Abstract
The type I melanoma antigen gene (MAGE) proteins CT7 (MAGE-C1) and MAGE-A3 are commonly expressed in multiple myeloma (MM), and their expression correlates with increased plasma cell proliferation and poor clinical outcome. They belong to the cancer-testis antigen (CTAg) group of tumor-associated proteins, some of which elicit spontaneous immune responses in cancer patients. CT7 and MAGE-A3 are promising antigenic targets for therapeutic tumor vaccines in myeloma; therefore, it is critical to determine if they are immunogenic in MM patients. We analyzed cellular and humoral immune responses against CTAgs in patients with plasma cell dyscrasias: MM, monoclonal gammopathy of undetermined significance (MGUS), and Waldenström's macroglobulinemia (WM). Bone marrow lymphocytes from two of four untreated MM patients exhibited CT7-specific cellular immune responses as measured by an autologous cellular immunity assay, the first such immune response to CT7 to be reported in cancer patients. Sera from 24 patients were screened by ELISA for humoral immune responses to CTAgs. Two patients with MM demonstrated positive titers, one for MAGE-A1 and the other for SSX1. These data demonstrate that CTAgs, particularly CT7, are immunogenic in MM patients and merit further exploration as targets of immunological therapy in MM.
Keywords: human, multiple myeloma, CT antigens, cellular immunity, humoral immunity
Introduction
The cancer-testis antigen (CTAg) group of tumor-associated proteins is expressed in a broad range of human cancers. They are normally restricted to immune-privileged tissues such as developing germ cells and placenta [reviewed in (1)]. The first CTAg, the type I melanoma antigen gene (MAGE) protein MAGE-A1, was originally identified because it elicited a spontaneous cellular immune response in a melanoma patient (2). T cells from this patient were used to clone the gene encoding MAGE-A1 (3). For these reasons, therapeutic tumor vaccines targeting CTAgs have been investigated in a diverse array of delivery strategies and have demonstrated clinical activity in priming cellular and humoral immune responses (4).
The type I MAGE proteins MAGE-A3 and CT7 (MAGE-C1) are commonly expressed in multiple myeloma (MM), an incurable malignancy of plasma cells (5). Immunohistochemical (IHC) analysis of bone marrow (BM) specimens from MM patients first showed that 81% of patient samples stained positively for CT7 and 75% for MAGE-A3 (6). There was a positive correlation between CT7 expression and advanced stage of disease, and patient samples with higher percentages of CT7- or MAGE-A3-positive tumor cells also had higher percentages of Ki-67-positive cells, demonstrating a correlation with plasma cell proliferation. These findings suggested that CT7 and MAGE-A3 play pathogenic roles in MM, possibly in the dysregulation of the cell cycle that characterizes this disease (7).
Consistent with these initial observations, CT7 and MAGE-A3 were detected by IHC in an independent study of myeloma BM specimens, and in two of these patients, humoral and/or cellular immunity against the CTAgs SSX2 and NY-ESO-1/LAGE-1 were detected after allogeneic hematopoietic stem cell transplantation (8). Gene expression profiling (GEP) using microarrays showed a correlation between the level of CTAg mRNA in BM plasma cells and proliferative gene expression signatures in patients with hyperdiploid MM (9). Longitudinal analysis of CTAg expression by RT-PCR and Western blotting through the natural history of the disease demonstrated that expression levels of certain CTAgs (CT7, MAGE-A3, CT10/MAGE-C2, and SSX2) had a strong correlation with the clinical status and outcome of MM patients (10). Furthermore, expression of a greater number of CTAgs by GEP and RT-PCR array analyses of BM plasma cells at diagnosis correlated with worse clinical outcomes (11, 12). These data indicate that MAGE-A3 and CT7 are promising targets for vaccine immunotherapy in MM.
In pursuit of MAGE vaccine immunotherapy, it is critical to demonstrate that CT7 and MAGE-A3 are immunogenic in MM patients. We investigated cellular immunity against CT7 in bone marrow lymphocytes from MM patients using an autologous ELISpot-based assay. Two of four patients demonstrated CT7-specific IFN-γ secretion, the first cellular immune response against this protein reported in cancer patients. We examined humoral immunity to a broad panel of CTAgs by screening serum and plasma samples from 18 MM patients, as well as patients with related plasma cell dyscrasias (four with MGUS and two with WM) by ELISA with recombinant CTAg or CTAg fragments. Two MM patients demonstrated positive titers, one against MAGE-A1 and another against SSX1. Interestingly, they were the same two patients who had CT7-specific cellular immune responses. These data indicate that CTAgs can elicit cellular (CT7) and humoral (MAGE-A1, SSX1) immune responses in MM patients and further supports investigation of vaccine strategies targeting these proteins.
Results
Patient characteristics
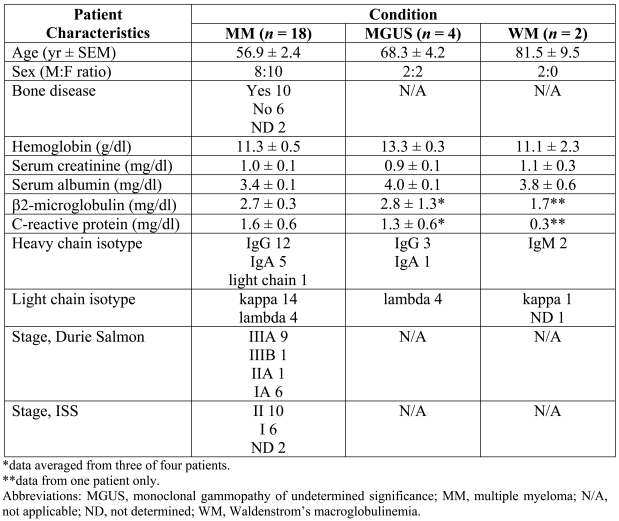
Bone marrow aspirates and serum samples for 24 plasma cell dyscrasia patients were used in this study. The patient characteristics are provided in Table 1. The median age of the MM group is slightly below average, but otherwise these patients are representative of the typical plasma cell dyscrasia population.
