Abstract
Mutations of the VHL tumor suppressor gene occur in patients with VHL disease and in the majority of sporadic clear cell renal carcinomas (VHL−/− RCC). Loss of VHL protein function is associated with constitutive expression of mRNAs encoding hypoxia-inducible proteins, such as vascular endothelial growth factor. Overproduction of angiogenic factors might explain why VHL−/− RCC tumors are so highly vascularized, but whether this overproduction is sufficient for oncogenesis still remains unknown. In this report, we examined the activity of transforming growth factor-α (TGF-α), another VHL-regulated growth factor. We show that TGF-α mRNA and protein are hypoxia-inducible in VHL−/− RCC cells expressing reintroduced VHL. In addition to its overexpression by VHL−/− RCC cells, TGF-α can also act as a specific growth-stimulatory factor for VHL−/− RCC cells expressing reintroduced wild-type VHL, as well as primary renal proximal tubule epithelial cells, the likely site of origin of RCC. This role is in contrast to those of other growth factors overexpressed by VHL−/− RCC cells, such as vascular endothelial growth factor and TGF-β1, which do not stimulate RCC cell proliferation. A TGF-α-specific antisense oligodeoxynucleotide blocked TGF-α production in VHL−/− RCC cells, which led to the dependence of those cells on exogenous growth factors to sustain growth in culture. Growth of VHL−/− RCC cells was also significantly reduced by a drug that specifically inhibits the epidermal growth factor receptor, the receptor through which TGF-α stimulates proliferation. These results suggest that the generation of a TGF-α autocrine loop as a consequence of VHL inactivation in renal proximal tubule epithelial cells may provide the uncontrolled growth stimulus necessary for the initiation of tumorigenesis.
VHL disease is an autosomal dominantly inherited cancer syndrome. Inheritance of a mutated form of the VHL tumor suppressor predisposes individuals to development of a wide variety of tumors in target tissues such as the kidneys, central nervous system, and retinas (1–3). Tumors arise from cells in which the remaining wild-type copy of VHL acquires a somatic inactivating mutation. Biallelic inactivating mutations of the VHL gene are also found in most sporadic clear cell renal cell carcinoma (RCC; hereafter referred to as VHL−/− RCC cells) (1–3), the most common malignancy of the kidney. Reintroduction of wild-type VHL protein in VHL−/− RCC cell lines (hereafter referred to as VHL-positive RCC cells) results in growth suppression of tumors in nude mice (4), consistent with the putative gatekeeper tumor suppressor function of VHL (5).
VHL is a 213-aa protein that forms a stable complex with four other proteins, elongin B, elongin C, cullin-2, and rbx-1, a complex that displays E3-ubiquitin ligase activity in vitro (6–9). The α subunits of hypoxia-inducible transcription factors-1 and -2 α (referred to as HIFα) are substrates for VHL-directed ubiquitination and degradation (10–14). It is suggested that the VHL complex mediates the degradation of HIFα at normal oxygen levels (normoxia), whereas HIFα degradation is inhibited in hypoxia, leading to an induction of a hypoxic response. In VHL−/− RCC, HIFα accumulates to high levels regardless of oxygen concentration, causing a constitutive overexpression of HIFα-regulated target genes such as VEGF and other angiogenic factors. Angiogenic factors play a physiological role in the recruitment of blood vessels, and their overexpression may well explain why VHL−/− RCC tumors are highly vascularized. However, overexpression of molecules exclusively involved in angiogenesis is unlikely to fully account for the tumorigenesis that follows the loss of VHL function. It was recently shown that the well-characterized VHL−/− human RCC cell line 786-0 showed increased expression of another secreted growth and angiogenic factor, transforming growth factor-α (TGF-α). On reintroduction of wild-type VHL, TGF-α levels decreased, indicating that TGF-α is also negatively regulated by VHL (15). An interesting characteristic of TGF-α is that aside from its established role in angiogenesis (16), it can also act as a specific growth-stimulatory factor for primary renal proximal tubule epithelial cells (RPTECs; refs. 17 and 18), which are thought to give rise to RCC. In situ hybridization studies have revealed that RCC tumors overexpress TGF-α mRNA compared with adjacent normal tissue (19–20), supporting a role for TGF-α in RCC proliferation in vivo (21–24). We recently found that VHL−/− RCC cells, like most cancer cells, grow even under serum-deprived conditions, whereas reintroduction of wild-type VHL restored their serum-dependent growth and ability to properly exit the cell cycle on serum withdrawal (25). However, both VHL-positive and VHL-negative RCC cells cease to proliferate under conditions of contact inhibition (25). These results suggest that the overexpression of a secreted factor may be responsible for the altered growth characteristics of VHL−/− RCC cells. Therefore, we hypothesized that VHL inactivation and resulting TGF-α overproduction may be the event that confers growth advantage on RCC cells.
