Abstract
HS-27a human bone stromal cells, in 2D or 3D coultures, induced cellular plasticity in human prostate cancer ARCaPE and ARCaPM cells in an EMT model. Cocultured ARCaPE or ARCaPM cells with HS-27a, developed increased colony forming capacity and growth advantage, with ARCaPE exhibiting the most significant increases in presence of bone or prostate stroma cells. Prostate (Pt-N or Pt-C) or bone (HS-27a) stromal cells induced significant resistance to radiation treatment in ARCaPE cells compared to ARCaPM cells. However pretreatment with anti-E-cadherin antibody (SHEP8-7) or anti-alpha v integrin blocking antibody (CNT095) significantly decreased stromal cell-induced radiation resistance in both ARCaPE- and ARCaPM-cocultured cells. Taken together the data suggest that mesenchymal-like cancer cells reverting to epithelial-like cells in the bone microenvironment through interaction with bone marrow stromal cells and reexpress E-cadherin. These cell adhesion molecules such as E-cadherin and integrin alpha v in cancer cells induce cell survival signals and mediate resistance to cancer treatments such as radiation.
1. Introduction
Prostate cancer is the most frequent tumor in men, afflicting African American males to a greater degree than Caucasians. Morbidity and mortality are mainly attributable to metastasis; yet the mechanisms associated with progression are largely unknown. Localized carcinomas are readily removed surgically, but once a tumor has established metastases, current therapies are not curative and prolong survival by only a few years. Metastasis occurs through a multistep process, where metastatic cells must intravasate local tissues and enter into and survive in the blood stream. These cells then extravasate into the secondary tissue and initiate and maintain micrometastases at distant sites, with the end result being the development of a metastatic tumor [1, 2]. During each step of this process, cancer cells exhibit transdifferentiation properties that allow both the spatial and temporal expression of epithelial and mesenchymal properties in response to microenvironment signals and its own basic survival needs (e.g., motility and invasion versus proliferation). Thus, a model of cellular transitions, as opposed to a continual progression to permanent differentiation state, is emerging as a significant mechanism during metastasis. A greater understanding of these mechanisms will result in clinical improvements and a better control of the metastasis process.
Epithelial-mesenhymal transition (EMT) was first described during development [3, 4]; however an EMT-like phenotypic change has been observed in a number of solid tumors [5–7]. This transition is typically characterized by a loss in E-cadherin and cytokeratin expression. EMT in cancer, as in development, is associated with an increase in cell proliferation [8, 9] and the acquisition of a mesenchymal phenotype that includes vimentin, N-cadherin, and osteopontin expression. In both normal development EMT and cancer-associated EMT, the loss of E-cadherin is critical to the differentiation and maintenance of the epithelial phenotype and provides a structural link between adjacent cellular cytoskeletons, which is important for tissue architecture. Cells that have undergone EMT (E-cadherin negative mesenchymal cells) subsequently become more migratory and invasive and proceed to traverse underlying basement membranes, with an acquired ability to intravaste the surrounding local tissue and gain access to vascular conduits. As such, the loss of E-cadherin is rate limiting for EMT [10, 11]. Recent reports from this laboratory and others have described a mesenchymal to epithelial reverting transition (MErT) to occur, where mesenchymal-like prostate cancer cell lines reexpress E-cadherin to become epithelial-like, and reestablish cellular adhesion during colonization within the liver tumor microenvironment [12, 13]. These findings are shared in clinical metastases of various cancer origins including breast, colon, and bladder, where robust membrane expression of E-cadherin was observed, and the paired more differentiated primary tumors were E-cadherin negative [6, 14]. Thus, a reversion of the mesenchymal phenotype appears to be important in latter stages of metastasis.
Numerous studies have shown that the underlying influence of these cellular transitions is a consequence of tumor-stromal interactions [15, 16]. Coculture studies have found that the survival and proliferation of cancer cells are intimately linked to the soluble factors in the microenvironment, such as EGF, TGF-β, IGF-l that contribute to survival and the subsequent formation of macrometastasis [17–20]. However, these factors are not likely to have a direct effect during initial metastatic colonization, and thus heterotypic and homotypic cellular adhesion has been proposed to provide the necessary survival signals for successful colonization [21, 22]. Current state-of-the-art technology does not provide the necessary resolution to determine at the single cell level in patients or experimental in vivo systems, individual cells that have successfully colonized the secondary site. However, numerous reports have firmly established that cancer-stromal interactions in vitro or in three-dimensional (3D) assays accurately mimic the drug sensitivity/resistance behavior of those cells found within solid tumors in vivo in a preclinical or clinical setting [23]. Thus, we employed a novel coculture assay to determine the cellular plasticity of cancer cells promoted by the bone stroma and the effect of tumor-stromal interactions on irradiation therapy in prostate cancer.
The ARCaP model is the only robust prostate cancer bone metastatic model which demonstrates epithelial to mesenchymal transition(EMT). The ARCaP progression model consists of ARCaPE (epithelial) and ARCaPM (mesenchymal), where the ARCaPE cells have a bone metastatic potential of 12.5% and the ARCaPM cells have a bone metastatic potential of 100%. The ARCaPE and ARCaPM cells express the classical markers of EMT [24, 25]. Herein we present findings that ARCaPM cells undergo MErT when cocultured within the bone microenvironment in 3D and 2D cultures. Additionally, ARCaPE cells that retained an epithelial phenotype exhibited a measurable growth advantage and retained ability to form colonies, however only under coculture conditions with bone stroma. Furthermore, blocking the ability of ARCaPE or ARCaPM cells from E-cadherin-mediated cell-cell adhesion or integrin alpha v beta-associated adhesion significantly affected ARCaP cell survival within bone stroma and sensitized these cells to radiation treatment.