Table 1.
Patient characteristics.
Cellular immune responses against CT7 in MM patients
We assayed CT7-specific cellular immunity in patient bone marrow samples by interferon-gamma (IFN-γ) ELISpot. We chose to examine bone marrow lymphocytes rather than peripheral blood lymphocytes based on the hypothesis that the tumor microenvironment would be enriched for tumor antigen-specific T cells. CT7 was chosen because it was the most commonly detected CTAg in MM (6), and the yield of myeloid dendritic cells (mDCs) from each patient was likely to limit the number of test antigens in each assay to one. However, a conventional peptide-based ELISpot assay could not be applied in this system as it depends upon defined antigenic peptides presented by a small number of well-characterized MHC class I haplotypes such as HLA-A*0201. This approach can only be used for a subset of patients and will not detect responses against the diverse array of dominant and subdominant epitopes that may be represented in the effector pool. Furthermore, antigenic epitopes for CT7 have not been described. Finally, a typical 10 ml bone marrow aspirate does not contain sufficient numbers of effector cells for an ELISpot assay with appropriate controls and duplicates.
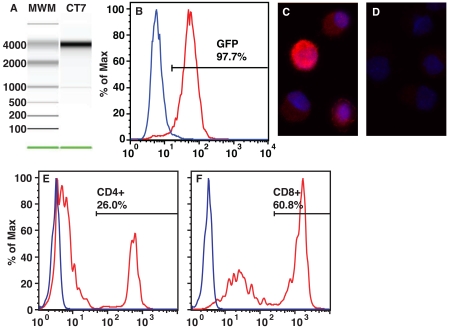
To overcome these limitations, we developed a method that can be applied to any patient regardless of MHC class I haplotype. This autologous system utilized mDCs generated from peripheral blood mononuclear cells (PBMCs) as antigen-presenting cells (APCs) (13). They were loaded in vitro by electroporation with transcribed mRNA coding for full-length antigen (14), similar to other methods for generating autologous, antigen-loaded APCs from monocytes or mDCs (15-17). We loaded mDCs with CT7 mRNA or the positive control antigen influenza matrix protein (Flu MP), or mDCs were mock electroporated without mRNA as a negative control (Figure 1A). Electroporation with mRNA coding for green fluorescent protein (GFP) demonstrated high transfer efficiency and protein expression at 24 hours (Figure 1B). CT7 protein expression was detected by immunofluorescence after 24 hours in culture, indicating that the mRNA was efficiently translated into protein (Figure 1, panels C and D).
Figure 1.
Preparation of cellular components for immune response assay. (A) In vitro transcribed mRNA coding for CT7 was synthesized and analyzed for size and purity. A representative electropherogram of elution from an Agilent 2100 Bioanalyzer showing CT7 mRNA appearing as a single band with the expected size of 4200 bases is shown. (B) Mature PBMC-derived DCs were electroporated with in vitro transcribed mRNA coding for GFP, and fluorescence was analyzed by flow cytometry at intervals up to 72 hours. Blue curve, mock electroporated control. Red curve, GFP mRNA transduction, 24-hour time point. (C and D) DCs were transduced with in vitro transcribed mRNA coding for CT7 and incubated for 24 hours. CT7 protein expression was then analyzed by immunofluorescence (IF). (C) IF with CT7-33 primary mAb (specific for CT7). (D) IF with isotype control primary Ab. Crimson fluorophore denotes Ag-specific staining. Blue fluorescence is DAPI nuclear counterstaining. Magnification 40x. Results are representative of two experiments. (E and F) Expansion of bone marrow T cells with lymphocyte stimulator beads. Mononuclear cells from MM patients were incubated with lymphocyte stimulator beads and recombinant human IL-2 for 14 days, and the resulting CD4+ (E) and CD8+ (F) subsets were analyzed by flow cytometry. The 2:1 (CD4:CD8) ratio typically observed in the periphery was inverted after expansion. Blue curve, isotype control mAb. Red curve, CD4- or CD8-specific mAb. Results are representative of four independent T cell expansions.
To generate a polyclonal pool of effector cells sufficient for an ELISpot assay, we co-cultured lymphocytes from the bone marrow compartment with anti-CD3 and anti-CD28 mAbs coated paramagnetic beads in the presence of recombinant human IL-2 (rhu-IL-2), which expands T cells several hundred-fold (18). These were expanded without antigen, excluding the possibility of in vitro priming of T cell activity. The resultant polyclonal pool of lymphocytes was characterized by an inversion in the CD4:CD8 ratio (the normal ratio is approximately 2:1; after expansion we typically observe a ratio of approximately 1:2) normally observed in bone marrow or peripheral blood (Figure 1, panels E and F).
