Abstract
Objective
The objective of the study was to determine the frequency and clinical significance of intraamniotic inflammation in asymptomatic women with a sonographic short cervix (SCX) in the midtrimester.
Study Design
This cohort study included 47 asymptomatic women (14-24 weeks) with an SCX (≤15 mm) who underwent amniocentesis. Women with multiple gestation, cerclage, or cervical dilatation greater than 2 cm were excluded. Intraamniotic inflammation was defined as an elevated amniotic fluid (AF) matrix metalloproteinase-8 concentration (>23 ng/mL).
Results
(1) intraamniotic infection was found in 4.3% of patients; (2) among patients with a negative AF culture, the prevalence of intraamniotic inflammation was 22.2%; and (3) patients with a negative AF culture, but with intraamniotic inflammation, had a higher rate of delivery within 7 days (40% vs 5.7%; P = .016) and a shorter median diagnosis-to-delivery interval than those without intraamniotic inflammation (18 vs 42 days; P = .01).
Conclusion
Twenty-two percent of patients with a midtrimester SCX have intraamniotic inflammation. The risk of preterm delivery within 7 days for these patients is 40%.
Keywords: cervical length, intraamniotic inflammation, matrix metalloproteinase-8, MMP-8, pregnancy, prematurity, preterm delivery
A sonographic short cervix (SCX) in the midtrimester of pregnancy is a powerful predictor of a subsequent spontaneous preterm delivery.1-9 The shorter the sonographic cervical length, the higher is the risk of spontaneous preterm labor/delivery,1-4,6 and asymptomatic women between 14 and 24 weeks of gestation with a cervical length of 15 mm or less have a nearly 50% rate of spontaneous preterm delivery at 32 weeks or less.4,6
The importance of identifying asymptomatic patients with a sonographic SCX in the midtrimester derives from the evidence that these patients, whether or not they have a history of preterm birth, may be candidates for therapeutic interventions such as vaginal progesterone,10-12 cervical cerclage,13-16 antibiotics,17 or indomethacin.18 However, these therapeutic measures may not be beneficial in all patients.15,18-22 Thus, the clinical management of an asymptomatic pregnant woman with a sonographic SCX in the midtrimester is still an obstetrical challenge.
A plausible explanation for the lack of a single effective treatment is that a sonographic SCX is a syndrome that may be caused by multiple etiologies.23,24 Of note, intraamniotic infection has been recently implicated in the pathophysiology of asymptomatic short cervix. Indeed, the subclinical microbial invasion of the amniotic cavity has been detected in 9% of patients with a sonographic cervical length less than 25 mm.17
Among patients with preterm labor and intact membranes25 or with preterm prelabor rupture of membranes (PROM),26 intraamniotic inflammation, regardless of the microbial status of the amniotic cavity, has been associated with similar adverse pregnancy outcomes as in cases with a proven intraamniotic infection. Recently an association between an SCX (≤5 mm) in the midtrimester and elevated concentrations of inflammatory cytokines in amniotic fluid has been proposed.27
Matrix metalloproteinase (MMP)-8, also known as neutrophil collagenase, is a sensitive marker of inflammation.28,29 Amniotic fluid MMP-8 concentration has been demonstrated to be a superior index of intraamniotic inflammation than amniotic fluid white blood cell (WBC) count or interleukin (IL)-6.30
The aims of this study were to determine the frequency and clinical significance of intraamniotic inflammation (defined by amniotic fluid MMP-8 concentration) in asymptomatic women in the midtrimester of pregnancy with a sonographic SCX and to examine whether there is a relationship between amniotic fluid and maternal plasma MMP-8 concentrations.
Materials and Methods
Study population
This retrospective cohort study consisted of women with a singleton pregnancy admitted to Hutzel Women's Hospital, between January 2002 and June 2008, with the diagnosis of an asymptomatic SCX. Using a computer-based search of our clinical and sonographic databases, consecutive patients with a cervical length of 15 mm or less diagnosed between 14 and 24 weeks of gestation, as determined by a documented transvaginal ultrasound examination (TVUS) and those who underwent an amniocentesis within 1 week of diagnosis were identified.
Patients with 1 or more of the following conditions were excluded: (1) multifetal pregnancy; (2) preterm labor or preterm PROM at the time of diagnosis; (3) a cervical cerclage (placed before or after the diagnosis of an SCX); (4) cervical dilatation greater than 2 cm by digital examination; (5) placenta previa; and (6) fetuses with chromosomal and/or congenital anomalies.
Patients included in this study were enrolled in a longitudinal protocol whose aim was to identify biochemical markers for the prediction of adverse pregnancy outcomes. As part of this protocol, patients are followed with TVUS every 2-4 weeks beginning as early as 14 weeks of gestation. A fraction of patients included in this study were diagnosed with an SCX during a routine TVUS examination and were sent to labor and delivery for further evaluation. Patients who agreed to participate were enrolled in our protocol and a repeat TVUS examination was performed. Digital assessment of the cervix was performed in all patients. The ultrasound findings were recorded and stored in a dedicated database. After the diagnosis of an SCX was made, the patients were referred to the labor and delivery ward for further evaluation and management. Both the sonographic cervical length and the result of the digital vaginal examination were available to the managing physicians.
