Volatile anesthetics, such as isoflurane, have cardioprotective properties against ischemia and reperfusion injury that were previously associated with their effects on mitochondria [2]. This includes a moderate increase in reactive oxygen species (ROS) production that triggers prosurvival signaling [3]. Cellular signaling of isoflurane-induced protection is complex, and includes various prosurvival kinases and mitochondrial and sarcolemmal potassium channels[4,5]. Mitochondria act as upstream trigger and as downstream effector of volatile anesthetic-induce cardioprotection. However, the reports on isoflurane's effects on mitochondria are contradictory, ranging from mitochondrial uncoupling to inhibition of various complexes of the ETC [2,6,7,8]. We have shown that cardiac mitochondria isolated from isoflurane-preconditioned rats exhibit mild depolarization and uncoupling, which may limit the damaging matrix Ca2+ accumulation without adversely affecting ATP production.[2] These events likely act as effectors of APC, reducing the extent of mitochondrial damage after hypoxic stress. In addition, volatile anesthetics, by nature of their lipid solubility, may also directly alter mitochondrial function, including oxidative phosphorylation, reactive oxygen species (ROS) production and ion transport, constituting the triggering phase of the APC. The complexity of mitochondrial function that is under regulation of other cellular compartments, such as cytosol and endoplasmic reticulum [9,10] is often overlooked when reductionist models like isolated mitochondria or permeabilized cells are used. Ratiometric redox fluorometry of endogenous pyridine nucleotides NAD(P)H and flavoproteins (FPs) allows to assess the function of mitochondria within their natural milieu of intact cells. Changes in the NADH/NAD+ redox couple dominate the NAD(P)H fluorometry [11,12]. Upon NADH oxidation, electrons are transferred to complex I and, via ubiquinone (UQ), proceed downstream to O2, the final electron acceptor (Figure 6A). The overall FP fluorescence signal originates from three sources: 1) lipoamide dehydrogenase (LipDH), where the redox state of the flavin moiety is coupled to NADH/NAD+, 2) electron transfer flavoprotein (ETF), in redox contact with UQ via ETF-ubiquinone oxidoreductase (ETF-QO), and 3) non-specific FPs which are not in redox contact with the ETC and contribute only ~25% to the overall signal in rat liver mitochondria [13,14,15].
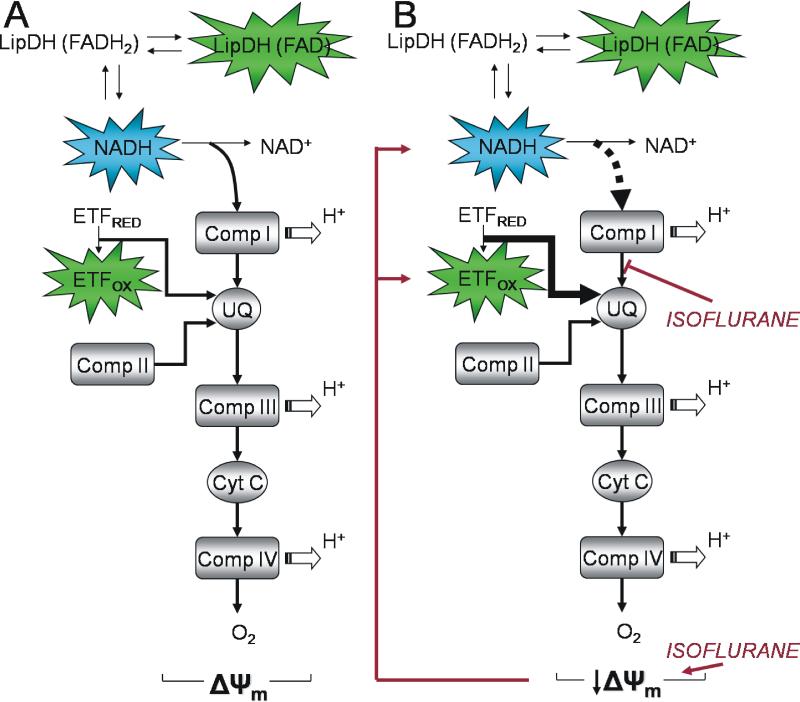
Figure 6. Electron pathways through the ETC from NADH and fluorescent FPs and the effects of isoflurane.
(A) NADH is oxidized to NAD+ while shuttling electrons to complex I, UQ, and downstream to the final electron acceptor O2. LipDH flavoprotein is in redox equilibrium with the NADH/NAD+ redox couple and its fluorescence intensity follows changes in NADH fluorescence. ETF is in redox contact with UQ pool. After oxidation of ETF electrons are transferred to UQ via ETF-QO (not shown). Separate entry sites of electrons arriving from NADH and ETF result in their independent redox and fluorescence responses to distinct alternations of the electron flow within the ETC. (B) A dual effect of isoflurane on the ETC: 1) an uncoupling-induced mitochondrial depolarization that is observed as increased oxidation of NAD(P)H and FPs and correlates with increased rate of cardiomyocyte O2 consumption at lower isoflurane concentrations, and 2) inhibition of electron flux at the level of complex I that is observed as attenuation of NAD(P)H oxidation compared to oxidation of FPs (ETF) and correlates with the attenuation of cardiomyocyte O2 consumption at higher isoflurane concentrations. (LipDH-lipoamid dehydrogenase, Comp.-complex, ETF-electron transfer flavoprotein, UQ-ubiquinone, Cyt C- cytochrome C, ΔΨm-mitochondrial membrane potential).
Based on different electron pathways originating from NADH and ETF, we used simultaneous NAD(P)H-FP fluorometry to assess changes of electron fluxes along different parts of the ETC in non-permeabilized cardiomyocytes. This approach was undertaken to determine the specific sites of action of the isoflurane within the ETCusing a whole-cell approach that was supported by measurements in isolated mitochondria. Our results indicate that isoflurane exhibits dual, dose-dependent effects on the ETC in cardiomyocytes and thereby alters electron pathways, enhancing ROS production and triggering cardioprotective signals. NAD(P)H-FP fluorometry is a useful technique capable of delineating pharmacological agent's specific sites of action within the ETC of intact cells.
2. Materials and methods
The animal use and experimental protocols of this study were approved by the Institutional Animal Use and Care Committee of the Medical College of Wisconsin, Milwaukee, WI.
2.1. Isolation of cardiomyocytes
Ventricular cardiomyocytes were obtained from male Wistar rats (180-250 g), as reported previously [3]. In brief, hearts from rats anesthetized with sodium thiobutabarbital (Inactin, Sigma-Aldrich, St. Louis, MO; 150 mg/kg intraperitoneally) were excised and perfused retrogradely through an aortic cannula with a buffer containing a mixture of collagenase and protease. After dissociation, myocytes were placed in Tyrode's solution containing (in mM): 132 NaCl, 10 HEPES, 5 glucose, 5 KCl, 1 CaCl2, 1.2 MgCl2, pH 7.4, and experiments were conducted within 5 hours after cell isolation.