2. Methods
2.1. Cell Culture
The human prostate cancer cell lines, ARCAPE, ARCaPM, the HS-27a bone stromal cells (ATCC, Manasss, VA) and the Pt-N or Pt-C human prostate stromal cell. Isolation and characterization of the human prostate cancer RFP-ARCaP cell lines has been reported [26]. Red Fluorescent Protein- (RFP-) transfected cells were maintained in G418 (350 mg/mL) prior to experimentation. All cell lines were grown in a 5% CO2 incubator at 37°C in media consisting of T-medium (Invitrogen, Carlsbad, CA) supplemented with 5% (v/v) fetal bovine serum and 1% Penicillin-Streptomycin.
2.2. Cocultures
Initial cocultures were performed as previously described [12, 13] with modifications. Cocultures consisted of 50 000 cells/cm2 of HS-27a bone marrow stromal cells and 2000 cells/cm2 prostate cancer cells. Cocultures were maintained in serum-free T-media and plated on tissue culture dishes.
2.3. Clonogenic Assay
Cells were plated at low densities in six-well plates for 24 hours and then were irradiated with the appropriate radiation dose. Twenty-four hours later, the media were changed and cells were incubated until they formed colonies having at least 50 or more cells. Seventeen days later colonies were rinsed with PBS, stained with methanol/crystal violet dye, and counted. The colony formation ability was calculated as a ratio of the number of colonies formed, divided by the total number of cells plated, times the plating efficiency [(# of colonies formed ÷ total # cells plated) × plating efficiency]. For experiments with cocultures, cells were initially incubated on a mat of stromal cells for 24 hours and radiated; 4 hours later clonogenic assay was performed. For antibody-based experiments using anti-E-cadherin (15 μg/mL, DECMA or SHEP8-7, Sigma) and anti-integrin alpha-v (20 μg/mL, CNT095) antibody, cancer cells were treated with respective antibodies for 24 hours prior to plating them on a mat of stromal cells.
2.4. Radiation
External beam radiation was delivered on a 600 Varian linear accelerator (Varian Medical Systems, Inc.Palo Alto, CA) with a 6 MV photon beam. A 40 × 40 cm field size was utilized and Petri dishes were placed on 1.5 cm of superflab bolus. Monitor units (MUs) were calculated to deliver the dose to a depth of dmax at a dose rate of 600 MU/min.
2.5. Statistical Analysis
Representative findings are shown for all experiments, which were performed in triplicate, repeated a minimum of three times. Student's t-test was used to determine the statistical significance between groups.
3. Results
3.1. ARCaP EMT Model Undergoes a Mesenchymal-to-Epithelial Reverting Transition (MErT)
Recently, the ARCaP model has been described to closely mimic the patho-physiology of advanced clinical human prostate cancer bone metastasis [25]. The ARCaPE cells were derived from single-cell dilutions of the ARCaP cells. These cells exhibit a cuboidal-shaped epithelial morphology with high expression of epithelial markers, such as cytokeratin 18 and E-cadherin. The lineage-derived ARCaPM cells have a spindle-shaped mesenchymal morphology and phenotype. ARCaPM cells have decreased expression of E-cadherin and cytokeratins 18 and 19 but increased expression of N-cadherin and vimentin. These cells have decreased cell adhesion and increased metastatic propensity to bone and adrenal glands [27]. The morphologic and phenotypic changes observed in the ARCaPM cells closely resemble those of cells undergoing EMT.
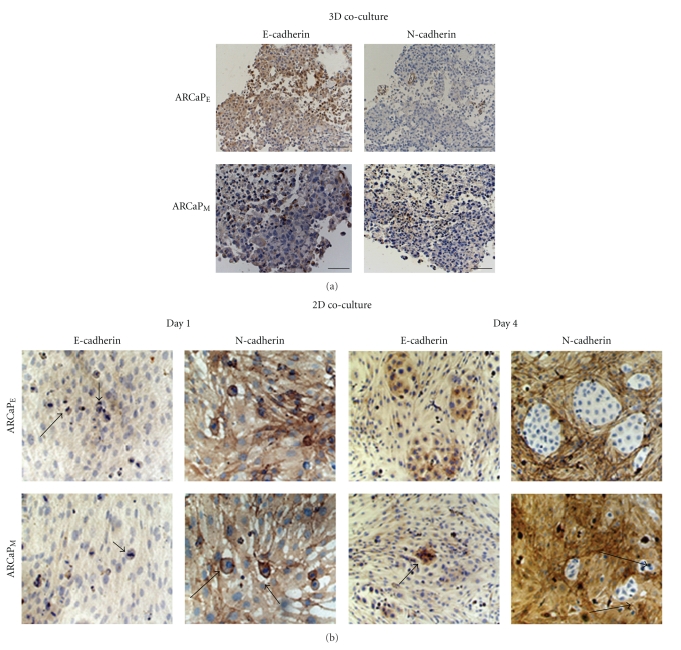
Previously, we have demonstrated a Mesenchymal to Epithelial reverse Transition (MErT) of metastatic prostate cancer cell lines within an experimental coculture model and confirmed in patients with liver metastasis [13, 28]. Our findings have recently been confirmed in prostate cancer bone metastasis where E-cadherin and β-catenin were robustly expressed in late stage carcinomas [29]. Therefore we sought to identify the significance of the bone microenvironment within the experimental ARCaP model. To assess cellular plasticity of the ARCaP EMT model, we coultured ARCaP cells with HS-27a cells in 3D RWV (rotary wall vessel) system for 3 days. ARCaPE cells formed larger prostate organoids than ARCaPM cells (data not shown). Upon immunohistochemical examination of organoids, we observed that both ARCaPE and ARCaPM express E-cadherin and lack N-cadherin expression (Figure 1(a)). To further examine the influence of tumor-stroma interactions over a multiday period we utilized a similar 2D cocultures method. Utilizing immunoctyochemical analysis, we observed a lack E-cadherin and robust N-cadherin staining after 1 day in both ARCAPE and ARCaPM cocultures. However by day 4, both ARCaPE and ARCaPM cells formed tumor nest that express E-cadherin and lack N-cadherin staining (Figure 1(b)). It is worthy to note that ARCaPM tumor nest appeared to develop at much smaller extent, compared to ARCaPE cocultures.
