Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by high autoantibody levels and multiorgan tissue damage, including kidney and skin. Cutaneous manifestations are frequent in patients with SLE, yet the etiology and pathogenesis of skin injury in SLE remains unclear. We reasoned that lupus serum containing high levels of autoreactive Ig contributes to skin injury. In this article, we report that serum from SLE patients and lupus-prone mice induces skin inflammation following intradermal injection into normal mice. Lupus serum depleted of IgG failed to cause skin inflammation. Monocytes, but not lymphocytes, were found to be crucial in the development of lupus serum-induced skin inflammation, and lupus serum IgG induced monocyte differentiation into dendritic cells (DCs). TNF-α and TNFR1, but not TNFR2, were required for the development of lupus serum-induced skin inflammation. TNFR1, not TNFR2, represented the main molecule expressed in the skin lesions caused by injected lupus serum. Our studies demonstrated that lupus serum IgG causes skin injury by involving the TNFR1 signaling pathway and monocyte differentiation to DCs. Accordingly, disruption of the TNFR1-mediated signaling pathway and blockade of DC generation may prove to be of therapeutic value in patients with cutaneous lupus erythematosus.
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by high levels of autoantibody and multiorgan tissue damage, including kidney and skin (1, 2). Skin is involved in three quarters of patients with SLE (3), whereas, in one fifth, skin lesions represent the first manifestation (4). Cutaneous lupus erythematosus (LE) has been reported as the third most common cause of industrial disability among dermatological diseases (5). The molecular and cellular mechanisms involved in the expression of cutaneous LE remain unclear (3, 6, 7).
There are three clinical subtypes of cutaneous LE: acute cutaneous LE (ACLE), subacute cutaneous LE, and chronic cutaneous LE (also known as discoid LE). Localized ACLE is often referred to as the malar rash or butterfly rash of SLE, whereas generalized ACLE is frequently referred to as the SLE rash (8). They share the following features of lichenoid tissue reaction: hyperkeratosis; epidermal atrophy; liquefactive degeneration of the epidermal basal cell layer; mononuclear cell infiltrates focused at the dermo-epidermal junction, perivascular areas, and perifollicular areas; thickening of the basal membrane; and melanin pigment incontinence. ACLE typically presents abruptly in the context of a systemic disease, and almost all patients develop SLE (9).
Production of autoantibodies directed against nuclear Ags and myriad other autoantigens characterize SLE. The cellular debris released as a result of apoptosis represents the source of nuclear and cytoplasmic Ags in SLE (2). Also, during apoptosis, Ags that are normally buried within the cells are exposed on the cell surface, and they may trigger an immune response (6). In the skin, the invariably observed keratinocyte apoptosis may result in the cell surface expression and release of self-Ags, including DNA, Ro/Sjögren syndrome A, La/Sjögren syndrome B, and histones (10). Although exposure to UV light and other environmental triggers may contribute to the initiation of cutaneous LE by triggering apoptosis of keratinocytes, it is unclear how the inflammatory process begins and is sustained (10). IFN-α was reported to be important in the pathogenesis of cutaneous LE (11), and increased numbers of IFN-producing plasmacytoid dendritic cells (pDCs) have been found in skin lesions (12, 13).
Tissue damage in SLE is associated with autoantibody production and immune complex formation and deposition (2). Anti-dsDNA Ab has been linked to kidney (2) and brain pathology (14). Anti-Ro Abs are associated with photosensitive skin disease (3) and have been found deposited in the skin of patients with SLE (15). It was proposed that they bind to keratinocytes undergoing apoptosis and contribute to the inflammatory process (2). These observations indicate that autoantibodies may play an important role in the expression of cutaneous lesions in SLE; however, the cellular and cytokine requirements for the initiation and propagation of skin injury have not been investigated.
We report that skin inflammation develops in normal mice following intradermal injection of serum from patients with SLE and lupus prone-mice but not from healthy controls and normal mice. The development of skin lesions required the presence of functional TNFR1 and the differentiation of monocytes into dendritic cells (DCs). The identified cytokine and cellular components of the lupus serum-induced skin lesions strongly suggest the exploitation of agents that block TNFR1 signaling and monocyte differentiation in the treatment of skin lesions in patients with SLE.
Materials and Methods
Mice and reagents
The mice (C57BL/6, BALB/c, Swiss, MRL/lpr, Rag1−/−, TNF-α−/−, TNFR1−/−, TNFR2−/−, and MCP-1−/−) were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were housed in the animal facility of the Beth Israel Deaconess Medical Center. Etoposide was purchased from Sigma-Aldrich (St. Louis, MO). Oligonucleotide 1668 (CpG DNA) and inhibitory oligonucleotide (5′-GCTCAAGCTTGAGGGG-3′) were synthesized by primmBiotech (Boston, MA). FITC-conjugated anti-mouse F4/80 Ab was purchased form BioLegend (San Diego, CA).
Sera were collected from patients and controls. Eighteen patients fulfilling ≥4 of 11 revised criteria of the American College of Rheumatology for the classification of SLE were studied. All patients had SLE disease-activity index (SLEDAI) scores ranging from 0–12. Medications were discontinued ≥24 h before venipuncture. Ten normal women served as controls in this study. Sera were collected from MRL/lpr mice of different ages and normal C57BL/6 mice. Animal- and human-use protocols were approved by appropriate Beth Israel Deaconess Medical Center committees.
Injection protocol and cell-depletion procedures
Lupus serum or CpG oligonucleotide (CpG DNA) was injected intradermally in the back of the neck of different mice strains. Macrophage depletion was accomplished by s.c. injection of etoposide (12.5 mg/kg body weight, in a volume of 100 μl) into the nuchal region on three consecutive days before and two consecutive days after the injection of lupus serum; control mice received the same volume of the vehicle diluted in PBS (16–18). To determine IgG deposition in the skin of normal C57BL/6 mice, we treated mice with an intradermal injection of lupus MRL/lpr serum and a repeated treatment of etoposide. Skin was removed for immunohistochemical staining. To determine whether etoposide depletes monocytes/macrophages, we performed experiments using the same protocol. Spleens of C57BL/6 mice treated with etoposide (n = 3) or PBS (n = 2) were collected. We investigated macrophages in spleen using FITC-conjugated anti-F4/80 Ab. Macrophages were measured in spleens using immunofluorescent staining and were evaluated in mononuclear cells isolated from spleens using flow cytometry. Macrophage apoptosis in spleens was determined with a TUNEL Apoptosis Detection Kit (GenScript USA, Piscataway, NJ).