2.2. Isolation of cardiac mitochondria
Mitochondria were isolated following a previously reported procedure [2]. Hearts were excised from anesthetized rats and left ventricles were minced in isolation buffer (in mM): 50 sucrose, 200 mannitol, 5 KH2PO4, 1 EGTA, 5 3-(N-morpholino)propanesulfonic acid, 0.1% bovine serum albumin and adjusted to pH 7.3. The tissue was homogenized twice for 5 s with a T 25 disperser (IKA-Werke, Staufen, Germany), and mitochondria were isolated by differential centrifugation. The final mitochondrial pellet was resuspended in isolation buffer without EGTA, stored on ice, and used for experiments within 4 h. Protein concentration was determined by a modified Lowry assay kit (Bio-Rad, Hercules, CA).
2.3. NAD(P)H-FP fluorometry in cardiomyocytes
A laser-scanning confocal microscope (Leica TCS SP5, Mannheim, Germany) with a 63x/1.4 Apo oil objective was utilized to monitor NAD(P) and FP fluorescence intensities in cardiomyocytes at 30°C. Consecutive laser-scans at 730 nm (titanium:sapphire laser, Spectra Physics, Mountain View, CA) and 488 nm (argon laser) were used for quasi-simultaneous two-photon NAD(P)H excitation and single-photon FP excitation, respectively. Each 512×512 pixel image represents an average of six scans taken with a resonant scanner at 8000 Hz. Emission bands of NAD(P)H and FPs were collected at 390-490 nm and 500-530 nm wavelength, respectively. This allowed sufficient separation between NAD(P)H and FP emission bands for independent quantification of their fluorescence intensities [16]. Data were processed with LAS AF software (Leica). The excitation power was controlled with acousto-optic tunable filters (AOTFs) whereby the excitation power of the titanium:sapphire laser was set at 12.5% of maximum. The power supply of the argon laser was set to 20% of maximum and the excitation line was attenuated to 15% of maximum. At these excitation powers, no photodamage was observed, that would be indicated by a change in cell behavior or fluorescence intensities. Based solely on the emitted fluorescence, the three major groups of fluorescent FPs cannot be distinguished with certainty, although the emission wavelengths were set to match emission spectrum of the ETF [17]. Nonetheless, a functional distinction between fluorescence of LipDH and ETF can be obtained [15,18,19], as discussed later. Fluorescence intensity data are normalized to baseline, expressed as 100%. The change in fluorescence (ΔF) is calculated as difference between baseline value and the effect of drug after reaching a plateau, where each value is an average of five to ten consecutive data points.
2.4. Mitochondrial membrane potential (ΔΨm) measurements in cardiomyocytes
Cardiomyocytes were loaded with the potentiometric fluorescent indicator tetramethylrhodamine ethyl ester (TMRE, 100 nM, Invitrogen, Carlsbad, CA) for 10 min in the absence or presence of isoflurane. TMRE fluorescence intensity was acquired using the confocal microscope at excitation and emission wavelengths: λex/λem=543/570-610.
2.5. Cardiomyocyte O2 consumption
Respiration of isolated cardiomyocytes supported by endogenous substrates was measured in Tyrode's solution at 37°C using an O2 electrode (Hansatech Instruments, Norfolk, UK). While other experiments were conducted at 30°C, these measurements were performed at higher temperature to enhance respiration and thereby increase the ability to detect changes. In the cell suspensions, more than 70% of cardiomyocytes were alive and rhod-shaped. After establishing baseline rate of O2 consumption, isoflurane was added at incremental concentrations and results were normalized to baseline values.
2.6. Isolated mitochondria O2 consumption
Mitochondrial O2 consumption was measured with an O2 electrode at 30°C in respiration buffer (130 mM KCl, 5 mM K2HPO4, 20 mM MOPS, 2.5 mM EGTA, 1 μM Na4P2O7 and 0.1% BSA, pH 7.4) containing 1.0 mg/ml mitochondrial protein in the presence or absence of isoflurane [2]. State 2 respiration was initiated with 5 mM pyruvate and malate or 5 mM succinate and 2 μM rotenone. State 3 respiration was measured in the presence of 250 μM ADP, and after all ADP was consumed, state 4 respiration was determined.
2.7. Cardiomyocyte ROS production
ROS production in isolated cardiomyocytes was monitored at 30°C using the confocal microscope. The ROS-sensitive indicator 5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA, 2 μM, Invitrogen), was loaded for 20 min, followed by 10 min dye washout [3]. Oxidation of deesterified CM-H2DCF by ROS yields fluorescent CM-DCF (λex/λem=488/500-550 nm). The CM-DCF fluorescence intensity was assessed 10 min after exposure to isoflurane or vehicle (control).
2.8. Isolated mitochondria ROS production
Mitochondrial ROS production was measured at state 2 respiration using Amplex Red (12.5 μM, Invitrogen) and 0.1 U/ml horseradish peroxidase at 30°C. Amplex Red reacts with H2O2 in the presence of peroxidase to produce the fluorescent resorufin. Resorufin fluorescence intensity was measured with a spectrofluorometer (Photon Technology International, Birmingham, NJ), λex/λem=530/583 nm. ROS measurements were conducted in the presence of 5 mM pyruvate and 5 mM malate in respiration buffer containing mitochondria at a final concentration of 1 mg protein/ml. Measurements were calibrated with known concentrations of H2O2.
2.9. Administration of isoflurane
The appropriate volumes of stock solution of isoflurane dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich) were added to experimental buffers, to achieve a desired concentrations of isoflurane (0.25, 0.5, or 1 mM). At the end of each experiment isoflurane concentration was analyzed by gas chromatography, and it varied ±10% of the reported values. DMSO alone was added in appropriate controls, its concentrations were below 0.2% and it did not affect measured parameters.
2.10. Statistical analysis
Data are presented as means ± SEM, where N represents the number of independent experiments. Results were analyzed using analysis of variance with the Bonferroni's post hoc test, and Student t test for individual isoflurane concentrations in NAD(P)H-FP fluorometry experiments. Differences at P<0.05 were considered significant.