Figure 1.
3D cocultures of ARCaPE or ARCaPM with HS-27a cells show E-cadherin expression. (a) 1 × 107 ARCaPE or ARCaPM were cocultured with HS-27a cells in RWV for 3 days. Immunohistochemistry of organoids was stained with anti-E-cadherin or N-cadherin antibody. (b) 2D Cocultures of HS-27a were preformed utilizing a total of 50,000 cm2/HS-27a fibroblasts, after which 20,000 cm2 ARCaPE or ARCaPM were seeded on top of the fibroblast monolayer. The cocultures were maintained in serum-free medium for 1 or 4 days. Immunocytochemistry of cocultures over these time periods was performed utilizing anti-E-cadherin and N-cadherin antibodies. Shown are the EMT/MET of ARCaPEcells (top panels) and MErT of ARCaPMcells (bottom panels).
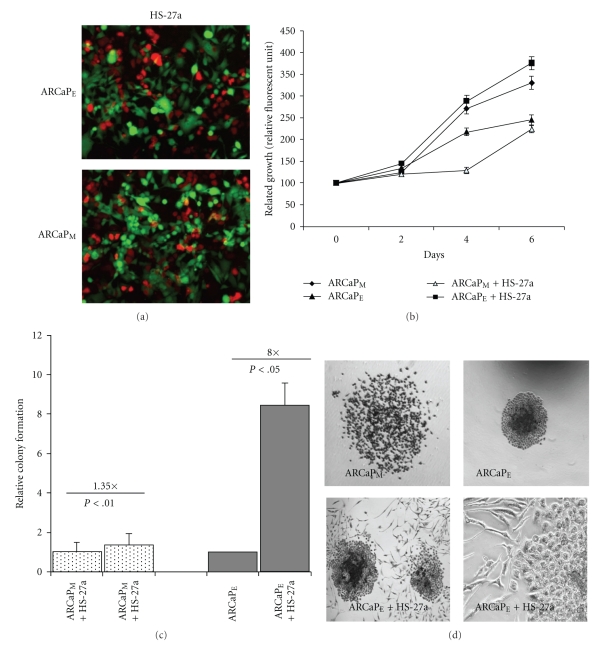
Since ARCaPE cells formed larger tumor nest and spheroids when cocultured with HS-27a cells compared to ARCaPM cells, we sought to further assess if HS-27a cells preferentially stimulated the growth of ARCaPE cells versus ARCaPM cells. Utilizing GFP-transfected HS-27a bone marrow stromal cells and RFP-transfected ARCaPE or ARCaPM cells (Figure 2(a)), we examined the proliferative ability of ARCaP cells in homotypic and coculture conditions. Growth of RFP-transfected ARCaPE and ARCaPM cells, respectively, was quantified by relative fluorescent units (RFU) of transfected cell lines over a 6-day period in homotypic cultures and coculture conditions (Figure 2(a)). As previously reported, homotypic cultured ARCaPM shows significant growth compared to ARCaPE homotypic cultures; however cocultures reversed this trend with ARCaPE cells demonstrating the most significant growth (Figure 2(b)). We also confirmed these findings in ARCaPE cells in coculture using clonogenic assay. Although ARCaPM cells have a higher plating efficiency than ARCaPE cells, ARCaPE cells exhibited an 8-fold increase in their ability to form colonies after coculture compared to 1.35-fold increase of cocultured ARCaPM cells (Figure 2(c)). Phase-contrast microscopy of colonies after coculture shows that ARCaPM colonies appear loosely adherent, while ARCaPE cells are compact and interact physically with few of the bone stromal fibroblast (Figure 2(d)). Taken together, these results demonstrate that ARCaPM cells reexpress E-cadherin when grown with bone stromal cells for longer periods. Additionally, ARCaPE cells which have high levels of E-cadherin gain enhanced growth and self-renewal ability when cocultured with bone stromal cells.
Figure 2.