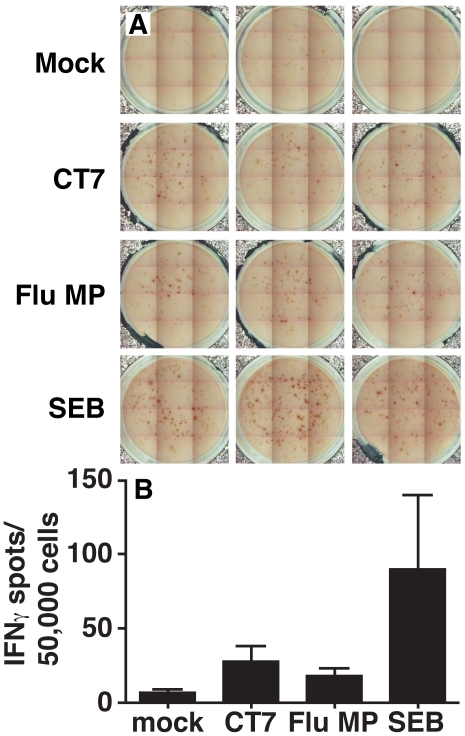
Expanded bone marrow lymphocytes were then co-cultured with DCs transduced with in vitro transcribed CT7 mRNA or the controls for 48 hours and assayed for IFN-γ production by the ELIspot assay. A positive response was defined as generating at least twenty spots per well and at least twice the number of spots detected in the mock control (Figure 2). As a positive control, in vitro transcribed Flu MP mRNA was efficiently translated after electroporation and processed for presentation in the context of MHC class I on DCs. In some cases, Flu MP was excluded due to limited numbers of mDCs. In addition, staphylococcal enterotoxin B (SEB; a T cell superantigen) stimulation of bone marrow lymphocytes was included as a control for the viability and responsiveness of the T cell effector pool.
Figure 2.
CT7-specific cellular immunity in MM patient 2 bone marrow lymphocytes. (A) Well images from IFN-γ ELIspot analysis of expanded bone marrow T cell pools co-incubated for 48 hours with autologous DC stimulator cells prepared as shown in Figure 1. Image enhanced for contrast only. Triplicate results from a single individual experiment shown. (B) Average spot forming units. Results are shown as average ± standard error of the mean for the triplicate wells in (A). Results are representative of four different patients.
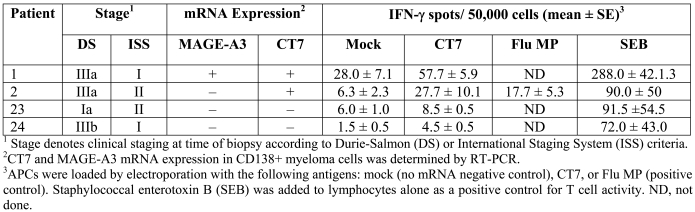
Two of the four untreated patients analyzed by this method demonstrated CT7-specific IFN-γ secretion (Table 2). Both patients expressed CT7 mRNA in their CD138+ myeloma cells by RT-PCR. In contrast, the two patients that did not exhibit CT7-specific cellular immunity did not express CT7 in their myeloma cells.
Table 2.
Cellular immune responses against CT7 in myeloma patients.
Humoral immune responses against CTAgs in MM patients
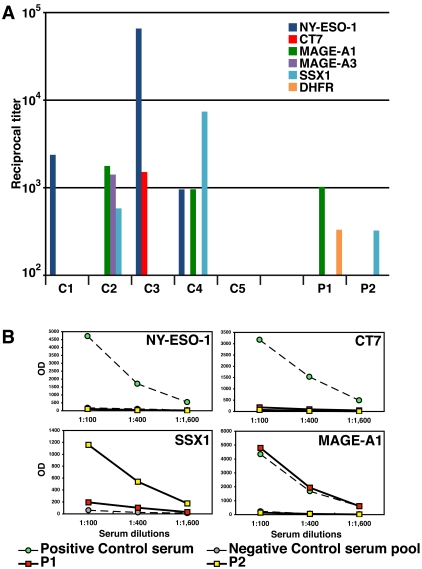
Serum or heparinized plasma from 24 patients (Table 1) with plasma cell dyscrasias were analyzed by ELISA for humoral immunity against a panel of recombinant full length or truncated CTAgs. Sera from patients with known humoral immunity against specific CTAgs (NY-ESO-1, MAGE-A1, multiple MAGE-A family members, SSX1, and CT7) served as positive controls (Figure 3A). Positive antibody titers against CTAgs were detected in two patients, one with a 1:1024 titer against MAGE-A1 (patient 1) and a second patient with a 1:324 titer against SSX1 (patient 2). The titration curves and controls for the positive patients are shown in Figure 3B. No other significant CTAg-specific titers were detected in any of the other MM patients. The WM and MGUS patients did not exhibit serologic reactivity against the CTAgs assayed. Interestingly, patients 1 and 2 were the same ones with T cell responses against CT7 (Table 2). It is also notable that patient 1 expressed MAGE-A3 but not MAGE-A1 by RT-PCR in CD138+ myeloma cells recovered from the patient's bone marrow (Table 2).
Figure 3.
Serological analysis of CTAg humoral immunity in MM patients. (A) Sera from cancer patients with known positive titers against the indicated CTAgs served as positive controls (C1-C4) for the ELISA assay, along with a normal donor negative control (C5). Controls: C1, positive control for NY-ESO-1; C2, MAGE-A family and SSX1; C3, NY-ESO-1 and CT7; C4, NY-ESO-1, SSX1, and MAGE-A1. Two patients (1 and 2) demonstrated positive titers against a CTAg. (B) ELISA with sera of MM patients against CT antigens. Sera from cancer patients with known positive titers against the CTAg indicated served as positive controls (green circles), and a serum pool from healthy donors served as negative control (grey circles) for the ELISA assay. Sera were tested in 3 four-fold dilutions, as indicated. Serum from patient 1 (red squares) had a positive titer against MAGE-A1 and patient 2 (yellow squares) had a positive titer against SSX1. A positive control for SSX1 was not available for this test run; however, a repeat assay with a positive control confirmed the positive titer (data not shown). Results are representative of two ELISA assays.
Discussion
The results reported here demonstrate the existence of spontaneous cellular immune responses against CT7 in some untreated MM patients. This is the first report of cellular immune responses against CT7 in cancer patients. Although the sample size is too small for statistical analysis, it is significant in establishing that CT7 is immunogenic in MM patients. In conjunction with our previous report of common expression of CT7 in this disease (6), these results indicate that CT7 is an excellent candidate antigen for vaccine formulation in this disease.
