Abstract
Context
Chronic psychological distress has deleterious effects on many of the body’s physiological systems. In experimental animal models, chronic stress leads to neuroanatomic changes in the hippocampus, in particular a decrease in the length and branching of dendrites as well as a decrease in the number of dendritic spines.
Objectives
To examine whether analogous distress-related neuroanatomic changes occur in humans and whether such changes might also be related to cognitive dysfunction observed in older people who report greater psychological distress.
Design
Postmortem study of brain tissues from participants of the Religious Orders Study, an ongoing population-based clinicopathological study of aging and cognition.
Setting
The Rush University Religious Orders Study and the University of Pennsylvania Cellular and Molecular Neuropathology Program.
Participants
Seventy-two deceased participants of the Religious Orders Study.
Main Outcome Measures
Densities of microtubule-associated protein 2–immunolabeled dendrites and synaptopodin-immunolabeled dendritic spines in the CA3 subfield of the hippocampus, quantified using semiautomated image acquisition and analysis.
Results
Higher levels of trait anxiety and longitudinal depression scores were associated with decreased densities of dendrites and spines in CA3. Dendrite and spine densities did not correlate with an index of global cognition or with densities of common age-related pathological changes.
Conclusions
Regressive neuronal changes occur in humans who experience greater psychological distress. These changes are analogous to neuronal changes in animal models of chronic stress.
Chronic psychological distress has harmful effects on many of the body’s physiological systems, including the cardiovascular, endocrine, immune, and central nervous systems.1–4 Anxiety, depression, and other negative emotional states have been associated with increased risk of hypertension, diabetes mellitus, myocardial infarction, stroke, infection, and cancer, along with mortality due to all causes.5 It has long been recognized that psychological distress also has deleterious effects on cognition. In humans and experimental animal models, distress is associated with impairments in learning, memory, attention, executive functions, and other aspects of cognition. Furthermore, our group and others have reported that conditions of chronic psychological distress increase the risk of developing Alzheimer disease (AD)–like dementias in late life.4,6–15 A history of major depression increases risk of clinical AD 2-to 3-fold, whereas longitudinal studies of aging persons without psychiatric illness have found that those who tend to experience more psychological distress in their day-to-day lives are at increased risk of incident mild cognitive impairment and clinical AD and have an accelerated rate of cognitive decline compared with those who experience lower degrees of distress.8,12
Neuroanatomic investigations in human mental illnesses and animal models have identified structural and cellular morphological changes in the brain associated with high psychological distress. The hippocampus, an area important for cognition, emotion, and regulation of neuroendocrine response, has been one of the principal areas of interest. In humans, brain magnetic resonance imaging volumetric studies in depression and posttraumatic stress disorder have reported decreased hippocampal volumes that correlate with duration or severity of illness.16–20 Distress does not appear to cause the death of neurons because postmortem studies of major depression have not found significant neuron loss in the hippocampus.21 In addition, experiments in mice, rats, and other species have not found neuron loss to be associated with chronic environmental stress or exposure to high-dose stress hormones (ie, glucocorticoid).21–25 However, chronic stress exposure in mice, rats, tree shrews, and other animals have led to significant decreases in apical dendrite length and branching and in dendritic spine density in the hippocampal formation. These changes appear to be very prominent in the CA3 subfield of the hippocampus, but they also have been reported in the dentate gyrus, the CA1 subfield of the hippocampus, the medial prefrontal cortex, and some amygdala nuclei.26–31 It has been presumed that these dendritic changes are responsible for the learning and memory deficits of chronic stress exposure because the neuroanatomic and neurobehavioral phenomena occur concordantly, although some investigators have questioned this.32,33
It is not known whether similar distress-related dendritic changes occur in humans and whether such changes are related to the greater cognitive decline seen in people with greater psychological distress. Thus, we investigated dendritic morphometric correlates of anxiety, depression, and cognition in the CA3 subfield of hippocampi from participants in the Religious Orders Study (ROS), a longitudinal clinicopathological study of aging and cognition in elderly Catholic nuns, priests, and brothers who had annual medical, neurological, cognitive, and psychological assessments annually until death.
METHODS
PARTICIPANTS
All clinical and pathological data are from participants in the ROS34 conducted by the Rush University Alzheimer’s Disease Center with approval from the institutional review board at Rush University. Its participants are older Catholic nuns, priests, and brothers without dementia who agreed after a complete description of the longitudinal study to annual clinical evaluation and brain autopsy at death.
The 72 cases used in this study were selected from the approximately 350 ROS participants who had died and undergone autopsy by 2007 (autopsy rate exceeds 90%). Demographic, clinical, and postmortem data on these participants are presented in Table 1. Cases were chosen by random selection stratification according to distress scores and the global cognitive score most proximate to death. This stratified random sampling procedure was intended to enrich the sample with a range of variables of interest without introducing bias. Preceding analyses showed that the cohort used in this study did not differ from the larger unselected group of autopsied cases from which it was drawn in terms of age, sex, education, last Mini-Mental State Examination (MMSE) score, postmortem interval, dementia diagnoses, anxiety and depression scores, or neuropathological features.7,35–40 Complete clinical data were obtained in all cases and included annual clinical evaluations until the time of death, information about the proximal cause and acuity of death and underlying medical illnesses, and usage of psychotropic and other medications.
Table 1.
Characteristics of the Entire Sample and by Clinical Cognitive Diagnosis
| Characteristic | All (N=72) | Normal Cognition (n=28) | MCI (n=20) | AD (n=24) | P Valuea |
|---|---|---|---|---|---|
| Demographic | |||||
| Age, mean (SD), y | 84.6 (6.4) | 81.4 (6.0) | 85.3 (6.5) | 87.9 (4.9) | <.001 |
| Female sex, No. (%) | 42 (58) | 16 (57) | 10 (50) | 16 (67) | .53 |
| Education, mean (SD), y | 18.6 (3.5) | 19.1 (3.4) | 18.6 (3.9) | 17.9 (3.2) | .42 |
| Postmortem interval, mean (SD), h | 7.1 (5.6) | 7.6 (5.6) | 7.6 (7.0) | 6.2 (4.4) | .63 |
| Psychiatric, mean (SD) | |||||
| Anxiety-depression index | −0.06 (0.73) | −0.21 (0.50) | 0.08 (0.84) | 0.0 (0.85) | .36 |
| Anxiety Trait Scale score | 3.2 (3.7) | 2.6 (3.0) | 3.5 (3.9) | 3.7 (4.2) | .55 |
| Depression sum score | 1.4 (1.2) | 1.2 (0.8) | 1.7 (1.5) | 1.4 (1.3) | .27 |
| Cognitive, mean (SD) | |||||
| MMSE score | 24.2 (5.8) | 27.9 (1.5) | 26.3 (2.7) | 18.3 (6.2) | <.001 |
| Global cognition score | −0.65 (0.74) | −0.04 (0.60) | −0.53 (0.38) | −1.47 (0.60) | <.001 |
| Episodic memory score | −0.67 (1.1) | 0.26 (0.36) | −0.50 (0.50) | −1.89 (0.95) | <.001 |
| Medication exposure, ever, No. (%) | |||||
| Antidepressant | 27 (38) | 10 (36) | 8 (40) | 9 (38) | .95 |
| Anxiolytic | 12 (17) | 7 (25) | 1 (5) | 4 (17) | .21 |
| Antipsychotic | 8 (11) | 2 (7) | 1 (5) | 5 (21) | .22 |
| Estrogensb | 13/42 (31) | 5/16 (31) | 2/10 (20) | 6/16 (37) | .69 |
| Neuroanatomic, mean (SD) | |||||
| Neuron density in CA3, neurons/mm3 | 2.25 × 106 (3.9 × 105) | 2.23 × 106 (3.9 × 105) | 2.17 × 106 (2.9 × 105) | 2.33 × 106 (4.7 × 105) | .42 |
| MAP2 dendrite index in CA3 | 0.96 (0.34) | 1.05 (0.37) | 0.92 (0.30) | 0.89 (0.32) | .20 |
| Synaptopodin spine index in CA3 | 51.8 (22.0) | 54.6 (24.4) | 47.1 (19.9) | 52.3 (21.0) | .50 |
| Neuropathological | |||||
| PHF-tau tangles in CA1, mean (SD), tangles/mm3 | 15.0 (18.6) | 5.8 (8.2) | 12.7 (17.2) | 27.8 (21.4) | <.001 |
| β-Amyloid plaques in CA1, mean (SD), % of area | 0.5 (1.0) | 0.3 (0.7) | 0.4 (1.3) | 0.8 (1.0) | .24 |
| Infarcts, any, No. (%) | 20 (28) | 5 (18) | 5 (25) | 10 (42) | .15 |
| Lewy bodies, any, No. (%) | 13 (18) | 4 (14) | 4 (20) | 5 (21) | .79 |
Abbreviations: AD, Alzheimer disease; MAP2, microtubule-associated protein 2; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; PHF-tau, paired helical filament tau.