To test this hypothesis, we used VHL−/− and VHL-positive RCC cells and tested their growth characteristics in culture and dependence on TGF-α for proliferation. We found that TGF-α mRNA is hypoxia-inducible in VHL-positive cells, in a manner reminiscent of VEGF. VHL−/− RCC cells were able to proliferate in the absence of exogenous growth factors, whereas VHL-positive RCC cells were dependent on exogenous TGF-α for growth. VHL−/− RCC cells grew in the absence of exogenous growth factors but displayed a reduced ability to proliferate when treated with an antisense oligodeoxynucleotide against TGF-α. Growth of VHL−/− RCC cells was also significantly reduced by a drug that inhibits the epidermal growth factor receptor (EGFR), the receptor through which TGF-α stimulates growth. These results indicate that loss of VHL function and subsequent TGF-α overexpression cause the establishment of an autocrine TGF-α/EGFR stimulatory system leading to the formation of RCC, providing the first link between VHL and growth control.
Materials and Methods
Cells and Cell Culture.
The human sporadic RCC cell line 786-0 contains a single mutated VHL allele predicted to encode a truncated VHL protein (amino acids 1–104; ref. 4). 786-0 cells were transfected with a cytomegalovirus-based expression vector containing wild-type VHL (WT8 cells) as described elsewhere (4). These cell lines as well as A498, another VHL−/− RCC cell line, were maintained in DMEM containing 10% FBS. Growth factors (Calbiochem) were added to cell cultures at the indicated concentrations. Primary cultures of human RPTECs (Clonetics, San Diego) were maintained in defined medium recommended by the supplier.
Flow Cytometry and BrdUrd Labeling.
Cells were plated at low density and incubated for at least 16 h in DMEM plus 10% FBS, or for the primary cultures in defined growth media. Cells were washed in PBS and incubated under various growth conditions, as indicated. For flow cytometry, cells were incubated with 10 μM BrdUrd for the indicated time, trypsinized, washed, and fixed in 70% ethanol at −20°C. Cells were then washed in PBS, incubated with a solution containing an anti-BrdUrd antibody (Roche Molecular Biochemicals) for 30 min at 37°C, washed, and incubated with an anti-mouse secondary FITC-conjugated antibody. Cells were then incubated in the presence of 50 μg/ml RNase A for 30 min at 4°C followed by incubation for 30 min with 2 μg/ml propidium iodide at 4°C. Cells were analyzed with the use of a fluorescence-activated cell sorter (FACScan) and cell quest software. In some experiments, cells were grown on coverslips and incubated for the indicated time in the presence of 10 μM BrdUrd. Cells were fixed in 70% ethanol in 50 mM glycine (pH 2.0) for at least 30 min at −20°C. Cells were washed and incubated with a solution containing an anti-BrdUrd antibody for 30 min at 37°C and washed and incubated with an anti-mouse FITC-conjugated secondary antibody. Cells were counterstained with Hoechst reagent to identify all nuclei and the percentage of BrdUrd-labeled cells (FITC-stained cells/Hoechst-stained cells) was determined with a Zeiss fluorescence microscope and digital imaging.
Antisense Oligonucleotide Experiments.
Phosphorothioate-modified 23-mer antisense oligodeoxynucleotide (5′-TCCAGCCGAGGGGACCATTTTAC-3′), targeted against the initiation (AUG) codon of TGF-α mRNA, was as described previously (26). A random oligodeoxynucleotide of the same base composition as the antisense (5′-GATGAATTACGTAACTGGCCCGCCC-3′) was used as a control (26). Oligodeoxynucleotides were added at a final concentration of 12.5 μM.
Cell Hypoxia Treatment and Reverse Transcription–PCR.
RCC cells were grown on 100-mm tissue culture dishes in DMEM plus 10% FBS in a humidified 10% CO2/room air atmosphere. When cultures reached ≈60–75% confluence, the medium was replenished, and parallel cultures were either maintained in 10% CO2/room air or transferred to a chamber connected to a 10% CO2/1% O2 supply tank. Cultures were maintained for an additional 16 h, and total RNA was harvested. Total RNA (1 μg) was used for reverse transcription with random primers and a first-strand cDNA kit (Life Technologies, Grand Island, NY). PCR was performed with 1 μl of the first-strand cDNA product in a reaction volume of 50 μl. PCR reactions contained 1× PCR buffer; 2.5 units Taq polymerase (Perkin–Elmer); 200 μM each of dGTP, dATP, dCTP, and TTP; and 10 pmol of each primer. TGF-α primers used to amplify a 533-bp fragment were as follows: sense, 5′-CTGCTGCCCGCCCGCCCGGTAAAATGGT-3′; antisense, 5′-CACCTGGCCAAACTCCTCCTCTGGGCT-3′. Amplification conditions were 95°C, 2 min; 22 cycles of 95°C, 30 s, 68°C, 1 min, 72°C, 1 min; and a final extension of 72°C, 10 min. Primers for β-actin amplification and reaction conditions were described previously (27). These conditions were within the linear range of TGF-α and β-actin amplification (data not shown). Products were analyzed on a 3% NuSieve 3:1 agarose gel (FMC) and photographed on a Bio-Rad gel documentation system.
Measurement of TGF-α Expression in Cell Lysates.
VHL−/− and VHL-positive RCC cells were plated approaching confluence, in DMEM and 10% FCS. After 24 h cells were washed with PBS and maintained in DMEM and ITS (insulin, transferrin, and selenium) for 24 h. Cells were then transferred to hypoxic conditions (1% O2/10% CO2) or remained in normoxic conditions (10% CO2 plus room air) for 24 h before harvesting supernatants. Or VHL−/− cells were treated with antisense or random oligodeoxynucleotide before supernatants were harvested. Cells were washed, trypsinized, counted, and lysed according to ELISA kit instructions (Calbiochem). ELISA was performed on 100,000 cells per assay, as described in the manufacturer's protocol.