There is an abundance of evidence that cellular immune responses against tumor-associated antigens may impact the natural history of plasma cell dyscrasias. A recent report showed that 19 of 53 untreated myeloma patients demonstrated peripheral blood CD8+ cells reactive to peptide epitopes of MAGE-A1, -A2, or -A3 (19). In this study, MAGE-specific cells represented between 0.001% to 0.03% of the CD8+ pool. These results are similar to our finding that CT7-specific T cells were a relatively small population in the bone marrow lymphocyte compartment (Table 2). Cellular immune responses against the stem cell-associated gene SOX2 in the bone marrow lymphocytes of MGUS patients was correlated with better clinical outcome (20). MM patients demonstrated de novo immunity against the CTAg NY-ESO-1 after allogeneic hematopoietic stem cell transplantation, indicating that CTAgs may be among the target antigens of the graft-vs.-myeloma effect (8). This is particularly notable since immunotherapy in the form of an allogeneic transplant is the only known curative therapy for this disease (21, 22); however, due to age and medical restrictions, this treatment option is not available to the vast majority of MM patients. In addition, a MAGE-A3 recombinant protein vaccine induced Ag-specific humoral and cellular immunity in a syngeneic stem cell donor, although the clinical impact of this activity on the MM patient who received the hematopoietic stem cell transplant is not yet known (23). Analysis of cellular immunity against a broader array of CTAgs in the bone marrow microenvironment coupled with prospective analysis of clinical outcome may shed more light on the impact of CTAg-specific immunity in MM.
Previous studies of CTAg-specific immunity in other cancers demonstrated concordance between humoral and cellular immune responses for a given CTAg (24). We did not observe concordance in this patient set: humoral immunity against CT7 was not detected in either patient with CT7-specific T cells in the marrow, although it is notable that these two patients also had serologic responses to other CTAgs. Unfortunately, we were unable to test for MAGE-A1- or SSX1-specific T cells due to the limited number of mDCs from each patient, so it is possible that corresponding cellular immunity may be present in the context of humoral immune responses. However, given the well-described perturbations in humoral immunity in MM patients (25), it is possible that cellular immunity against some of these antigens may develop in the absence of detectable humoral immunity.
We did not detect titers against CT7 in any of our patient samples. This contrasts with the findings of Curioni-Fontecedro and colleagues (26) who reported that 50% of MM patients they examined had humoral immune responses against CT7. An explanation for this marked discrepancy in results is not readily apparent. Therefore, further serologic analysis of independent sets of patient samples is required to determine the true frequency of humoral immunity to CT7 and other CTAgs in MM.
The cellular immune response assay employed here has several advantages. First, it is an autologous system that can be applied to any patient regardless of MHC class I haplotype. Similar assays rely upon pre-defined MHC class I-restricted peptides for well-characterized haplotypes, rendering them useless to a substantial majority of patients. Since full-length mRNAs coding for the antigens of interest are used, the DCs process and present the class I-restricted peptides in a physiologically relevant manner. Second, this method generates large polyclonal pools of T cells from relatively small samples, allowing for efficient screening and subcloning of antigen-specific clones. The major limitation is the supply of autologous mDCs as APCs. Small volumes of peripheral blood generally yield enough mature DCs for a single ELISpot assay, but further analysis requires acquisition of additional PBMCs from the patient. Therefore, methods for generating larger pools of APCs would enhance the utility of this assay. One potential strategy is to use autologous B cell pools expanded by co-culture with CD40 ligand and recombinant human IL-4 (27). If a sufficient supply of APCs can be obtained, this method may have value in monitoring cellular immune responses in cancer vaccine clinical trials.
We were unable to analyze discreet CD4 and CD8 T cell populations for this study due to the limited number of mDCs available, further highlighting the need to efficiently generate larger pools of autologous APCs. In addition, although the predominant population after expansion was T cells (>85%; Figure 1, panels E and F), it is possible that non-specific IFN-γ secretion by contaminating NK cells may have been responsible for some spot formation. However, given the low background in the mock-transfected controls, it is likely that NK cell activity contributed little, if anything, to the final results. The number of Flu MP-specific T cells from patient 2 (Table 2) was relatively low. This may have been due to the impairment of anti-viral immune responses that has been reported in multiple myeloma patients (28). As noted in the results, polyclonal expansion by the method presented resulted in inversion of the CD4:CD8 ratio typically observed in bone marrow and peripheral blood, similar to results observed in other studies (29). The physiological basis for this phenomenon is unknown. In this setting, the CD8+ T cell population is relatively over-represented, and therefore, this type of assay may underestimate the contribution of CD4+ cells to the cellular immune response.
Vaccines that efficiently stimulate CT7- and MAGE-A3-specific immunity may have significant activity in MM through expansion of Ag-specific T and B cells in patients with pre-existing immunity or by priming of de novo cellular and humoral activity. It is important to broaden the analysis of cellular immunity to MAGE-A3 and other MM-associated CTAgs, as a multi-targeted vaccine may be more broadly applicable in the patient population and result in more robust tumor-specific immunity. Ultimately, such vaccines should be tested in clinical trials that include monitoring of cellular and humoral immunity by design, to demonstrate positive correlations between the induction of CTAg-specific immunity and clinical outcome. The results reported here add to the body of evidence supporting the exploration of this strategy in MM.
Abbreviations
- CTAg
cancer-testis antigen
- mDC
myeloid dendritic cell
- MM
multiple myeloma
Acknowledgements
HJC was supported by a Compound Validation Award from the Multiple Myeloma Research Foundation and by NCI K01-CA115917.
