Abstract
In retina, an ischemic injury-resistant condition (ischemic tolerance) can be induced by a sub-lethal ischemic treatment (preconditioning) prior to an otherwise injurious ischemic insult. In this work, we compared retinal proteomic changes under three different ischemic conditions, as a means to identify the effector mechanisms that underlie retinal ischemic tolerance. Transient retinal ischemia was induced by elevating the intraocular pressure (IOP) in three groups of adult rats as follows: Group 1, ischemic-preconditioned, 110 mmHg for 8 minutes followed by 48 hours reperfusion; Group 2, ischemic-injured, 110 mmHg for 60 minutes followed by 24 hours reperfusion; Group 3, ischemic-tolerant, preconditioning treatment followed by another 60 minutes of 110 mmHg and 24 hours reperfusion. Protein quantities in retinas from each of the afore-mentioned retinal ischemic conditions, as determined by quantitative mass spectrometry, were compared with that of the contralateral control eyes (sham-treated). As a result, a total of 328 proteins were identified and quantified; among them, 30–60% of proteins showed a change in abundance under one or more retinal ischemic conditions. In particular, in ischemic-tolerant retinas, histone proteins H2B, H3 and H4 demonstrated an increase in abundance, whereas histone H2A showed a decrease in abundance. Further immunohistochemical analyses confirmed the results of proteomic analyses, and detected an up regulation of tri-methylated histone H3, mono-ubiquitinated histone H2A and Polycomb group protein RING2. Together, these results suggest a role of epigenetic regulation in the induction of retinal ischemic tolerance that involves histone and polycomb proteins.
Keywords: Neuroprotection, ischemia, epigenetics, proteomics, retina, high intraocular pressure
Introduction
Retinal and optic nerve head ischemia, a condition that can be experimentally modeled by elevating the intraocular pressure (IOP), may contribute to the onset of multiple disorders in the visual system including glaucomatous damage. Studies have shown that retinal injury caused by acute high IOP (HIOP) can be prevented by exposing the retina to a brief preconditioning ischemia or other forms of non-injurious ischemic or hypoxic insults, prior to an otherwise injurious ischemia - a condition termed ischemic tolerance [1-3] (for simplicity, HIOP conditions are referred as ischemic conditions in this work). Hence, a preconditioning ischemia in the retina produces an endogenous protection against ischemic injury. The effectors of this inducible neuroprotective mechanism in the retina are unknown. Work by Kamphuis et al. [4, 5] and Thiersch et al. [6] have shown that preconditioning ischemia in the retina results in increased expression of genes involved in amino acid transport, transcription regulation, antioxidative pathways and cell death regulation. In none of these studies, however, was the ischemic-tolerant retina, the condition in which the effectors of tolerance are at play, included.
In a recent study on ischemic-tolerant rodent brains, we have found that a group of epigenetic regulator proteins including several histone and Polycomb group (PcG) proteins are up regulated, and an alteration in the PcG protein level has a profound impact on the outcome of ischemic stroke [7]. PcG proteins are epigenetic gene repressor proteins; they exert their roles in epigenetic regulation by modifying histone proteins. Accordingly, in brain, a PcG protein-mediated epigenetic mechanism that underlies precondition ing-induced neuroprotection against ischemic brain injury has been elucidated [7]. As the first step in understanding the molecular mechanisms that underlie retinal ischemic tolerance, we conducted an unbiased, quantitative proteomic study on rat retinas under different ischemic conditions including ischemic-tolerant retinas. The proteomic results revealed differential and condition-specific changes of histone proteins, including changes that are either similar to or different from those found in brain. Results of follow-up immunohis-tochemical analyses demonstrated increased abundance of PcG protein RING2 in the ischemic-tolerant retina. Thus an involvement of histone and PcG proteins in the induction of ischemic tolerance in retina is implicated by the results of this study.
Matherials and methods
Retinal ischemia in rats
All animals were treated in accordance with the National Institutes of Health Guide for the use of animals in research, and all protocols were approved by the local Institutional Animal Care and Use Committee. Adult Sprague-Dawley rats (250 g–300 g) were purchased from Charles River Laboratories (Wilmington, MA). The animals were housed in a temperature- and humidity-controlled room with a 12-hour light: 12-hour dark cycle and provided with food and water ad libitum.