In our institution, the majority of patients newly diagnosed with a sonographic cervical length less than 25 mm in the midtrimester undergo an initial evaluation during which patients are assessed for signs and symptoms of preterm labor. If preterm labor is ruled out, tocolysis and prophylactic antibiotics are not administered. In addition, these patients are offered an amniocentesis to determine the microbial status of the amniotic cavity, as previous observations suggested an association between SCX and histologic chorioamnionitis31 and intraamniotic infection.17,20 Patients were counseled by clinicians, and those who agreed to undergo an amniocentesis as part of their clinical management were asked to donate amniotic fluid and allow collection of clinical information for research purposes. Further management of these patients was at the discretion of the attending physician.
All participating women provided written informed consent prior to the collection of amniotic fluid and maternal blood samples. The use of clinical and ultrasound data and the utilization of samples for research purposes was approved by the institutional review boards of Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (National Institutes of Health, Department of Health and Human Services). Some of these samples have been used previously to study microbial invasion of the amniotic cavity and other biological markers of disease.
Definitions and study procedures
Gestational age was determined by the last menstrual period or by ultrasound if the sonographic determination of gestational age was not consistent with the last menstrual period dating by more than 1 week. Gestational age at diagnosis was defined as the earliest gestational age at which a cervical length of 15 mm or less was recorded.
Data regarding pregnancy outcomes were obtained from the clinical and research records. Patients who were lost to follow-up and for whom delivery data were not available were censored from the last available follow-up visit. Spontaneous preterm labor was defined by the presence of regular uterine contractions occurring at a frequency of at least 2 every 10 minutes associated with cervical changes before 37 completed weeks of gestation.
Intraamniotic infection was defined as a positive amniotic fluid culture for microorganisms (aerobic/anaerobic bacteria or genital mycoplasmas). Intraamniotic inflammation was defined as an amniotic fluid MMP-8 concentration greater than 23 ng/mL,32-34 as determined by enzyme-linked immunosorbent assay. Based on the results of the amniotic fluid culture and the amniotic fluid MMP-8 concentration, patients were classified as having 1 of the following conditions: (1) intraamniotic infection; (2) intraamniotic inflammation without intraamniotic infection; and (3) no intraamniotic infection or inflammation. Histologic chorioamnionitis was diagnosed based on the presence of inflammatory cells in the chorionic plate and/or chorioamniotic membranes,35 and acute funisitis was diagnosed by the presence of neutrophils in the wall of the umbilical vessels and/or Wharton's jelly, using the criteria previously described.36
Sonographic assessment of the cervix
Transvaginal ultrasound examination was conducted with commercially available 2-dimensional and 3-dimensional ultrasound systems (Acuson Sequoia; Siemens Medical Systems, Mountain View, CA, and Voluson E8; GE Healthcare, Milwaukee, WI) equipped with endovaginal transducers with frequency ranges of 5–7.5 and 5–9 MHz, respectively. All sonographic examinations of the cervical length were performed by registered diagnostic medical sonographers using a technique previously described1,2 and reviewed by an experienced physician.
Sample collection
Amniotic fluid samples were obtained by transabdominal amniocentesis, transported to the laboratory in a sterile capped syringe, and cultured for aerobic/anaerobic bacteria and genital mycoplasmas. WBC count, glucose concentration, and Gram stain were also performed shortly after the amniotic fluid collection. The results of these tests were used for the clinical management of the patients. Amniotic fluid not required for clinical assessment was centrifuged for 10 minutes at 4°C, and the supernatant was aliquoted and stored at −70°C until analysis.
Maternal blood samples were collected into Vacutainer (Becton Dickinson, Franklin Lakes, NJ) tubes immediately before or after the amniocentesis. The samples were then centrifuged at 1300 × g for 10 minutes at 4°C and the obtained plasma was stored at −70°C until assayed.
Following delivery, the placenta, umbilical cord, and chorioamniotic membranes were collected and the presence or absence of histologic chorioamnionitis and/or funisitis was assessed.
Determination of MMP-8 concentration
Amniotic fluid and maternal plasma concentrations of MMP-8 were determined by sensitive enzyme-linked immunoassays (GE Healthcare/Amersham Pharmacia Biotech Inc, Piscataway, NJ). Immunoassays were carried out according to the manufacturer's recommendations. The calculated inter- and intraassay coefficients of variation for MMP-8 immunoassays in our laboratory were 1.7% and 3.9%, respectively, and the sensitivity was 0.101 ng/mL.
Statistical analysis
Shapiro-Wilk and Kolmogorov-Smirnov tests were used to test for normal distribution of the data. As amniotic fluid and maternal plasma MMP-8 concentrations were not normally distributed, Mann-Whitney U test was used for comparisons of continuous variables. Comparisons of proportions among groups were performed using Fisher's exact test for categorical variables.
Correlations between amniotic fluid MMP-8 concentrations and cervical length at diagnosis, gestational age at amniocentesis, diagnosis-to-delivery interval, and maternal plasma MMP-8 concentrations were examined using Spearman's rank correlation test. Multivariate logistic regression analysis was performed to determine the relationship between the amniotic fluid MMP-8 concentrations (as a categorical variable of ≤23 ng/mL and >23 ng/mL), gestational age at diagnosis, and cervical length in the prediction of preterm delivery within 7 days from diagnosis.
A Kaplan-Meier survival analysis was performed to assess the diagnosis-to-delivery interval according to the presence or absence of intraamniotic inflammation. A P < .05 was considered statistically significant. The statistical package used was SPSS version 12.0 (SPSS Inc, Chicago, IL).