Based on 1-way analyses of variance for quantified variables and Pearson χ2 or Fisher exact test for categorical variables.
Data available for 42 cases.
CLINICAL EVALUATIONS
On recruitment into the longitudinal study (ie, at baseline) and annually thereafter, a standardized evaluation was conducted of each participant. This included completion of a medical history, neurological examination, cognitive testing, and ratings on psychiatric scales. The evaluations most relevant to the present study were ratings on well-established scales of anxiety (at baseline) and depression (at baseline and annually thereafter). For anxiety, we used a modified version14 of the Anxiety Trait Scale of the State-Trait Anxiety Inventory,41 a measure of anxiety proneness. The participants were read a series of 20 brief statements about anxious feelings; they responded yes to statements that indicated how they generally felt and no otherwise. We modified the test by using a yes-no response option instead of the original 4-point scale to reduce response burden. The score was the number of questions answered in a direction indicative of anxiety (range, 0–20), which has been associated with mortality in previous research.14 For depression, we used the 10-item version of the Center for Epidemiological Studies Depression Scale (CES-D),42,43 a measure of current level of depressive symptoms. The participants were asked whether they had experienced any of 10 symptoms much of the time in the past week. The number of symptoms reported (0–10) was averaged across evaluations to provide a measure of the enduring tendency to experience depressive symptoms. A mean CES-D score was generated across all evaluations before death because scores do not systematically change over time.38 We found that Anxiety Trait Scale and CES-D longitudinal scores were significantly correlated (r=0.66; P<.001); therefore, they were combined into a composite anxiety-depression index of psychological distress for primary analyses, followed by secondary analyses with each measure separately.
Cognition was assessed annually with a battery of 21 cognitive performance tests, as previously described.44 Data from the annual examination proximate to death for each participant (all within 1 year of death) were used in the analyses herein. The MMSE was used to describe participants but was not used in the analyses, and a different test was used in diagnostic classification. The remaining 19 tests consisted of 7 tests of episodic memory, 4 tests of semantic memory, 4 tests of working memory, 2 tests of perceptual speed, and 2 tests of visuospatial ability. For each participant, the raw scores on individual tests were converted to z scores by means of the baseline mean and SD in the entire ROS cohort; z scores on component tests were averaged to yield the composite global cognition index score. Detailed information on the individual tests and on the derivation and correlates of the composite measures is contained in previous publications.9,34,45
At each evaluation, participants were also asked to bring in all prescription and over-the-counter medications, which were inspected. The name and dosage were recorded and classified according to the Medi-Span list46 independently by a pharmacy student and a physician, as previously described in this and other cohorts.47,48
TISSUE PROCESSING, HISTOLOGY, AND IMMUNOHISTOCHEMISTRY
Participants’ brains were removed in a standard fashion as described previously.40,49 After weighing, each brain was cut coronally into 1-cm-thick coronal slabs, immersion fixed in 4% paraformaldehyde for 48 to 72 hours, and then placed in graded glycerol-dimethylsulfoxide in phosphate-buffered saline for storage (final dilutions, 20% glycerol and 2% dimethylsulfoxide). The hippocampal formation was dissected from the coronal slabs into 0.5-cm-thick coronal blocks and embedded in paraffin. Sections measuring 20 μm thick were cut from each block for cytoarchitectural assessment following staining of Nissl substance with a 1% cresyl violet solution. On the basis of this assessment, 2 adjacent blocks from intermediate rostrocaudal levels of the hippocampal formation were selected from each case and analyzed at comparable cytoarchitectural levels of that structure (Figure 1). We used Figure 21.11 from Insausti and Amaral50 as our main reference, excluding the rostral and caudal sections (a–e and l). These Nissl preparations were also used to estimate neuron densities, as described in the “Image Acquisition and Analysis” section.
Figure 1.
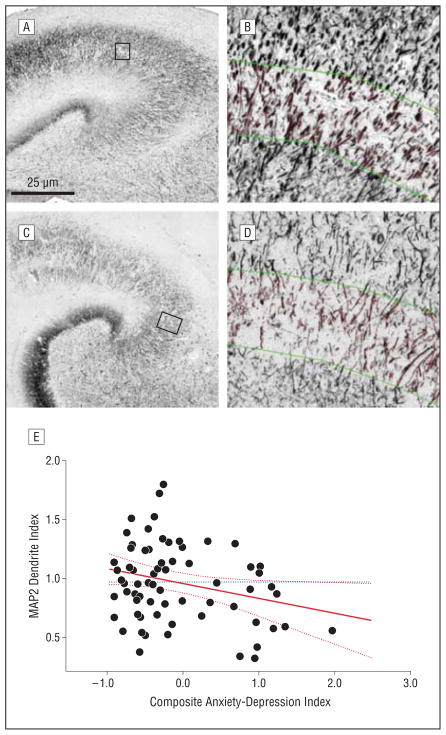
Microtubule-associated protein 2 (MAP2)–labeled dendrites in CA3. Photomicrographs from participants with low (A and B) and high (C and D) psychological distress immunolabeled for MAP2. Photomontages of the CA3 region are provided (A and C), with high-magnification insets of the stratum lucidum after image processing to delineate skeletonized dendrites (B and D). A leverage plot of the composite psychological distress score by dendrite index value is also provided (E). The solid red line depicts the regression line, the hatched red lines represent confidence intervals, and the dotted blue horizontal line shows the mean for all cases.