Retinal ischemia was induced by transiently and manometrically increasing the IOP. Briefly, rats were anesthetized with ketamine/xylazine (55/5 mg/kg). A HIOP condition was achieved by inserting a 30-gauge needle into the anterior chamber. The needle was connected to a saline -filled reservoir, which was positioned at a corresponding height above the eye to achieve a sustained IOP of 110 mmHg. Three groups of animals (n=4 each) were subjected to different durations and levels of HIOP and reperfusion as follows: (1) preconditioning - 8 minutes IOP at 110 mm Hg, 48 hours reperfusion; (2) injurious - 60 minutes IOP at 110 mm Hg, 24 hours reperfusion; (3) tolerant - 8 minutes IOP at 110 mm Hg, 48 hours reperfusion, followed by another 60 minutes of IOP at 110 mm Hgand 24 hours reperfusion. All contralateral eyes were treated as sham controls by setting the IOP at 20 mmHg for corresponding durations. At the termination of reperfusions, animals were anesthetized with ketamine/xylazine and euthanized by an intracardiac injection of Euthasol® (pentobarbital, 100 mg/kg). The eyes were enucleated, and the entire retinas were collected and kept at -80°C until further analyses.
Protein extraction and tryptic digestion
Retinal specimen were thawed on ice, boiled directly into 250 μL pre-heated ddH2O for 10 minutes, chilled on ice, and then homogenized with a hand-held homogenizer. After homogenization, an additional 250 μL of pre-chilled ddH2O was added, and samples were centri-fuged at 16,000 g for 30 minutes at 4°C. Protein concentrations in the cleared supernatants were determined by Bradford Assay. Next, for each IOP treatment group, samples from 4 animals were pooled. Twenty micrograms of protein from each pool were lyophilized and resuspended with 100 mM ammonium bicarbonate, pH 8.0, to a final protein concentration of 1 μg/ μL. Proteins were denatured by incubation at 95°C for 10 minutes in the presence of 0.05% RapiGest SF (Waters, Milford, MA) followed by incubation at 60°C for 30 minutes with 20 mM dithiothreitol, and a final incubation with 20 mM iodoacetamide for 10 minutes in the dark at the room temperature. The proteins were then digested with sequencing grade trypsin (2.25×106 unit/μL; Promega, Madison, WI) at 37°C overnight. The RapiGest SF in the digestion mixture was precipitated by the addition of trifluoroacetic acid to pH 2-2.5 and incubation at 37°C for 10 minutes.
Mass spectrometry (MS) and bioinformatic analyses
The tryptic digests from each pooled sample were analyzed by a non-labeling, quantitative MS method, as previously described [7], with 3 technical replications. Briefly, the MS system consisted of a nanoflow ultra performance liquid chromatography (UPLC) machine coupled inline to a Micromass Global Ultima Quadrupole-Time-of-light mass spectrometer (Waters). The UPLC included a 20 cm × 75 μm bridged-ethyl hybrid C18 (1.7 μm) analytical column. The separation of tryptic peptides by UPLC and the subsequent dual-energy MS identification and quantification of detected peptides, as managed by ProteinLynx Global Server version 2.3 (Waters) and with the use of a custom database of annotated, non-redundant rat proteins from The Universal Protein Resource (Unitprot, www.UniProt.org) were performed as previously described [7]. The fmol amounts for each identified protein in a sample were determined by comparison to an internal standard. For each sample (treatment group), protein quantities determined in each individual MS run were normalized using the total fmol numbers for each run. Proteins that were found in at least two of the three runs for each pool of retina sample were accepted as valid entries. For each accepted protein, a fmol ratio was established between the ischemic eye and the contra lateral control eye, and a ratio of > 1.5 (increased) or < -1.5 (decreased) was defined as a change in ischemic eyes.
For all regulated proteins, their known Gene Ontology (GO) terms were retrieved with the assistance of the batch retrieval tool provided by UniProt. An enriched presence of these proteins in particular biological processes, metabolic and signaling pathways were analyzed with the assistance of the MetaCore program (GeneGo, Inc. West Lothian, UK).
Antibodies
The following primary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA): histone H2A - rabbit polyclonal; his-tone H3 - rabbit polyclonal; histone H4 - mouse monoclonal; RING2 (a.k.a Ring1B) - mouse monoclonal. Monoclonal antibody against mono -ubiquitinated histone H2Awas purchased from Millipore (Billerica, MA), and monoclonal antibody against tri-methylated histone H3 at lysine 27 was from Abcam (Cambridge, MA). Fluo-rescein isothiocyanate (FITC)- or Cy3-conjugated secondary antibodies were from Jackson Immu-noResearch (West Grove, PA).