3. Results
3.1. Alterations in cardiomyocyte ETC electron pathways detected by NAD(P)H-FP fluorometry
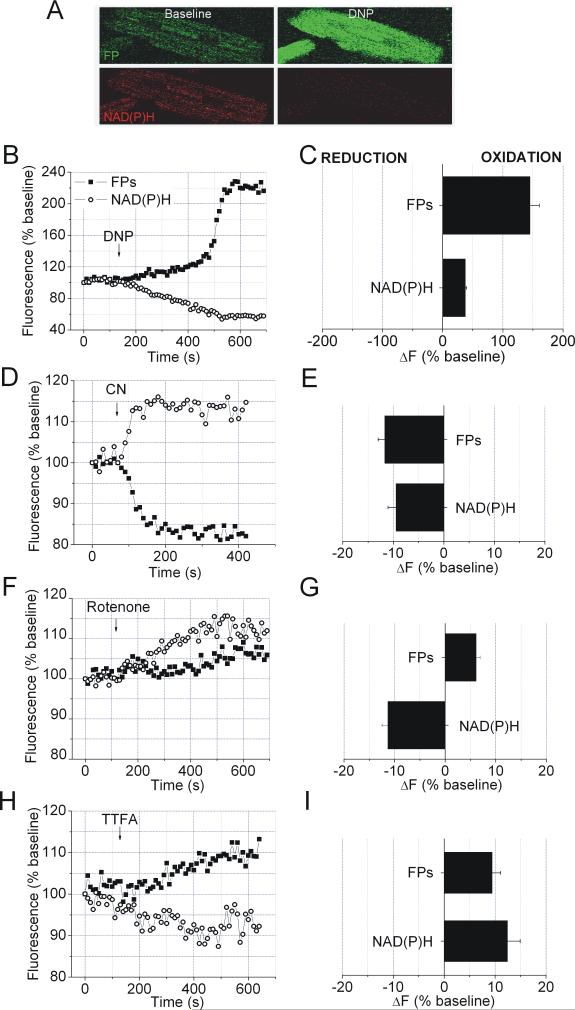
The protonophore 2,4-dinitrophenol (DNP) and ETC inhibitors were used to induce specific alterations in the electron pathways within the ETC, and changes in NAD(P)H and FP fluorescence intensities were recorded simultaneously. DNP decreased NAD(P)H and increased FP fluorescence intensity indicating oxidation of each fluorophore due to the increase in electron delivery into the ETC (Figure 1, A-C). Inhibition of complex IV by cyanide caused an increase in NAD(P)H and a decrease in FP fluorescence intensity, indicating blocked electron transfer into the ETC and the reduction of electron carriers by endogenous substrates (Figure 1, D and E). Rotenone, an inhibitor of complex I, increased NAD(P)H fluorescence intensity due to inhibited NADH oxidation (Figure 1, F and G). FP fluorescence intensities also slightly increased due to increased electron transfer from ETF to UQ caused by increased oxidation of UQ pool [15]. Thenoyltrifluoreroacetone (TTFA), a complex II inhibitor, induced decrease in NAD(P)H and increase in FP fluorescence intensity again due to oxidation of UQ pool and increased electron transfer to UQ from unobstructed pathways that involve NADH and ETF (Figure 1, H and I).
Figure 1. NAD(P)H-FP fluorometry and electron fluxes in the ETC.
Time courses, (B, D, F and H) and summary histogramms of relative fluorescence intensity change (ΔF), (C, E, G and I). Oxidation decreases NAD(P)H and increases FP fluorescence intensity and reduction induces the opposite response of each electron carrier. A: Representative images of NAD(P)H and FP fluorescence before and after treatment with mitochondrial uncoupler DNP(100 μM). (B and C): DNP increased FP and decreased NAD(P)H fluorescence intensity. (D and E): CN- (2 mM), a complex IV inhibitor, decreased FP and increased NAD(P)H fluorescence intensity. (F and G): Complex I inhibitor rotenone (1 μM) enhanced FP and NAD(P)H fluorescence intensity. (H and I): TTFA (50 μM), a complex II inhibitor, increased FP and decreased NAD(P)H fluorescence intensity. Data are means ± SEM, N=6-8/group.
3.2. In cardiomyocytes, isoflurane exhibits two distinct effects, mitochondrial uncoupling and inhibition of ETC at the level of complex I
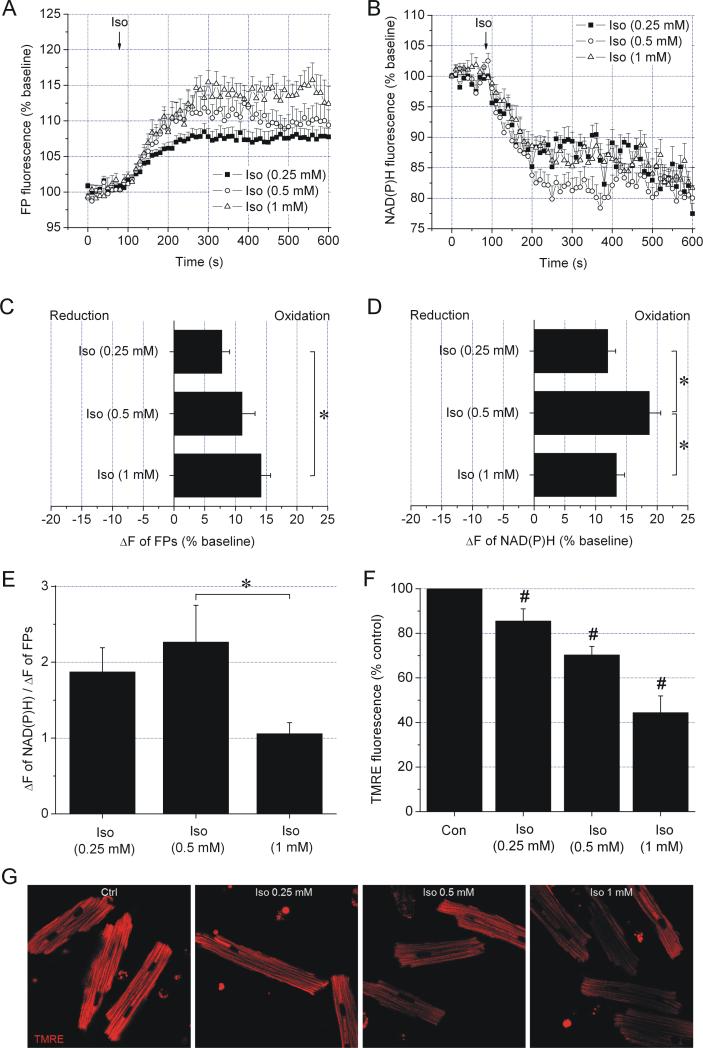
The effects of isoflurane on the ETC of cardiomyocytes were analyzed by NAD(P)H-FP fluorometry. At all concentrations, isoflurane increased FP and decreased NAD(P)H fluorescence intensities indicating oxidation of the respective fluorophore (Figure 2, A-D), which qualitatively resembled the effects of DNP and TTFA. Isoflurane also reduced TMRE fluorescence compared to control, indicating mitochondrial depolarization (Figure 2, F and G). While isoflurane dose-dependently increased FP oxidation, NADH oxidation was attenuated at 1 mM isoflurane. This indicated a partial obstruction of the ETC at the level of complex I, thus affecting the NADH but not the ETF pathway. The decrease in the ratio of fluorescence intensity change of NAD(P)H to FP at 1 mM isoflurane, which reflects the relative contribution of electron transfer in each pathway (NADH → complex I → UQ and ETF → ETF-QO → UQ) confirmed the obstruction of electron flow at the level of complex I (Figure 2E).