From the selected hippocampal blocks, 5-μm paraffin sections were cut for immunohistochemical labeling. In earlier studies, our group determined that 5-μm thin sections optimized the imaging and analysis of both proteins of interest and allowed sufficient immunolabeling of objects without excessive overlap that would obfuscate dendrite or spine resolution for image analysis. Dendrites were immunolabeled with the use of a rabbit polyclonal antibody against microtubule-associated protein 2 (MAP2) (AB5622; Millipore Corp, Billerica, Massachusetts) and the dendritic spine apparatus was labeled using a mouse monoclonal antibody against synaptopodin (Q44590M; Biodesign International Inc, Saco, Maine). Optimal results with both antibodies were obtained with heat-induced epitope retrieval but with different types of signal amplification (silver for MAP2 and tyramide for synaptopodin). Separate protocols were thus used for the 2 antigens of interest.
For MAP2 immunolabeling of dendrites, dewaxed sections were rehydrated in ethanol and soaked in 5% hydrogen peroxide in absolute methanol for 30 minutes to quench endogenous peroxidase activity. For antigen retrieval, sections were then boiled in 1mM EDTA in 0.1M TRIS buffer (pH 8.0) for 10 minutes, cooled for 20 minutes, and then immersed in 0.5% sodium boro-hydride for 5 minutes followed by 2 washes in TRIS-Triton (0.01% Triton X-100 in 0.1M TRIS-hydrochloride buffer, pH 7.6). Sections were blocked for 45 minutes in 2% horse serum dissolved in TRIS-Triton and incubated in the MAP2 primary antibody (1: 2000) at 4°C overnight (14–18 hours), as previously described.51 The primary antibody was detected by incubating sections with biotinylated secondary antibody (anti-mouse IgG) for 1 hour at room temperature, treated for another hour with an avidin-biotin-peroxidase complex made from a kit (Vectastain ABC; Vector Laboratories Inc, Burlingame, California), and developed for 10 minutes with the use of diaminobenzidine chromogen. For contrast intensification of the dendritic MAP2 immunoreactive product, we used a silver-gold protocol to maximize signal amplification, as previously described.51 This involved treatment with methenamine silver developer incubated in a 60°C oven for 60 minutes and later immersion in 0.1% gold chloride solution for 5 minutes. Sections were cleared in xylenes, dehydrated, and coverslipped with an acrylic resin (Cytoseal; Stephens Scientific, Wayne, New Jersey).
For synaptopodin immunolabeling of the dendritic spines, another protocol was used. After the dewaxing, peroxidase quenching, and antigen retrieval steps described in the MAP2 protocol, sections were rinsed twice in a TRIS hydrochloride–sodium chloride–Tween 20 washing buffer (pH 7.5), blocked for 30 minutes in a TRIS hydrochloride–sodium chloride–blocking reagent (TNB) buffer from a kit (TSA [tyramide signal amplification] Biotin System; PerkinElmer Inc, Waltham, Massachusetts), and incubated in the primary synaptopodin antibody (1:2000). The primary antibody was detected by incubating sections with biotinylated secondary antibody (anti-mouse IgG) for 1 hour at room temperature followed by a series of short incubations in TSA reagents according to kit instructions: 30 minutes in streptavidin-horseradish peroxidase (SA-HRP; 1:100 in TNB buffer), 10 minutes in biotinyl tyramide working solution (1:50 in a 10× amplification reagent provided in the kit), and 30 minutes in SA-HRP (1:100). Sections were then developed for 10 minutes with the use of nickel-enhanced diaminobenzidine chromogen, dehydrated, and coverslipped.
Our group has previously reported no association between AD lesions and psychological distress.39,40 To determine whether that was the case in the hippocampal samples in the present study, we immunolabeled sections with antibodies to paired helical filament (PHF)–tau (AT8; Innogenex Corp, San Ramon, California) for neurofibrillary tangles and β-amyloid (MO0872, 1:100; DAKO, Carpenteria, California) for senile plaques, as previously described.36,39,52 This also allowed us to assess any relationship or interaction effects of these common neuropathological lesions and dendrite or dendritic spine density in CA3 that might influence interpretation of our results.
IMAGE ACQUISITION AND ANALYSIS
If staining or other technical anomalies prohibited reliable measurement, the cases were excluded. Seventy-two cases remained after 8 exclusions from an original 80 cases selected randomly from the stratified cohort. Data were collected on 2 hippocampal regions: CA3 for the primary analyses of dendrites and dendritic spines and CA1/subiculum for estimates of hippocampal β-amyloid plaques and PHF-tau neurofibrillary tangle density. The rationale for measuring tangles and plaques in CA1/subiculum as opposed to CA3 was that these AD lesions are very sparse in CA3, even in severe AD, whereas CA1 is selectively vulnerable to their accumulation.53,54 Thus, measurement of these age- and disease-related lesions in CA1/subiculum is a more sensitive index of the degree to which they affected the hippocampus in these cases.
The boundaries of CA3 and CA1 are evident in the cytoarchitectural features of their pyramidal layers seen in Nissl sections, as specified by Amaral and Insausti.50 The internal border of CA3 is marked by a clear decrease in the density of its pyramidal cell layer as this enters the hilus of the dentate gyrus. The external border of CA3 is less clear but can still be approximated by the decrease in thickness of the pyramidal layer as it merges with the thinner, more densely packed pyramidal cell layer of CA2. The internal border of CA1 is approximated by a widening and less densely packed pyramidal cell layer compared with CA2. This layer of CA1 merges with the even wider pyramidal cell layer of the subiculum, the external border of which is indicated by the appearance of the presubiculum, which is characterized by a superficial layer of densely packed neurons much smaller than subicular pyramidal cells. Where the borders of CA3 or CA1/subiculum could only be approximated, we estimated their location conservatively to include only tissue unambiguously in the regions of interest. The borders selected for those regions in a Nissl section on a given tissue block guided recognition of the same borders in immunohistochemical sections from that block.
All image acquisition and analyses were conducted blind to case information with the use of previously optimized acquisition and image processing protocols. To quantify aspects of the CA3 area and stained objects of interest within it, gray-scale images of microscopic fields in CA3 of each section were captured via a light microscope (DMRB; Leica Microsystems GmbH, Wetzlar, Germany) equipped with a motorized stage (MAC 2000; Ludl Electronic Products Ltd, Hawthorne, New York) and digital camera (Retiga Exi/QEi; QImaging, Surrey, British Columbia, Canada). For Nissl and MAP2 preparations, a series of contiguous images captured at 100× magnification in each tissue section was assembled into a high-resolution composite image (ie, a photomontage) using Image-Pro Plus software (Media Cybernetics Inc, Silver Spring, Maryland). Each of these photomontages covered the stratum pyramidale in the case of Nissl preparations and both the strata pyramidale and the lucidum in the case of MAP2 preparations. For the synaptopodin preparations, random, systematic sampling was used to capture separate 1000× images in the stratum lucidum.
Quantitative analyses of tissue elements visualized in Nissl, MAP2, and synaptopodin preparations were conducted with algorithmic image filtering using Image-Pro Plus software (Figure 2). Neurons of the stratum pyramidale of CA3 are large with virtually no overlap in size with glial cells; therefore, after the automatic delineation of discrete objects, the software was engaged to apply a size parameter (>65 μm2) to distinguish neurons from glia and count these based on cross-sectional profiles. In addition, somal area size was averaged for all the neurons counted. To moderate the effect of a split-cell artifact, we applied a Floderus correction factor for neuron density.55 For the MAP2 and synaptopodin immunohistochemical preparations, we applied histogram-based optical density thresholding to limit analysis to those tissue elements exceeding background levels of immunoreactivity. More advanced applications of the software were then used to selectively identify tissue elements of interest.