Fluorojade-B staining and fluorescent immuno-histochemistry (IHC)
Whole eye globes from rats that underwent the HIOP and reperfusion procedures described earlier were removed immediately after euthani-zation, fixed in 4% paraformaldehyde in phosphate balanced saline (PBS) for 24 hours, and then frozen in 2-methylbutane. Sections at 12-μm thickness were prepared. Fluorojade-B staining was performed following standard protocols to reveal injury [8]. For immunohistochemical analyses, retinal sections were incubated with appropriate primary antibodies (dilutions are specified in figure legends) at 4°C overnight. The next day, the sections were washed three times with PBS, incubated for 1 hour with an appropriate secondary antibody, washed with ddH2O, dried and mounted with a 4',6-diamidino-2-phenylindole (DAPI)-containing mounting media to counterstain nuclei. The fluorescent images were examined and documented with an epifluorescence microscope (Leica Microsystems, Inc. Bannockburn, IL) attached to a Magnifire digital color camera (ChipCoolers, Warwick, RI), with the assistance of the BIOQUANT program (Bioquant Image Analysis, Nashville, TN).
Results and discussion
Modeling retinal ischemic tolerance in rats
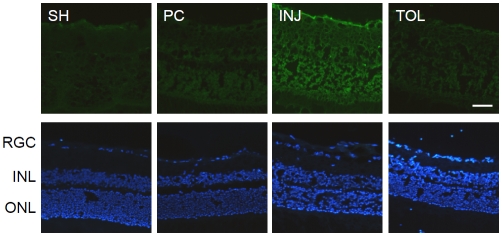
Preconditioning treatment with a brief or mild insult has been shown to have a protective effect against a subsequent, more severe insult in both the brain and in the retina. In retina, a decrease in ischemia-induced injury of multiple retinal cell layers has been reported for chemical-, HIOP (ischemic)- or hypoxic-preconditioned conditions [1-5, 9-15]. For ischemic preconditioning in rats, 130 -170 mmHg IOPs have been used to produce tolerance in published studies [4, 5, 16] . Though effective in producing retinal insults, such IOP levels are very high relative to what is observed under various pathophysiologi-cal conditions in human eyes. We attempted to apply an IOP level that is lower than 130 mmHg, but would still produce detectable retinal injuries by neuroanatomical means and at a relatively early time point, and would still be effective at inducing ischemic tolerance within a controlled period of time. Figure 1 and Table 1 present the experimental paradigm and HIOP conditions used in the present work. As demonstrated by the results of fluorojade-B staining shown in Figure 2, a 60-minute 110 mmHg HIOP produced injuries across multiple cell layers in the retina, when examined 24 hours after the HIOP treatment, and such injuries were greatly reduced in the eyes treated with a preconditioning HIOP (8 minutes of 110 mmHg at 48 hours prior to the injurious 110 mmHg). Therefore, these results verified the establishment of a HIOP (ischemic)-tolerant paradigm in rats using 110 mmHg. The exact retinal cell types that were protected in this experimental paradigm remain to be further defined with detailed IHC analyses for appropriate cell markers. It is apparent that the retinal ganglion cell (RGC) layer and the outer nuclear layer (ONL) are protected .
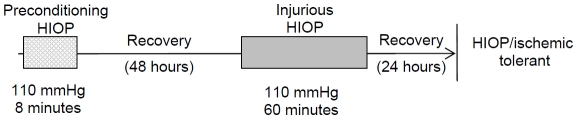
Figure 1.
Experimental paradigm of HIOP treatments. Ipsilateral eyes of rats were subjected to the following HIOP conditions with reperfusions: Preconditioning HIOP -110 mmHg for 8 minutes followed by 24 hours reperfusion; Injurious HIOP - 110 mmHg for 60 minutes followed by 24 hours reperfusion; For the tolerant condition: - 8 minutes preconditioning HIOP with 48 hours of reperfusion, followed by another 60 minutes of HIOP and 24 hours reperfusion. Retina samples were collected at the end of reperfusions (for the tolerant conditions, at the end of the second reperfusion). In each group, the IOP of contralateral eyes was sustained at 20 mmHgforthe same duration as that of the HIOP treatment of the ipsilateral eyes followed by the same duration of reperfusions.
Table 1.
Retinal HIOP conditions (IOP (mmHg)/duration (minutes))
| Conditions | n | Day 1 | Day 2 | Day 3 | Day 4 |
|---|---|---|---|---|---|
| Preconditioned | 4 | 110/8 | Harvest | ||
| Contralateral to Preconditioned | 4 | 20/8 | Harvest | ||
| Injured | 4 | 110/60 | Harvest | ||
| Contralateral to Injured | 4 | 20/60 | Harvest | ||
| Tolerant | 4 | 110/8 | 110/60 | Harvest | |
| Contralateral to Tolerant | 4 | 20/8 | 20/60 | Harvest |
Figure 2.