Tyrosine Kinase Inhibition Experiments.
PD153035 (Calbiochem), an EGFR tyrosine kinase inhibitor (28), and AG1296 (Calbiochem), a platelet-derived growth factor receptor and FGF receptor tyrosine kinase inhibitor (29), were resuspended in DMSO and added to VHL−/− RCC cells maintained in DMEM and ITS to achieve concentrations of 1 μM (PD153035) and 50 μM (AG1296).
Western Blot Analysis.
Cells were washed with PBS and lysed in 4% SDS in PBS, and the DNA was sheared with a 20-gauge needle. Protein concentration was quantified with a bicinchoninic acid assay (Pierce), and 200 μg of protein was analyzed on an SDS/8% polyacrylamide gel. Proteins were transferred to a poly(vinylidene difluoride) membrane (NEN). Before immunodetection the membrane was blocked in 3% (wt/vol) skim milk powder (Carnation, Glendale, CA) in a 0.2% Tween PBS solution for 1 h. Subsequently, membranes were incubated with a polyclonal anti-HIF2 α antibody (1:1000; Novus, Littleton, CO). After washing with 0.2% Tween PBS solution, membranes were incubated for 1 h with an anti-rabbit secondary antibody conjugated to horseradish peroxidase (1:5,000; Jackson ImmunoResearch) and detected by enhanced chemiluminescence (Pierce).
Fibronectin Deposition.
Detection of fibronectin deposition was essentially as described (30). Briefly, cells were placed on coverslips and maintained at confluency in stated conditions for 6 days. Cells were washed with PBS and fixed in 95% EtOH for 30 min at −80°C. Coverslips were air-dried at 4°C and then bathed in PBS. The coverslips were incubated in a solution (1% Triton, 10% FCS in PBS) containing the anti-fibronectin antibody (1:400; Dako) for 1 h. After washing with PBS coverslips were incubated with anti-rabbit secondary antibody labeled with cy3 (Jackson ImmunoResearch). Coverslips were counterstained with Hoecsht to identify all nuclei, and fibronectin deposition was assessed with a fluorescence microscope.
Results
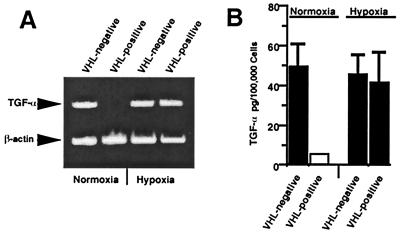
VHL acts as a negative regulator of TGF-α mRNA (15) as well as hypoxia-inducible mRNAs such as VEGF in normoxic conditions (31–33). We determined whether TGF-α mRNA and protein could also be induced by hypoxia in VHL−/− RCC cells that express reintroduced wild-type VHL. TGF-α mRNA expression was evaluated by reverse transcription–PCR with RNA isolated from 786-0 RCC cells expressing either wild type or a mutated, nonfunctional form of VHL. Cells were grown under either normoxic or hypoxic (1% O2) conditions for 16 h, as described previously (32). VHL−/− RCC cells overexpressed TGF-α mRNA relative to VHL-positive cells in normoxic growth conditions (Fig. 1A). TGF-α mRNA levels were elevated in VHL-positive cells grown under hypoxic conditions to levels similar to those of VHL−/− RCC cells. There was a concomitant change in TGF-α protein levels, as determined by ELISA (Fig. 1B). Therefore, TGF-α mRNA can be added to the list of hypoxia-inducible mRNA regulated by VHL.
Figure 1.
TGF-α mRNA and protein is hypoxia-inducible in VHL-positive RCC cells. (A) Semiquantitative reverse transcription–PCR was performed with primers specific for TGF-α or β-actin. RNA was prepared from VHL−/− RCC cells expressing wild-type VHL (VHL-positive) or mutated forms of VHL (VHL−/−). Cells were grown under either normoxic conditions (10% CO2 plus room air) or subjected to hypoxia (1% O2/10% CO2) for 16 h before harvesting of total cellular RNA. (B) TGF-α protein production as measured by ELISA (Calbiochem). VHL−/− and VHL-positive RCC cells were maintained in ITS for 24 h. Cells were then transferred to hypoxic conditions or remained in normoxic conditions for 24 h before supernatants were harvested. Cells were washed, trypsinized, counted, and lysed according to ELISA kit instructions. Protein concentration per 100,000 cells was then assayed. Shown is the mean of three independent experiments; the SEM is indicated. The white box indicates that TGF-α levels were below the detection limit.