ARCaPE cells show a growth and colony forming capacity advantage in presence of HS-27a cells. (a) and (b) ARCaPM cells were cocultured in the presence of GFP-HS-27a cells over a 6-day period. Growth of RFP. ARCaPE or ARCaPM human prostate cancer cells was assessed by RFUs (relative fluorescent units) in the presence cocultures over a 6-day period. Results are means ± SE of three independent experiments. *P < .05 (students t-test) compared to cell number at day 1 ± SEM. (c) Clonogenic colony forming capacity of ARCaPE and ARCaPM prostate cancer cell after coculture ± SEM. ARCaPM data were normalized to ARCaPM control, and ARCaPE data were normalized to ARCaPE control (Note HS-27a induced slightly (1.35x) the growth of ARCaPM cells but markedly (8x) the growth of ARCaPE cells.). (d) ARCaPE or ARCaPM cells were cocultured with HS-27a cells. Shown are phase contrast images of colonies formed in the clonogenic assay.
3.2. Stromal Cells Influence Radiation Treatment in Prostate Cancer Cells
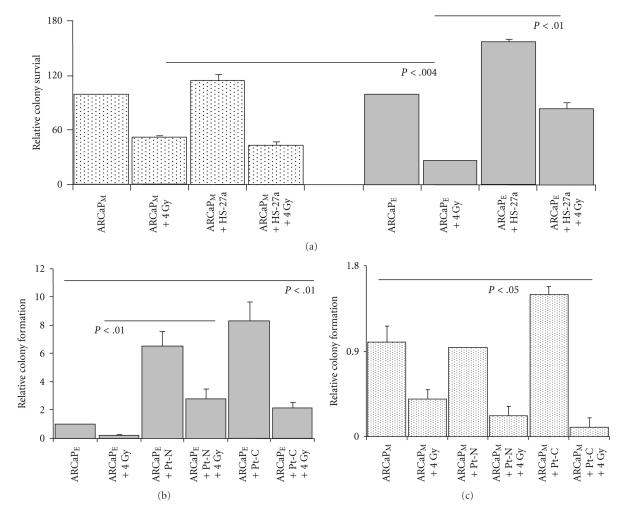
Mesenchymal cancer cells have been thought to be more tumorigenic, aggressive, and resistant to treatments when compared to epithelial cancer cells [30]. A similar trend was observed in both ARCaPE and ARCaPM cells after (4 Gy) irradiation treatment. ARCaPM homotypic cancer cells are more resistant to radiation treatment compared to ARCaPE homotypic cancer cells (Figure 3(a)). However, ARCaPM cocultures did not affect the radiation sensitivity of ARCaPM cancer cells. The highly sensitive ARCaPE cells exhibit a significant increased resistance to radiation therapy, up to 3-fold, as result of their interaction with bone stromal cells (Figure 3(a), P < .01).
Figure 3.
Cocultured ARCaPE cells gain cell colony forming capacity and radiation resistance when grown with bone and prostate stromal cells. (a) ARCaPE or ARCaPM cocultured cells were irradiated 24 hours after coculture with HS-27a cells and cancer cell colony forming capacity was assayed using clonogenic assay. Results are means ± SE of three independent experiments. ARCaPM experimental data are normalized to ARCaPM control and ARCaPE experimental data are normalized to ARCaPE control (a). ARCaPE cells cocultured with prostate stromal fibroblasts Pt-C (Cancer associated fibroblasts) or Pt-N (Normal/benign fibroblasts) were irradiated and compared to nonirradiated cocultures. Cell colony forming capacity was assayed by clonogenic assay. Data are normalized to ARCaPE control levels. (b) ARCaPM cells cocultured with Pt-C or Pt-N were irradiated and compared to nonirradiated cocultures (c). Cell colony forming capacity was assayed by clonogenic assay. Data are normalized to ARCaPM control levels.
To further assess the role of the prostate stromal cells on tumor-stromal interactions influencing ARCaP cellular behavior, we cocultured paired prostate stromal fibroblasts isolated either from normal (Pt-N) or from cancer-associated regions (Pt-C) [31]. Again, ARCaPE cells cocultured with (Pt-N) or (Pt-C) exhibited a 7-fold and 8-fold increase in colony formation, respectively (Figure 3(b), P < .01). We also saw a similar trend in a growth analysis assay (data not shown). However when measuring clonogenic ability after radiation treatment, ARCaPE cells cocultured with either Pt-N or Pt-C had increased radiation resistance, with a 2-fold difference observed between homotypic cultured cells. Although a significant increase in clonogenic formation was observed in Pt-C versus Pt-N cocultures (P < .05), this did not significantly effect the radiation sensitivity of ARCaPM cells (Figure 3(c)). Taken together, both bone and prostate stromal cell have a grown inductive effect on ARCaPE cancer cells and mediate radiation resistance (up to 2-3 fold) in epithelial cancer phenotype, but not in ARCaPM mesenchymal cancer cells.