Examination of HIOP-induced retinal injury by fluorojade B staining. Top: fluorojade B staining of retina sections. Bottom: DAPI staining of consecutive retinal sections to reveal nuclei. The scale bar represents 50 μm. SH: sham-treated (contralateral to HIOP-treated); PC: HIOP-preconditioned; INJ: HIOP-injured; TOL: HIOP-tolerant; RGC: retina ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer. Images in this figure and figures below are representative results of analyses of at least three animals under each HIOP treatment condition, and at least two sections from each eye.
Next, we proceeded to analyzing proteomes of ischemic-preconditioned, ischemic-injured and ischemic-tolerant retinas, as a means to identify potential effector proteins of retinal ischemic tolerance.
Retinal proteomic changes under different ischemic conditions
Table 2 provides the numbers of proteins that were identified and quantified in each group of eyes, and the numbers of proteins that were increased or decreased in abundance by at least 1.5 fold in ischemia-treated eyes when compared to that in their contralateral control eyes. A complete list of the proteins that were detected and quantified in each sample is provided in Supplemental Table 1.
Table 2.
Numbers of identified and quantified retinal proteins under each HIOP condition
| Preconditioned | Injured | Tolerant | |
|---|---|---|---|
| HIOP-treated | 176 | 181 | 165 |
| Contralateral control | 200 | 168 | 161 |
| Total for both* | 239 | 240 | 210 |
| > 1.5 fold change in abundance** | |||
| Increased | 83 (47.2%) | 92 (50.8%) | 99 (60.0%) |
| Decreased | 80 (40.0%) | 52 (31.0%) | 74 (46.0%) |
The numbers include proteins that were detected either in both eyes or only in one eye.
Proteins that were detected only in the HIOP-treated eyes are considered increased in abundance relevant to contralateral control eyes; likewise, proteins that were detected only in the control eyes are considered decreased in the HIOP-treated eyes.
Using a simple, one-step protein extraction protocol in this study, a total of 328 retinal proteins were identified and quantified; this number includes proteins that were detected only in one or few groups of eyes but not in others. While this number is far less than the predicted numbers of translated proteins in eukaryotic cells, it is comparable to the numbers reported in a limited number of published proteomic studies on retinas of different species, in which similar or even more comprehensive pre-MS preparation steps were involved [17-25]. Obviously, these proteins cannot possibly provide us a thorough description of the retinal proteome, which can only be achieved through more comprehensive proteomic studies in the future by employing additional protein enrichment protocols and MS procedures. Rather, the present proteomic data, as described below, provide an initial view of the most readily detectable proteomic changes in rat retinas under different ischemic conditions.
First, in the present study, relatively high percentages of retinal proteins showed a change in abundance in ischemia-treated eyes (Table 2). In the literature for retina and other tissues, the extent of ischemia-induced changes or changes induced by other forms of insults in gene transcripts or proteins, as determined by high throughput approaches such as cDNA microar-rays and quantitative MS, respectively, varies greatly, from just a few to several tens of percentages [7, 26-31]. Besides variations in ischemic models and post-ischemia time points at which samples are harvested, the choice of controls also differs (for example, contra lateral tissue of the same animal versus ipsilateral tissue of a different animal) [27]. Another important but often overlooked issue in high throughput proteomic studies is how to report proteins detected only in one or more but not all conditions, since no ratio numbers could be established for these proteins. The protein lists that we report in Table 2 include such proteins. This may explain, at least partially, the relatively high percentages of regulated proteins that we report here.
Table 3 reports the most significant biological processes (by GO terms) that are associated with up-regulated retinal proteins under different retinal ischemic conditions. In both ischemic -injured and ischemic-tolerant retinas, the most significantly up-regulated biological processes are those of glucose or hexose metabolism, with little difference between the two conditions, whereas in ischemic-preconditioned retinas, the top ten up-regulated biological processes are those of macromolecule and organelle organization. A possible increase in glucose and carbohydrate metabolism processes in ischemic-tolerant retinas, as noted above, is somehow a surprising result that is different from what is observed in ischemic tolerant brains, in which decreased energy metabolism processes has been suggested [7, 32]. At this time, the exact metabolic condition in the ischemic-tolerant retina is unknown. It is an important issue to be addressed in future studies using both biochemical and physiological approaches, and at different reperfusion time points following retinal ischemia.
Table 3.