Figure 2. Dual effect of isoflurane on the ETC detected by NAD(P)H-FP fluorometry.
FP (A and C) and NAD(P)H (B and D) fluorescence intensity recordings after addition of various concentrations of isoflurane (Iso). While FPs became progressively oxidized with increasing isoflurane concentrations, NAD(P)H oxidation exhibited a bell-shaped concentration dependence. Summary data in panels C and D are average time points in time frame 200-400 s. (E) The ratio between NAD(P)H and FP fluorescence intensity change decreased at 1 mM isoflurane suggesting a relative decrease of electron flux in the pathway NADH → complex I → UQ compared to the pathway ETF → ETF-QO → UQ. (F) Isoflurane dose-dependently decreased TMRE fluorescence intensity compared to control (Con), reflecting a decrease in ΔΨm. (G) Representative images of TMRE fluorescence in cardiomyocytes. Data are means ± SEM, N=10-12/group. *P<0.05; #P<0.05 vs. Con.
3.3. In cardiomyocytes, isoflurane dose-dependently alters respiration
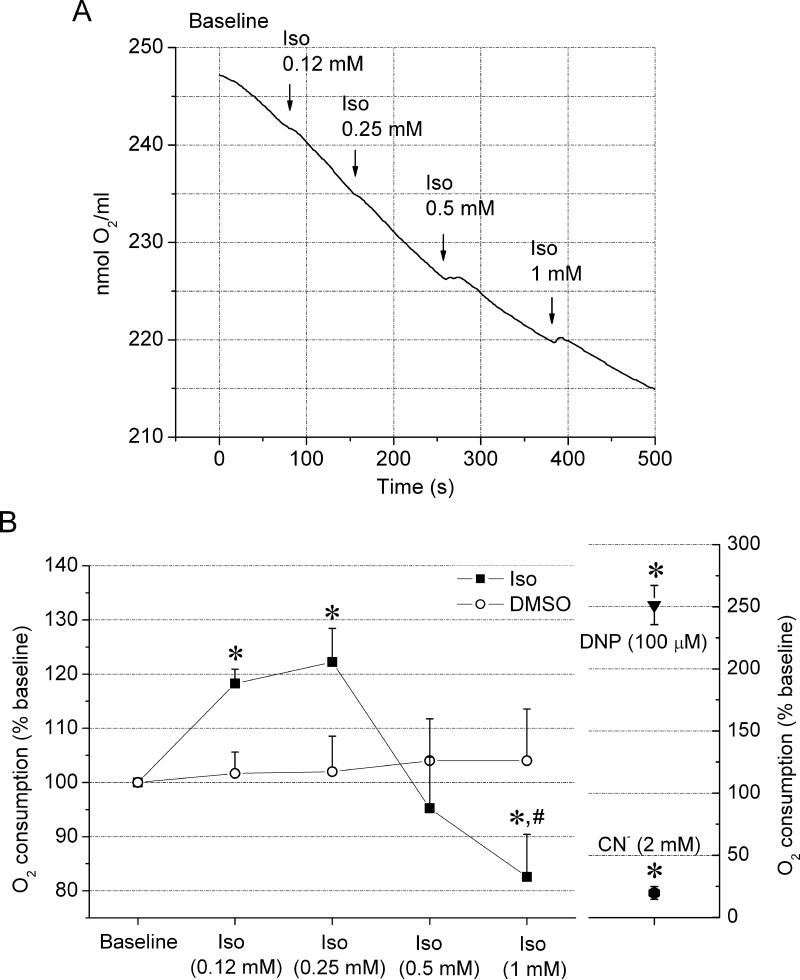
The O2 consumption rate was used as an indirect estimate of the overall rate of electron transfer along the ETC in the presence of isoflurane. At 0.25 mM concentration, isoflurane increased the rate of O2 consumption by 22±6% compared to baseline (Figure 3). However, higher concentrations of isoflurane 0.5 and 1 mM, reversed the trend and isoflurane attenuated O2 consumption by 5±9 and 17±8%, respectively. The increase in O2 consumption indicates increased electron flow through the ETC that can be caused by mitochondrial uncoupling [20], identified as one effect of isoflurane by NAD(P)H-FP fluorometry. The attenuation of O2 consumption at higher isoflurane concentrations indicates decrease in electron transfer along the ETC, identified by NAD(P)H-FP fluorometry as inhibition of the ETC at the level of complex I. Compared to maximal and minimal respiration stimulated by DNP and cyanide, respectively, the effects of isoflurane were mild.
Figure 3. Dose-dependent effect of isoflurane on cardiomyocyte O2 consumption.
(A) Representative signal trace of O2 consumption measurement of cardiomyocytes after the addition of incremental isoflurane (Iso) concentrations. (B) Summarized data from 14 experiments are expressed as the rate of O2 consumption normalized to baseline. At lower concentrations, isoflurane enhanced O2 consumption, but at higher concentrations, the trend was reversed and isoflurane attenuated O2 consumption. DMSO, a vehicle for isoflurane, did not significantly affect respiration. Maximal and minimal O2 consumption was induced in the presence of DNP (100 μM) and sodium cyanide (2 mM), respectively. Values are means ± SEM. *P<0.05 vs. Baseline; #P<0.05 vs. Iso 0.25 mM.