Figure 2.
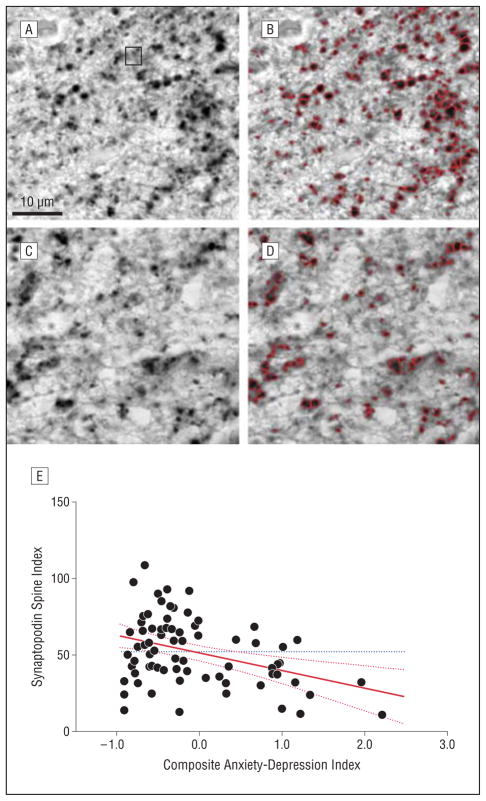
Synaptopodin-labeled dendritic spines in CA3 stratum lucidum. The images depict spines before and after image processing to identify synaptopodin-immunoreactive puncta from a low-distress individual (A and B) and a high-distress individual (C and D). The participants are the same as those in Figure 1. A leverage plot of the composite psychological distress score by spine index value is also shown (E). The solid red line depicts the regression line plot, the hatched red lines represent confidence intervals, and the dotted blue horizontal line shows the mean for all cases.
To obtain a reliable index of the dendrite density, we first applied optical density, pixel contiguity, and size (>5 μm2) threshold filters to delineate all MAP2-labeled dendritic processes in the CA3 strata pyramidale and lucidum. To attenuate the inherent noise of enzymatic-based immunohistochemical labeling, which could create variability in dendritic thickness from run to run, we applied a filter that “skeletonized” the dendrites, effectively reducing imaged dendrites of any thickness to a 1-pixel width. The software calculated the total length of the skeletonized dendrites and expressed this as a percentage of the area of the entire CA3. To adjust for the unpredictable variations in tissue shrinkage due to aldehyde fixation and other aspects of tissue processing,56 we then divided this value by the density of neurons obtained in adjacent sections to create a biologically meaningful, dimensionless index of dendrites per neuron for that case. For synaptopodin-labeled dendritic spines, we applied optical density, pixel contiguity, size (>0.05 μm2), and segmentation filters to automatically delineate dendritic spines, which appeared as labeled puncta (Figure 2). These were counted to determine the number of spines per unit of area, and this density was divided by the neuron density to create an index of spines per neuron. Finally, because 2 hippocampal blocks were analyzed per case, the indices of dendrites and spines per neuron for each block were averaged for each case to calculate 1 composite dendrite index and 1 composite spine index for each case. These indices were used for the statistical analyses.
It should be noted that the dendrites and spines we quantified arise from both the pyramidal neurons and interneurons of the stratum pyramidale. We did not attempt to differentiate between these neuronal origins. However, we conducted a separate analysis on our tissue set, immunolabeling for parvalbumin,57 a calcium-binding protein present in important spinous γ-aminobutyric acid–containing interneurons of the stratum pyramidale of CA3. The results of this analysis showed that less than 2% of all neurons in the stratum pyramidale of CA3 are parvalbumin-immunoreactive interneurons. Thus, we reasoned that most of the dendrites and spines measured were those of pyramidal neurons.
Our methods for high-throughput estimation of β-amyloid plaque load (the percentage of tissue area occupied by contiguous immunoreactive pixels) and PHF-tau neurofibrillary tangle density (per unit of area) have been described previously.39,52,58–60
STATISTICAL ANALYSIS
Our sample reflected older adults with a continuum of anxiety, depression, and cognition scores. Initial descriptive analyses examined correlations of age, sex, education, postmortem interval, psychotropic medication exposure, and other potential confounders with the principal neuroanatomic outcome variables. Significant correlations prompted inclusion in the primary regression analyses. Correlation and linear regression models were used as our primary approach to assess the relationships of the principal neuroanatomic outcome variables of interest, including neuron density, the MAP2 dendrite index, the synaptopodin spine index, and the PHF-tau neurofibrillary tangle and β-amyloid senile plaque densities with the psychiatric and cognition scores. Post hoc analyses were then conducted for the individual anxiety and depression measures. Multiple regression modeling was further used to assess the degree to which the relationship between psychological distress and cognition measures may be mediated or modified by dendrites and/or spines. One-way analysis of variance and Pearson χ2 or Fisher exact tests were used to examine differences among groups of participants diagnostically classified as having clinically normal cognition, mild cognitive impairment, or AD. All statistical analyses were conducted using JMP software (version 7.0.2; SAS Institute Inc, Cary, North Carolina).
RESULTS
SUBJECT CHARACTERISTICS
Descriptive data are presented in Table 1 for the entire sample of 72 participants and for groups clinically diagnosed as having normal cognition, mild cognitive impairment, and AD according to standard diagnostic criteria.61,62 As expected, 1-way analysis of variance demonstrated significant between-group differences for age, cognition (MMSE, global cognition, and episodic memory scores), and neurofibrillary tangle density in CA1 but not for other demographic, psychiatric, psychotropic medication, perimortem, or neuroanatomic and neuropathological variables.
RELATIONSHIP OF ANXIETY AND DEPRESSION TO NEUROANATOMIC AND NEUROPATHOLOGICAL MEASURES
As shown in Figures 1 and 2 and presented in Table 2, our analyses found significant relationships between the composite anxiety-depression index and the MAP2 dendrite and synaptopodin spine indices. Scores on this composite index of psychological distress were significantly associated with decreased densities of both the dendrites and spines. Post hoc analyses found that this was largely because of the contribution of the Anxiety Trait Scale measure for dendrites and the contributions of anxiety and depression for spines. Anxiety and depression were not related to neuron density, and there were no correlations between the psychiatric measures and hippocampal neurofibrillary tangle or senile plaque densities.
Table 2.