Biological processes associated with up-regulated proteins*
| Preconditioned | Injured | Tolerant | |
|---|---|---|---|
| Up-regulated | |||
| 1 | organelle organization | glycolysis | glycolysis |
| 2 | cellular macromolecular complex assembly | glucose catabolic process | glucose catabolic process |
| 3 | cellular component organization | hexose catabolic process | hexose catabolic process |
| 4 | cellular macromolecular complex subunit organization | monosaccharide catabolic process | monosaccharide catabolic process |
| 5 | cellular component assembly | cellular carbohydrate catabolic process | cellular carbohydrate catabolic process |
| 6 | cellular component biogenesis | alcohol catabolic process | alcohol catabolic process |
| 7 | anatomical structure formation | carbohydrate catabolic process | carbohydrate catabolic process |
| 8 | macromolecular complex assembly | glucose metabolic process | glucose metabolic process |
| 9 | macromolecular complex subunit organization | generation of precursor metabolites and energy | hexose metabolic process |
| 10 | nucleosome assembly | hexose metabolic process | monosaccharide metabolic process |
| Up-regulated only under specific conditions | |||
| 1 | muscle thin filament assembly | gluconeogenesis | anti-apoptosis |
| 2 | skeletal myofibril assembly | hexose biosynthetic process | respiratory burst during acute inflammatory response |
| 3 | cardiac myofibril assembly | response to misfolded protein | regulation of protein folding in endoplasmic reticulum |
| 4 | protein polymerization | monosaccharide biosynthetic process | production of molecular mediator of acute inflammatory response |
| 5 | cardiac cell development | glycolysis | negative regulation of apoptosis |
| 6 | cardiac muscle cell development | pyruvate metabolic process | negative regulation of programmed cell death |
| 7 | cytoskeleton organization | acute inflammatory response | negative regulation of cell death |
| 8 | cellular component assembly | alcohol biosynthetic process | oxygen transport |
| 9 | microtubule-based process | glucose catabolic process | regulation of apoptosis |
| 10 | cellular component organization | hexose catabolic process | gas transport |
All listed processes were significantly regulated with p values ≤ 0.01, as a result of bioinformatic analyses of up-regulated proteins with the MetaCore program. Please see Table S2 for down-regulated biological processes.
When proteins that were uniquely up-regulated under each of the three retinal ischemic conditions (that is, after excluding proteins that also changed in other conditions) were analyzed for their bioinformatics, an up regulation of anti-cell death processes was recognized in the ischemic -tolerant retina (Table 3). In light of our recent description of a gene repressor protein-mediated mechanism for ischemic tolerance in brain [7], and to consider the mechanism(s) that underlie the increased anti-cell death processes in ischemic-tolerant retinas, we paid attention to changes of histone proteins that were detected in our present proteomic datasets. We found an increase of variants of histone proteins H1, H2B, H3 and H4, and a decrease of histone H2A in the ischemic-tolerant retina (Table 4). The abundance of a post-translationally modified form of histone H2A (an epigenetic mark), however, as demonstrated next by results of immunohistochemical analyses, showed an increase in the ischemic-tolerant retina.
Table 4.
HIOP condition-specific changes in the abundance of select nuclear proteins
| Protein Name | Gene Name | Preconditioned | Injured | Tolerant |
|---|---|---|---|---|
| COP9 signalosome complex subunit 2 | Cops2 | ND | ND | Up(1) |
| Heterogeneous nuclear ribonucleoprotein D0 | Hnrnpd | Up(1) | UC(1) | UC(1) |
| Histone H1 | Hist1h1t/1c | Up (2) | UC(2) | UC(1), Up (1) |
| Histone H2A | H2afz, H2afj | Down (8) | UC(1), Up (7) | Down (8) |
| Histone H2B | Hist1h2ba | UC (1), Up (1) | UC(2) | Up (2) |
| Histone H3 | H3f3b | Up (2) | UC(2) | Up (2) |
| Histone H4 | Hist1h4b | UC(1) | Up(1) | Up(1) |
| Host cell factor 2 | Hcfc2 | Up(1) | ND | ND |
| Methyl CpG binding protein 2 | Mecp2 | UC(1) | ND | ND |
| Non-histone chromosomal protein HMG-17 | Hmgn2 | Up(1) | ND | ND |
| Nuclear protein Hcc-1 | otei | UC(1) | Up(1) | Down (1) |
| Nucleosome assembly protein 1-like 4 | Nap1l4 | Down (1) | Up(1) | ND |
The numbers in the parentheses designate the numbers of isoforms that were detected under each HIOP condition. UC: unchanged; ND: not detected.