Histopathological and immunohistochemical examination of skin
Histopathological examination of skin was done after routine fixation and paraffin embedding of the tissue. Tissue sections from skin were cut and stained with H&E. All slides were coded and evaluated in a blinded manner. Severity of skin inflammation was scored 0–4: grade 0, normal; grade 1, hyperplasia of epidermis; and grades 2–4, different amounts of infiltrating inflammatory cells in the skin.
After deparaffinization and Ag retrieval, samples were stained with Ab to CD4+ (GK1.5), CD8+ (53.6.7), NF-κB, MCP-1, and blood dendritic cell Ag-2 (BDCA-2) (Santa Cruz Biotechnology, Santa Cruz, CA), followed by incubation with biotinylated secondary Abs, avidin-biotin-peroxidase complexes, and 3-amino-9-ethyl-carbazole–containing H2O2. All sections were counterstained with Mayer’s hematoxylin.
Monocyte differentiation into DCs
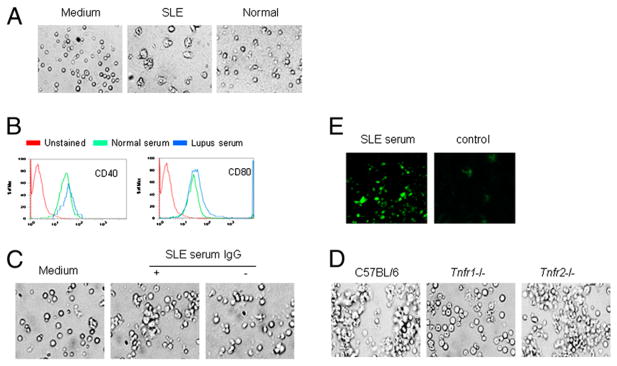
Spleens were removed from C57BL/6 or TNFR1- or TNFR2-deficient mice. Mononuclear cells were isolated from mouse spleens. Mononuclear cells were incubated in 24-well plates at 37°C. Monocytes were obtained after suspended lymphocytes were removed. Serum (40 μl) before or after IgG depletion from lupus patients or from healthy subjects, TNF-α (2 ng), and M-CSF (10 ng) were added to the plates (19). Differentiation of monocytes into DCs was examined by light microscopy. DCs were fixed, permeabilized, and stained with monoclonal anti-CD11c conjugated to FITC (Novus Biologicals, Littleton, CO). Cells were examined with an immunofluorescent microscope. Fixed cells were stained with anti-CD40 and -CD80 Ab conjugated to PE, and the expression of CD40 and CD80 was measured by flow cytometry. To investigate the role of TNFR2-Fc protein (R&D Systems, Minneapolis, MN) in lupus serum-induced monocyte differentiation into DCs, monocytes from C57BL/6 mouse were cultured with lupus serum with or without TNFR2-Fc protein (1.5 or 3 μg).
Statistical analysis
Statistical analyses were performed using the Student t test; p ≤ 0.05 was considered statistically significant.
Results
Lupus serum induces skin inflammation
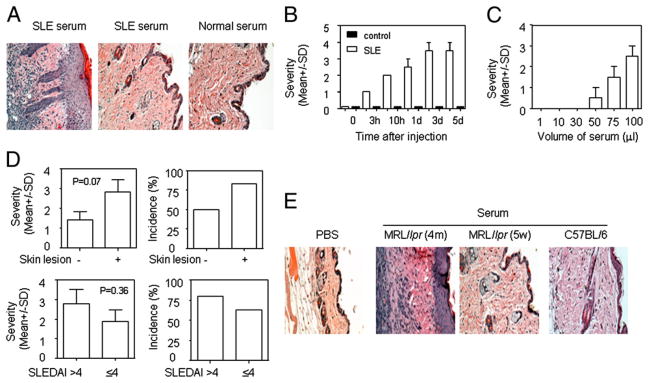
Tissue damage in SLE has been associated with the presence of autoantibody (1, 2). In MRL/lpr mice, skin injury develops after the appearance of autoimmunity and proteinuria (data not shown). Thus, we hypothesized that autoantibody-laden serum from SLE patients and lupus-prone mice may contribute to the expression of skin injury. To test this hypothesis, we injected serum from patients with SLE and healthy controls intradermally into normal C57BL/6 mice. Histopathological examination demonstrated that serum from some SLE patients induced skin inflammation, whereas serum from some SLE patients and all healthy controls did not (Fig. 1A). The lesions appeared 3 h after the injection and peaked 3 d after the injection (Fig. 1B). The skin lesions were characterized by keratinocyte hyperplasia and inflammatory cell infiltration. The severity of lupus serum-induced skin inflammation depended on the volume of the injected serum (Fig. 1C). To understand whether the severity of lupus serum-induced skin inflammation correlates with skin disease activity, we compared the severity of skin inflammation induced by lupus serum with or without skin manifestation. We found that lupus serum-induced skin inflammation closely relates to the presence of skin disease activity (Fig. 1D, Table I) but not to SLEDAI (Fig. 1D). Furthermore, we found that serum from MRL/lpr mice with skin injury (16 wk of age) induced skin inflammation, whereas serum from young MRL/lpr mice (5 wk of age) or normal C57BL/6 mice of the same age and PBS did not (Fig. 1E). We also found that serum from MRL/lpr mice with skin injury induced more severe skin inflammation than did serum from MRL/lpr mice without skin injury (data not shown). We did not obtain strong evidence that skin inflammation induced by lupus serum is related to levels of anti-Ro Ab or low C3/C4 in serum (Supplemental Fig. 1). We did not observe skin inflammation following i.v. or i. p. injection of the same volume of lupus serum (data not shown). We also found that lupus serum can induce skin inflammation in Swiss and BALB/c mice (data not shown); serum from NZB/W F1 mice with the established lupus pathology induced skin inflammation that was milder than that induced by the same volume of serum from MRL/lpr mice (data not shown). These data demonstrate that lupus serum causes skin inflammation.