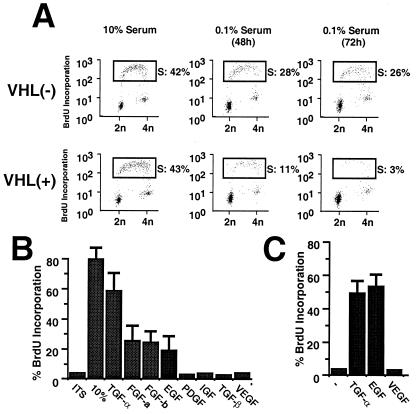
Exogenous TGF-α stimulates growth of RPTECs (17), which are believed to give rise to RCC. We attempted to determine whether the reintroduction of VHL in VHL−/− RCC cells restored their requirement for exogenous TGF-α for growth in culture. VHL−/− and VHL-positive RCC cells were grown at low density for 24 h in media supplemented with 10% FBS, and the cultures were washed and incubated for 0, 48, or 72 h in the presence of medium supplemented with ITS only. Growth rates and cell cycle profiles were measured with the use of either a BrdUrd incorporation assay or a double labeling method that consisted of BrdUrd incorporation and propidium iodine staining followed by flow cytometry. VHL−/− and VHL-positive RCC cells proliferated at similar rates when cultured in 10% FBS. However, when incubated in ITS (insulin, transferrin, and selenium) alone, VHL-positive RCC cells ceased to proliferate and became quiescent, whereas VHL−/− RCC cells continued to incorporate BrdUrd and showed DNA content greater than 2N (Fig. 2A). We then investigated which growth factors would be able to stimulate quiescent VHL-positive RCC cells to reenter the cell cycle. VHL-positive RCC cells were incubated for 72 h in media supplemented with ITS, washed, and then incubated in medium containing ITS alone, medium supplemented with 10% FBS, or medium supplemented with various recombinant growth factors for an additional 24 h. ITS alone was insufficient to stimulate quiescent VHL-positive RCC cells to incorporate BrdUrd, whereas a maximal stimulation was observed with 10% FBS, with approximately 80% of VHL-positive RCC cells incorporating BrdUrd (Fig. 2B). After 24 h of stimulation with TGF-α approximately 60% of quiescent VHL-positive RCC cells showed BrdUrd incorporation, representing about 75% of the effect seen with 10% FBS. Acidic or basic FGF, as well as EGF, was also able to stimulate proliferation of quiescent VHL-positive RCC cells. Growth factors such as platelet-derived growth factor-AB, insulin-like growth factor, TGF-β1, or VEGF exhibited no detectable growth-stimulatory effect on quiescent VHL-positive RCC cells (Fig. 2B and data not shown). The growth factor dependence of primary RPTEC cultures was also examined in these studies. Similar to our findings for VHL-positive RCC cells, TGF-α and EGF, but not VEGF, stimulated proliferation of RPTECs (Fig. 2C). No difference were observed when dose responses were determined with the use of up to 10-fold greater levels of growth factor (data not shown). These results demonstrate that reintroduction of wild-type VHL into VHL−/− RCC cells restored not only the dependence on serum for growth in tissue culture, but also their requirement for specific growth factors, including TGF-α.
Figure 2.
Reintroduction of wild-type VHL in VHL−/− RCC cells restores their dependency on specific growth factors to proliferate in culture. (A) Serum requirement for proliferation in culture of VHL−/− and VHL-positive RCC cells analyzed by two-dimensional flow cytometry. Cells were plated overnight in DMEM containing 10% FBS, washed, and then incubated in DMEM supplemented with 0.1% FBS for 48 or 72 h. Cells were then incubated for 2 h in the presence of 10 μM BrdUrd before fixation. Cells were prepared for two-dimensional flow cytometry by incubation with an anti-BrdUrd antibody and propidium iodine. Boxes indicate cell populations in S phase, and the percentages of cells in S phase are indicated. Similar results were seen after ITS was substituted for 0.1% FBS (data not shown). (B) Effects of addition of various growth factors on quiescent VHL-positive cells. VHL-positive cells were incubated for 72 h in the presence of ITS. Cells were washed and incubated for 24 h in the presence of DMEM supplemented with ITS, ITS + 10% FBS, and ITS with different growth factors: TGF-α (2.0 nM; 10 ng/ml), acidic FGF (0.5 nM; 10 ng/ml), basic FGF (0.5 nM; 10 ng/ml), EGF (2 nM; 10 ng/ml), insulin-like growth factor-1 (3 nM; 20 ng/ml), platelet-derived growth factor-AB (0.5 nM; 25 ng/ml), and TGF-β1 (0.5 nM; 5 ng/ml), as suggested by the manufacturer (Calbiochem). BrdUrd was added to the media 16 h before cells were analyzed by flow cytometry. Experiments were performed two times under the conditions described and one time with 5-fold higher growth factor concentrations, which did not significantly affect the relative growth-stimulatory activity shown here. (C) Dependence on TGF-α or EGF for RPTEC proliferation. RPTECs were grown in reconstituted media as described by the manufacturer (Clonetics). Cells were grown for 48 h in the presence of EGF as provided by the manufacturer, or EGF was replaced by TGF-α (2.0 nM; 10 ng/ml) or VEGF (1 nM; 50 ng/ml). A minus sign indicates cells that were grown in the absence of exogenous growth factors. Cells were incubated for 3–4 h in the presence of BrdUrd before fixing and staining. Error bars represent SEM.