Little is known about how expression levels and modifications of histone proteins are regulated in ischemic retinas. Recently, Crosson et al have reported that inhibition of histone deacety-lase (HDAC) protects retinas from ischemia (HIOP)-induced injury in rats [33], whereas work by Chen and Cepko shows that, in mice, HDAC4 activity is beneficial in retinal neuronal survival with the involvement of hypoxia-inducible factor 1a [34]. Histones H2A, H2B, H3 and H4 are all subject to acetylation. While the results of these studies do not include direct analyses of histone protein levels, they support a critical role of his-tones in retinal disorders through an epigenetic mechanism. Our current proteomic finding - the increased levels of several histone proteins in the ischemic-tolerant retina, points in the direction that a preconditioning ischemia in retina may induce an endogenous neuroprotective mechanism that includes an epigenetic component.
Enriched presence of epigenetic gene repressor proteins in ischemic-tolerant retinas
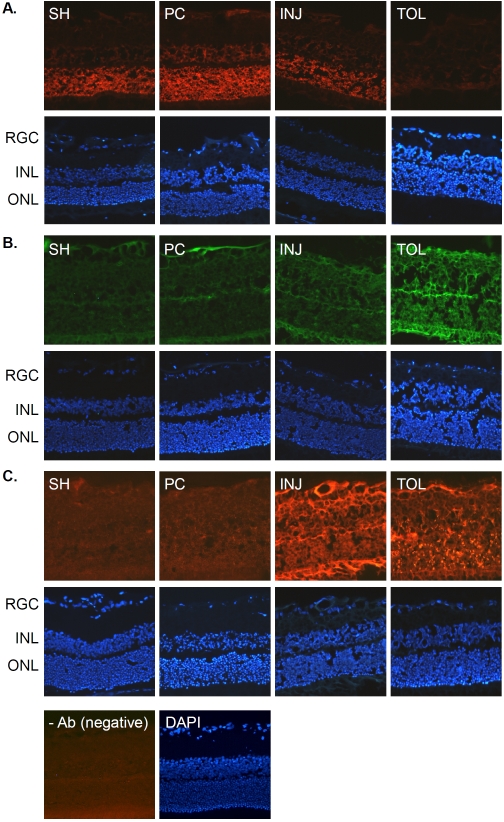
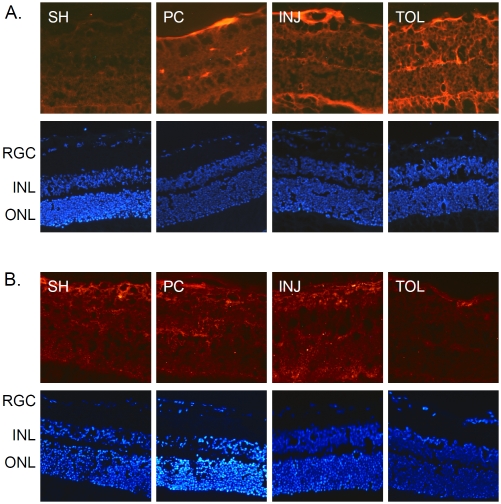
The results of the above-introduced proteomic characterization of ischemic-tolerant retinas prompted us to further examine changes of selected histone proteins and PcG proteins under different ischemic conditions by IHC, as a means to determine whether or not an epigenetic mechanism that is revealed in our recent studies on ischemic-tolerant brains [7] may also be at play in retinal ischemic tolerance Figure 3 shows the changes in immunoreactivity for histone proteins H2A, H3 and H4; the results were essentially in agreement with the results of MS analyses (Table 4), hence validating our proteomic results. Specifically, in ischemic -tolerant retinas, the immunoreactivity of histone H2A was greatly diminished, whereas the immunoreactivity of histone H3 and H4 was robustly up regulated.
Figure 3.
Histone protein immunohistochemistry. Retinal sections prepared from the same eyes described in Figure 2 were analyzed by immunohistochemical staining for his-tones H2A (A, rabbit polyclonal antibody), H3 (B, rabbit polyclonal antibody) and H4 (C, mouse monoclonal antibody) (all antibodies were used at 1:50 dilution). Abbreviations are the same as those indicated in Figure 2.