FIGURE 1.
Lupus serum induces skin inflammation. A, Representative histopathologic photomicrograph of skin of C57BL/6 mice sacrificed 3 d after intradermal inoculation of serum (100 μl) from a lupus patient with skin disease (SLE15, left panel), a lupus patient without skin disease (SLE6, middle panel), and a healthy control (right panel). Infiltration of mononuclear cells and proliferation of keratinocytes are apparent in the skin following intradermal injection of lupus serum. H&E, original magnification ×10. B, Kinetics of skin inflammation induced by serum from a lupus patient (SLE15) with skin disease and a healthy control. C, The severity of skin inflammation induced by various volumes of lupus serum from a patient (SLE12) with skin disease 3 d after intradermal injection. D, Severity and incidence of skin inflammation induced by lupus patients with (n = 6) or without (n = 12) skin disease and SLEDAI > 4 (n = 7) or SLEDAI ≤ 4 (n = 10). E, Histopathology of skin inflammation 3 d after intradermal injection of serum (100 μl) from lupus-prone MRL/lpr mice with skin injury and normal C57BL/6 mice or PBS (100 μl). H&E, original magnification ×10.
Table I.
Relevant clinical and serological information for SLE patients
| Patient ID No. | Anti-dsDNA Titer | Presence of SSA | C3/C4 Levels | History of Skin Lesions | History of Alopecia | SLEDAI |
|---|---|---|---|---|---|---|
| SLE1 | 1:20 | + | 116/20 | − | − | 4 |
| SLE2 | 253.9 | + | 89.1/19.4 | − | − | 8 |
| SLE3 | 1:20 | + | 112/7 (low) | − | − | 4 |
| SLE4 | 1:20 | + | 125/7 (low) | + | + | 8 |
| SLE5 | − | − | 101/25 | + | − | 2 |
| SLE6 | − | + | − | − | 12 | |
| SLE7 | 1:20 | + | 74/14 | − | + | 4 |
| SLE8 | 1:20 | + | 98/30 | − | + | 0 |
| SLE9 | 1:20 | + | 82/32 (low) | − | − | 2 |
| SLE10 | − | − | 78/15 (low) | − | − | 1 |
| SLE11 | 1:20 | − | − | + | 4 | |
| SLE12 | 1:20 | + | 125/41 | + | + | 4 |
| SLE13 | 1:640 | − | Historical | − | − | 4 |
| SLE14 | − | − | 111/29 | + | − | 2 |
| SLE15 | − | − | 180/37 | + | − | 14 |
| SLE16 | 1:20 | − | 75/8 (low) | + | − | 10 |
| SLE17 | 1:160 | + | − | − | − | 8 |
| SLE18 | 1:1280 | − | + | − | − | 36 |
| Normal l-9 | − | − | − | − | − | 0 |
+, positive; −, negative.
Monocytes are important in the development of skin inflammation induced by lupus serum
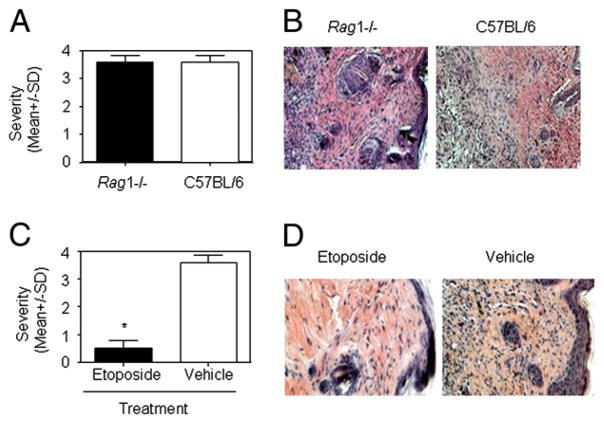
We observed that the lupus serum-induced skin inflammatory lesions displayed large numbers of infiltrating inflammatory cells. Because T cells were shown to be involved in skin inflammation in MRL/lpr mice (data not shown), we first evaluated the role of lymphocytes in lupus serum-induced skin inflammation by using Rag1−/− mice, which lack mature T and B cells but have intact monocytes/macrophages (20). Lymphocytes do not seem to be important in the development of lupus serum-induced skin inflammation, because the severity and incidence of the recorded lesions were not different between Rag1−/− mice and wild type mice (Fig. 2A, 2B). Next, we sought to determine the role of monocytes/macrophages in the development of lupus serum-induced skin inflammation. To this end, we treated mice with a cytotoxic drug (etoposide) that is known to selectively deplete monocytes/-macrophages (16–18). TUNEL staining demonstrated that etoposide induced macrophage, but not lymphocyte, apoptosis in spleens of C57BL/6 mice and depleted macrophages in spleens of C57BL/6 mice (Supplemental Fig. 2). Pretreatment of mice with etoposide led to abrogation of lupus serum-induced skin inflammation (Fig. 2C, 2D). Etoposide treatment did not affect IgG deposition in the skin (Supplemental Fig. 3). Monocytes were reported to contribute to the development of skin injury in MRL/lpr mice (21).
FIGURE 2.
Cellular requirement for lupus serum-induced skin inflammation. Severity (A) and photomicrographs (B) of skin inflammation 3 d after intradermal inoculation of serum (100 μl) from a patient with lupus with skin disease (SLE4) in Rag1−/− mice (n = 5 per group) and C57BL/6 mice (n = 5 per group). Severity (C) and photomicrographs (D) of skin inflammation 3 d after intradermal inoculation of serum (100 μl) from a lupus patient with skin disease (SLE4) in C57BL/6 mice with or without the depletion of monocytes following etoposide treatment (n = 5 per group). B and D, H&E, original magnification ×10. *p < 0.01.