3.4. In isolated cardiac mitochondria, isoflurane inhibits ETC predominantly at the level of complex I
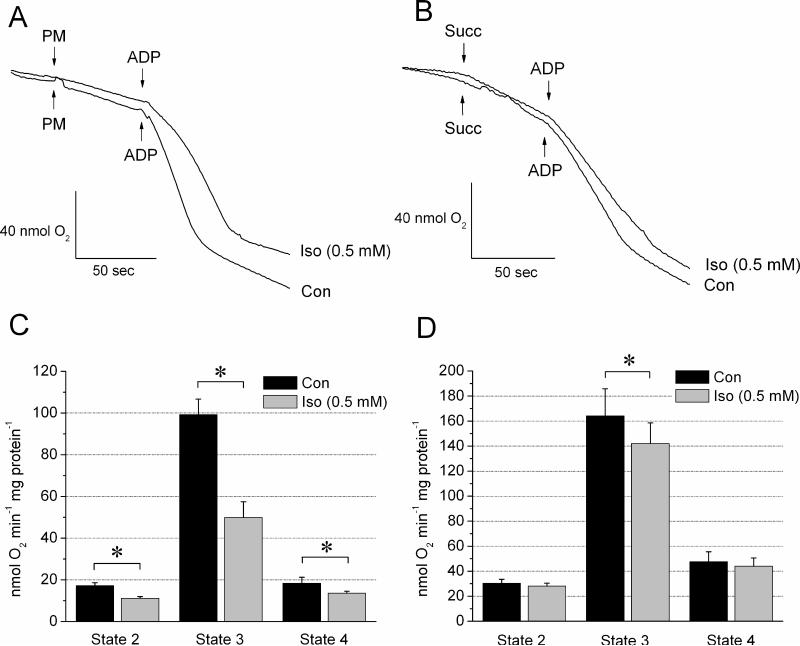
To confirm the specific site of ETC obstruction by isoflurane, the lowest concentration of 0.5 mM at which cardiomyocyte respiration was attenuated was tested in isolated mitochondria. The rate of O2 consumption was measured when delivering electrons into ETC via complex I using pyruvate/malate or via complex II using succinate and rotenone. As shown in Figure 4 A and C, isoflurane decreased the rates of respiration at states 2, 3, and 4 compared to control when mitochondria were fueled with pyruvate/malate (11.2±0.8 vs. 17.2±1.4, 49.9±7.6 vs. 99.3±7.4, and 13.6±1.0 vs. 18.4±3.0 nmol O2 min-1 mg protein-1, respectively). In contrast, with the complex II substrate succinate and in the presence of rotenone, O2 consumption was not significantly altered in the presence of 0.5 mM isoflurane at respiration states 2 and 4. There was an inhibition at state 3 respiration, but to lesser extent than with pyruvate/malate (isoflurane vs. control: 142.0±16.5 vs. 164.1±21.8 nmol O2 min-1 mg protein-1) (Figure 4, B and D). When measured in the absence of rotenone, succinate-induced respiration was 109±13.5 nmol O2 min-1 mg protein-1 without isoflurane, and 124.0± min-1 mg protein-1 with isoflurane. Overall, these data indicate a predominant obstruction of the ETC at the level of complex I by isoflurane and possibly a lesser pronounced obstruction between complexes II and IV.
Figure 4. Isoflurane inhibits ETC of isolated mitochondria primarily at the level of complex I.
Representative respiration recordings of isolated mitochondria in the absence (Con) and presence of isoflurane (Iso, 0.5 mM) using pyruvate/malate (PM) (A) or succinate (Succ) (B) as substrates. (C) Summarized data for PM show that compared to control, the rate of O2 consumption at states 2, 3 and 4 was attenuated in the presence of Iso. (D) With Succ as substrate, isoflurane slightly attenuated O2 consumption only at state 3. Data are means ± SEM, N=5-6/group. *P<0.05 vs. Con.
3.5. Isoflurane enhances ROS production in cardiomyocytes and in isolated mitochondria
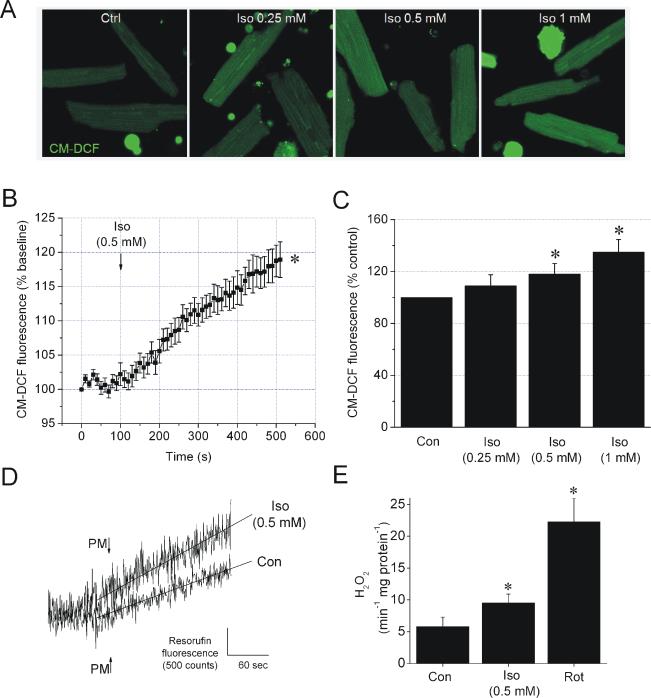
The production of ROS in the presence or absence of isoflurane was monitored in cardiomyocytes and in isolated mitochondria. In cardiomyocytes, 0.25, 0.5 and 1 mM isoflurane increased CM-DCF fluorescence intensity by 9±9, 18±8 and 35±10% of control, respectively, indicating a dose-dependent increase in ROS production (Figure 5, A-C). To test whether isoflurane-induced ROS production originated from mitochondria, isoflurane was applied to isolated mitochondria and ROS production was measured by spectrofluorometry. At state 2 respiration supported by pyruvate/malate, 0.5 mM isoflurane enhanced the rate of increase in resorufin fluorescence compared to control (5.8±1.5 vs. 9.5±1.4 pmol H2O2 min-1 mg protein-1, respectively), indicating stimulation of ROS production by isoflurane (Figure 5, D and E). Inhibition of complex I with rotenone induced a greaincrease in ROS production than isoflurane (22.3±3.7 pmol H2O2 min-1 mg protein-1), suggesting only a mild effect of isoflurane.
Figure 5. Isoflurane increases ROS production in cardiomyocytes and mitochondria.
(A) Representative images of CM-DCF fluorescence in cardiomyocytes. (B) Summary of signal traces of CM-DCF fluorescence intensity as indication of ROS production before and after addition of 0.5 mM isoflurane (Iso). (C) Summarized values of CM-DCF fluorescence intensity in control or after the treatment with various concentrations of Iso. (D) Representative traces of resorufin fluorescence intensity measurements in mitochondria with pyruvate and malate (PM) as substrate in the absence (Con) and presence of 0.5 mM Iso. (E) Summarized and calibrated rates of increase in resorufin fluorescence intensity in Con, Iso or rotenone (Rot, 1 μM)-treated mitochondria. Data are means ± SEM, N=6-10/group. *P<0.05 vs. Con.