Regression Analyses for Neuroanatomic and Neuropathological Variables on Psychiatric and Cognitive Measures
| Outcome Measure | r Value | Regression Coefficient (SE) | P Value |
|---|---|---|---|
| Neuroanatomic Outcomes | |||
| CA3 neuron density | |||
| Anxiety-depression index | 0.05 | 2738 (6414) | .67 |
| Anxiety Trait Scale score | 0.04 | 462 (1274) | .72 |
| Depressiona | 0.05 | 1524 (3850) | .69 |
| Global cognition index | −0.14 | −7192 (6248) | .25 |
| Episodic memory score | −0.05 | −5049 (4134) | .23 |
| CA3 MAP2 dendrite index | |||
| Anxiety-depression index | −0.25 | −0.13 (0.06) | .03 |
| Anxiety Trait Scale score | −0.31 | −0.03 (0.01) | .009 |
| Depressiona | −0.12 | −0.39 (0.37) | .29 |
| Global cognition index | 0.21 | 0.10 (0.06) | .09 |
| Episodic memory score | 0.17 | 0.06 (0.04) | .16 |
| CA3 synaptopodin spine index | |||
| Anxiety-depression index | −0.38 | −11.5 (3.3) | <.001 |
| Anxiety Trait Scale score | −0.33 | −1.97 (0.67) | .004 |
| Depressiona | −0.35 | −6.31 (2.02) | .003 |
| Global cognition index | 0.10 | 3.05 (3.52) | .39 |
| Episodic memory score | 0.10 | 1.91 (2.33) | .41 |
| Neuropathological Outcomes | |||
| CA1/subiculum PHF-tau neurofibrillary tangle densityb | |||
| Anxiety-depression index | −0.17 | −4.2 (2.8) | .15 |
| Anxiety Trait Scale score | −0.10 | −0.52 (0.56) | .36 |
| Depressiona | −0.21 | −2.87 (1.69) | .09 |
| Global cognition index | −0.42 | −0.01 (0.004) | .007 |
| Episodic memory score | −0.50 | −0.02 (0.006) | <.001 |
| CA1/subiculum β-amyloid senile plaque densityb | |||
| Anxiety-depression index | −0.05 | −0.08 (0.18) | .66 |
| Anxiety Trait Scale score | −0.07 | −0.02 (0.04) | .59 |
| Depressiona | −0.03 | −0.03 (0.10) | .80 |
| Global cognition index | −0.16 | −0.12 (0.07) | .10 |
| Episodic memory score | −0.30 | −0.33 (0.12) | .005 |
Abbreviations: MAP2, microtubule-associated protein 2; PHF-tau, paired helical filament tau.
Determined with the use of the Center for Epidemiological Studies Depression Scale.
Adjusted for age and education.
RELATIONSHIPS OF COGNITION TO ANXIETY AND DEPRESSION AND TO NEUROANATOMIC AND NEUROPATHOLOGICAL MEASURES
To test whether the significant effects of anxiety and depression on dendrites and spines might be related to cognition and thus mediate the relationship of psychological distress to cognition, we used linear regression modeling. First, we established the essential relationship of anxiety and depression and cognitive performance. As was previously reported in the larger ROS cohort,7,9,10,38–40 we found that higher scores on the anxiety-depression index in this current sample were associated with poorer performance on the global cognition index (adjusted for age and education, mean [SE] parameter estimate, −0.28 [0.10]; P=.006). Post hoc analyses found significant associations of global cognition with both Anxiety Trait Scale scores (P=.02) and longitudinal depression scores (P=.01). Next, we tested whether the dendrite or spine indices were related to global cognition. As seen in Table 2, we found no significant relationship between global cognition and our measures of dendrites or spines. Without these associations, no further mediation modeling was conducted. Thus, our hypothesis that the dendrite and spine atrophy associated with distress mediated the relationship between psychological distress and impaired cognition was not supported.
Next, we considered whether anxiety and depression might modify the significant relationship between age-related neuropathological features of AD and cognitive impairment. First, we established the relationship of AD lesions in the hippocampus to cognitive impairment. As seen in Table 2, global cognition and episodic memory were inversely related to neurofibrillary tangle density in the CA1/subiculum subfields of the hippocampus (with adjustment for age and education), and episodic memory was associated with β-amyloid deposits.
Next, we entered terms for the anxiety-depression index and the interaction of anxiety-depression and neurofibrillary tangles and β-amyloid deposits. This model yielded a marginally significant association of the interaction with global cognition (mean [SE] parameter estimate, −0.011 [0.006]; P=.05) and a significant interaction with episodic memory (parameter estimate, −0.021 [0.009]; P=.03) for neurofibrillary tangles. This suggests that psychological distress may amplify the toxic effects of neurofibrillary tangles on cognition. No interactions were found for hippocampal β-amyloid deposition.
Finally, we used the same approach to assess whether the CA3 dendrite or spine indices might interact with neurofibrillary tangles to modify their association with cognition. There was no interaction effect for cognition.
ASSESSMENT OF OTHER POTENTIAL CONFOUNDERS
We used correlation analyses and unpaired, 2-tailed t tests to assess possible relationships of our primary data with a large number of potentially confounding variables. We found no relationships between psychiatric or dendrite and spine measures with age; sex; education; history of cardiovascular disease, hypertension, diabetes mellitus, cancer, and other medical illnesses; proximal causes of death; postmortem interval; global measures of neurofibrillary tangles and senile plaques (the composite of densities in multiple brain regions); presence or absence of infarcts or Lewy bodies anywhere in the brain; and current or past treatment with antidepressants, anxiolytics, antipsychotics, other psychotropic medications, or hormone therapy (Table 1).
COMMENT
To our knowledge, this is the first study to report a relationship between chronic psychological distress and regressive dendrite and dendritic spine changes in humans, specifically a reduction in MAP2-immunoreactive dendrites and synaptopodin-immunoreactive dendritic spines in the CA3 subfield of the hippocampal formation. Our results are reminiscent of the extensive data in jewel fish, mice, rats, and tree shrews, which have shown that chronic stress induces atrophic changes in the architecture of neuronal dendrites and spines.26,28–31,63–66
Dendrites and spines are crucial components for synaptic function and plasticity. Changes in their density or morphological features can result in significant alterations in the connectivity of neural systems67,68 and would be expected to result in changes in the neurobehavioral functions subserved by those systems. Chronic stress exposure in rodents causes reversible atrophy of dendrites and spines in the dentate gyrus and hippocampus, as well as impairments in hippocampal-based spatial memory.69–72 Whether these anatomic and functional effects of chronic stress are causally related or are parallel phenomena has not been established yet. Although a direct pathophysiological relationship has been widely assumed because of the similar time courses of anatomic and functional changes with experimental, environmental, or pharmacological stress manipulations, important exceptions to this relationship have been recognized (for review, see Conrad32). It is also important to note that our measures of psychological distress may be analogous but not equivalent to the presumed effects of chronic stressors used in animal experiments. Although our study found a similar association between distress and our anatomic variables of interest, we cannot assume that the pathophysiological mechanisms are similar.
Human postmortem research bears many uncontrollable factors that may confound data or their interpretation. Nevertheless, such research plays an important role in translating findings from preclinical studies to humans. Intraspecific biological and environmental variability, the reliability and fidelity of clinical assessment measures, changes that may occur during the intervals between death and the clinical assessments of participants, comorbid medical illnesses and pharmacotherapy, cause and manner of death, postmortem interval between death and tissue fixation, and other factors all may affect sensitive and dynamic neuroanatomic structures and molecular integrity. With regard to these factors, our sample has some notable strengths as well as potential weaknesses.