TNF and TNFR1 play important roles in development of skin inflammation induced by lupus serum
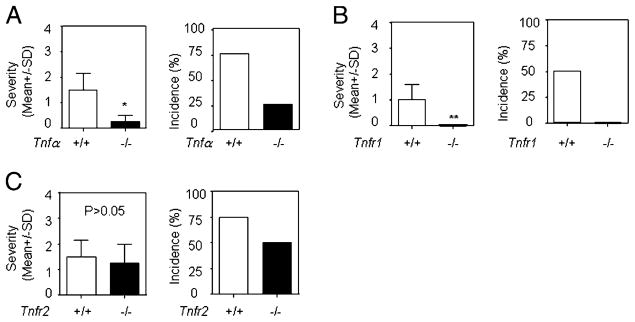
TNF-α is a proinflammatory cytokine produced mainly by monocytes/macrophages, and it is known to play an important role in the pathogenesis of inflammatory diseases (16, 18, 22). We used TNF-α–deficient mice to evaluate the role of TNF-α in lupus serum-induced skin inflammation. We found that the severity and incidence of lupus serum-induced skin inflammation were significantly decreased in TNF-α–deficient mice compared with wild type mice (Fig. 3A). TNF-α exerts its effect by binding to TNFR1 and TNFR2 (22). We assessed the role of TNFR1 and TNFR2 in the development of lupus serum-induced skin inflammation by using Tnfr1- or Tnfr2-deficient mice. Lupus serum was injected intradermally into Tnfr1- or Tnfr2-deficient mice and wild type mice. We found that skin inflammation was abolished in Tnfr1-deficient mice but not in Tnfr2-deficient mice (Fig. 3B, 3C). These data demonstrate that lupus serum mediated the development of skin inflammation through the TNFR1 signaling pathway.
FIGURE 3.
TNF–TNFR signaling requirements for lupus serum-induced skin inflammation. Severity and incidence of skin inflammation 3 d after intradermal inoculation of serum (100 μl) from lupus patients in mice with TNF-α (A; n = 5 per group), TNFR1 (B; n = 10 per group), or TNFR2 (C; n = 5 per group) gene deficiency and control mice. Sera were from six lupus patients, including two patients with skin disease (SLE4 and SLE15) and four patients without skin disease (SLE1, SLE3, SLE8, and SLE10). One lupus serum sample was used for one gene-deficient mouse and one wild type control mouse in these experiments. *p < 0.05; **p < 0.01.
IgG is a major contributor to the development of skin inflammation induced by lupus serum
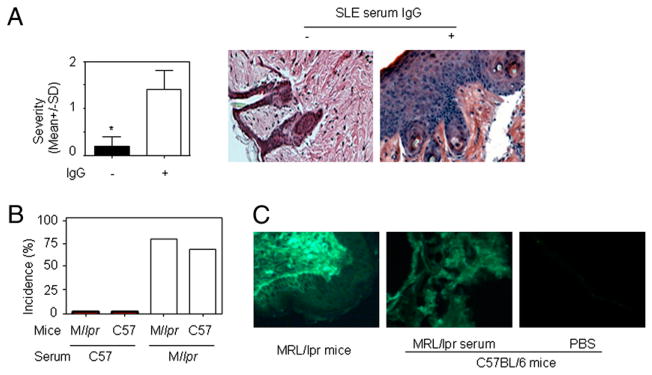
While performing these experiments, we observed that the same volume of serum from different lupus patients induced skin lesions of varying degrees of severity. To determine whether IgG present in lupus sera is a major contributor to lupus serum-induced skin inflammation, we used protein G agarose beads (23) to remove IgG from lupus sera. IgG depletion was confirmed by electrophoresis (data not shown). We found that skin inflammation was abrogated in mice injected with lupus sera depleted of IgG or IgG-containing complex (Fig. 4A). We injected IgG or IgG-containing complexes isolated from protein G agarose beads intradermally in mice and observed that they also induced skin inflammation (data not shown). These data indicate that IgG or IgG-containing complexes are required for lupus serum-induced skin inflammation. When we injected C57BL/6 mice and MRL/lpr mice (6 wk old) with serum from normal C57BL/6 or MRL/lpr mice with established (5 mo old) lupus pathology, only serum from 5-mo-old MRL/lpr mice caused skin inflammation (Fig. 4B). To determine whether the same dose of IgG from lupus serum and healthy serum mediated similar skin inflammation, we treated normal C57BL/6 mice with an intradermal injection of 500 μg IgG from lupus serum and healthy controls. Both IgGs induced the development of skin inflammation, but IgG from lupus serum mediated more severe skin inflammation than did IgG from healthy controls (Supplemental Fig. 4). In addition, we noted that large amounts of IgG were deposited in skin lesions and normal skin of lupus MRL/lpr mice (Fig. 4C); IgG deposition was also detected in the skin of C57BL/6 mice injected intradermally with MRL/lpr serum (Fig. 4C). Depletion of monocytes using etoposide did not remove IgG deposition induced by intradermal injection of MRL/lpr serum in the skin of C57BL/6 mice (Supplemental Fig. 4). Altogether, these data indicated that IgG or IgG-containing complexes represent major contributors to the development of skin inflammation induced by lupus serum.
FIGURE 4.
The role of IgG in lupus serum-induced skin inflammation. A, Severity and representative photomicrograph of skin inflammation 3 d after intradermal inoculation of 100 μl lupus serum (SLE1) with or without IgG depletion. *p <0.05. B, Incidence of skin inflammation 3 d after intradermal inoculation of serum (100 μl) from lupus-prone MRL/lpr mice (16 wk old) and normal C57BL/6 mice (16 wk old) in MRL/lpr mice (6 wk old) and C57BL/6 mice (6 wk old). C, Immunofluorescent staining of IgG deposition in skin lesion of MRL/lpr mouse at age 21 wk (left panel) and skin of normal C57BL/6 mice with intradermal injection of serum from MRL/lpr mice at age 21 wk (middle panel) or from normal C57BL/6 mice (right panel). Original magnification ×20.