References
- 1.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 2.Knuth A, Wölfel T, Klehmann E, Boon T, Meyer zum Buschenfelde KH. Cytolytic T-cell clones against an autologous human melanoma: specificity study and definition of three antigens by immunoselection. Proc Natl Acad Sci U S A. 1989;86:2804–2808. doi: 10.1073/pnas.86.8.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 4.Jäger D, Jäger E, Knuth A. Immune responses to tumour antigens: implications for antigen specific immunotherapy of cancer. J Clin Pathol. 2001;54:669–674. doi: 10.1136/jcp.54.9.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jungbluth AA, Ely S, DiLiberto M, Niesvizky R, Williamson B, Frosina D, Chen YT, Bhardwaj N, Chen-Kiang S, Old LJ, Cho HJ. The cancer-testis antigens CT7 (MAGE-C1) and MAGE-A3/6 are commonly expressed in multiple myeloma and correlate with plasma-cell proliferation. Blood. 2005;106:167–174. doi: 10.1182/blood-2004-12-4931. [DOI] [PubMed] [Google Scholar]
- 7.Ely S, Di Liberto M, Niesvizky R, Baughn LB, Cho HJ, Hatada EN, Knowles DM, Lane J, Chen-Kiang S. Mutually exclusive cyclin-dependent kinase 4/cyclin D1 and cyclin-dependent kinase 6/cyclin D2 pairing inactivates retinoblastoma protein and promotes cell cycle dysregulation in multiple myeloma. Cancer Res. 2005;65:11345–11353. doi: 10.1158/0008-5472.CAN-05-2159. [DOI] [PubMed] [Google Scholar]
- 8.Atanackovic D, Arfsten J, Cao Y, Gnjatic S, Schnieders F, Bartels K, Schilling G, Faltz C, Wolschke C, Dierlamm J, Ritter G, Eiermann T, Hossfeld DK, Zander AR, Jungbluth AA, Old LJ, Bokemeyer C, Kröger N. Cancer-testis antigens are commonly expressed in multiple myeloma and induce systemic immunity following allogeneic stem cell transplantation. Blood. 2007;109:1103–1112. doi: 10.1182/blood-2006-04-014480. [DOI] [PubMed] [Google Scholar]
- 9.Chng WJ, Kumar S, Vanwier S, Ahmann G, Price-Troska T, Henderson K, Chung TH, Kim S, Mulligan G, Bryant B, Carpten J, Gertz M, Rajkumar SV, Lacy M, Dispenzieri A, Kyle R, Greipp P, Bergsagel PL, Fonseca R. Molecular dissection of hyperdiploid multiple myeloma by gene expression profiling. Cancer Res. 2007;67:2982–2989. doi: 10.1158/0008-5472.CAN-06-4046. [DOI] [PubMed] [Google Scholar]
- 10.Atanackovic D, Luetkens T, Hildebrandt Y, Arfsten J, Bartels K, Horn C, Stahl T, Cao Y, Zander AR, Bokemeyer C, Kröger N. Longitudinal analysis and prognostic effect of cancer-testis antigen expression in multiple myeloma. Clin Cancer Res. 2009;15:1343–1352. doi: 10.1158/1078-0432.CCR-08-0989. [DOI] [PubMed] [Google Scholar]
- 11.Andrade VC, Vettore AL, Felix RS, Almeida MS, Carvalho F, Oliveira JS, Chauffaille ML, Andriolo A, Caballero OL, Zago MA, Colleoni GW. Prognostic impact of cancer/testis antigen expression in advanced stage multiple myeloma patients. Cancer Immun. 2008;8:2. http://www.cancerimmunity.org/v8p2/071018.htm [PMC free article] [PubMed] [Google Scholar]
- 12.Condomines M, Hose D, Raynaud P, Hundemer M, De Vos J, Baudard M, Moehler T, Pantesco V, Moos M, Schved JF, Rossi JF, Rème T, Goldschmidt H, Klein B. Cancer/testis genes in multiple myeloma: expression patterns and prognosis value determined by microarray analysis. J Immunol. 2007;178:3307–3315. doi: 10.4049/jimmunol.178.5.3307. [DOI] [PubMed] [Google Scholar]
- 13.O'Neill D, Bhardwaj N. Generation of autologous peptide- and protein-pulsed dendritic cells for patient-specific immunotherapy. Methods Mol Med. 2005;109:97–112. [PubMed] [Google Scholar]
- 14.Kavanagh DG, Kaufmann DE, Sunderji S, Frahm N, Le Gall S, Boczkowski D, Rosenberg ES, Stone DR, Johnston MN, Wagner BS, Zaman MT, Brander C, Gilboa E, Walker BD, Bhardwaj N. Expansion of HIV-specific CD4+ and CD8+ T cells by dendritic cells transfected with mRNA encoding cytoplasm- or lysosome-targeted Nef. Blood. 2006;107:1963–1969. doi: 10.1182/blood-2005-04-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreiter S, Konrad T, Sester M, Huber C, Türeci O, Sahin U. Simultaneous ex vivo quantification of antigen-specific CD4+ and CD8+ T cell responses using in vitro transcribed RNA. Cancer Immunol Immunother. 2007;56:1577–1587. doi: 10.1007/s00262-007-0302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teufel R, Carralot JP, Scheel B, Probst J, Walter S, Jung G, Hoerr I, Rammensee HG, Pascolo S. Human peripheral blood mononuclear cells transfected with messenger RNA stimulate antigen-specific cytotoxic T-lymphocytes in vitro. Cell Mol Life Sci. 2005;62:1755–1762. doi: 10.1007/s00018-005-5067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knights AJ, Nuber N, Thomson CW, de la Rosa O, Jäger E, Tiercy JM, van den Broek M, Pascolo S, Knuth A, Zippelius A. Modified tumour antigen-encoding mRNA facilitates the analysis of naturally occurring and vaccine-induced CD4 and CD8 T cells in cancer patients. Cancer Immunol Immunother. 2009;58:325–338. doi: 10.1007/s00262-008-0556-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maus MV, Thomas AK, Leonard DG, Allman D, Addya K, Schlienger K, Riley JL, June CH. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat Biotechnol. 2002;20:143–148. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 19.Goodyear O, Piper K, Khan N, Starczynski J, Mahendra P, Pratt G, Moss P. CD8+ T cells specific for cancer germline gene antigens are found in many patients with multiple myeloma, and their frequency correlates with disease burden. Blood. 2005;106:4217–4224. doi: 10.1182/blood-2005-02-0563. [DOI] [PubMed] [Google Scholar]