Results
Demographic and clinical characteristics of the study population
During the study period, 53 asymptomatic patients who met the inclusion criteria were identified. Amniotic fluid samples for retrospective analysis of MMP-8 concentration were not available for 6 patients; thus, 47 women (88.7%) were included in the final analysis.
The overall rate of intraamniotic inflammation was 23.4% (11/47). Intraamniotic infection was identified in 2 of 47 patients (4.3%). Amniotic fluid WBC count of 100 cells/mm3 or greater was found in 3 of 47 patients (6.4%). Among patients with a negative amniotic fluid culture for microorganisms, the rate of intraamniotic inflammation was 22.2% (10/45), whereas only 4.4% (2/45) had an amniotic fluid WBC count of 100 cells/mm3 or greater.
Of the 2 patients with intraamniotic infection, 1 had a positive amniotic fluid culture for Fusobacterium nucleatum. This patient developed clinical chorioamnionitis shortly after admission and labor was induced. The second patient had a positive amniotic fluid culture for ureaplasma urealyticum (without intraamniotic inflammation) and received intravenous azithromycin for 7 days with a subsequent term delivery.
Of the 45 patients without intraamniotic infection, 1 patient was lost to follow-up from 32 weeks of gestation, 8 patients (18.2%) delivered at term, and 36 (81.8%) had a spontaneous preterm delivery before 37 completed weeks of gestation. Spontaneous preterm delivery followed a spontaneous preterm labor in 24 patients (67%) and preterm PROM in 12 patients (33%) (2 patients were induced following preterm PROM because of clinical chorioamnionitis).
Table 1 displays the demographic and clinical characteristics of patients with a negative amniotic fluid culture, with (n = 10) and without (n = 35) intraamniotic inflammation. The gestational age range of patients included in this study was 16 weeks and 3 days to 24 weeks of gestation. Patients with intraamniotic inflammation had a shorter gestational age at diagnosis and a lower cervical length than those without intraamniotic inflammation.
TABLE 1. Characteristics of patients with a negative amniotic fluid culture.
| Characteristic | Without intraamniotic inflammation (n = 35) | With intraamniotic inflammation (n = 10) | P value |
|---|---|---|---|
| Age, y | 23 (20–29) | 26.5 (21.6-37.6) | .17 |
| African-American ethnic origin | 94.3 (33) | 80 (8) | .21 |
| Smoker | 18.2 (6/33) | 33.3 (3/9) | .37 |
| Prepregnancy BMI, kg/m2 | 28.3 (24.1–29.4) | 28.3 (22.4–32.3) | .73 |
| Nulliparity | 57.1 (20) | 50 (5) | .7 |
| History of spontaneous PTD <35 wks | 28.6 (10) | 50 (5) | .26 |
| Prophylactic 17α-hydroxyprogesterone caproate | 9.4 (3) | 10 (1) | 1.0 |
| Genital infection during pregnancy | 25.7 (9) | 20 (2) | 1.0 |
| Bacterial vaginosis | 11.4 (4) | 20 (2) | |
| Chlamidia trachomatis | 8.6 (3) | 0 (0) | |
| Gonorrhea | 0 (0) | 0 (0) | |
| Trichomonas | 5.7 (2) | 0 (0) | |
| GA at diagnosis, wks | 21.1 (19.1–23.1) | 23.2 (21.9–23.5) | .044 |
| Cervical length at diagnosis, mm | 11 (4–13) | 1.5 (0–6.5) | .005 |
| GA at amniocentesis, wks | 21.1 (19.1–23.1) | 23.2 (22.1–23.5) | .045 |
| Amniotic fluid WBC count ≥50 cells/mm3 | 5.7 (2) | 20 (2) | .2 |
| Amniotic fluid WBC count ≥100 cells/mm3 | 2.9 (1) | 10 (1) | .4 |
| GA at delivery, wks | 28.1 (23.3–36.9) | 25.8 (23–30.7) | .12 |
| Birthweight, g | 1098 (500–2680) | 763 (578–1482) | .18 |
| Diagnosis-to-delivery interval, d | 42 (23–107) | 21.5 (5.5–51.5) | .026 |
| Delivery within 7 d | 5.7 (2) | 40 (4) | .016 |
| Spontaneous preterm delivery <32 wks | 57.1 (20) | 80 (8) | .17 |
| Spontaneous preterm delivery <37 wks | 72.2 (26/34) | 100 (10) | .17 |
Numbers are presented as median (interquartile range) or percentage (number).
BMI, body mass index; GA, gestational age; PTD, preterm delivery.
Pregnancy outcome of women with a negative amniotic fluid culture, with and without intraamniotic inflammation
Among women with a negative amniotic fluid culture for microorganisms, patients with intraamniotic inflammation had a shorter median diagnosis-to-delivery interval than those without this condition (21.5 days; interquartile range [IQR], 5.5–51.5 vs 42 days; IQR, 22.7–108; P = .035). None of the patients delivered within 48 hours of amniocentesis.
Women with intraamniotic inflammation had a higher rate of preterm delivery within 1 week than those without intraamniotic inflammation (40% [4/10] vs 5.7% [2/35]; P = .016). The rate of preterm delivery less than 32 weeks of gestation, although higher among patients with intraamniotic inflammation, did not differ significantly between the 2 groups of patients (those with intraamniotic inflammation: 80% [8/10] vs those without intraamniotic inflammation: 57% [20/35]; P = .17] (Table 1).