3.3. Blocking Adhesive Contact Effects Radiation Sensitivity of Cocultured ARCaP Cells
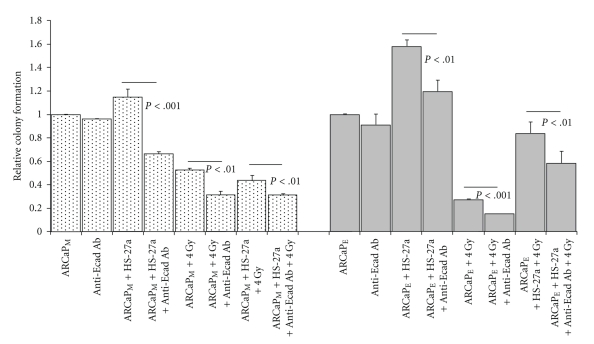
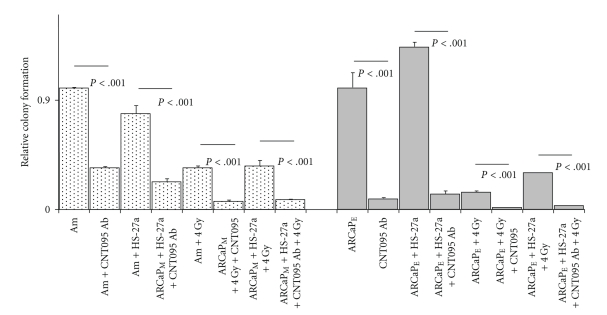
The importance of cell adhesion (i.e., cell-cell and cell-ECM adhesion) on the survival of disseminated cancer cells has been well documented as a requirement for colonization and survival within the metastatic microenvironment [32–34]. Therefore we utilized a well-known E-cadherin blocking antibody (SHEP8-7) and a pan-integrin antibody (CNT095) that targets human alpha-v-integrin and also was shown to block prostate tumor growth within bone [35]. Since ARCaPE cells express high levels of the epithelial marker E-cadherin, and ARCaPM cells can be microenvironmentally induced to express E-cadherin, we tested whether either of these blocking antibodies would affect the colony forming ability of either ARCaPE or ARCaPM bone stroma-cocultured cells. Pretreatment with E-cadherin antibody did not affect the colony forming capacity of either ARCaPE or ARCaPM homotypic cultured cells; however it significantly reduced the ability of ARCaPM- (P < .001) and ARCaPE- (P < .01) coultured cells to form colonies (Figure 4). Additionally, E-cadherin blocking antibody pretreatments further increased sensitivity to radiation treatment of ARCaPM cells in homotypic and cocultured conditions, similarly (P < .01). E-cadherin blocking antibody-pretreated ARCaPE cells showed the most significant increased sensitivity to radiation treatment in homotypic compared cocultured conditions (P < .001), however a significant reduction in colony formation, to a lesser extent, was observed in ARCaPE cocultured cells (Figure 4, P < .01). Therefore, targeting E-cadherin limited both epithelial and mesenchymal cells ability to form colonies after coculture with bone stromal cells.
Figure 4.
Effect of Anti-E-cadherin antibody on tumor-stroma interactions. A. ARCaPM and ARCaPE, cells were pretreated with Anti-E-cadherin antibody (SHEP8-7), cocultured with HS-27a stromal cells for 24 hours, and radiated with 4 Gy. Cell colony forming capacity was assayed using clonogenic assay. ARCaPM data are normalized to ARCaPM control levels, and ARCaPE data are normalized to ARCaPE control levels.
To determine the influence of intergin alpha v cell adhesion with bone microenvironment, we performed similar clonogenic formation assay. Pretreatment with CNT095 antibody significantly decreased the clonogenic ability of both ARCaPM and ARCaPE cells in homotyic cultures (Figure 5, P < .001). Additionally, CNT095 significantly decreased bone stroma-induced radiation resistance in cancer cells in both ARCaPM (P < .001) and ARCaPE (P < .001) cancer cells, with the most significant reduction in cocultured conditions (P < .001) (Figure 5). Taken together, these results suggest that bone stroma-induced radiation resistance is mediated through both E-cadherin and integrin alpha v beta signaling in epithelial and mesenchymal cells. Thus, E-cadherin and integrin alpha v beta appear to present novel targets for metastatic and radiation resistant cells.
Figure 5.
Effect of Anti-alpha v integrin (CNT095) on tumor-stroma interactions. ARCaPM and ARCaPE, cells were pretreated with CNT095 antibody was cocultured with HS-27a stromal cells for 24 hours, and radiated with 4 Gy. Cell colony forming capacity was assayed using clonogenic assay. ARCaPM data are normalized to ARCaPM control levels, and ARCaPE data are normalized to ARCaPE control levels.
4. Discussion
It is well documented in prostate and others cancers that EMT is associated with initial transformation from encapsulated to invasive carcinomas. The mesenchymal phenotype, which is required for dissemination, has been suggested to revert to an epithelial phenotype in distant metastasis [13, 14, 29, 36]. This has been evidenced in the primary tumors which lack E-cadherin expression and, showing nuclear β-catenin expression, show strong membrane staining for both E-cadherin and β-catenin in metastatic liver [13] or bone microenvironment [28, 29]. We have previously shown, in commonly utilized prostate cancer cells lines DU-145 and PC-3, that reexpression of E-cadherin and reversion of the mesenchymal phenotype is a rate limiting for metastatic seeding of primary rat hepatocytes [13]. Since bone metastasis is most prevalent in prostate cancers, we sought to extent these finding utilizing the ARCaP model, which is the first prostate cancer EMT model demonstrating histomorphological features and classical markers in a lineage-derived series of cells, to determine the functional relationship of this cellular transition. Whether this is accomplished through exposure to soluble growth factors or the bone microenvironment, the end result decreased differentiation with increased metastatic potential [25, 27, 37].