TNFR1 regulates lupus serum-induced monocyte differentiation into DCs
In addition to becoming macrophages during an inflammatory process, monocytes represent an important source of DCs (24–29). We investigated whether sera from SLE patients and lupus-prone mice promoted monocyte differentiation into DCs (19). We found that serum from SLE patients induced monocyte differentiation into DCs, but serum from healthy individuals and normal C57BL/6 mice did not (Fig. 5A). To confirm whether lupus serum promotes monocyte differentiation into DCs, we analyzed CD40 and CD80 marker on monocytes treated with lupus serum and healthy control serum using flow cytometry. The results demonstrated that serum from lupus patients promoted the expression of CD40 and CD80 on monocytes (Fig. 5B). Because IgG or IgG-containing complexes are a major contributor in lupus serum-induced skin inflammation, we evaluated whether IgG or IgG-containing complexes present in lupus serum are required for lupus serum-induced monocyte differentiation into DCs. We found that depletion of IgG or IgG complexes from SLE sera rendered them unable to cause monocyte differentiation into DCs (Fig. 5C). Because TNFR1 was found to play a crucial role in the development of lupus serum-induced skin inflammation, we assessed whether TNFR1 exerts an important role in lupus serum-induced monocyte differentiation into DCs. The results showed that monocyte differentiation into DCs was significantly limited in Tnfr1-deficient, but not Tnfr2-deficient, mice (Fig. 5D), which confirms the importance of TNFR1 in the expression of lupus serum-mediated skin inflammation. Because TNFR1 is the receptor for TNF-α, we also determined whether TNF-α promotes monocyte differentiation into DCs. Monocytes from spleen of C57BL/6 mice were stimulated or not with TNF-α. We observed enhanced differentiation of monocytes into DCs in the presence of TNF-α compared with the absence of TNF-α (data not shown). TNF inhibitor delayed lupus serum-induced monocyte differentiation into DCs (Supplemental Fig. 5), which indicates that TNF-α is important in promoting monocyte differentiation into DCs. To confirm the identity of DCs identified by light microscopy, we performed immunofluorescent staining using anti–CD11c-FITC Ab to determine the expression of CD11c, a common marker of DCs. Immunofluorescent staining confirmed that the cells identified by light microscopy as DCs were CD11c+ cells (data not shown). To determine whether the expression of CD11c is increased in the skin of C57BL/6 mice following the intradermal injection of lupus serum, we evaluated the expression of CD11 in the skin of C57BL/6 mice using immunofluorescent staining. We found increased numbers of CD11c+ cells in skin lesions induced by SLE serum (Fig. 5E). To determine whether pDCs are involved in the skin inflammation induced by lupus serum, we performed immunohistochemical staining of pDCs, using anti–BDCA-2+ Ab, in the skin lesions induced by lupus serum, but we did not find any (data not shown). These data indicate that TNFR1 regulates lupus serum containing IgG-induced monocyte differentiation into DCs.
FIGURE 5.
Lupus serum-induced DC differentiation requires TNFR1 signaling. A, Monocytes from C57BL/6 mice were cultured with serum from a lupus patient (SLE2) or healthy controls for 40 h. Original magnification ×20. B, Expression of CD40 and CD80 on monocytes treated with serum from a lupus patient (SLE2) or healthy controls for 40 h, as measured by flow cytometry. C, Monocytes from C57BL/6 mice were cultured with serum from a lupus patient (SLE2), with or without IgG depletion, for 40 h. D, Monocytes from mice with TNFR1 or TNFR2 gene deficiency and wild type mice were cultured with serum from a lupus patient (SLE2) for 40 h. E, Immunofluorescent staining of DCs in C57BL/6 mice skin with FITC-conjugated anti-CD11c Ab 4 d after intradermal injection of serum (100 μl) from a lupus patient (SLE2) or normal control. C–E, original magnification ×20.
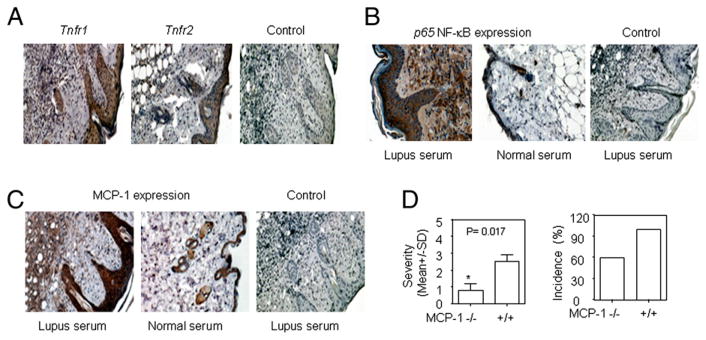
Enhanced expression of TNFR1, NF-κB, and MCP-1 in skin lesion induced by lupus serum
Our studies demonstrated that TNFR1, but not TNFR2, is required for lupus serum-induced skin inflammation. To further understand the role of TNFR1 in the development of lupus serum-induced skin inflammation, we determined the expression of TNFR in skin lesions using immunohistochemical staining. We found that TNFR1 is expressed more prominently than TNFR2 in the lupus serum-induced skin lesions (Fig. 6A); the pattern was similar to that observed in skin lesions from MRL/lpr mice (G.-M. Deng, L. Liu, and G.C. Tsokos, unpublished observations). This finding corroborates our observation that Tnfr1-deficient, but not Tnf2-deficient, mice fail to develop skin lesions following the injection of lupus serum.
FIGURE 6.
Expression of MCP-1 and NF-κB in lupus serum-induced skin inflammation. A, Immunohistochemical staining for TNFR1 and TNFR2 expression in skin lesions 3 d after intradermal inoculation of serum (100 μl) from a lupus patient (SLE1) in C57BL/6 mice. Immunohistochemical staining shows NF-κB (B) and MCP-1 (C) expression in skin lesions 3 d after intradermal injection of serum from a lupus patient (SLE15) and a healthy control in C57BL/6 mice. Anti-human IgG Ab was used as control staining. A–C, H&E, original magnification ×10. D, Severity and incidence of skin inflammation induced by serum from a lupus patient (SLE4) in MCP-1–deficient mice (n = 5) and wild type mice (n = 5). *p < 0.05.
Because increased expression of TNFR1 and MCP-1 skin lesions were induced by lupus serum, and NF-κB is an important regulator of genes involved in the inflammatory response (30), we determined whether NF-κB is upregulated in the skin lesions caused by the injection of lupus serum. Indeed, as shown in Fig. 6B, p65NF-κB expression was markedly increased in inflammatory lesions caused by lupus serum compared with healthy serum.