Among patients without intraamniotic infection, the concentration of MMP-8 in amniotic fluid inversely correlated with cervical length at diagnosis (r = −0.41; P = .005) and with diagnosis-to-delivery interval (r = −0.42; P = .004). There was no significant correlation between amniotic fluid MMP-8 concentrations and gestational age at amniocentesis (r = 0.21; P = .16).
Kaplan-Meier survival curves of patients with a negative amniotic fluid culture, with and without intraamniotic inflammation, are displayed in the Figure. One patient was lost to follow-up and was censored at 32 weeks (the last available follow-up visit). None of the patients underwent labor induction before 37 weeks because of conditions not directly related to the diagnosis of a short cervix such as clinical chorioamnionitis or preterm PROM. All undelivered patients were censored at term (37 weeks of gestation). The survival curves of patients with and without intraamniotic inflammation differed significantly (log rank, P = .0018).
FIGURE. Kaplan-Meier survival curves.
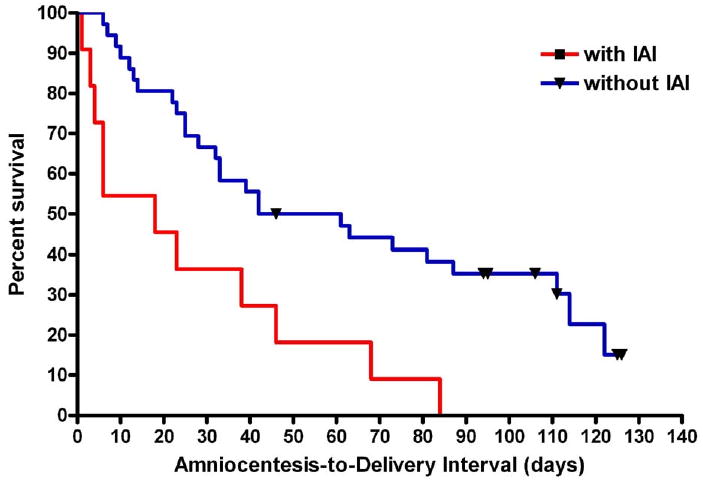
Kaplan-Meier survival curves of patients with a negative amniotic fluid culture, with and without intraamniotic inflammation (IAI). One patient was censored at 32 weeks because of loss to follow-up, and all other patients were censored at 37 completed weeks of gestation (black symbols at censored observations). The 2 curves differed significantly (log rank, P = .0018)
A multivariate logistic regression analysis was used to explore the relationship between intraamniotic inflammation (amniotic fluid MMP-8 concentration >23 ng/mL), cervical length (millimeters), and gestational age at diagnosis (weeks) in the prediction of preterm delivery within 7 days. In this model, only intraamniotic inflammation was independently associated with a preterm delivery within 1 week after adjustment for gestational age at diagnosis and cervical length (Table 2). In this model there was no significant interaction between cervical length (millimeters) and MMP-8 concentration greater than 23 ng/mL for the prediction of preterm delivery within 7 days.
TABLE 2. Logistic regression for preterm delivery within 7 days of amniocentesis.
| Explanatory variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Gestational age at diagnosis of short cervix, wks | 0.46 | 0.16–1.37 | .16 |
| Cervical length, mm | 0.29 | 0.02–3.8 | .34 |
| Amniotic fluid MMP-8 concentration >23 ng/mL | 55.4 | 1.1–2807 | .045 |
CI, confidence interval.
MMP-8 in maternal plasma samples of patients with a short cervix with and without intraamniotic inflammation
Maternal blood samples taken within 72 hours from amniocentesis were available for 37 patients (8/11 of those with and 29/36 of those without intraamniotic inflammation). A 72 hour interval was chosen to preserve a meaningful temporal relationship between amniotic fluid and maternal plasma MMP-8 concentrations. The median maternal plasma MMP-8 concentration did not differ significantly between women with and without intraamniotic inflammation (51.9 ng/mL; IQR, 31.5–115.1 vs 42.5 ng/mL; IQR, 29–56.7; respectively; P = .41). In addition, there was no significant correlation between amniotic fluid and maternal plasma MMP-8 concentrations (r = 0.22; P = .2).
Intraamniotic inflammation and histologic chorioamnionitis
Placental histopathologic examination was available for 91% of patients (41/45) with a negative amniotic fluid culture. All patients with intraamniotic inflammation (9/9) had placental lesions consistent with histologic chorioamnionitis, whereas only 59% of patients (19/32) without intraamniotic inflammation had such lesions (P = .02).
Comment
Principal findings of this study
The principal findings of the study included the following: (1) almost a fourth of asymptomatic patients with a sonographic short cervix (≤15 mm) diagnosed between 14 and 24 weeks of gestation had intraamniotic inflammation, and 4.3% of the patients had proven intraamniotic infection; (2) 40% of patients with intraamniotic inflammation, but a negative amniotic fluid culture, delivered within 1 week from amniocentesis; (3) women with intraamniotic inflammation had a shorter median diagnosis-to-delivery interval than those without this condition; (4) amniotic fluid MMP-8 concentrations inversely correlated with cervical length and diagnosis-to-delivery interval; and (5) intraamniotic inflammation was not reflected in maternal plasma.