Our initial results show that ARCaPM cells maintained in 3D Rotary Wall Vessel (RWV) or 2D cocultures underwent MErT when cocultured with HS-27a bone stromal cells, as shown through expression of E-cadherin and of N-cadherin expression (Figures 1(a) and 1(b)). Moreover ARCaPE cells show a significant enhancement in colony formation (8×) and significant growth pattern comparable to ARCaPM (1.35×) cocultures (Figures 2(b) and 2(c)). A recent report has shown through RFP cell tracking that selected ARCaPE clones after in vivo inoculation into the bone microenvironment gives rise to both ARCaPE and ARCaPM populations [37]. These findings coupled with our observed reversion of ARCaPM cells to ARCAPE like cells suggest that tumor-stromal-induced cellular plasticity gives rise to distinct populations of cancer cells within bone microenvironment, the mesenchymal phenotype and its kinetic characteristics (motility/invasive), and the epithelial characteristics necessary for secondary tumor development. The fact that the ARCaPM cells have an increase propensity for metastasis compared to ARCaPE cells suggest that dissemination from the primary tumor mass requires the mesenchymal phenotype. However a mesenchymal to epithelial transition is associated with initial metastatic seeding and subsequent formation of a cohesive tumor mass within the bone microenvironment. This hypothesis is supported in a bladder cancer model, where lineage-derived series of EMT-transformed mesenchymal-like cells exhibit increased lung metastasis in vivo; however secondary tumor formation is predominantly enhanced by the presence of epithelial cells compared to mesenchymal cells [38].
Since epithelial reversion enhances the growth of tumor cells in bone microenvironment, and this is observed in multiple experimental models and clinical metastases, there is a question of whether this transition is required for metastatic seeding and therefore an avenue for therapeutic intervention(s). To gain insight into the importance of this reversion, we utilized ionizing radiation on ARCaPE and ARCaPM homotypic and cocultured cells. Our results show that ARCaPE homotypic cultures when compared to ARCaPM homotypic cultures are more sensitive to radiation treatment (Figure 3(a)). However in the presence of bone or prostate stromal cells, ARCaPE cells gained increased radiation resistance, with increased proliferative and colony forming capacity (Figures 2(b) and 2(c)). This phenomenon was not observed in the ARCaPM cocultures. To determine the underlining causes of this observation, we hypothesized that cell-cell interactions through E-cadherin or cell-ECM interactions through integrins may mediate the stromal induced proliferative effect and radiation resistance in ARCaPE cancer cells. Using E-cadherin neutralizing antibody (SHEP8-7) and pan-anti-integrin alpha v antibody (CNT095), we were able to significantly block the stromal induced colony forming ability on ARCaPE cancer cells (Figures 4 and 5). Additionally, both antibodies significantly blocked the radiation resistance of ARCaPE in cocultured conditions (Figures 4 and 5). The E-cadherin neutralizing antibody also had an effect on homotypic ARCaPM-radiated cells and ARCaPM cells within cocultures (Figure 4). Thus it appears that blocking bone stroma-induced reexpression of E-cadherin in ARCaPM in the presence of bone stromal cells reduced the colony forming capacity of these cells (Figure 4). The decreased radiation sensitivity of E-cadherin expressing cells compared to cells lacking E-cadherin expression has recently been demonstrated in a cocultured model of MCF-7 (E-cad positive) and MDA-MB-231 (E-cad negative) cells with normal and radiation-induced senescent fibroblast [39], where radiation in MCF-7 cells showed enhanced resistance to radiation treatment compared to MDA-MB-231 cells. These findings are consent with our model of a reepithelization requirement within tumor microenvironment.
CNT095 antibody was toxic to both ARCaPM and ARCaPE homotypic and cocultured cells. Additionally CNT095 increased radiation sensitivity, even to a greater extent than E-cadherin neutralizing antibody treatment (Figure 5). These findings are consistent with our results of CTN095 treatment that causes a significantly reduced number of tumors generated by C4-2B cells, along with a concomitant increase of cortical bone in mice (unpublished data). Although C4-2B cells have not been observed to undergo EMT, this would suggest that targeting the cell-ECM in vitro and in vivo could be limiting the cell cohesiveness necessary for metastatic tumor formation.
Targeting of cell adhesion as a therapeutic approach has been proposed previously. E-cadherin neutralizing antibody (SHEP8-7) has been shown to sensitize multicellular spheroids to microtubule binding therapies in the taxane family in HT29 human colorectal adenocarcinoma cells [23]. A more recent observation is that survival of androgen receptor-expressing differentiated prostate cells is dependent on E-cadherin and PI3K, but not on androgen, AR, or MAPK [40]. Given the predominate role for PI3K in cell survival and reports that PI3K is rapidly recruited to cell membrane to stabilize E-cadherin junctions [40] and that PI3K activation requires integrin alpha v activity [41] suggests that PI3K is possibly responsible for the increased growth and colony formation gained within the tumor microenvironment. Thus in the absence of stimulating growth factors, it is possible that E-cadherin/PI3K or integrin alpha v/PI3K is involved in a signaling cascade that is initiated by the tumor microenvironment, at least during initial metastatic seeding.
In conclusion, our data demonstrate that the E-cadherin and integrin alpha nctional adhesive interaction is a possible adjuvant therapy avenue for patients treated with radiation. Although an in-depth in vivo exploration of targeting epithelial-like versus mesenchymal-like cells is necessary to translate these findings to the clinical situation, our results indeed raise critical questions as to how we view prostate cancer metastasis and subsequently target metastatic tumor cells for therapy. Additionally, we have generated an in vitro model, that closely mimics the clinical situation, to delineate in a stepwise manner the dynamic tumor-host interaction(s) that promote cellular plasticity in the later stages of metastasis. The identification of further key molecules driving MErT in this system holds promise for the development of preventative and therapeutic strategies to minimize metastatic disease.