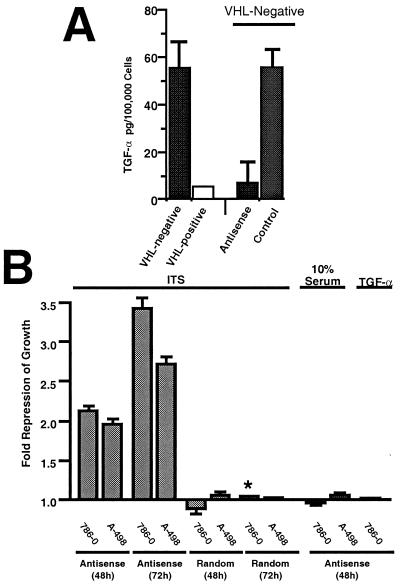
Results presented in Fig. 2 suggest that VHL-positive RCC cells require exogenous growth factors, including TGF-α, to grow in culture. Because VHL−/− RCC cells overproduce TGF-α (but not EGF or FGF), we asked whether this overproduction might explain why these cells are able to grow in culture in the absence of serum or exogenous growth factors. Two VHL−/− RCC cell lines (786-0 and A-498) were grown in ITS or 10% FBS in the presence of an antisense oligodeoxynucleotide to TGF-α, or a control, random oligodeoxynucleotide (26). ELISA experiments revealed that treatment with the antisense TGF-α oligodeoxynucleotide reduced levels of TGF-α overproduced by VHL−/− 786-0 RCC cells to levels comparable to those of VHL-positive cells, whereas the control oligodeoxynucleotide had no effect (Fig. 3A). VHL−/− 786-0 and A-498 RCC cells grown in ITS in the presence of the antisense TGF-α oligodeoxynucleotide showed approximately a 2.2-fold and 1.8-fold repression of proliferation after 48 h, respectively, as compared with VHL−/− RCC cells grown in ITS, but without addition of oligodeoxynucleotide (Fig. 3B). A stronger growth-inhibitory effect was observed after 72 h of incubation in ITS in the presence of the antisense oligodeoxynucleotide. The control oligodeoxynucleotide had no effect on the proliferation of the VHL−/− RCC cells grown in either ITS. Expectedly, VHL−/− RCC cells incubated with the antisense TGF-α oligodeoxynucleotide in the presence of 10% FBS or purified TGF-α continued to proliferate in a manner similar to that of cells treated with the control oligodeoxynucleotide or untreated cells. Therefore, antisense TGF-α oligodeoxynucleotide treatment induced a specific inhibition of the proliferation of VHL−/− RCC cells when they were grown in culture in the absence of exogenous growth factors, an effect that was abolished on the addition of serum or purified TGF-α (Fig. 3B). We also noticed several other striking effects of the antisense TGF-α oligodeoxynucleotide on VHL−/− RCC cells grown in ITS. These cells showed a flatter morphology and survived longer than those cells incubated with the control oligodeoxynucleotide or untreated cells (data not shown). The most likely explanation for these findings is that the VHL−/− RCC cells that were treated with antisense TGF-α oligodeoxynucleotide entered quiescence and survived, whereas the untreated or control RCC cells continued to proliferate to a point where the growth medium was exhausted and the cells eventually died. These observations are similar to those previously reported for VHL−/− RCC cells expressing wild-type VHL, which showed longer survival times in the absence of serum compared with VHL−/− 786-0 or A-498 RCC cells (25).
Figure 3.
Effects of TGF-α antisense oligodeoxynucleotide treatment on the growth of VHL−/− RCC cells. (A) Effect of antisense oligonucleotide on TGF-α protein production as measured by ELISA (Calbiochem). VHL−/− RCC cells were incubated for 48 h in ITS with 12.5 μM antisense oligodeoxynucleotide to TGF-α or 12.5 μM random control oligodeoxynucleotide, as described in Materials and Methods. Cells were washed, trypsinized, counted, and lysed according to ELISA kit instructions. TGF-α protein concentration per 100,000 cells was then assayed. Shown is the mean of three independent experiments; the SEM is indicated. Data for untreated VHL-positive and VHL−/− RCC cells incubated in ITS for 48 h are also shown. The white box indicates that TGF-α levels were below the detection limit of the assay. (B) VHL−/− RCC cells were incubated for 48 or 72 h in ITS or 10% FBS with 12.5 μM antisense oligodeoxynucleotide to TGF-α or 12.5 μM random control oligodeoxynucleotide, as described in Materials and Methods. BrdUrd was added during the last 3 h before analysis. Cells were then fixed and stained with anti-BrdUrd and Hoescht reagent. Ratios of BrdUrd-positive nuclei/Hoescht-stained nuclei were compared with VHL−/− RCC cells incubated for 48 h in ITS, but without any oligodeoxynucleotide. Notice that the 72-h data obtained for 786-0 cells with the antisense to TGF-α oligodeoxynucleotide (indicated with an asterisk) are compared with 48-h untreated 786-0 cells, because there was significant cell death for untreated 786-0 cells at 72 h. Error bars represent the SEM of three to nine independent assays.