The role of intraamniotic infection and/or inflammation in preterm parturition has been a subject of extensive discussion in the literature. Space limitation precludes presentation of the evidence concerning the causative link between intraamniotic infection and preterm parturition, and the interested readers are kindly referred to review articles addressing this issue.37,38
Intraamniotic inflammation, regardless of the presence or absence of intraamniotic infection, has been reported to be associated with adverse pregnancy outcomes in several obstetrical conditions including preterm labor,25,39,40 preterm PROM,26,30 and cervical insufficiency.34 Furthermore, elevated markers of intraamniotic inflammation at the time of genetic amniocentesis were associated with subsequent pregnancy loss or preterm delivery.32,41,42
The value of MMP-8 concentration in amniotic fluid as a predictor of intraamniotic infection and/or inflammation and adverse pregnancy outcome is well established,30,32,43,44 and its superior performance over other inflammatory markers (ie, WBC count, IL-6, angiogenin) in the detection of intraamniotic infection/inflammation has been previously reported.30,32,40
The association between intraamniotic infection and an asymptomatic SCX has been first proposed by our group.17 In that study, subclinical microbial invasion of the amniotic cavity (MIAC) was evident in 9% of patients (5/57) with a sonographic SCX (defined as <25 mm) diagnosed at 14-24 weeks. Recently Kiefer et al27 proposed an association between a cervical length of 5 mm or less in the midtrimester and increased amniotic fluid concentrations of inflammatory cytokines such as monocyte chemotactic protein (MCP)-1 and IL-6. Keeler and colleagues45 in a subsequent study of 44 patients in the midtrimester with a cervical length of 25 mm or less, in which those with intraamniotic infection and/or signs of preterm labor were not excluded, reported that amniotic fluid MCP-1 was predictive of spontaneous preterm delivery. Of note, 16% of these patients delivered spontaneously within 48 hours.45
In contrast, the results of the present study provide information concerning pregnancy outcome of patients with intraamniotic inflammation in the presence of a negative amniotic fluid culture (including for Ureaplasma urealyticum and Mycoplasma hominis, which are prevalent microorganisms causing intraamniotic infection17,26,32,46,47). Furthermore, our study specifically concentrated on asymptomatic patients without intraamniotic infection, advanced cervical dilatation, or premature contractions/labor (conditions that have been previously linked to intraamniotic inflammation25,34,39,40), and the fact that none of the patients in our study delivered within 48 hours supports the notion that these patients were completely asymptomatic.
This study demonstrates that intraamniotic inflammation, even in the absence of MIAC, is a risk factor for preterm delivery within 7 days of the diagnosis of a short cervix in asymptomatic patients in the second trimester of pregnancy. Indeed, intraamniotic inflammation has been identified in 22% of patients with a negative amniotic fluid culture, which is similar to the rate described for preterm labor (23%; 44/185),25 whereas it was lower than in those with preterm PROM (30%; 51/169)26 or cervical insufficiency (79%; 38/48).34
Kiefer et al27 reported that the concentrations of the inflammatory cytokines MCP-1, IL-6, and IL-8 appeared to become elevated at a cervical length of 5 mm or less. This finding is in agreement with the results of our study, which showed that amniotic fluid MMP-8 concentration significantly correlated with cervical length at diagnosis and that 73% (8/11) of patients with intraamniotic inflammation had a cervical length of 5 mm or less.
This information may be of clinical importance when counseling these patients regarding therapeutic interventions. Indeed, despite the promising results of some randomized clinical trials,10,48 other studies have reported no beneficial effect of therapeutic measures used in patients with an asymptomatic SCX.15,18-22 Fonseca et al10 reported an overall 44% reduction in the rate of preterm birth in women with a cervical length of 15 mm or less. In women with an extremely SCX (<5 mm), however, progesterone administration was less effective (10%) (K. H. Nicolaides, personal communication49).
One plausible explanation for the high failure rate in patients with a very SCX is that this subset of women may have already developed asymptomatic intrauterine infection and/or inflammation and may already have entered into the irreversible phase of parturition, in which available interventions to prolong pregnancy such as cervical cerclage or progesterone administration are ineffective. This view is supported by our observation that 40% of the patients with intraamniotic inflammation without proven MIAC delivered within 7 days. This association remained significant after adjusting for gestational age and cervical length at diagnosis, both of which had previously been reported to be inversely correlated with the risk of preterm delivery.2,50,51 Importantly, MMP-8 concentration in maternal plasma was not informative of the status of the amniotic cavity, and there was no significant correlation between the concentrations of MMP-8 in the maternal and fetal compartments, further demonstrating the value of amniocentesis because of the independent nature of these 2 compartments.
The etiologic factors responsible for the intraamniotic inflammation in the presence of a negative amniotic fluid culture in patients with an asymptomatic SCX remain to be elucidated. However, several possible explanations can account for our findings. First, it is possible that some microbial invasion of the amniotic cavity cannot be detected by standard microbiological cultivation techniques. Of note, only about 1% of the whole microbial world can be detected by cultivation techniques.52-54 Indeed, using molecular microbiology techniques, microbial footprints can be detected in women with a negative amniotic fluid culture.55-61 Second, some women with a negative amniotic fluid culture may have intrauterine infection of the extraamniotic space with an intraamniotic inflammatory response.37,62 Third, recently the presence of amniotic fluid sludge,63-66 a cluster of particulate matter in the amniotic fluid in close proximity to the cervix, has been shown to represent, at least in some cases, microbial biofilms.67 Detection of microbial invasion of the amniotic cavity in the presence of biofilms is challenging, and current cultivation techniques are inadequate to identify such infections.67 Finally, the inflammatory response detected in amniotic fluid may be a consequence of a sterile inflammatory response activated by nonmicrobial endogenous danger signals such as cellular alarm signals from distressed and injured cells or tissues.68-72 It remains to be elucidated which of the aforementioned suggested mechanisms is indeed associated with intraamniotic inflammation and whether therapeutic interventions can modulate the inflammatory response detected in asymptomatic patients with an SCX.