Monocytes migrate to inflammatory sites from blood vessels in response to increased expression of chemokines (31). MCP-1 was shown to contribute to the attraction of monocytes to the inflamed skin in MRL/lpr mice (32). We investigated the expression of MCP-1 in skin lesions induced by lupus serum using immunohistochemical staining. MCP-1 expression was largely increased in the skin at the site of intradermal injection of SLE serum compared with healthy serum in normal mice (Fig. 6C). To further investigate the role of MCP-1 in lupus serum-induced skin inflammation, we injected MCP-1–deficient mice with lupus and observed that the severity of the induced skin inflammation was decreased (Fig. 6D).
Additive effect of lupus serum and CpG DNA in inducing skin inflammation
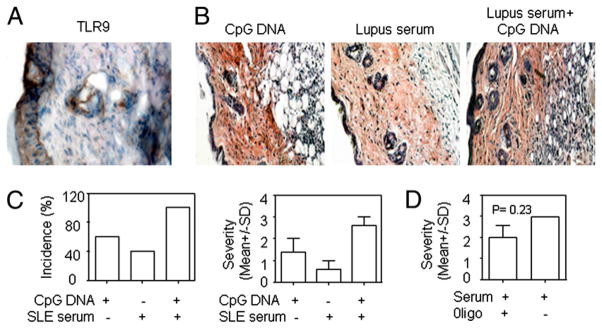
Considering that TLRs were found to regulate autoimmunity and autoimmune pathology and that apoptotic material in the skin may engage TLRs, we investigated whether CpG DNA and lupus serum act synergistically to induce skin inflammation because we found that keratinocytes express TLR9 in skin lesions of MRL/lpr mice (Fig. 7A). There were a high incidence and severity of skin inflammation when a suboptimal volume of lupus serum (50 μl) and CpG DNA (5 μg) were injected simultaneously; a lower incidence was noted after they were injected individually (Fig. 7B, 7C). We also found that inhibitor of CpG DNA oligonucleotide reduced the severity of lupus serum-induced skin inflammation, but the difference was not statistically significant (Fig. 7D).
FIGURE 7.
CpG DNA and lupus serum act additively in the induction of skin inflammation. A, Immunohistochemical staining of TLR9 in skin lesions of lupus-prone MRL/lpr mice. Histopathology (B) and severity and incidence (C) of skin inflammation 3 d after intra-dermal injection of CpG ODN 1668 (5 μg), lupus serum (50 μl), or a combination of CpG ODN 1668 (5 μl) and lupus serum (50 μl) in C57BL/6 mice. Serum was from a lupus patient with anti-dsDNA Ab (SLE3). D, Severity of skin inflammation induced by lupus serum (100 μl) with anti-dsDNA Ab (SLE3), with or without inhibitory oligonucleotide (10 μg).
Discussion
Skin inflammation developed following the local injection of lupus serum but not after the systemic injection of lupus serum. The possibility of traumatic/surgical injury can be excluded because the injection of PBS or normal serum did not result in skin inflammation. It is apparent that local injection, compared with systemic injection, should result in greater concentrations of IgG in the skin, and the intensity of the inflammatory lesion correlated with the volume of injected serum (Fig. 1C). IgG deposition in the skin was detected in normal mice following intradermal injection of MRL/lpr serum but not after the injection of normal C57BL/6 mouse serum. In addition, IgG deposition was consistently detected in the skin lesions of MRL/lpr mice, documenting the involvement of IgG in skin inflammation. Because IgG was found deposited in the normal skin of MRL/lpr mice, it is apparent that IgG deposition alone may not be sufficient to instigate skin injury.
Certain autoantibodies have been connected to distinct pathologies in SLE patients. For example, anti-TCR/CD3 autoantibodies present in SLE sera were shown to suppress IL-2 production (33). Anti-dsDNA Abs were demonstrated to contribute to CNS (14) and kidney damage (34). Although anti-dsDNA+ human lupus sera (included in our study) caused skin injury, we cannot conclude that anti-DNA Abs are directly involved in the expression of skin injury; the injection of monoclonal anti-DNA Abs and the injection of sera with progressively higher titers of anti-DNA Abs failed to induce significant skin inflammation. In addition, anti-DNA Ab-negative sera were able to cause skin damage. It is possible that other autoantibodies, including anti-Ro, anti-histone, or anti-phospholipid Abs, elicit skin inflammation (2, 35). Anti-Ro Abs were shown to accumulate in skin lesions in lupus patients and human skin grafted in SCID mice (3, 15). Anti-Ro Abs to keratinocytes expressing Ro Ag on the surface membrane were reported, and it was postulated that they facilitate Ab-dependent cell cytotoxicity by infiltrating cytotoxic cells (36).
Skin lesions in humans and mice with SLE display abundant infiltration of lymphocytes (37). We were surprised to observe that injection of lupus serum into the skin of lymphocyte-deficient mice (Fig. 2A, 2B) caused inflammation comparable to that observed in normal mice. It is possible that lymphocytes are attracted to the inflamed skin and contribute to the chronicity of the lesions but are not required for the initiation of the pathology.
Lupus sera have been widely claimed to contain IFN-α defined by their ability to promote the expression of genes responsive to IFN (38). In addition, lupus serum IFN-α was shown to promote monocyte differentiation into DCs (19). Differentiation of DCs requires TNFR1 signaling (39). TNFR1 is critical for TNF-α to stimulate keratinocyte apoptosis (40) and skin inflammation (41) and the propagation of a specific immune response after they travel to local lymph nodes to stimulate T cells (42). In this article, we showed preferential expression of TNFR1 over TNFR2 in the skin lesions of MRL/lpr mice and the need for functional TNFR1, rather than TNFR2, for the expression of skin lesions after the intradermal injection of lupus serum. Differentiation of monocytes into DCs in the presence of lupus serum depended on the presence of functional TNFR1, thus increasing the complexity of requirements for the differentiation of monocytes into DCs in SLE patients beyond the reported role of IFN-α (19). Although skin of lupus patients is frequently characterized by the infiltration by pDCs (12, 13), we did not find any pDCs in mouse skin lesions induced by lupus serum. This may result from differences in mouse and human pDC maturation (43) or the BDCA-2 Ab used, which might be not sensitive enough to detect mouse pDCs in our mouse model.