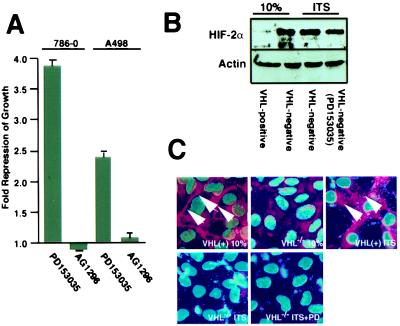
TGF-α binds to the epidermal growth factor receptor (EGFR) and subsequently stimulates growth through the activation of the EGFR tyrosine kinase activity. Consequently, drugs that specifically inhibit EGFR tyrosine kinase activity would be predicted to have a growth-inhibitory effect on VHL−/− RCC cells similar to those observed with the antisense oligodeoxynucleotide to TGF-α. VHL−/− 786-0 and A-498 RCC cells showed about a 2.5- to 3-fold reduction of growth in ITS in the presence of an EGFR inhibitor compared with untreated cells (Fig. 4A). In contrast, growth of VHL−/− RCC cells in ITS was unaffected by an inhibitor of platelet-derived growth factor receptor and FGF receptor tyrosine kinase activity. VHL−/− RCC cells overproduce HIFα and are unable to assemble an extracellular fibronectin matrix (30). We asked whether blocking the TGF-α/EGFR autocrine loop with the EGFR inhibitor, which has an effect on the growth of VHL−/− RCC cells in ITS, would also have an effect on these defects of VHL−/− RCC cells. Western blot analysis revealed that the EGFR tyrosine kinase inhibitor did not have a significant effect on levels of HIFα in VHL−/− 786-0 RCC cells (Fig. 4B). Furthermore, the EGFR inhibitor failed to restore the ability of these cells to produce an extracellular fibronectin matrix (Fig. 4C). These results suggest that blocking the TGF-α/EGFR growth stimulatory activity specifically corrected the loss of growth control of VHL−/− RCC cells in low serum without correcting other defects associated with the loss of VHL function.
Figure 4.
Effects of EGF-R tyrosine kinase inhibitor treatment on the growth of VHL−/− RCC cells in low serum. (A) Proliferation of VHL−/− RCC cells treated with tyrosine kinase inhibitors was measured by BrdUrd labeling. Cells were plated overnight in DMEM supplemented with 10% FCS, washed, and then incubated in DMEM supplemented with ITS in the presence of either PD153035 (1 μM) or AG1296 (50 μM) for 48 h. BrdUrd was added during the last 3 h before analysis. Cells were then fixed and stained with anti-BrdUrd and Hoecsht reagent. Ratios of BrdUrd-positive nuclei/Hoecsht-stained nuclei were compared with VHL−/− RCC cells incubated for 48 h in ITS. Shown is the mean of three fields counted for at least three independent experiments; the SEM is indicated. (B) VHL−/− RCC cells and VHL-positive cells were grown in DMEM supplemented with 10% FCS, ITS, or ITS and PD153035 (1 μM) for 48 h. HIFα levels were determined by immunoblot analysis. (C) VHL−/− RCC cells and VHL-positive cells were grown at confluency in DMEM supplemented with 10% FCS, ITS, or ITS and PD153035 (1 μM) for 6 days. Nuclear areas were revealed by Hoecsht stain, and fibronectin deposition was determined by immunofluorescence and is identified by arrows. Images were superimposed with the use of adobe photoshop.
Discussion
Gatekeeper tumor suppressor proteins are thought to be directly involved in the control of cell growth or death and are likely to be central to the tumor initiation process (5). It has been hypothesized that VHL may exert gatekeeper function in RPTECs, the cells believed to give rise to RCC (25). Progress has been made in the understanding of VHL cellular function and its role in the control of hypoxia-inducible factors and angiogenesis. Although the overexpression of hypoxia-inducible genes such as VEGF may explain the high degree of vascularization of VHL−/− RCC tumors, VEGF itself does not stimulate RPTEC or RCC cell growth. Results presented here identify TGF-α as a bona fide growth factor that confers growth advantage on RCC cells on the loss of VHL function. Dependence on TGF-α by primary RPTEC proliferation in culture has previously been demonstrated (17), as has regulation of TGF-α expression by VHL (15). We have shown here that TGF-α mRNA is hypoxia-inducible in RCC cells reexpressing wild-type VHL, in a manner similar to that of VEGF (31–33). It is likely that loss of normal regulatory controls for TGF-α or VEGF expression in VHL−/− RCC cells occurs through a common mechanism and may not require the loss of two independent functional pathways. Therefore, we propose a mechanism by which the loss of VHL function causes RCC cells to undergo a constitutive hypoxia-like response, most likely the consequence of stabilization of HIFα (Fig. 5). This stabilization of HIFα causes the accumulation of factors, such as VEGF and TGF-α, that would otherwise only be induced on hypoxic conditions. Because RPTECs are sensitive to TGF-α for proliferation, we suggest that overproduction of TGF-α might be the event that promotes growth of RCC cells through an autocrine TGF-α/EGFR circuit. Reintroduction of VHL into VHL−/− RCC cells serves to restore the requirement for exogenous growth factors for proliferation in culture by negatively regulating levels of TGF-α in normoxia. This effect appears to be specific, inasmuch as TGF-α but not other growth factors that are also regulated by VHL (e.g., VEGF, TGF-β1) stimulated proliferation of VHL-positive RCC cells. These results also suggest that the loss of VHL function does not cause an intrinsic cellular defect in growth control per se. Consistent with this model is the observation that interfering with the TGF-α/EGFR circuit significantly reduced the ability of VHL−/− RCC cells to grow in low serum without correcting other cellular defects associated with the loss of VHL function, such as HIFα overexpression and failure to assemble an extracellular fibronectin matrix. Inhibition of the EGFR receptor also prevents RCC tumor formation in nude mice, in agreement with our data (34, 35). However, it remains to be shown whether the unregulated growth of VHL−/− RCC cells is solely the consequence of the establishment of a TGF-α/EGFR growth-stimulatory circuit or whether other defects are involved in this process. It will be important to determine whether other VHL-related tumors, such as hemangioblastomas of the retinas or central nervous system, may also be initiated through a TGF-α autocrine circuit.