First, the ROS cohort from which this sample was drawn differs from the general US population in terms of educational, social, cultural, and other lifestyle factors. Many potential sources of variability are reduced in this group, possibly increasing the precision of the associations, but the generalizability of findings to other populations also may be limited. For instance, ROS participants are highly educated, generally maintain a healthy lifestyle, and are largely without histories of major psychiatric illness. The “cerebral reserve” that education and other lifestyle factors purportedly confer or reflect may have allowed study participants’ brains to tolerate more distress before anatomic changes or cognitive effects were manifest. Furthermore, although the participants we studied exhibited a range of scores on the psychological distress rating scales administered, these were generally mild. Therefore, because this study showed a significant association between distress measures and changes in hippocampal anatomy, we consider that the general population may present changes that are as great or even greater. Associations of psychological distress with cognition have been found in other cohorts, including the Rush Memory and Aging Project, a lay cohort of similar study design,6,73 and the Chicago Health and Aging Project, a longitudinal population-based study in a biracial community.15 These data suggest that, despite differences in some risk factors, associations with cognition are generally replicable.
Most postmortem neuroanatomic and molecular studies in psychiatry are typically conducted in clinical samples of patients with major psychiatric illness. Our study is unique in that it investigates the neuroanatomic and cognitive correlates of anxiety and depressive symptoms in people who have no history of major psychiatric illness. It is curious that, despite CA3 and the mossy fiber terminal zone being such a prominent focus of anatomic and molecular changes in animal models of chronic stress, there have been relatively few postmortem studies of this important hippocampal subfield in psychiatric illness. In schizophrenia, findings in CA3 have included decreased densities of mossy fiber synapses,74,75 decreased spinophilin messenger RNA expression,76 decreased dysbindin 1 protein expression in the mossy fiber terminal zone,51 decreased chromogranin B and synapsin I in mossy fibers,77 and a variety of neurotransmitter receptor abnormalities.78–81 Such changes may be specific to schizophrenia or, alternatively, they may be nonspecific effects of the psychological distress that accompanies this severe mental illness. To our knowledge, there have been no similar postmortem studies in major depression or anxiety disorders that might shed light on this. Thus, it is noteworthy that, even in our nonclinical sample, we observed an association of dendrite and spine densities with trait anxiety and longitudinal symptoms of depression.
The dendrite and spine atrophy that occurs with chronic stress exposure in experimental animals is dynamic. It occurs over days and, upon discontinuation of the stress, reverts to normal.26 In our human sample, there are likely to have been various stressors unknown to us occurring in the weeks before death. Illnesses culminating in natural deaths are major stressors, and it is not clear what effects these may have had on dendritic and spinal architecture.
Also of particular interest are medications that participants may have been taking, such as antidepressants, anxiolytics, antipsychotics, and estrogens, which have well-established though complex effects on dendrites and spines.66,82–85 We considered these medication exposures specifically, as well as a variety of other potentially confounding variables such as illnesses, causes of death, acuity of death, coexistent neuropathological lesions (eg, infarction, AD lesions, and Lewy bodies), and various other medication exposures. Each of these may affect neuron, dendrite, or spine morphometry in vivo or even exert differential effects on tissue shrinkage during postmortem tissue processing.56 We statistically examined our neuroanatomic data for associations with each of these potential confounders but found none. Nonetheless, caution is advised in attributing the relative atrophy of hippocampal dendrites and spines solely to the enduring effects of trait anxiety and enduring depressive symptoms without applying other analytic approaches and replication in other samples.
In interpreting our investigation of dendrites and spines in light of studies of chronic stress in animal models, it is important to emphasize that we investigated the association of symptoms and traits of psychological distress and not stress per se. Exposure to stressors—whether experimental or naturally occurring, acute or chronic—should not necessarily be conflated with the experience of psychological distress because the neurophysiological, molecular, and anatomic correlates are diverse and complex.
We used a novel approach to estimate the densities of dendrites and spines in this study. Most morphometric studies of dendrites and spines in experimental animals and in human postmortem brain tissues have used Golgi staining with Scholl analyses or other labor-intensive approaches to estimate the number, lengths, and patterns of labeled processes and objects of interest in comparatively small samples. In the present study, we used a more high-throughput approach consisting of immunohistochemical labeling in thin sections for proteins that are highly enriched in the neuronal compartments of interest, ie, MAP2 in dendrites and synaptopodin in dendritic spines. For quantitation, we used semiautomated algorithmic image analysis to segment and estimate the densities of neuronal profiles, dendrites, and spines. There are relative advantages and disadvantages to each approach. Morphological detail with Golgi staining can be exquisite, and much of the full extent of a given neuron’s dendritic tree can be measured. However, Golgi staining labels the somata, axons, dendrites, and spines of only some neurons, and which neurons it labels is unpredictable. Finally, Golgi staining is technically lengthy and challenging, variable from case to case, and does not lend itself well to larger-scale studies. In contrast, immunohistochemistry with antibodies directed at MAP2 and synaptopodin respectively label all dendrites and spines that express those proteins, is technically more uniform and robust, can be conducted in variously fixed and processed tissues, and is suitable for high-throughput analysis of relatively large samples. On the other hand, limitations of this method can include variability in enzymatic reaction product contrast and resolution, especially for dense and overlapping dendrites and spines, different shrinkages of tissue with heat-induced or other epitope retrieval methods, and vulnerability to split-object artifact inherent in 2-dimensional quantitation. We attempted to attenuate these limitations by using computer-assisted algorithmic size, shape, optical density, and pixel contiguity filtering to delineate and then measure standard 1-pixel-wide lengths of skeletonized dendrites or count segmented synaptopodin-immunoreactive puncta. These methods allowed for more reliable measurement, although they precluded measurement of certain data that may be informative, such as thickness, shape, or staining intensity. We also applied a correction factor to moderate split-cell artifact bias in our 2-dimensional neuron density determination. Finally, we “normalized” our dendrite and spine densities to the densities of neurons in CA3, thus eliminating the potential confounder of variable tissue shrinkage.
It has been suggested that chronic stress in animals causes memory impairment via stress-induced synaptic changes. Accordingly, we initially hypothesized that atrophy of dendrites and spines associated with anxiety and depression would mediate the relationship between these distress traits and cognitive impairment that our group has previously described in the ROS and Memory and Aging Project cohorts.6–9,11,12,15,38–40,73 Our analyses did not support this. One possible explanation is that our study was underpowered to detect a relationship with cognition. Another explanation is that dendrite and spine atrophy in CA3 is only one of many factors contributing to cognitive decline. Common neuropathological lesions in older people, especially neurofibrillary tangles and amyloid plaques, may have more powerful effects on cognition, obscuring the lesser effects of distress-related neuroanatomic changes in CA3. Indeed, while there was no relationship between anxiety and depression and densities of tangles and plaques in this sample, the relationship between these lesions and cognition was strong, as expected. Finally, another possibility is that dendrite and spine atrophy does not lie in a causal chain linking chronic stress and cognitive impairment, as has been proposed.32 Chronic stress in animals may cause neuroanatomic and behavioral changes via parallel but independent mechanisms that may not be the same in humans.
One final consideration is that human postmortem studies are limited in that they can only give a snapshot of the brain as it was at the time of death. As such, postmortem studies are useful for showing associations of clinical and brain data but cannot ascertain causal relatedness with certainty. Therefore, although animal studies of stress have shown causality in one direction, the results found in this study could imply causality in the opposite direction for humans. In other words, inherently reduced numbers of dendrites and spines could be one factor that makes a person more susceptible to distress than another.
Acknowledgments
Funding/Support: This study was supported by grants P30AG10161, R01AG15819, R01AG024871, and P30AG10124 from the National Institute on Aging.
Footnotes
Financial Disclosure: None reported.