Acknowledgments
The project described was supported by Grant nos. PC073977 Department of Defense and PO-1 CA-098912 NIH/NCI. The authors would like to thank Drs. Hayien Zhau, Daquig Wu, and Wolfgang Cerwinka for insightful comments and discussions.
References
- 1.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nature Reviews Cancer. 2002;2(8):563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 2.Comoglio PM, Trusolino L. Invasive growth: from development to metastasis. Journal of Clinical Investigation. 2002;109(7):857–862. doi: 10.1172/JCI15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veltmaat JM, Orelio CC, Ward-Van Oostwaard D, Van Rooijen MA, Mummery CL, Defize LHK. Snail is an immediate early target gene of parathyroid hormone related peptide signaling in parietal endoderm formation. The International Journal of Developmental Biology. 2000;44(3):297–307. [PubMed] [Google Scholar]
- 4.Ciruna B, Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Developmental Cell. 2001;1(1):37–49. doi: 10.1016/s1534-5807(01)00017-x. [DOI] [PubMed] [Google Scholar]
- 5.Machado JC, Oliveira C, Carvalho R, et al. E-cadherin gene (CDH1) promoter methylation as the second hit in sporadic diffuse gastric carcinoma. Oncogene. 2001;20(12):1525–1528. doi: 10.1038/sj.onc.1204234. [DOI] [PubMed] [Google Scholar]
- 6.Thiery JP. Epithelial-mesenchymal transitions in tumor progression. Nature Reviews Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 7.Bates RC, Mercurio AM. The epithelial-mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biology and Therapy. 2005;4(4):365–370. doi: 10.4161/cbt.4.4.1655. [DOI] [PubMed] [Google Scholar]
- 8.Brabletz T, Jung A, Reu S, et al. Variable β-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(18):10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poser I, Domínguez D, de Herreros AG, Varnai A, Buettner R, Bosserhoff AK. Loss of E-cadherin expression in melanoma cells involves up-regulation of the transcriptional repressor Snail. The Journal of Biological Chemistry. 2001;276(27):24661–24666. doi: 10.1074/jbc.M011224200. [DOI] [PubMed] [Google Scholar]
- 10.Lowy AM, Knight J, Groden J. Restoration of E-cadherin/β-catenin expression in pancreatic cancer cells inhibits growth by induction of apoptosis. Surgery. 2002;132(2):141–148. doi: 10.1067/msy.2002.125168. [DOI] [PubMed] [Google Scholar]
- 11.Strathdee G. Epigenetic versus genetic alterations in the inactivation of E-cadherin. Seminars in Cancer Biology. 2002;12(5):373–379. doi: 10.1016/s1044-579x(02)00057-3. [DOI] [PubMed] [Google Scholar]
- 12.Yates C, Shepard CR, Papworth G, et al. Novel three-dimensional organotypic liver bioreactor to directly visualize early events in metastatic progression. Advances in Cancer Research. 2007;97:225–246. doi: 10.1016/S0065-230X(06)97010-9. [DOI] [PubMed] [Google Scholar]
- 13.Yates CC, Shepard CR, Stolz DB, Wells A. Co-culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. British Journal of Cancer. 2007;96(8):1246–1252. doi: 10.1038/sj.bjc.6603700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saha B, Chaiwun B, Imam SS, et al. Overexpression of E-cadherin protein in metastatic breast cancer cells in bone. Anticancer Research. 2007;27(6 B):3903–3908. [PubMed] [Google Scholar]
- 15.Oh HS, Moharita A, Potian JG, et al. Bone marrow stroma influences transforming growth factor-β production in breast cancer cells to regulate c-myc activation of the preprotachykinin-I gene in breast cancer cells. Cancer Research. 2004;64(17):6327–6336. doi: 10.1158/0008-5472.CAN-03-3122. [DOI] [PubMed] [Google Scholar]
- 16.Bhowmick NA, Moses HL. Tumor-stroma interactions. Current Opinion in Genetics and Development. 2005;15(1):97–101. doi: 10.1016/j.gde.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackland ML, Newgreen DF, Fridman M, et al. Epidermal growth factor-induced epithelio-mesenchymal transition in human breast carcinoma cells. Laboratory Investigation. 2003;83(3):435–448. doi: 10.1097/01.lab.0000059927.97515.fd. [DOI] [PubMed] [Google Scholar]
- 18.Andl CD, Mizushima T, Nakagawa H, et al. Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. The Journal of Biological Chemistry. 2003;278(3):1824–1830. doi: 10.1074/jbc.M209148200. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong AP, Miller RE, Jones JC, Zhang J, Keller ET, Dougall WC. RANKL acts directly on RANK-expressing prostate tumor cells and mediates migration and expression of tumor metastasis genes. Prostate. 2008;68(1):92–104. doi: 10.1002/pros.20678. [DOI] [PubMed] [Google Scholar]
- 20.Odero-Marah VA, Wang R, Chu G, et al. Receptor activator of NF-κB Ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cells. Cell Research. 2008;18(8):858–870. doi: 10.1038/cr.2008.84. [DOI] [PubMed] [Google Scholar]
- 21.Mason MD, Davies G, Jiang WG. Cell adhesion molecules and adhesion abnormalities in prostate cancer. Critical Reviews in Oncology/Hematology. 2002;41(1):11–28. doi: 10.1016/s1040-8428(01)00171-8. [DOI] [PubMed] [Google Scholar]