- 20.Spisek R, Kukreja A, Chen LC, Matthews P, Mazumder A, Vesole D, Jagannath S, Zebroski HA, Simpson AJ, Ritter G, Durie B, Crowley J, Shaughnessy JD Jr, Scanlan MJ, Gure AO, Barlogie B, Dhodapkar MV. Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med. 2007;204:831–840. doi: 10.1084/jem.20062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gahrton G, Tura S, Ljungman P, Belanger C, Brandt L, Cavo M, Facon T, Granena A, Gore M, Gratwohl A, Löwenberg B, Nikoskelainen J, Reiffers JJ, Samson D, Verdonck L, Volin L. Allogeneic bone marrow transplantation in multiple myeloma. N Engl J Med. 1991;325:1267–1273. doi: 10.1056/NEJM199110313251802. for the European Group for Bone Marrow Transplantation. [DOI] [PubMed] [Google Scholar]
- 22.Bensinger WI, Buckner CD, Anasetti C, Clift R, Storb R, Barnett T, Chauncey T, Shulman H, Appelbaum FR. Allogeneic marrow transplantation for multiple myeloma: an analysis of risk factors on outcome. Blood. 1996;88:2787–2793. [PubMed] [Google Scholar]
- 23.Szmania S, Gnjatic S, Tricot G, Stone K, Zhan F, Moreno A, Thuro B, Melenhorst J, Barrett J, Shaughnessy J, Old LJ, Barlogie B, Brichard VG, van Rhee F. Immunization with a recombinant MAGE-A3 protein after high-dose therapy for myeloma. J Immunother. 2007;30:847–854. doi: 10.1097/CJI.0b013e318158fcff. [DOI] [PubMed] [Google Scholar]
- 24.Jäger E, Nagata Y, Gnjatic S, Wada H, Stockert E, Karbach J, Dunbar PR, Lee SY, Jungbluth A, Jäger D, Arand M, Ritter G, Cerundolo V, Dupont B, Chen YT, Old LJ, Knuth A. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. Proc Natl Acad Sci U S A. 2000;97:4760–4765. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broder S, Humphrey R, Durm M, Blackman M, Meade B, Goldman C, Strober W, Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975;293:887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- 26.Curioni-Fontecedro A, Knights AJ, Tinguely M, Nuber N, Schneider C, Thomson CW, von Boehmer L, Bossart W, Pahlich S, Gehring H, Moch H, Renner C, Knuth A, Zippelius A. MAGE-C1/CT7 is the dominant cancer-testis antigen targeted by humoral immune responses in patients with multiple myeloma. Leukemia. 2008;22:1646–1648. doi: 10.1038/leu.2008.43. [DOI] [PubMed] [Google Scholar]
- 27.Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004;103:2046–2054. doi: 10.1182/blood-2003-07-2379. [DOI] [PubMed] [Google Scholar]
- 28.Maecker B, Anderson KS, von Bergwelt-Baildon MS, Weller E, Vonderheide RH, Richardson PG, Schlossman RL, Menezes IA, Xia Z, Munshi NC, Anderson KC, Nadler LM, Schultze JL. Viral antigen-specific CD8+ T-cell responses are impaired in multiple myeloma. Br J Haematol. 2003;121:842–848. doi: 10.1046/j.1365-2141.2003.04375.x. [DOI] [PubMed] [Google Scholar]
- 29.Rapoport AP, Stadtmauer EA, Aqui N, Vogl D, Chew A, Fang HB, Janofsky S, Yager K, Veloso E, Zheng Z, Milliron T, Westphal S, Cotte J, Huynh H, Cannon A, Yanovich S, Akpek G, Tan M, Virts K, Ruehle K, Harris C, Philip S, Vonderheide RH, Levine BL, June CH. Rapid immune recovery and graft-versus-host disease-like engraftment syndrome following adoptive transfer of Costimulated autologous T cells. Clin Cancer Res. 2009;15:4499–4507. doi: 10.1158/1078-0432.CCR-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee AW, Truong T, Bickham K, Fonteneau JF, Larsson M, Da Silva I, Somersan S, Thomas EK, Bhardwaj N. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: implications for immunotherapy. Vaccine. 2002;20(Suppl 4):A8–A22. doi: 10.1016/s0264-410x(02)00382-1. [DOI] [PubMed] [Google Scholar]
- 31.Gnjatic S, Old LJ, Chen YT. Autoantibodies against cancer antigens. Methods Mol Biol. 2009;520:11–19. doi: 10.1007/978-1-60327-811-9_2. [DOI] [PubMed] [Google Scholar]
Materials and methods
Patient samples
Bone marrow aspirate and biopsy specimens for this study were obtained from patients at Weill-Cornell Medical Center, New York, NY and St. Vincent's Comprehensive Cancer Center, New York, NY. These specimens were obtained under Institutional Review Board-approved sample collection protocols (Weill Medical College of Cornell University IRB #1000-422, and St. Vincent's Comprehensive Cancer Center IRB #53-03). All patients received counseling by an investigator, and IRB-approved consent forms and HIPAA compliance statements (for patients enrolled after July 2003) were signed in the presence of a witness prior to procedures.