Strengths and limitations
One of the strengths of this study is that it is the first to provide evidence that intraamniotic inflammation in asymptomatic patients with an SCX, in the absence of intraamniotic infection, is associated with adverse pregnancy outcome. These findings can be beneficial to clinicians in the counseling and clinical management of these challenging patients.
The limitations of this study rest on its relatively small sample size. However, the prevalence of a sonographic cervical length of 15 mm or less at 14-24 weeks in our population is around 0.6%.6 This relatively low prevalence and the fact that not all patients had amniocentesis coupled with the strict inclusion criteria used in this study make it difficult to obtain a much larger sample size.
Another potential limitation is that not all women agreed to undergo an amniocentesis. The reasons that some women agreed to have an amniocentesis and others do not are unknown. Several attending clinicians were involved in counseling patients regarding amniocentesis, and although there is no evidence that a bias was introduced by the individual who counseled the patient, neither can we exclude such a bias. In addition, because of the retrospective nature of this study, amniotic fluid and maternal plasma samples for MMP-8 assay were not available for all patients.
Conclusions
One of every 4 patients with a sonographic SCX (≤ 15 mm) before 24 weeks has subclinical intraamniotic infection and/or inflammation. The risk of spontaneous preterm delivery within 7 days among patients without intraamniotic infection but with intraamniotic inflammation is 40%. These findings have implications for the counseling and management of patients with an asymptomatic SCX.
Acknowledgments
This study was supported in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services.
Footnotes
Presented at the 30th Annual Meeting of the Society for Maternal-Fetal Medicine, Chicago, IL, Feb. 1-6, 2010.
References
- 1.Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol. 1990;163:859–67. doi: 10.1016/0002-9378(90)91084-p. [DOI] [PubMed] [Google Scholar]
- 2.Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334:567–72. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 3.Watson WJ, Stevens D, Welter S, Day D. Observations on the sonographic measurement of cervical length and risk of premature birth. J Maternal Fetal Med. 1999;8:17–9. doi: 10.1002/(SICI)1520-6661(199901/02)8:1<17::AID-MFM4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 4.Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12:312–7. doi: 10.1046/j.1469-0705.1998.12050312.x. [DOI] [PubMed] [Google Scholar]
- 5.Berghella V, Daly SF, Tolosa JE, et al. Prediction of preterm delivery with transvaginal ultrasonography of the cervix in patients with high-risk pregnancies: does cerclage prevent prematurity? Am J Obstet Gynecol. 1999;181:809–15. doi: 10.1016/s0002-9378(99)70306-6. [DOI] [PubMed] [Google Scholar]
- 6.Hassan SS, Romero R, Berry SM, et al. Patients with an ultrasonographic cervical length less than or equal to 15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–67. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 7.Owen J, Yost N, Berghella V, et al. Can shortened midtrimester cervical length predict very early spontaneous preterm birth? Am J Obstet Gynecol. 2004;191:298–303. doi: 10.1016/j.ajog.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Tekesin I, Eberhart LH, Schaefer V, Wallwiener D, Schmidt S. Evaluation and validation of a new risk score (CLEOPATRA score) to predict the probability of premature delivery for patients with threatened preterm labor. Ultrasound Obstet Gynecol. 2005;26:699–706. doi: 10.1002/uog.2633. [DOI] [PubMed] [Google Scholar]
- 9.To MS, Skentou CA, Royston P, Yu CK, Nicolaides KH. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: a population-based prospective study. Ultrasound Obstet Gynecol. 2006;27:362–7. doi: 10.1002/uog.2773. [DOI] [PubMed] [Google Scholar]
- 10.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–9. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 11.DeFranco EA, O'Brien JM, Adair CD, et al. Vaginal progesterone is associated with a decrease in the risk of early preterm birth and an improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:697–705. doi: 10.1002/uog.5159. [DOI] [PubMed] [Google Scholar]
- 12.Dodd JM, Flenady VJ, Cincotta R, Crowther CA. Progesterone for the prevention of preterm birth: a systematic review. Obstet Gynecol. 2008;112:127–34. doi: 10.1097/AOG.0b013e31817d0262. [DOI] [PubMed] [Google Scholar]
- 13.Heath VC, Souka AP, Erasmus I, Gibb DM, Nicolaides KH. Cervical length at 23 weeks of gestation: the value of Shirodkar suture for the short cervix. Ultrasound Obstet Gynecol. 1998;12:318–22. doi: 10.1046/j.1469-0705.1998.12050318.x. [DOI] [PubMed] [Google Scholar]
- 14.Althuisius SM. The short and funneling cervix: when to use cerclage? Curr Opin Obstet Gynecol. 2005;17:574–8. doi: 10.1097/01.gco.0000188727.53622.f9. [DOI] [PubMed] [Google Scholar]
- 15.Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cerclage for short cervix on ultrasonography: meta-analysis of trials using individual patient-level data. Obstet Gynecol. 2005;106:181–9. doi: 10.1097/01.AOG.0000168435.17200.53. [DOI] [PubMed] [Google Scholar]