4. Discussion
We demonstrated a dual effect of isoflurane on the mitochondrial ETC in cardiomyocytes: uncoupling-induced mitochondrial depolarization and inhibition of the ETC at the level of complex I. This novel application of NAD(P)H-FP fluorometry allows monitoring of electron fluxes along distinct sites of ETC in intact cells. With this method the effects of pharmacological agents on specific sites of the ETC can be analyzed with the advantage of encompassing mechanisms involving other cellular organelles. The uncoupling-induced mitochondrial depolarization revealed by NAD(P)H-FP fluorometry corresponds to the enhanced rates of O2 consumption by cardiomyocytes at lower isoflurane concentrations, and the inhibition of the ETC at the level of complex I corresponds to the attenuation of O2 consumption by cardiomyocytes at higher isoflurane concentrations. In isolated mitochondria, substrate-dependent O2 consumption measurements confirm that complex I is the predominant site of ETC inhibition by isoflurane. The identified alteration of electron fluxes within the ETC caused by isoflurane is likely to be responsible for the enhanced ROS production, an important trigger for cardioprotective signaling.
NAD(P)H-FP fluorometry and ETC
We have demonstrated that simultaneous recording of NAD(P)H and FP fluorescence intensity changes gives distinct responses to specific alterations of electron flow within the ETC. This is in accord with previous studies that characterized the NAD(P)H-FP fluorometric responses to manipulation with the ETC in isolated mitochondria and permeabilized cells [15]. The fluorescence intensity of each fluorophore is in linear correlation with its redox state, tying the changes in fluorescence intensities to alterations of electron transfer via these electron carriers [21,22]. Consistent with the model depicted in Figure 6A, we showed that an uncoupling agent oxidized NAD(P)H and FPs. A decrease in the ΔΨm and the proton gradient across the inner mitochondrial membrane accelerates electron transfers into ETC via upstream electron carriers, causing oxidation of both NADH and FPs (LipDH and ETF) [23]. Conversely, cyanide reduced both NAD(P)H and FP due to the obstruction of electron flow at complex IV, causing accumulation of the reduced electron carriers. Similarly, it has been shown that the depletion of the final electron acceptor O2, or obstruction of complex III with myxothiazol, slows oxidation of electron carriers in the upstream pathways that converge at UQ, leading to more reduced FPs and NADH [24,25]. Complex I inhibitor rotenone reduced NAD(P)H to a similar extent as cyanide [26]. However, rotenone also slightly increased FP oxidation, which we attributed to the increased fluorescence of ETF. This observation is supported by Kunz et al. who demonstrated that the UQ/ubiquinol (UQH2) couple equilibrates with ETF and controls the rate of electron transfer from ETF to ETC [15]. Therefore, an increased UQ/UQH2 ratio caused by rotenone (due to attenuated reduction of UQ by electrons arriving from complex I) increases electron transfer rate from ETF to UQ causing ETF oxidation [15]. While rotenone blocks oxidation of LipDH [26,27], we observed that the oxidation of ETF predominated in the overall FP fluorescence intensity change. Under baseline conditions in energized cells FPs, including LipDH, are almost completely reduced (as observed in Fig 1: DNP induces a substantially greater FP fluorescence intensity change than cyanide). Thus, only a small portion of predominantly reduced LipDH flavin can be further reduced by rotenone, compared to the greater portion of ETF that becomes oxidized. Lastly, complex II inhibition by TTFA oxidized NAD(P)H and FP. We presume that similar to the effect of rotenone, complex II inhibition attenuated UQ reduction by blocking electron flux from complex II, thereby increasing the UQ/UQH2 ratio, which in turn increases electron transfer to UQ from unobstructed pathways, involving NADH and both FPs.
Isoflurane and NAD(P)H-FP fluorometry
The observed oxidation of NAD(P)H and FPs induced by isoflurane was qualitatively comparable to the effects of DNP or TTFA. Isoflurane-induced depolarization of mitochondria and oxidation of FPs has been associated with opening of mitochondrial ATP-sensitive K+ channels [2], suggesting that electron carrier oxidation observed here is caused by uncoupling-induced mitochondrial depolarization (Figure 6B). However, the possibility of a mild complex II inhibition cannot be excluded since isoflurane partly obstructed O2 consumption in isolated mitochondria respiring on succinate, as discussed below. The attenuation of NAD(P)H oxidation at 1 mM isoflurane compared to the lower doses, together with dose-dependent FP oxidation, suggests the obstruction in the pathway NADH → complex I → UQ as an additional effect of isoflurane. The possibility that complex I inhibition by isoflurane is alone responsible for the decrease in ΔΨm is excluded since isoflurane and rotenone caused opposite NAD(P)H responses. Isoflurane caused oxidation of FP as well as NAD(P)H due to concomitant uncoupling, while rotenone caused a reduction in NAD(P)H and mild FP oxidation solely due to complex I inhibition. Cardiomyocyte respiration measurements verified the existence of a dual effect of isoflurane, as discussed below.
O2 consumption
While measurements of O2 consumption in cardiomyocytes reflect only the overall rate of electron flux through the ETC, these results were in agreement with our NAD(P)H-FP fluorometry results. At lower concentrations, isoflurane increased the rate of O2 consumption, indicating increase in the overall rate of electron flow along the ETC caused by uncoupling. However, at higher concentrations, isoflurane reversed this trend and attenuated O2 consumption, suggesting a decrease in electron flow through the ETC due to an inhibitory effect. This correlates with the inhibition of ETC at the level of complex I identified by NAD(P)H-FP fluorometry. In isolated mitochondria, when electrons were delivered to the ETC via complex I, isoflurane substantially attenuated O2 consumption at all respiration states. When electrons were delivered downstream of complex I, by succinate via complex II, only a minor inhibition of mitochondrial respiration at state 3 by isoflurane was observed. This indicates that isoflurane inhibited ETC primarily at the level of complex I. Measurements with succinate in the absence of rotenone supported this connotation. Oxalacetate, formed in the citrate cycle when NADH levels decrease in the absence of rotenone, is a potent inhibitor of succinate dehydrogenase. In addition, reverse electron flow from complex II to complex I is known to stimulate production of reactive oxygen species under these conditions. Isoflurane reduced the decline in state 3 respiration observed in the absence of rotenone. This may be explained by isoflurane-induced complex I inhibition and thus, increasing NADH level and less oxalacetate production, as well as reduced reverse electron flow. However the possibility of an additional mild inhibition of other complexes cannot be excluded. The absence of isoflurane-induced increase in state 4 respiration that would be expected in the presence of uncoupling, may be due to concomitant inhibition of ETC overriding the effect of uncoupling on the overall rate of electron flow. Alternatively, mitochondrial uncoupling may result from an action of isoflurane that involves presence of other cellular compartments. The later is suggested by previous findings that isoflurane induces translocation of protein kinase C from the cytosol to mitochondria [4] and subsequent activation of mitochondrial ATP-sensitive K+ channels, causing uncoupling [28,29].