Additional Contributions: The authors express their great appreciation to the participants of the Religious Orders Study.
References
- 1.Selye H. Stress in Health and Disease. Boston, MA: Butterworth; 1976. [Google Scholar]
- 2.Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: new perspectives from psychoneuroimmunology. Annu Rev Psychol. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- 3.Sobel RM, Markov D. The impact of anxiety and mood disorders on physical disease: the worried not-so-well. Curr Psychiatry Rep. 2005;7(3):206–212. doi: 10.1007/s11920-005-0055-y. [DOI] [PubMed] [Google Scholar]
- 4.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prince M, Patel V, Saxena S, Maj M, Maselko J, Phillips MR, Rahman A. No health without mental health. Lancet. 2007;370(9590):859–877. doi: 10.1016/S0140-6736(07)61238-0. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- 7.Wilson RS, Barnes LL, Mendes de Leon CF, Aggarwal NT, Schneider JS, Bach J, Pilat J, Beckett LA, Arnold SE, Evans DA, Bennett DA. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59(3):364–370. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- 8.Wilson RS, Bennett DA, Mendes de Leon CF, Bienias JL, Morris MC, Evans DA. Distress proneness and cognitive decline in a population of older persons. Psychoneuroendocrinology. 2005;30(1):11–17. doi: 10.1016/j.psyneuen.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology. 2003;61(11):1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- 10.Wilson RS, Fleischman DA, Myers RA, Bennett DA, Bienias JL, Gilley DW, Evans DA. Premorbid proneness to distress and episodic memory impairment in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75(2):191–195. [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson RS, Mendes De Leon CF, Bennett DA, Bienias JL, Evans DA. Depressive symptoms and cognitive decline in a community population of older persons. J Neurol Neurosurg Psychiatry. 2004;75(1):126–129. [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- 13.Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry. 1999;56(5):425–430. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]
- 14.Wilson RS, Bienias JL, Mendes de Leon CF, Evans DA, Bennett DA. Negative affect and mortality in older persons. Am J Epidemiol. 2003;158(9):827–835. doi: 10.1093/aje/kwg224. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RS, Barnes LL, Bennett DA, Li Y, Bienias JL, Mendes de Leon CF, Evans DA. Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurology. 2005;64(2):380–382. doi: 10.1212/01.WNL.0000149525.53525.E7. [DOI] [PubMed] [Google Scholar]
- 16.MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahias C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100(3):1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160(8):1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 18.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19(12):5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30 (7):1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57(10):925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 21.Müller MB, Lucassen PJ, Yassouridis A, Hoogendijk WJ, Holsboer F, Swaab DF. Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci. 2001;14(10):1603–1612. doi: 10.1046/j.0953-816x.2001.01784.x. [DOI] [PubMed] [Google Scholar]
- 22.Leverenz JB, Wilkinson CW, Wamble M, Corbin S, Grabber JE, Raskind MA, Peskind ER. Effect of chronic high-dose exogenous cortisol on hippocampal neuronal number in aged nonhuman primates. J Neurosci. 1999;19(6):2356–2361. doi: 10.1523/JNEUROSCI.19-06-02356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czéh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007;257(5):250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- 24.Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 1995;15(1 pt 1):61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vollmann-Honsdorf GK, Flugge G, Fuchs E. Chronic psychosocial stress does not affect the number of pyramidal neurons in tree shrew hippocampus. Neurosci Lett. 1997;233(2–3):121–124. doi: 10.1016/s0304-3940(97)00647-2. [DOI] [PubMed] [Google Scholar]
- 26.Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97(2):253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 27.Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995;69 (1):83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- 28.Magariños AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16(10):3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965(1–2):290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- 30.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125(1):1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav Cogn Neurosci Rev. 2006;5(1):41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conrad CD, McLaughlin KJ, Harman JS, Foltz C, Wieczorek L, Lightner E, Wright RL. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. J Neurosci. 2007;27(31):8278–8285. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson RS, Bienias JL, Evans DA, Bennett DA. Religious Orders Study: overview and change in cognitive and motor speed. Aging Neuropsychol Cogn. 2004;11(2–3):280–303. doi: 10.1080/13825580490511125. [DOI] [Google Scholar]
- 35.Bennett DA. Postmortem indices linking risk factors to cognition: results from the Religious Order Study and the Memory and Aging Project. Alzheimer Dis Assoc Disord. 2006;20(3 suppl 2):S63–S68. doi: 10.1097/00002093-200607001-00009. [DOI] [PubMed] [Google Scholar]
- 36.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Education modifies the association of amyloid but not tangles with cognitive function. Neurology. 2005;65(6):953–955. doi: 10.1212/01.wnl.0000176286.17192.69. [DOI] [PubMed] [Google Scholar]
- 37.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 38.Wilson RS, Arnold SE, Beck TL, Bienias JL, Bennett DA. Change in depressive symptoms during the prodromal phase of Alzheimer disease. Arch Gen Psychiatry. 2008;65(4):439–445. doi: 10.1001/archpsyc.65.4.439. [DOI] [PubMed] [Google Scholar]
- 39.Wilson RS, Arnold SE, Schneider JA, Li Y, Bennett DA. Chronic distress, age-related neuropathology, and late-life dementia. Psychosom Med. 2007;69(1):47–53. doi: 10.1097/01.psy.0000250264.25017.21. [DOI] [PubMed] [Google Scholar]
- 40.Wilson RS, Schneider JA, Bienias JL, Arnold SE, Evans DA, Bennett DA. Depressive symptoms, clinical AD, and cortical plaques and tangles in older persons. Neurology. 2003;61(8):1102–1107. doi: 10.1212/01.wnl.0000092914.04345.97. [DOI] [PubMed] [Google Scholar]
- 41.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 42.Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D) Arch Intern Med. 1999;159(15):1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- 43.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 44.Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17(2):179–193. [PubMed] [Google Scholar]
- 45.Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287(6):742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 46.Medi-Span master drug data base. Version 2.5. Wolters-Kluwer; [Accessed January 4, 2008]. Web site. http://www.medispan.com/master-drug-database.aspx. [Google Scholar]
- 47.Shah RC, Wilson RS, Bienias JL, Arvanitakis Z, Evans DA, Bennett DA. Relation of blood pressure to risk of incident Alzheimer’s disease and change in global cognitive function in older persons. Neuroepidemiology. 2006;26(1):30–36. doi: 10.1159/000089235. [DOI] [PubMed] [Google Scholar]
- 48.Wilson RS, Gilley DW, Bennett DA, Beckett LA, Evans DA. Person-specific paths of cognitive decline in Alzheimer’s disease and their relation to age. Psychol Aging. 2000;15(1):18–28. doi: 10.1037//0882-7974.15.1.18. [DOI] [PubMed] [Google Scholar]