Information generated from the use of TNF-α blockade in patients with inflammatory disorders revealed the expression of autoimmune manifestations primarily of increased anti-nuclear Ab titers (44). Although TNF-α blockade has been suggested for the treatment of lupus patients (45), formal trials have not been attempted. Our study demonstrates a clear dependence of the lupus serum-induced skin lesions on the presence of TNFR1 and provides the first evidence of different roles for TNFR1 and TNFR2 in the expression of skin damage in SLE. Indeed, infusion of preligand assembly domain 60 (TNFR1), a protein that blocks TNFR1 signaling by interfering with its trimerization on the cell surface membrane (22), but not infusion of preligand assembly domain 80 (TNFR2), a protein that inhibits trimerization of TNFR2, can effectively block the expression of skin lesions in the MRL/lpr mouse (G.-M. Deng, L. Liu, and G.C. Tsokos, unpublished observations).
In conclusion, TNF-α/TNFR1 signaling has an important role in lupus serum-induced skin inflammation. Lupus serum containing IgG complexes activates skin resident DCs and enhances the expression of proinflammatory cytokines and chemokines, which attract monocytes to inflammatory sites from blood vessels. DCs may travel to local lymph notes to promote the autoimmune response while instigating a local inflammatory response. Our data suggest that specific blockade of TNFR1, rather than TNF-α signaling, and monocyte differentiation may represent rational targets in the treatment of cutaneous skin lesions. Patients with recalcitrant skin lupus have fared well following treatment with azathioprine, a monocyte-differentiation blocker (46).
Supplementary Material
Acknowledgments
This work was supported by the Public Health Service, National Institutes of Health Grant RO1 AI 42269 and a Mary Kirkland Scholar Award.
Abbreviations in this paper
- ACLE
acute cutaneous lupus erythematosus
- BDCA-2
blood dendritic cell Ag-2
- DC
dendritic cell
- LE
lupus erythematosus
- pDC
plasmacytoid dendritic cell
- SLE
systemic lupus erythematosus
- SLEDAI
systemic lupus erythematosus disease-activity index
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Fu SM, Castillo JD, Deshmukh US, Lewis JE, Waters ST, Gaskin F. Autoantibodies and glomerulonephritis in systemic lupus erythematosus. Lupus. 2003;12:175–180. doi: 10.1191/0961203303lu352xx. [DOI] [PubMed] [Google Scholar]
- 2.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 3.Lee HJ, Sinha AA. Cutaneous lupus erythematosus: understanding of clinical features, genetic basis, and pathobiology of disease guides therapeutic strategies. Autoimmunity. 2006;39:433–444. doi: 10.1080/08916930600886851. [DOI] [PubMed] [Google Scholar]
- 4.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, Doménech I, Aydintug AO, Jedryka-Góral A, de Ramón E, et al. The European Working Party on Systemic Lupus Erythematosus. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. Medicine (Baltimore) 1993;72:113–124. [PubMed] [Google Scholar]
- 5.Prystowsky SD, Gilliam JN. Discoid lupus erythematosus as part of a larger disease spectrum. Correlation of clinical features with laboratory findings in lupus erythematosus. Arch Dermatol. 1975;111:1448–1452. [PubMed] [Google Scholar]
- 6.Werth VP. Cutaneous lupus: insights into pathogenesis and disease classification. Bull NYU Hosp Jt Dis. 2007;65:200–204. [PubMed] [Google Scholar]
- 7.Crowson AN, Magro C. The cutaneous pathology of lupus erythematosus: a review. J Cutan Pathol. 2001;28:1–23. doi: 10.1034/j.1600-0560.2001.280101.x. [DOI] [PubMed] [Google Scholar]
- 8.Sontheimer RD. Skin manifestations of systemic autoimmune connective tissue disease: diagnostics and therapeutics. Best Pract Res Clin Rheumatol. 2004;18:429–462. doi: 10.1016/j.berh.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Yung A, Oakley A. Bullous systemic lupus erythematosus. Australas J Dermatol. 2000;41:234–237. doi: 10.1046/j.1440-0960.2000.00426.x. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn A, Bijl M. Pathogenesis of cutaneous lupus erythematosus. Lupus. 2008;17:389–393. doi: 10.1177/0961203308090019. [DOI] [PubMed] [Google Scholar]
- 11.Wenzel J, Zahn S, Bieber T, Tting T. Type I interferon-associated cytotoxic inflammation in cutaneous lupus erythematosus. Arch Dermatol Res. 2009;301:83–86. doi: 10.1007/s00403-008-0892-8. [DOI] [PubMed] [Google Scholar]
- 12.Blomberg S, Eloranta ML, Cederblad B, Nordlin K, Alm GV, Rönnblom L. Presence of cutaneous interferon-alpha producing cells in patients with systemic lupus erythematosus. Lupus. 2001;10:484–490. doi: 10.1191/096120301678416042. [DOI] [PubMed] [Google Scholar]
- 13.Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid dendritic cells (natural interferon- alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol. 2001;159:237–243. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7:1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 15.Lee LA, Gaither KK, Coulter SN, Norris DA, Harley JB. Pattern of cutaneous immunoglobulin G deposition in subacute cutaneous lupus erythematosus is reproduced by infusing purified anti-Ro (SSA) autoantibodies into human skin-grafted mice. J Clin Invest. 1989;83:1556–1562. doi: 10.1172/JCI114052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng GM, I, Nilsson M, Verdrengh M, Collins LV, Tarkowski A. Intra-articularly localized bacterial DNA containing CpG motifs induces arthritis. Nat Med. 1999;5:702–705. doi: 10.1038/9554. [DOI] [PubMed] [Google Scholar]
- 17.Deng GM, Liu ZQ, Tarkowski A. Intracisternally localized bacterial DNA containing CpG motifs induces meningitis. J Immunol. 2001;167:4616–4626. doi: 10.4049/jimmunol.167.8.4616. [DOI] [PubMed] [Google Scholar]