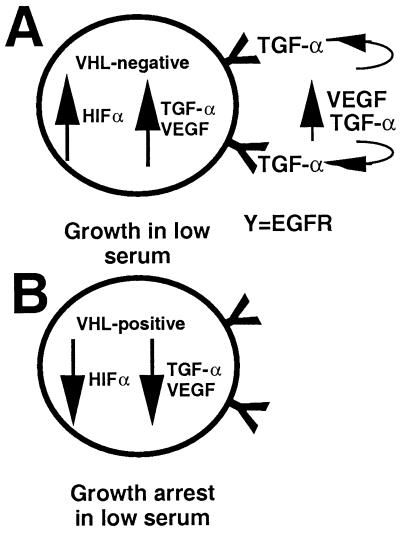
Figure 5.
Model of TGF-α as the factor that might confer a growth advantage on RCC cells on the loss of VHL function. (A) VHL−/− RCC cells abnormally overproduce factors, such as TGF-α and VEGF, that would otherwise accumulate only in hypoxic conditions. This overproduction may be because of HIFα stabilization. Because RPTECs are sensitive to TGF-α but not to VEGF for growth, we suggest that these cells might engage in a TGF-α-mediated autocrine/paracrine circuit loop that can stimulate their growth. This loop might be an important oncogenic event on the loss of VHL function, explaining why VHL−/− RCC cells are able to grow in low serum. (B) Reintroduction of VHL into VHL−/− RCC causes levels of TGF-α to be down-regulated, possibly explaining why these cells do not grow in low serum and do not form tumors in nude mice.
The results presented here support a model that VHL acts as a gatekeeper in RPTECs by regulating levels of a bona fide growth factor, TGF-α. Although it has not yet been determined whether additional growth factors may be overproduced by VHL−/− RCC cells, our data may provide a basis for studies focusing on TGF-α activity in VHL disease and related sporadic tumors.
Acknowledgments
This work was supported in part by grants from the Medical Research Council of Canada and the Kidney Foundation of Canada (to S.L.) and grants from the von Hippel–Lindau Family Alliance and the Murray Foundation (to J.R.G.) A.P. was supported by the Max Planck Society. S.L. is a Scholar of the Medical Research Council.
Abbreviations
- TGF
transforming growth factor
- RCC
renal cell carcinoma
- VHL
von Hippel–Lindau
- FGF
fibroblast growth factor
- EGF
epidermal growth factor
- EGFR
EGF receptor
- HIFα
α-subunit of hypoxia-inducible factor
- ITS
insulin, transferrin, and selenium
- VEGF
vascular endothelial growth factor
- RPTECs
renal proximal tubule epithelial cells
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.031587498.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.031587498
References
- 1.Humphrey J S, Klausner R D, Linehan W M. In: Diagnosis and Treatment of Genitourinary Malignancies. Pienta K J, editor. Boston: Kluwer; 1996. pp. 13–39. [Google Scholar]
- 2.Linehan W M, Lerman M I, Zbar B. J Am Med Assoc. 1995;273:564–570. [PubMed] [Google Scholar]
- 3.Gnarra J R, Duan D R, Weng Y, Humphrey J S, Chen D Y, Lee S, Pause A, Dudley C F, Latif F, Kuzmin I. Biochim Biophys Acta. 1996;1242:201–210. doi: 10.1016/0304-419x(95)00012-5. [DOI] [PubMed] [Google Scholar]
- 4.Iliopoulos O, Kibel A, Gray S, Kaelin W G., Jr Nat Med. 1995;1:822–826. doi: 10.1038/nm0895-822. [DOI] [PubMed] [Google Scholar]
- 5.Kinzler K W, Vogelstein B. Nature (London) 1997;386:761–763. doi: 10.1038/386761a0. [DOI] [PubMed] [Google Scholar]
- 6.Lisztwan J, Imbert G, Wirbelauer C, Gstaiger M, Krek W. Genes Dev. 1999;13:1882–1833. doi: 10.1101/gad.13.14.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwai K, Yamanaka K, Kamura T, Minato N, Conaway R C, Conaway J W, Klausner R D, Pause A. Proc Natl Acad Sci USA. 1999;96:12436–12441. doi: 10.1073/pnas.96.22.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tyers M, Rottapel R. Proc Natl Acad Sci USA. 1999;96:12230–12232. doi: 10.1073/pnas.96.22.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamura T, Koepp D M, Conrad M N, Skowyra D, Moreland R J, Iliopoulos O, Lane W S, Kaelin W G, Jr, Elledge S J, Conaway R C, et al. Science. 1999;284:657–661. doi: 10.1126/science.284.5414.657. [DOI] [PubMed] [Google Scholar]