- 22.Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. The EMBO Journal. 2004;23(8):1739–1748. doi: 10.1038/sj.emboj.7600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green SK, Karlsson MCI, Ravetch JV, Kerbel RS. Disruption of cell-cell adhesion enhances antibody-dependent cellular cytotoxicity: implications for antibody-based therapeutics of cancer. Cancer Research. 2002;62(23):6891–6900. [PubMed] [Google Scholar]
- 24.Zhau HE, Li C-L, Chung LWK. Establishment of human prostate carcinoma skeletal metastasis models. Cancer. 2000;88(12):2995–3001. doi: 10.1002/1097-0142(20000615)88:12+<2995::aid-cncr15>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Wang R, Xie ZH, et al. Prostate cancer metastasis: role of the host microenvironment in promoting epithelial to mesenchymal transition and increased bone and adrenal gland metastasis. Prostate. 2006;66(15):1664–1673. doi: 10.1002/pros.20488. [DOI] [PubMed] [Google Scholar]
- 26.He H, Yang X, Davidson AJ, et al. Progressive epithelial to mesenchymal transitions in ARCaPE prostate cancer cells during xenograft tumor formation and metastasis. Prostate. 2010;70(5):518–528. doi: 10.1002/pros.21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhau HE, Odero-Marah V, Lue H-W, et al. Epithelial to mesenchymal transition (EMT) in human prostate cancer: lessons learned from ARCaP model. Clinical & Experimental Metastasis. 2008;25(6):601–610. doi: 10.1007/s10585-008-9183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells A, Yates C, Shepard CR. E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clinical & Experimental Metastasis. 2008;25(6):621–628. doi: 10.1007/s10585-008-9167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saha B, Kaur P, Tsao-Wei D, et al. Unmethylated E-cadherin gene expressionis significantly associated with metastatic human prostate cancer cells in bone. Prostate. 2008;68(15):1681–1688. doi: 10.1002/pros.20836. [DOI] [PubMed] [Google Scholar]
- 30.Li L-N, Zhang H-D, Yuan S-J, Yang D-X, Wang L, Sun Z-X. Differential sensitivity of colorectal cancer cell lines to artesunate is associated with expression of beta-catenin and E-cadherin. European Journal of Pharmacology. 2008;588(1):1–8. doi: 10.1016/j.ejphar.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 31.Sung S-Y, Hsieh C-L, Law A, et al. Coevolution of prostate cancer and bone stroma in three-dimensional coculture: implications for cancer growth and metastasis. Cancer Research. 2008;68(23):9996–10003. doi: 10.1158/0008-5472.CAN-08-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ. Cellular growth and survival are mediated by β1 integrins in normal human breast eqithelium but not in breast carcinoma. Journal of Cell Science. 1995;108(5):1945–1957. doi: 10.1242/jcs.108.5.1945. [DOI] [PubMed] [Google Scholar]
- 33.Fornaro M, Plescia J, Chheang S, et al. Fibronectin protects prostate cancer cells from tumor necrosis factor-α-induced apoptosis via the AKT/survivin pathway. The Journal of Biological Chemistry. 2003;278(50):50402–50411. doi: 10.1074/jbc.M307627200. [DOI] [PubMed] [Google Scholar]
- 34.Li G, Satyamoorthy K, Herlyn M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Research. 2001;61(9):3819–3825. [PubMed] [Google Scholar]
- 35.Bisanz K, Yu J, Edlund M, et al. Targeting ECM-integrin interaction with liposome-encapsulated small interfering RNAs inhibits the growth of human prostate cancer in a bone xenograft imaging model. Molecular Therapy. 2005;12(4):634–643. doi: 10.1016/j.ymthe.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 36.Rubin MA, Mucci NR, Figurski J, Fecko A, Pienta KJ, Day ML. E-cadherin expression in prostate cancer: a broad survey using high-density tissue microarray technology. Human Pathology. 2001;32(7):690–697. doi: 10.1053/hupa.2001.25902. [DOI] [PubMed] [Google Scholar]
- 37.He H, Yang X, Davidson AJ, et al. Progressive epithelial to mesenchymal transitions in ARCaPE prostate cancer cells during xenograft tumor formation and metastasis. The Prostate. 2010;70(5):518–528. doi: 10.1002/pros.21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Research. 2006;66(23):11271–11278. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 39.Tsai KK, Stuart J, Chuang YY, Little JB, Yuan ZM. Low-dose radiation-induced senescent stromal fibroblasts render nearby breast cancer cells radioresistant. Radiation Research. 2009;172(3):306–313. doi: 10.1667/RR1764.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamb LE, Knudsen BS, Miranti CK. E-cadherin-mediated survival of androgen-receptor-expressing secretory prostate epithelial cells derived from a stratified in vitro differentiation model. Journal of Cell Science. 2010;123(2):266–276. doi: 10.1242/jcs.054502. [DOI] [PubMed] [Google Scholar]
- 41.Matsuo M, Sakurai H, Ueno Y, Ohtani O, Saiki I. Activation of MEK/ERK and PI3K/Akt pathways by fibronectin requires integrin αv-mediated ADAM activity in hepatocellular carcinoma: a novel functional target for gefitinib. Cancer Science. 2006;97(2):155–162. doi: 10.1111/j.1349-7006.2006.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]