- 49.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64(5):834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 50.Amaral DG, Insausti R. Hippocampal formation. In: Paxinos G, editor. The Human Nervous System. San Diego, CA: Academic Press; 1990. pp. 711–755. [Google Scholar]
- 51.Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ, Hahn CG, Siegel SJ, Trojanowski JQ, Gur RE, Blake DJ, Arnold SE. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest. 2004;113(9):1353–1363. doi: 10.1172/JCI20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitchell TW, Mufson EJ, Schneider JA, Cochran EJ, Nissanov J, Han LY, Bienias JL, Lee VM, Trojanowski JQ, Bennett DA, Arnold SE. Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early Alzheimer’s disease. Ann Neurol. 2002;51(2):182–189. doi: 10.1002/ana.10086. [DOI] [PubMed] [Google Scholar]
- 53.Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex. 1991;1(1):103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 54.Fukutani Y, Kobayashi K, Nakamura I, Watanabe K, Isaki K, Cairns NJ. Neurons, intracellular and extracellular neurofibrillary tangles in subdivisions of the hippocampal cortex in normal ageing and Alzheimer’s disease. Neurosci Lett. 1995;200(1):57–60. doi: 10.1016/0304-3940(95)12083-g. [DOI] [PubMed] [Google Scholar]
- 55.Weibel ER. Stereological Methods: Practical Methods for Biological Morphometry. London, England: Academic Press; 1979. [Google Scholar]
- 56.Oorschot DE. Are you using neuronal densities, synaptic densities or neurochemical densities as your definitive data? there is a better way to go. Prog Neurobiol. 1994;44(3):233–247. doi: 10.1016/0301-0082(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 57.Celio MR. Parvalbumin in most gamma-aminobutyric acid–containing neurons of the rat cerebral cortex. Science. 1986;231(4741):995–997. doi: 10.1126/science.3945815. [DOI] [PubMed] [Google Scholar]
- 58.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61(3):378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- 59.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Berry-Kravis E, Arnold SE. Amyloid mediates the association of apolipoprotein E e4 allele to cognitive function in older people. J Neurol Neurosurg Psychiatry. 2005;76(9):1194–1199. doi: 10.1136/jnnp.2004.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell TW, Nissanov J, Han LY, Mufson EJ, Schneider JA, Cochran EJ, Bennett DA, Lee VM, Trojanowski JQ, Arnold SE. Novel method to quantify neuropil threads in brains from elders with or without cognitive impairment. J Histochem Cytochem. 2000;48(12):1627–1638. doi: 10.1177/002215540004801206. [DOI] [PubMed] [Google Scholar]
- 61.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 62.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 63.Burgess JW, Coss RG. Effects of chronic crowding stress on midbrain development: changes in dendritic spine density and morphology in jewel fish optic tectum. Dev Psychobiol. 1982;15(5):461–470. doi: 10.1002/dev.420150508. [DOI] [PubMed] [Google Scholar]
- 64.Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69(1):89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588(2):341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 66.Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531(1–2):225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- 67.Segal M. Dendritic spines and long-term plasticity. Nat Rev Neurosci. 2005;6(4):277–284. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- 68.Chen LY, Rex CS, Casale MS, Gall CM, Lynch G. Changes in synaptic morphology accompany actin signaling during LTP. J Neurosci. 2007;27(20):5363–5372. doi: 10.1523/JNEUROSCI.0164-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sandi C, Davies HA, Cordero MI, Rodriguez JJ, Popov VI, Stewart MG. Rapid reversal of stress induced loss of synapses in CA3 of rat hippocampus following water maze training. Eur J Neurosci. 2003;17(11):2447–2456. doi: 10.1046/j.1460-9568.2003.02675.x. [DOI] [PubMed] [Google Scholar]
- 71.Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110(6):1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- 72.Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639(1):167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 73.Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, Tang Y, Bennett DA. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234–240. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- 74.Kolomeets NS, Orlovskaya DD, Rachmanova VI, Uranova NA. Ultrastructural alterations in hippocampal mossy fiber synapses in schizophrenia: a postmortem morphometric study. Synapse. 2005;57(1):47–55. doi: 10.1002/syn.20153. [DOI] [PubMed] [Google Scholar]
- 75.Kolomeets NS, Orlovskaya DD, Uranova NA. Decreased numerical density of CA3 hippocampal mossy fiber synapses in schizophrenia. Synapse. 2007;61(8):615–621. doi: 10.1002/syn.20405. [DOI] [PubMed] [Google Scholar]
- 76.Law AJ, Weickert CS, Hyde TM, Kleinman JE, Harrison PJ. Reduced spinophilin but not microtubule-associated protein 2 expression in the hippocampal formation in schizophrenia and mood disorders: molecular evidence for a pathology of dendritic spines. Am J Psychiatry. 2004;161(10):1848–1855. doi: 10.1176/ajp.161.10.1848. [DOI] [PubMed] [Google Scholar]
- 77.Nowakowski C, Kaufmann WA, Adlassnig C, Maier H, Salimi K, Jellinger KA, Marksteiner J. Reduction of chromogranin B–like immunoreactivity in distinct subregions of the hippocampus from individuals with schizophrenia. Schizophr Res. 2002;58(1):43–53. doi: 10.1016/s0920-9964(01)00389-9. [DOI] [PubMed] [Google Scholar]
- 78.Benes FM, Khan Y, Vincent SL, Wickramasinghe R. Differences in the subregional and cellular distribution of GABAA receptor binding in the hippocampal formation of schizophrenic brain. Synapse. 1996;22(4):338–349. doi: 10.1002/(SICI)1098-2396(199604)22:4<338::AID-SYN5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 79.Benes FM, Todtenkopf MS, Kostoulakos P. GluR5,6,7 subunit immunoreactivity on apical pyramidal cell dendrites in hippocampus of schizophrenics and manic depressives. Hippocampus. 2001;11(5):482–491. doi: 10.1002/hipo.1065. [DOI] [PubMed] [Google Scholar]
- 80.Dean B, Scarr E, Bradbury R, Copolov D. Decreased hippocampal (CA3) NMDA receptors in schizophrenia. Synapse. 1999;32(1):67–69. doi: 10.1002/(SICI)1098-2396(199904)32:1<67::AID-SYN9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 81.Kerwin R, Patel S, Meldrum B. Quantitative autoradiographic analysis of glutamate binding sites in the hippocampal formation in normal and schizophrenic brain post mortem. Neuroscience. 1990;39(1):25–32. doi: 10.1016/0306-4522(90)90219-t. [DOI] [PubMed] [Google Scholar]
- 82.McEwen BS, Magarinos AM, Reagan LP. Structural plasticity and tianeptine: cellular and molecular targets. Eur Psychiatry. 2002;17(suppl 3):318–330. doi: 10.1016/s0924-9338(02)00650-8. [DOI] [PubMed] [Google Scholar]
- 83.Hajszan T, MacLusky NJ, Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur J Neurosci. 2005;21(5):1299–1303. doi: 10.1111/j.1460-9568.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- 84.Brake WG, Alves SE, Dunlop JC, Lee SJ, Bulloch K, Allen PB, Greengard P, McEwen BS. Novel target sites for estrogen action in the dorsal hippocampus: an examination of synaptic proteins. Endocrinology. 2001;142(3):1284–1289. doi: 10.1210/endo.142.3.8036. [DOI] [PubMed] [Google Scholar]
- 85.McLaughlin KJ, Wilson JO, Harman J, Wright RL, Wieczorek L, Gomez J, Korol DL, Conrad CD. Chronic 17β-estradiol or cholesterol prevents stress-induced hippocampal CA3 dendritic retraction in ovariectomized female rats: possible correspondence between CA1 spine properties and spatial acquisition [published online July 31, 2009] Hippocampus. 2009 doi: 10.1002/hipo.20678. [DOI] [PMC free article] [PubMed] [Google Scholar]