- 10.Maxwell P H, Wiesener M S, Chang G W, Clifford S C, Vaux E C, Cockman M E, Wykoff C C, Pugh C W, Maher E R, Ratcliffe P J. Nature (London) 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 11.Ohh M, Park C W, Ivan M, Hoffman M A, Kim T Y, Huang L E, Pavletich N, Chau V, Kaelin W G., Jr Nat Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 12.Cockman M E, Masson N, Mole D R, Jaakkola P, Chang G W, Clifford S C, Maher E R, Pugh C W, Ratcliffe P J, Maxwell P H. J Biol Chem. 2000;275:25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- 13.Tanimoto K, Makino Y, Pereira T, Poellinger L. EMBO J. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway R C, Conaway J W. Proc Natl Acad Sci USA. 2000;97:10430–10445. doi: 10.1073/pnas.190332597. . (First Published September 5, 2000; 10.1073/pnas.190332597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knebelmann B, Ananth S, Cohen H T, Sukhatme V P. Cancer Res. 1998;58:226–231. [PubMed] [Google Scholar]
- 16.Lee D C, Fenton S E, Berkowitz E A, Hissong M A. Pharmacol Rev. 1995;47:51–85. [PubMed] [Google Scholar]
- 17.Humes H D, Beals T F, Cieslinski D A, Sanchez I O, Page T P. Lab Invest. 1991;64:538–545. [PubMed] [Google Scholar]
- 18.Gomella L G, Sargent E R, Wade T P, Anglard P, Linehan W M, Kasid A. Cancer Res. 1989;49:6972–6975. [PubMed] [Google Scholar]
- 19.Mydlo J H, Michaeli J, Cordon-Cardo C, Goldenberg A S, Heston W D, Fair W R. Cancer Res. 1989;49:3407–3411. [PubMed] [Google Scholar]
- 20.Derynck R, Goeddel D V, Ullrich A, Gutterman J U, Williams R D, Bringman T S, Berger W H. Cancer Res. 1987;47:707–712. [PubMed] [Google Scholar]
- 21.Perera A D, Kleymenova E V, Walker C L. Clin Cancer Res. 2000;6:1518–1523. [PubMed] [Google Scholar]
- 22.Petrides P E, Bock S, Bovens J, Hofmann R, Jakse G. Cancer Res. 1990;50:3934–3939. [PubMed] [Google Scholar]
- 23.Atlas I, Mendelsohn J, Baselga J, Fair W R, Masui H, Kumar R. Cancer Res. 1992;52:3335–3339. [PubMed] [Google Scholar]
- 24.Walker C, Everitt J, Freed J J, Knudson A G, Whiteley L O. Cancer Res. 1991;51:2973–2978. [PubMed] [Google Scholar]
- 25.Pause A, Lee S, Lonergan K M, Klausner R D. Proc Natl Acad Sci USA. 1998;95:993–998. doi: 10.1073/pnas.95.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sizeland A M, Burgess A W. Mol Biol Cell. 1992;3:1235–1243. doi: 10.1091/mbc.3.11.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gnarra J R, Tory K, Weng Y, Schmidt L, Wei M H, Li H, Latif F, Liu S, Chen F, Duh F M, et al. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 28.Fry D W, Kraker A J, McMichael A, Ambroso L A, Nelson J M, Leopold W R, Connors R W, Bridges A J. Science. 1994;265:1093–1095. doi: 10.1126/science.8066447. [DOI] [PubMed] [Google Scholar]
- 29.Kovalenko M, Gazit A, Bohmer A, Rorsman C, Ronnstrand L, Heldin C H, Waltenberger J, Bohmer F D, Levitzki A. Cancer Res. 1994;54:6106–6114. [PubMed] [Google Scholar]
- 30.Ohh M, Yauch R L, Lonergan K M, Whaley J M, Stemmer-Rachamimov A O, Louis D N, Gavin B J, Kley N, Kaelin W G, Jr, Iliopoulos O. Mol Cell. 1998;7:959–968. doi: 10.1016/s1097-2765(00)80096-9. [DOI] [PubMed] [Google Scholar]
- 31.Gnarra J R, Zhou S, Merrill M J, Wagner J R, Krumm A, Papavassiliou E, Oldfield E H, Klausner R D, Linehan W M. Proc Natl Acad Sci USA. 1996;93:10589–10594. doi: 10.1073/pnas.93.20.10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iliopoulos O, Levy A P, Jiang C, Kaelin W G, Jr, Goldberg M A. Proc Natl Acad Sci USA. 1996;93:10595–10599. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seimeister G, Weidel K, Mohrs K, Barleon B, Martiny-Baron G, Marne D. Cancer Res. 1996;56:2299–2301. [PubMed] [Google Scholar]
- 34.Ciardiello F, Caputo R, Bianco R, Damiano V, Pomatico G, Pepe S, Bianco A R, Agrawal S, Mendelsohn J, Tortora G. J Natl Cancer Inst. 1998;90:1087–1094. doi: 10.1093/jnci/90.14.1087. [DOI] [PubMed] [Google Scholar]
- 35.Prewett M, Rothman M, Waksal H, Feldman M, Bander N H, Hicklin D J. Clin Cancer Res. 1998;4:2957–2966. [PubMed] [Google Scholar]