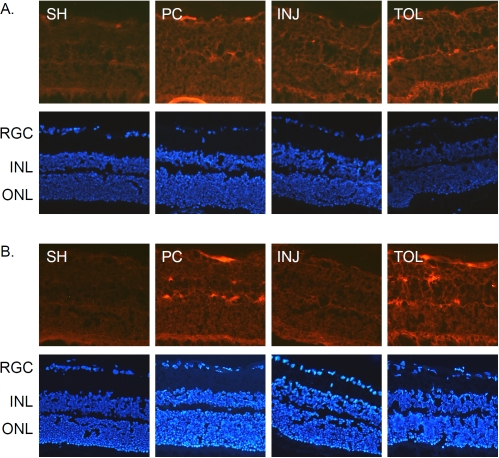
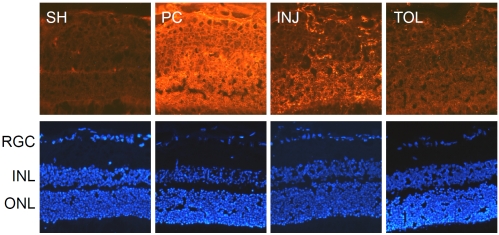
As introduced earlier, histone H2A and H3 are subject to mono-ubiquitination (at lysine 119) and tri-methylation (at lysine 27), respectively, by the action of PcG proteins. A concerted H2A mono-ubiquitination and H3 methylation are critical for epigenetic transcriptional suppression (for review, see Bantignies and Cavalli, 2006 [35]). While a decreased level of H2A seen in the ischemic-tolerant retina, as revealed by IHC using an antibody that does not distinguish modified forms of H2A, does not seem to support a role of neuroprotection against ischemic retinal injury for H2A, it is possible that there may be an increase in the abundance of mono-ubiquitinated H2A, especially if in the ischemic-tolerant retina there is an increase of PcG proteins. Indeed, results of IHC analyses for mono-ubiquitinated H2A demonstrate an increase of its abundance in the tolerant retina (Figure 4A). The mechanism that underlies an overall decrease in the level of histone H2A in the ischemic-tolerant retina is unknown. One possible explanation is that there is an increased rate of conversion of H2A to its mono-ubiquitinated form and/or a change in H2A metabolic rate. This will be an issue to be addressed in future studies. Interestingly, the abundance of tri-methylated H3 was also increased in the ischemic-tolerant retina (Figure 4B). In other words, in ischemic-tolerant retinas, the abundance of both mono-ubiquitinated H2A and tri-methylated H3 was increased. This suggests a possible involvement of PcG proteins in the induction of the retinal ischemic-tolerance. Figure 5 presents the results of IHC analyses for PcG proteins RING2 (a.k.a. Ring1B), an E3 li-gase that mediates H2A mono-ubiquitination, and EZH1, a PcG protein mediating tri-methylation for histone H3 at lysine 27. RING2 has been shown to play a role in retinal development [36]. Similar to the results of our previous studies on ischemic-tolerant brains, the immu-noreactivity of RING2 was increased in the ischemic-tolerant retina (Figure 5A). Unlike the changes in tri-methylated H3, the abundance of EZH1 did not show an increase in the ischemic-tolerant retina. Instead, an overall decrease across the retina was observed (Figure 5B). This observation does not seem to correspond to the increase of tri-methylated H3 in the ischemic-tolerant retina. In literature, elevated levels of EZH1 has been reported for cancer cells, and an anti-apoptotic role has been proposed for it [37]. It remains to be examined whether or not there may be a change in EZH1 biosynthesis or metabolism. Ischemia-induced changes in retinal levels of EZH1 and tri-methylated H3 may also exhibit different temporal orders.
Figure 4.
Immunohistochemical analyses of modified histone H2A and H3 proteins. The analyses were performed using mouse monoclonal antibodies against mono-uniquitinated H2A (A, 1:100) and tri-methylated H3 at lysine 27 (B, 1:50), respectively, and with appropriate Cy3-conjugated secondary antibodies (1:760).
Figure 5.
Immunohistochemical analyses of PcG proteins. The analyses were performed using a mouse monoclonal antibody against RING2 (A, 1:100) or a rabbit polyclonal antibody against EZH1 (B, 1:500) and with appropriate Cy3-conjugated secondary antibodies, respectively, at 1:760.
A histone and PcG protein-mediated mechanism in retinal ischemic tolerance was further implicated by an up regulation of COPS9 sig-nalosome complex subunit 2 (CSN2, a.k.a. TRIP15 in humans and Alien in drosophilia) that was detected in ischemic-tolerant (Table 4) and ischemic-preconditioned retinas (Figure 6) in the present study. Recently, CSN2 has been shown to interact with E3 ubiquitin ligases, and Alien was shown to be a chromatin-associated protein that binds specifically to histones H3 and H4 and participates in gene repression [38, 39], although it is not known at this time whether or not CSN2/TRIP15 may interact with PcG proteins directly.
Figure 6.
Immunohistochemical analyses of CSN2/TRIP15. The analyses were performed on retinal sections prepared from the same eyes used in Figures. 2-5. A goat polyclonal antibody against CSN2/TRIP15 was used (1:50).
Taken together, the results of the present study revealed ischemic condition-specific changes of the retinal proteome, with marked increase in anti-cell death processes and the abundance of several histone proteins and a PcG protein in the ischemic-tolerant retina. Future studies are needed to establish the retinal cell populations in which histone and PcG proteins are endoge-nously expressed and regulated by ischemic stress. A possible essential role of these gene repressor proteins in the retinal neuroprotection against ischemic insults remains to be demonstrated by approaches such as gene knockdown or over-expression.