Plasma cell isolation
Plasma cells were isolated from bone marrow aspirate specimens using anti-CD138-conjugated magnetic beads and magnetic media columns according to the manufacturer's instructions (Miltenyi Biotec Inc., Auburn, CA). Isolated plasma cells were washed three times in RPMI (Mediatech Inc., Manassas, VA), pelleted by centrifugation, snap frozen in liquid nitrogen, and stored at -80˚C until RNA isolation. CD138- bone marrow mononuclear cells (BMMCs) were cryopreserved in 8% DMSO (Sigma-Aldrich, St. Louis, MO) in heat inactivated pooled human AB serum (PHS; Omega Scientific, Inc, Tarzana, CA) and stored at -150˚C until use. Lymphocytes from the CD138- fraction were expanded as described further on.
Peripheral blood preparation
Heparinized whole blood from multiple myeloma and MGUS patients was separated into peripheral blood mononuclear cells (PBMCs) and plasma fractions by Ficoll (GE Healthcare, Inc., Uppsala, Sweden) density centrifugation, and the resultant heparinized plasma was recovered. In some cases the blood was collected in non-heparinized tubes and allowed to clot overnight, after which serum was separated from the clot by centrifugation. Aliquots were stored at -80˚C until serologic testing.
Myeloid DC isolation and maturation
The procedure for in vitro differentiation and maturation of myeloid dendritic cells (mDCs) has been described previously (13). Briefly, peripheral blood mononuclear cells (PBMCs) from myeloma patients were plated in 10 cm tissue culture plates (BD Biosciences, Franklin Lakes, NJ) at 37˚C. Adherent monocytes were cultured in the RPMI supplemented with 1% autologous plasma, GM-CSF (Immunex, Seattle WA), and IL-4 (R&D Systems, Minneapolis, MN) for five days, followed by maturation for two days with a cytokine cocktail consisting of IL-1β, IL-6, TNF-α and PGE2 (all from R&D Systems, Minneapolis, MN) (30).
In vitro mRNA synthesis
Template plasmid pCT7 (1 µg) containing the CT7 cDNA coding sequence under the control of the T7 promoter in the pGEM vector (Promega) was linearized by digestion with SpeI (New England BioLabs) at 37˚C for one hour. Messenger RNA was synthesized from the linearized template using the mMachine mMessage T7 polymerase kit (Ambion) according to the manufacturer's instructions. Excess reagents were removed using RNA cleanup kits (Qiagen) and the resultant mRNA products were analyzed for size and purity with an Agilent 2100 BioAnalyzer (Agilent Biotechnologies) and for concentration with a Nanodrop 2000 UV spectrophotometer (Thermo Scientific, Wilmington, DE). Messenger RNA was stored in 10 µg aliquots at -20˚C until electroporation.
mDC electroporation
Mature mDCs were resuspended at 106 cells/300 µl in OptiMEM media at 37˚C. Cells were transferred to 2 mm gap electroporation cuvettes (BioRad) and 10 µg in vitro transcribed mRNA (CT7, FluMP, or GFP) was added with gentle mixing. Cells were electroporated by square-wave protocol, 300 V, 0.5 ms pulse in a Gene Pulser II apparatus (BioRad). 700 µl of warmed 5% PHS was added to the cuvette and the cell suspension transferred to a 6-well plate (Falcon). The volume of each well was brought up to 4 ml with 5% PHS and the cells were incubated at 37˚C until transfer to an ELISpot plate.
Immunofluorescence
Electroporated DCs were incubated for 24 h at 37˚C, 5% CO2, harvested and resuspended at 5 x 106 cells/ml in 5% PHS. 100 µl of cell suspension was applied to poly-L-lysine coated Superfrost slides (Thermo Scientific) and allowed to settle for 15 min at room temperature (RT). The slides were washed once in PBS and excess solution was removed by gentle blotting. Cells were fixed in 4% paraformaldehyde (Sigma-Aldrich) at RT for 10 min. The slides were washed in PBS for 5 min, and the cells were then permeabilized with 0.2% Triton X-200 at RT for 2 min. The slides were washed three times with PBS for 5 min then blocked with a blocking solution of 1% BSA, 5% goat serum in PBS for 30 min at RT. Primary antibody (CT7-33 or IgG isotype control, the latter from BD-Pharmingen) was diluted to a final concentration of 10 µg/ml in blocking solution and 200 µl of primary antibody was applied to the slides. Slides were incubated in a humidified chamber at 4˚C overnight. Slides were washed four times with PBS for 5 min each. Secondary antibody [Cy3-conjugated goat anti-mouse IgG (H + L), Invitrogen] was diluted 1:200 (v:v) in blocking solution and 200 µl of this solution was applied to the slides which were then incubated for 30 min at 4˚C in a light-protected chamber. Slides were washed five times with PBS for 5 min each. One drop of anti-fade (ProLong Gold Anti-fade reagent with DAPI, Invitrogen) was applied and a coverslip was placed over each slide. Slides were incubated at 37˚C for two hours then the coverslips were sealed with clear nail polish. Cells were imaged on a DMIRB/E inverted microscope (Leica, Wetzlar, Germany), using a 1.32 NA ×63 oil-immersion objective. Image stacks consisting of 15 to 20 planes spaced 0.5 µm were collected with a Photometrics CoolSNAP HQ camera (Roper Scientific, Tucson, AZ) operated by the Slidebook software (Intelligent Imaging Innovations, Denver, CO). The image stacks were deconvoluted using the nearest-neighbor algorithm of Slidebook.
In vitro T cell expansion
BMMCs were resuspended at 5 x 105 cells/ml in T-cell media (X-Vivo 15, BioWhittaker) supplemented with 10% PHS, 50 IU/ml rhuIL-2 (R&D Systems), 20 µg/ml gentamycin (Gibco BRL), and 1 ml aliquots were added to each well of a 24-well plate (Falcon). Anti-CD3/CD28 mAb-coated paramagnetic beads (Dynabeads, Invitrogen) were resuspended in T-cell media at a ratio of 1.25 µl bead suspension to 1 ml media, and 1 ml of this suspension was added to each well containing PBMCs. The cells were incubated at 37˚C, 5% CO2.