- 18.Deng GM, Verdrengh M, Liu ZQ, Tarkowski A. The major role of macrophages and their product tumor necrosis factor alpha in the induction of arthritis triggered by bacterial DNA containing CpG motifs. Arthritis Rheum. 2000;43:2283–2289. doi: 10.1002/1529-0131(200010)43:10<2283::AID-ANR16>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 20.Mudter J, Amoussina L, Schenk M, Yu J, Brüstle A, Weigmann B, Atreya R, Wirtz S, Becker C, Hoffman A, et al. The transcription factor IFN regulatory factor-4 controls experimental colitis in mice via T cell-derived IL-6. J Clin Invest. 2008;118:2415–2426. doi: 10.1172/JCI33227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menke J, Hsu MY, Byrne KT, Lucas JA, Rabacal WA, Croker BP, Zong XH, Stanley ER, Kelley VR. Sunlight triggers cutaneous lupus through a CSF-1-dependent mechanism in MRL-Fas(lpr) mice. J Immunol. 2008;181:7367–7379. doi: 10.4049/jimmunol.181.10.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng GM, Zheng L, Chan FK, Lenardo M. Amelioration of inflammatory arthritis by targeting the pre-ligand assembly domain of tumor necrosis factor receptors. Nat Med. 2005;11:1066–1072. doi: 10.1038/nm1304. [DOI] [PubMed] [Google Scholar]
- 23.Jury EC, Kabouridis PS, Flores-Borja F, Mageed RA, Isenberg DA. Altered lipid raft-associated signaling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J Clin Invest. 2004;113:1176–1187. doi: 10.1172/JCI20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, Caux C, Aït-Yahia S, Vicari A, Kaiserlian D, Dubois B. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 26.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, Randolph GJ, Merad M. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 28.León B, López-Bravo M, Ardavín C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against. Leishmania Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 30.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 31.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tesch GH, Maifert S, Schwarting A, Rollins BJ, Kelley VR. Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-Fas(lpr) mice. J Exp Med. 1999;190:1813–1824. doi: 10.1084/jem.190.12.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juang YT, Wang Y, Solomou EE, Li Y, Mawrin C, Tenbrock K, Kyttaris VC, Tsokos GC. Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. J Clin Invest. 2005;115:996–1005. doi: 10.1172/JCI200522854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajagopalan S, Zordan T, Tsokos GC, Datta SK. Pathogenic anti-DNA autoantibody-inducing T helper cell lines from patients with active lupus nephritis: isolation of CD4-8- T helper cell lines that express the gamma delta T-cell antigen receptor. Proc Natl Acad Sci USA. 1990;87:7020–7024. doi: 10.1073/pnas.87.18.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleming SD, Egan RP, Chai C, Girardi G, Holers VM, Salmon J, Monestier M, Tsokos GC. Anti-phospholipid antibodies restore mesenteric ischemia/reperfusion-induced injury in complement receptor 2/complement receptor 1-deficient mice. J Immunol. 2004;173:7055–7061. doi: 10.4049/jimmunol.173.11.7055. [DOI] [PubMed] [Google Scholar]
- 36.Norris DA. Pathomechanisms of photosensitive lupus erythematosus. J Invest Dermatol. 1993;100:58S–68S. doi: 10.1111/1523-1747.ep12355599. [DOI] [PubMed] [Google Scholar]
- 37.Kuhn A, Sontheimer RD. Cutaneous lupus erythematosus: molecular and cellular basis of clinical findings. Curr Dir Autoimmun. 2008;10:119–140. doi: 10.1159/000131451. [DOI] [PubMed] [Google Scholar]
- 38.Crow MK. Type I interferon in systemic lupus erythematosus. Curr Top Microbiol Immunol. 2007;316:359–386. doi: 10.1007/978-3-540-71329-6_17. [DOI] [PubMed] [Google Scholar]
- 39.Le Hir M, Bluethmann H, Kosco-Vilbois MH, Müller M, di Padova F, Moore M, Ryffel B, Eugster HP. Differentiation of follicular dendritic cells and full antibody responses require tumor necrosis factor receptor-1 signaling. J Exp Med. 1996;183:2367–2372. doi: 10.1084/jem.183.5.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwarz A, Bhardwaj R, Aragane Y, Mahnke K, Riemann H, Metze D, Luger TA, Schwarz T. Ultraviolet-B-induced apoptosis of keratinocytes: evidence for partial involvement of tumor necrosis factor-alpha in the formation of sunburn cells. J Invest Dermatol. 1995;104:922–927. doi: 10.1111/1523-1747.ep12606202. [DOI] [PubMed] [Google Scholar]
- 41.Pasparakis M, Courtois G, Hafner M, Schmidt-Supprian M, Nenci A, Toksoy A, Krampert M, Goebeler M, Gillitzer R, Israel A, et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature. 2002;417:861–866. doi: 10.1038/nature00820. [DOI] [PubMed] [Google Scholar]
- 42.Randolph GJ, Ochando J, Partida-Sánchez S. Migration of dendritic cell subsets and their precursors. Annu Rev Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- 43.Hochrein H, O’Keeffe M, Wagner H. Human and mouse plasma-cytoid dendritic cells. Hum Immunol. 2002;63:1103–1110. doi: 10.1016/s0198-8859(02)00748-6. [DOI] [PubMed] [Google Scholar]
- 44.Eriksson C, Engstrand S, Sundqvist KG, Rantapää-Dahlqvist S. Autoantibody formation in patients with rheumatoid arthritis treated with anti-TNF alpha. Ann Rheum Dis. 2005;64:403–407. doi: 10.1136/ard.2004.024182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aringer M, Smolen JS. The role of tumor necrosis factor-alpha in systemic lupus erythematosus. Arthritis Res Ther. 2008;10:202. doi: 10.1186/ar2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsokos GC, Caughman SW, Klippel JH. Successful treatment of generalized discoid skin lesions with azathioprine. Its use in a patient with systemic lupus erythematosus. Arch Dermatol. 1985;121:1323–1325. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.