In the presence of 0.5 mM isoflurane, respiration at state 3 supported by pyruvate/malate exhibits an unsteady character (Fig. 4A), similar to the biphasic response of the NAD(P)H-FP fluorometric measurement at the same isoflurane concentration (Fig. 2, A and B). We speculate that the effect of complex I inhibition and uncoupling are comparable at 0.5 mM isoflurane, as suggested by cardiomyocyte respiration experiments (Fig. 3B), where uncoupling and ETC inhibition exist in parallel. Fluctuations in the extent of uncoupling or complex I inhibition and the transient predominance of each effect may be responsible for less stable signals at 0.5 mM isoflurane. These fluctuations could be potentiated by a time-dependent redistribution of lipophilic isoflurane between membrane compartments.
ROS production
Previous reports that isoflurane inhibits complex I [7], other complexes of the ETC [30] or induces mitochondrial uncoupling via mitochondrial K+ channels [2] brought some confusion in elucidating the effects of volatile anesthetics on mitochondria, which is important for cardioprotection [1]. A moderate increase in ROS production was suggested to trigger these cardioprotective mechanisms [3]. Cardioprotective agents, including volatile anesthetics may generate ROS by opening mitochondrial K+ channels [31,32,33]. Moreover, complex III was proposed as the site of isoflurane-induced ROS production [34]. The dual effect of isoflurane on the ETC observed in our study, that is mild uncoupling combined with inhibition of the ETC at the level of complex I could readily be considered as mechanism for ROS generation at complex I [35,36], or potentially at complex III. The later is implied by a study by Drose et al. showing that an increased UQ/UQH2 ratio, here generated by obstruction of complex I, enhances ROS production at complex III [37].
In conclusion, we demonstrated that simultaneous NAD(P)H-FP fluorometry can be used as simple and useful approach for the analysis of electron fluxes along the ETC in intact cardiomyocytes to delineate specific sites of action of drugs within the ETC. Using this approach, we identified a complex action of isoflurane that included uncoupling-induced mitochondrial depolarization and inhibition of complex I. These effects are likely the base for the mechanism by which isoflurane moderately enhances ROS production, an important trigger of cardioprotective pathways.
Acknowlwdgments
We thank Dr. Paul Brookes for the very helpful discussion. We also thank Mary Ziebell for the technical assistance and Teri Misorski for editorial help. Supported in part by grants P01GM066730 and R01HL034708 (ZJB) from the NIH, Bethesda, Maryland.
Abbreviations
- FP
flavoprotein
- ETC
electron transport chain
- UQ
ubiquinone
- LipDH
lipoamide dehydrogenase
- ETF
electron transfer flavoprotein
- ETF-QO
electron transfer flavoprotein-ubiquinone oxidoreductase
- ROS
reactive oxygen species
- AOTF
acousto-optic tunable filter
- ΔΨm
mitochondrial membrane potential
- TMRE
tetramethylrhodamine ethyl ester
- CM-H2DCFDA
5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester
- DMSO
dimethyl sulfoxide
- DNP
2,4-dinitrophenol
- TTFA
thenoyltrifluoreroacetone
- UQH2
ubiquinol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Camara AK, Lesnefsky EJ, Stowe DF. Potential Therapeutic Benefits of Strategies Directed to Mitochondria. Antioxid. Redox. Signal. 2010;13:279–347. doi: 10.1089/ars.2009.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ljubkovic M, Mio Y, Marinovic J, Stadnicka A, Warltier DC, Bosnjak ZJ, Bienengraeber M. Isoflurane preconditioning uncouples mitochondria and protects against hypoxia-reoxygenation. Am. J. Physiol. Cell Physiol. 2007;292:C1583–1590. doi: 10.1152/ajpcell.00221.2006. [DOI] [PubMed] [Google Scholar]
- 3.Sedlic F, Pravdic D, Ljubkovic M, Marinovic J, Stadnicka A, Bosnjak ZJ. Differences in production of reactive oxygen species and mitochondrial uncoupling as events in the preconditioning signaling cascade between desflurane and sevoflurane. Anesth. Analg. 2009;109:405–411. doi: 10.1213/ane.0b013e3181a93ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pravdic D, Sedlic F, Mio Y, Vladic N, Bienengraeber M, Bosnjak ZJ. Anesthetic-induced preconditioning delays opening of mitochondrial permeability transition pore via protein Kinase C-epsilon-mediated pathway. Anesthesiology. 2009;111:267–274. doi: 10.1097/ALN.0b013e3181a91957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marinovic J, Bosnjak ZJ, Stadnicka A. Preconditioning by isoflurane induces lasting sensitization of the cardiac sarcolemmal adenosine triphosphate-sensitive potassium channel by a protein kinase C-delta-mediated mechanism. Anesthesiology. 2005;103:540–547. doi: 10.1097/00000542-200509000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Jiang MT, Nakae Y, Ljubkovic M, Kwok WM, Stowe DF, Bosnjak ZJ. Isoflurane activates human cardiac mitochondrial adenosine triphosphate-sensitive K+ channels reconstituted in lipid bilayers. Anesth. Analg. 2007;105:926–932. doi: 10.1213/01.ane.0000278640.81206.92. [DOI] [PubMed] [Google Scholar]
- 7.Hanley PJ, Ray J, Brandt U, Daut J. Halothane, isoflurane and sevoflurane inhibit NADH:ubiquinone oxidoreductase (complex I) of cardiac mitochondria. J. Physiol. 2002;544:687–693. doi: 10.1113/jphysiol.2002.025015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riess ML, Kevin LG, McCormick J, Jiang MT, Rhodes SS, Stowe DF. Anesthetic preconditioning: the role of free radicals in sevoflurane-induced attenuation of mitochondrial electron transport in Guinea pig isolated hearts. Anesth. Analg. 2005;100:46–53. doi: 10.1213/01.ANE.0000139346.76784.72. [DOI] [PubMed] [Google Scholar]
- 9.Maack C, O'Rourke B. Excitation-contraction coupling and mitochondrial energetics. Basic Res. Cardiol. 2007;102:369–392. doi: 10.1007/s00395-007-0666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogbi M, Chew CS, Pohl J, Stuchlik O, Ogbi S, Johnson JA. Cytochrome c oxidase subunit IV as a marker of protein kinase Cepsilon function in neonatal cardiac myocytes: implications for cytochrome c oxidase activity. Biochem. J. 2004;382:923–932. doi: 10.1042/BJ20040468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klingenberg M, Slenczka W, Ritt E. Comparative biochemistry of the pyridine nucleotide system in the mitochondria of various organs. Biochem. Z. 1959;332:47–66. [PubMed] [Google Scholar]