- 16.Owen J, Hawkins G, Iams JD, et al. Multi-center randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201:375.e1–8. doi: 10.1016/j.ajog.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassan S, Romero R, Hendler I, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–9. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berghella V, Rust OA, Althuisius SM. Short cervix on ultrasound: does indomethacin prevent preterm birth? Am J Obstet Gynecol. 2006;195:809–13. doi: 10.1016/j.ajog.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Hassan SS, Romero R, Maymon E, et al. Does cervical cerclage prevent the preterm delivery in patients with a short cervix? Am J Obstet Gynecol. 2001;184:1325–9. doi: 10.1067/mob.2001.115119. [DOI] [PubMed] [Google Scholar]
- 20.Rust OA, Atlas RO, Reed J, van GJ, Balducci J. Revisiting the short cervix detected by transvaginal ultrasound in the second trimester: why cerclage therapy may not help. Am J Obstet Gynecol. 2001;185:1098–105. doi: 10.1067/mob.2001.118163. [DOI] [PubMed] [Google Scholar]
- 21.Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol. 2004;191:1311–7. doi: 10.1016/j.ajog.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 22.Fox NS, Chervenak FA. Cervical cerclage: a review of the evidence. Obstet Gynecol Surv. 2008;63:58–65. doi: 10.1097/OGX.0b013e31815eb368. [DOI] [PubMed] [Google Scholar]
- 23.Romero R. Prenatal medicine: the child is the father of the man. 1996. J Matern Fetal Neonatal Med. 2009;22:636–9. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 24.Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol. 2006;194:1–9. doi: 10.1016/j.ajog.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–6. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 26.Shim SS, Romero R, Hong JS, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339–45. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 27.Kiefer DG, Keeler SM, Rust OA, Wayock CP, Vintzileos AM, Hanna N. Is midtrimester short cervix a sign of intraamniotic inflammation? Am J Obstet Gynecol. 2009;200:374–5. doi: 10.1016/j.ajog.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 28.Tschesche H. Human neutrophil collagenase. Methods Enzymol. 1995;248:431–49. doi: 10.1016/0076-6879(95)48028-5. [DOI] [PubMed] [Google Scholar]
- 29.Chen HY, Cox SW, Eley BM, Mantyla P, Ronka H, Sorsa T. Matrix metalloproteinase-8 levels and elastase activities in gingival crevicular fluid from chronic adult periodontitis patients. J Clin Periodontol. 2000;27:366–9. doi: 10.1034/j.1600-051x.2000.027005366.x. [DOI] [PubMed] [Google Scholar]
- 30.Maymon E, Romero R, Chaiworapongsa T, et al. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. Am J Obstet Gynecol. 2001;185:1143–8. doi: 10.1067/mob.2001.118166. [DOI] [PubMed] [Google Scholar]
- 31.Guzman ER, Shen-Schwarz S, Benito C, Vintzileos AM, Lake M, Lai YL. The relationship between placental histology and cervical ultrasonography in women at risk for pregnancy loss and spontaneous preterm birth. Am J Obstet Gynecol. 1999;181:793–7. doi: 10.1016/s0002-9378(99)70303-0. [DOI] [PubMed] [Google Scholar]
- 32.Yoon BH, Oh SY, Romero R, et al. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol. 2001;185:1162–7. doi: 10.1067/mob.2001.117678. [DOI] [PubMed] [Google Scholar]
- 33.Park JS, Romero R, Yoon BH, et al. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol. 2001;185:1156–61. doi: 10.1067/mob.2001.117679. [DOI] [PubMed] [Google Scholar]
- 34.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198:633–8. doi: 10.1016/j.ajog.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 35.Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med. 2006;11:296–301. doi: 10.1016/j.siny.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 37.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greci LS, Gilson GJ, Nevils B, Izquierdo LA, Qualls CR, Curet LB. Is amniotic fluid analysis the key to preterm labor? A model using interleukin-6 for predicting rapid delivery. Am J Obstet Gynecol. 1998;179:172–8. doi: 10.1016/s0002-9378(98)70269-8. [DOI] [PubMed] [Google Scholar]
- 40.Maymon E, Romero R, Chaiworapongsa T, et al. Amniotic fluid matrix metalloproteinase-8 in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185:1149–55. doi: 10.1067/mob.2001.118165. [DOI] [PubMed] [Google Scholar]
- 41.Wenstrom KD, Andrews WW, Tamura T, DuBard MB, Johnston KE, Hemstreet GP. Elevated amniotic fluid interleukin-6 levels at genetic amniocentesis predict subsequent pregnancy loss. Am J Obstet Gynecol. 1996;175:830–3. doi: 10.1016/s0002-9378(96)80007-x. [DOI] [PubMed] [Google Scholar]
- 42.Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, DuBard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol. 1998;178:546–50. doi: 10.1016/s0002-9378(98)70436-3. [DOI] [PubMed] [Google Scholar]
- 43.Maymon E, Romero R, Pacora P, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183:94–9. doi: 10.1067/mob.2000.105344. [DOI] [PubMed] [Google Scholar]