After seven days in culture with anti-CD3/CD28 beads, each well was resuspended and the beads were removed using a magnetic field. The T cells were pooled and washed in RPMI, resuspended at 5 x 105 cells/ml in T-cell media, and fresh Dynabeads were added as indicated in the previous paragraph. This process was repeated every seven days until sufficient numbers of T cells were obtained for ELISpot assay. T cells were stored by cryopreservation in pooled human AB serum with 8% DMSO (Sigma-Aldrich).
IFN-γ ELIspot assay
Interferon (IFN)-γ ELIspot assays were performed as previously described (14). Briefly, 96-well Elispot plates (Millipore) were coated overnight at 4˚C with anti-IFN-γ mAb (clone 1-D1K, MABtech Inc., Mariemont, OH) diluted to 2.5 µg/ml in sterile PBS. Triplicate sets of effector (expanded bone marrow T cells) and antigen presenting (autologous mDCs electroporated with in vitro transcribed mRNA) cells prepared as indicated in the previous sections were plated at a DC:lymphocyte ratio of 1:20 with 5 x 104 lymphocytes per well. One set of effector cells was stimulated with 2.5 µg/ml SEB (Sigma-Aldrich) as a positive control for overall lymphocyte activity. After a 48-hour incubation at 37˚C, 5% CO2, the plates were developed with a biotinylated anti-IFN-γ secondary mAb (clone 7-B6-1, MABtech Inc.) at 2.5 µg/ml in PBS with 0.5% bovine serum albumin (Sigma-Aldrich) for two hours at 37˚C, followed by streptavidin conjugated to horseradish peroxidase (HRP) (Vector Laboratories, Burlingame, CA). IFN-γ-forming spots were visualized with the NovaRED HRP substrate kit (Vector Laboratories) according to the manufacturer's instructions. Spot forming units (sfu) were counted using a KS Elispot digital imaging and analysis suite (Carl Zeiss Micro Imaging Inc., Thornwood, NY). Positive response criteria were >20 spots/well and a >2-fold differential between the antigen stimulation and the negative controls.
Flow cytometry
Expanded bone marrow lymphocytes were washed and incubated with mouse anti-human CD4 or CD8 mAb or isotype control conjugated with PE (BD Biosciences). Cells were analyzed on a BD FACScaliber flow cytometer, and flow data was analyzed with the FlowJo 6.4.7 for Mac software suite (Tree Star, Inc., Palo Alto, CA).
CTAg ELISA
Serological analysis of CTAg humoral immune responses was performed as previously described (31). Serum samples were analyzed by ELISA for seroreactivity to bacterially produced full-length recombinant proteins NY-ESO-1, MAGE-A4, SSX1, SSX2, SSX4, p53, CT10/MAGE-C2, and CT7 (gift of A. Knuth) or to truncated proteins MAGE-A1 (57-219), MAGE-A3 (64-226). Each ELISA plate included positive and negative control antigens and positive and negative control sera. The positive control sera were previously titrated against the relevant antigens, and extrapolated titers were based on the negative control sera. A reciprocal titer was calculated for each plasma sample as the maximal dilution still significantly reacting to a specific antigen. Specificity was determined by comparing seroreactivity among various antigens tested. A positive result was defined as extrapolated reciprocal titers >100. Each serum sample was tested twice.
RNA isolation
Frozen plasma cell pellets were solubilized in 1 ml TRI reagent (MRC, Cincinnati, OH) and then sonicated for 10 s on ice with a Branson Sonifier 150 ultrasonic cell disruptor (Branson Scientific, Danbury CT). Each sample was mixed with 100 µl bromochlorophenol (MRC) and incubated at room temperature for 10 min, centrifuged at 18,000 x g, 4˚C for 10 min, and the aqueous phase transferred to a fresh tube. RNA was precipitated by adding 500 µl isopropanol (Sigma-Aldrich, St. Louis, MO) and incubating at room temperature for 10 min, followed by pelleting by centrifugation at 13,000 x g, 4˚C for 15 min. The RNA pellet was washed with 75% ethanol (Sigma-Aldrich) and centrifuged again as in the previous step. The RNA pellet was air-dried and then resuspended in 20 µl DEPC-H2O and incubated at 70˚C for 5 min. The concentration was determined by measuring the optical density of diluted samples at 260 nm in a Beckman Coulter DU530 spectrophotometer (Beckman Coulter Inc., Fullerton, CA).
RT-PCR
RT-PCR of total RNA patient plasma cells was performed as previously described (6). Pooled human testis cDNA (Invitrogen) served as a positive control. PCR primers (Invitrogen) for CT antigens were as follows: MAGE-A3, forward 5'-GAA GCC GGC CCA GGC TCG-3' and reverse 5'-GGA GTC CTC ATA GGA TTG GCT-3'; CT7 forward 5'-GAC GAG GAT CGT CTC AGG TCA GC-3' and reverse 5'-ACA TCC TCA CCC TCA GGA GGG-3'; p53 (positive control) forward 5'-TAC TCC CCT GCC CTC AAC AG-3' and reverse 5'-CTC AGG CGG CTC ATA GGG-3'. Samples were run on a 4% agarose gel in TAE buffer (Eppendorf), stained with ethidium bromide (Sigma-Aldrich) and imaged by UV fluorescence. Results were scored as positive or negative based on comparison with controls.
Data analysis
Statistical analysis was performed with Prism 4.0 (GraphPad Software, San Diego, CA) for Mac OS X (Apple Inc., Cupertino, CA). Data analysis and graphing of serological response data was performed with Excel 12.1.5 (Microsoft Corporation, Redmond, WA) for Mac OS X.