- 12.Estabrook RW. Fluorometric measurement of reduced pyridine nucleotide in cellular and subcellular particles. Anal. Biochem. 1962;4:231–245. doi: 10.1016/0003-2697(62)90006-4. [DOI] [PubMed] [Google Scholar]
- 13.Toogood HS, Leys D, Scrutton NS. Dynamics driving function: new insights from electron transferring flavoproteins and partner complexes. FEBS J. 2007;274:5481–5504. doi: 10.1111/j.1742-4658.2007.06107.x. [DOI] [PubMed] [Google Scholar]
- 14.Beckmann JD, Frerman FE. Reaction of electron-transfer flavoprotein with electron-transfer flavoprotein-ubiquinone oxidoreductase. Biochemistry. 1985;24:3922–3925. doi: 10.1021/bi00336a017. [DOI] [PubMed] [Google Scholar]
- 15.Kunz WS, Kunz W. Contribution of different enzymes to flavoprotein fluorescence of isolated rat liver mitochondria. Biochim. Biophys. Acta. 1985;841:237–246. doi: 10.1016/0304-4165(85)90064-9. [DOI] [PubMed] [Google Scholar]
- 16.Huang S, Heikal AA, Webb WW. Two-photon fluorescence spectroscopy and microscopy of NAD(P)H and flavoprotein. Biophys. J. 2002;82:2811–2825. doi: 10.1016/S0006-3495(02)75621-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunz WS. Spectral properties of fluorescent flavoproteins of isolated rat liver mitochondria. FEBS Lett. 1986;195:92–96. doi: 10.1016/0014-5793(86)80137-5. [DOI] [PubMed] [Google Scholar]
- 18.Chorvat D, Jr., Kirchnerova J, Cagalinec M, Smolka J, Mateasik A A, Chorvatova A. Spectral unmixing of flavin autofluorescence components in cardiac myocytes. Biophys. J. 2005;89:L55–57. doi: 10.1529/biophysj.105.073866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunz WS, Gellerich FN. Quantification of the content of fluorescent flavoproteins in mitochondria from liver, kidney cortex, skeletal muscle, and brain. Biochem. Med. Metab. Biol. 1993;50:103–110. doi: 10.1006/bmmb.1993.1051. [DOI] [PubMed] [Google Scholar]
- 20.Vrbacky M, Krijt J, Drahota Z, Melkova Z. Inhibitory effects of Bcl-2 on mitochondrial respiration. Physiol. Res. 2003;52:545–554. [PubMed] [Google Scholar]
- 21.Qiu L, Zhao W, Sick T. Quantitative analysis of brain NADH in the presence of hemoglobin using microfiber spectrofluorometry: a pre-calibration approach. Comput. Biol. Med. 2005;35:583–601. doi: 10.1016/j.compbiomed.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Hustad S, Ueland PM, Schneede J. Quantification of riboflavin, flavin mononucleotide, and flavin adenine dinucleotide in human plasma by capillary electrophoresis and laser-induced fluorescence detection. Clin. Chem. 1999;45:862–868. [PubMed] [Google Scholar]
- 23.Brand MD, Hafner RP, Brown GC. Control of respiration in non-phosphorylating mitochondria is shared between the proton leak and the respiratory chain. Biochem. J. 1988;255:535–539. [PMC free article] [PubMed] [Google Scholar]
- 24.Frerman FE. Reaction of electron-transfer flavoprotein ubiquinone oxidoreductase with the mitochondrial respiratory chain. Biochim. Biophys. Acta. 1987;893:161–169. doi: 10.1016/0005-2728(87)90035-1. [DOI] [PubMed] [Google Scholar]
- 25.Eaton S, Pourfarzam M, Bartlett K. The effect of respiratory chain impairment of beta-oxidation in rat heart mitochondria. Biochem. J. 1996;319(Pt 2):633–640. doi: 10.1042/bj3190633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocheleau JV, Head WS, Piston DW. Quantitative NAD(P)H/flavoprotein autofluorescence imaging reveals metabolic mechanisms of pancreatic islet pyruvate response. J. Biol. Chem. 2004;279:31780–31787. doi: 10.1074/jbc.M314005200. [DOI] [PubMed] [Google Scholar]
- 27.Romashko DN, Marban E, O'Rourke B. Subcellular metabolic transients and mitochondrial redox waves in heart cells. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1618–1623. doi: 10.1073/pnas.95.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaburek M, Costa AD, Burton JR, Costa CL, Garlid KD. Mitochondrial PKC epsilon and mitochondrial ATP-sensitive K+ channel copurify and coreconstitute to form a functioning signaling module in proteoliposomes. Circ. Res. 2006;99:878–883. doi: 10.1161/01.RES.0000245106.80628.d3. [DOI] [PubMed] [Google Scholar]
- 29.Stadnicka A, Marinovic J, Ljubkovic M, Bienengraeber MW, Bosnjak ZJ. Volatile anesthetic-induced cardiac preconditioning. J. Anesth. 2007;21:212–219. doi: 10.1007/s00540-006-0486-6. [DOI] [PubMed] [Google Scholar]
- 30.Riess ML, Eells JT, Kevin LG, Camara AK, Henry MM, Stowe DF. Attenuation of mitochondrial respiration by sevoflurane in isolated cardiac mitochondria is mediated in part by reactive oxygen species. Anesthesiology. 2004;100:498–505. doi: 10.1097/00000542-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka K, Weihrauch D, Ludwig LM, Kersten JR, Pagel PS, Warltier DC. Mitochondrial adenosine triphosphate-regulated potassium channel opening acts as a trigger for isoflurane-induced preconditioning by generating reactive oxygen species. Anesthesiology. 2003;98:935–943. doi: 10.1097/00000542-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Pain T, Yang XM, Critz SD, Yue Y, Nakano A A, Liu GS, Heusch G, Cohen MV, Downey JM. Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circ. Res. 2000;87:460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 33.Andrukhiv A, Costa AD, West IC, Garlid KD. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am. J. Physiol. Heart. Circ. Physiol. 2006;291:H2067–2074. doi: 10.1152/ajpheart.00272.2006. [DOI] [PubMed] [Google Scholar]
- 34.Ludwig LM, Tanaka K, Eells JT, Weihrauch D, Pagel PS, Kersten JR, Warltier DC. Preconditioning by Isoflurane Is Mediated by Reactive Oxygen Species Generated from Mitochondrial Electron Transport Chain Complex III. Anesth. Analg. 2004;99:1308–1315. doi: 10.1213/01.ANE.0000134804.09484.5D. [DOI] [PubMed] [Google Scholar]
- 35.Batandier C, Guigas B, Detaille D, El-Mir MY, Fontaine E, Rigoulet M, Leverve XM. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J. Bioenerg. Biomembr. 2006;38:33–42. doi: 10.1007/s10863-006-9003-8. [DOI] [PubMed] [Google Scholar]
- 36.Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- 37.Drose S, Brandt U. The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J. Biol. Chem. 2008;283:21649–21654. doi: 10.1074/jbc.M803236200. [DOI] [PubMed] [Google Scholar]