- 44.Nien JK, Yoon BH, Espinoza J, et al. A rapid MMP-8 bedside test for the detection of intraamniotic inflammation identifies patients at risk for imminent preterm delivery. Am J Obstet Gynecol. 2006;195:1025–30. doi: 10.1016/j.ajog.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 45.Keeler SM, Kiefer DG, Rust OA, et al. Comprehensive amniotic fluid cytokine profile evaluation in women with a short cervix: which cytokine(s) correlates best with outcome? Am J Obstet Gynecol. 2009;201:276.e1–6. doi: 10.1016/j.ajog.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 46.Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–24. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 47.Romero R, Mazor M, Morrotti R, et al. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol. 1992;166:129–33. doi: 10.1016/0002-9378(92)91845-2. [DOI] [PubMed] [Google Scholar]
- 48.Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, van Geijn HP. Final results of the Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2001;185:1106–12. doi: 10.1067/mob.2001.118655. [DOI] [PubMed] [Google Scholar]
- 49.Romero R. Prevention of spontaneous preterm birth: the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment. Ultrasound Obstet Gynecol. 2007;30:675–86. doi: 10.1002/uog.5174. [DOI] [PubMed] [Google Scholar]
- 50.Berghella V, Roman A, Daskalakis C, Ness A, Baxter JK. Gestational age at cervical length measurement and incidence of preterm birth. Obstet Gynecol. 2007;110:311–7. doi: 10.1097/01.AOG.0000270112.05025.1d. [DOI] [PubMed] [Google Scholar]
- 51.Vaisbuch E, Romero R, Erez O, et al. The clinical significance of early (<20 weeks) versus late (20-24 weeks) detection of a sonographic short cervix in asymptomatic women. Ultrasound Obstet Gynecol. 2008;32:276. doi: 10.1002/uog.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–69. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Relman DA. The search for unrecognized pathogens. Science. 1999;284:1308–10. doi: 10.1126/science.284.5418.1308. [DOI] [PubMed] [Google Scholar]
- 54.Ranjard L, Poly F, Nazaret S. Monitoring complex bacterial communities using culture-independent molecular techniques: application to soil environment. Res Microbiol. 2000;151:167–77. doi: 10.1016/s0923-2508(00)00136-4. [DOI] [PubMed] [Google Scholar]
- 55.Jalava J, Mantymaa ML, Ekblad U, et al. Bacterial 16S rDNA polymerase chain reaction in the detection of intra-amniotic infection. Br J Obstet Gynaecol. 1996;103:664–9. doi: 10.1111/j.1471-0528.1996.tb09835.x. [DOI] [PubMed] [Google Scholar]
- 56.Hitti J, Riley DE, Krohn MA, et al. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin Infect Dis. 1997;24:1228–32. doi: 10.1086/513669. [DOI] [PubMed] [Google Scholar]
- 57.Yoon BH, Romero R, Kim M, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183:1130–7. doi: 10.1067/mob.2000.109036. [DOI] [PubMed] [Google Scholar]
- 58.Yoon BH, Romero R, Lim JH, et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189:919–24. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]
- 59.Gardella C, Riley DE, Hitti J, Agnew K, Krieger JN, Eschenbach D. Identification and sequencing of bacterial rDNAs in culture-negative amniotic fluid from women in premature labor. Am J Perinatol. 2004;21:319–23. doi: 10.1055/s-2004-831884. [DOI] [PubMed] [Google Scholar]
- 60.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PloS One. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DiGiulio DB, Romero R, Kusanovic JP, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010 Mar 21; doi: 10.1111/j.1600-0897.2010.00830.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, Cassell GH. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol. 1995;173:606–12. doi: 10.1016/0002-9378(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 63.Espinoza J, Goncalves LF, Romero R, et al. The prevalence and clinical significance of amniotic fluid ‘sludge’ in patients with preterm labor and intact membranes. Ultrasound Obstet Gynecol. 2005;25:346–52. doi: 10.1002/uog.1871. [DOI] [PubMed] [Google Scholar]
- 64.Bujold E, Pasquier JC, Simoneau J, et al. Intra-amniotic sludge, short cervix, and risk of preterm delivery. J Obstet Gynaecol Can. 2006;28:198–202. doi: 10.1016/S1701-2163(16)32108-9. [DOI] [PubMed] [Google Scholar]
- 65.Kusanovic JP, Espinoza J, Romero R, et al. Clinical significance of the presence of amniotic fluid ‘sludge’ in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007;30:706–14. doi: 10.1002/uog.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romero R, Kusanovic JP, Espinoza J, et al. What is amniotic fluid ‘sludge’? Ultrasound Obstet Gynecol. 2007;30:793–8. doi: 10.1002/uog.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Romero R, Schaudinn C, Kusanovic JP, et al. Detection of a microbial biofilm in intraamniotic infection. Am J Obstet Gynecol. 2008;198:135.e1–5. doi: 10.1016/j.ajog.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 69.Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126:659–62. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 70.Pedra JH, Cassel SL, Sutterwala FS. Sensing pathogens and danger signals by the inflammasome. Curr Opin Immunol. 2009;21:10–6. doi: 10.1016/j.coi.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pineles BL, Romero R, Montenegro D, et al. “The Inflammasone” in human parturition. Reprod Sci. 2007;14(supp):59A. [Google Scholar]
- 72.Gotsch F, Romero R, Chaiworapongsa T, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21:605–16. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]