Acknowledgments
The authors thank Dr. Roger Simon for critical reading of the manuscript and helpful discussions, and Chelsea Piper for general laboratory assistance. The study was supported by grants from National Eye Institute (1R21EY017345, A.Z.) and the Glaucoma Research Foundation (The Shaffer Fund Grant SG-10[Zhou], A.Z.). For this work, Cheri Stowell was partially supported by the Discoveries In Sight Laboratories of Legacy Research. None of the material has been published or is under consideration elsewhere, including the internet.
Glossary
Abbreviations:
- IOP
intraocular pressure
- HIOP
high IOP
- PcG
Polycomb group
- MS
mass spectrometry
- UPLC
ultra performance liquid chromatography
- GO
gene ontology
- FITC
fluorescein isothiocyanate;
- IHC
immunohistochemistry
- PBS
phosphate balance saline
- DAPI
4',6-diamidino-2-phenylindole
- HDAC
histone deacetylase
- CSN2
COPS9 signalosome complex subunit
- RGC
retina ganglion cells
- INL
inner nuclear layer
- ONL
outer nuclear layer
References
- 1.Roth S, Li B, Rosenbaum PS, Gupta H, Goldstein IM, Maxwell KM, Gidday JM. Preconditioning provides complete protection against retinal ischemic injury in rats. Invest Ophthalmol Vis Sci. 1998;39(5):777–85. [PubMed] [Google Scholar]
- 2.Zhang C, Rosenbaum DM, Shaikh AR, Li Q, Rosenbaum PS, Pelham DJ, Roth S. Ischemic preconditioning attenuates apoptotic cell death in the rat retina. Invest Ophthalmol Vis Sci. 2002;43(9):3059–66. [PubMed] [Google Scholar]
- 3.Zhu Y, Zhang Y, Ojwang BA, Brantley MA, Gidday JM., Jr Long-term tolerance to retinal ischemia by repetitive hypoxic preconditioning: role of HIF-1alpha and heme oxygenase-1. Invest Ophthalmol Vis Sci. 2007;48(4):1735–43. doi: 10.1167/iovs.06-1037. [DOI] [PubMed] [Google Scholar]
- 4.Kamphuis W, Dijk F, Bergen AA. Ischemic preconditioning alters the pattern of gene expression changes in response to full retinal ischemia. Mol Vis. 2007;13:1892–901. [PubMed] [Google Scholar]
- 5.Kamphuis W, Dijk F, van Soest S, Bergen AA. Global gene expression profiling of ischemic preconditioning in the rat retina. Mol Vis. 2007;13:1020–30. [PMC free article] [PubMed] [Google Scholar]
- 6.Thiersch M, Raffelsberger W, Frigg R, Samardzija M, Wenzel A, Poch O, Grimm C. Analysis of the retinal gene expression profile after hypoxic preconditioning identifies candidate genes for neuroprotection. BMC Genomics. 2008;9:73. doi: 10.1186/1471-2164-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stapels M, Piper C, Yang T, Li M, Stowell C, Xiong ZG, Saugstad J, Simon RP, Geromanos S, Langridge J, Lan JQ, Zhou A. Polycomb group proteins as epigenetic mediators of neuroprotection in ischemic tolerance. Sci Signal. 3(111):ra15. doi: 10.1126/scisignal.2000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmued LC, Hopkins KJ. Fluoro-Jade: novel fluorochromes for detecting toxicant-induced neuronal degeneration. Toxicol Pathol. 2000:28. doi: 10.1177/019262330002800111. [DOI] [PubMed] [Google Scholar]
- 9.Lin J, Roth S. Ischemic preconditioning attenuates hypoperfusion after retinal ischemia in rats. Invest Ophthalmol Vis Sci. 1999;40(12):2925–31. [PubMed] [Google Scholar]
- 10.Casson RJ, Wood JP, Melena J, Chidlow G, Osborne NN. The effect of ischemic preconditioning on light-induced photoreceptor injury. Invest Ophthalmol Vis Sci. 2003;44(3):1348–54. doi: 10.1167/iovs.02-0368. [DOI] [PubMed] [Google Scholar]
- 11.Ozbay D, Ozden S, Muftuoglu S, Kaymaz F, Yaylali V, Yildirim C, Tatlipinar S. Protective effect of ischemic preconditioning on retinal ischemia-reperfusion injury in rats. Can J Ophthalmol. 2004;39(7):727–32. [PubMed] [Google Scholar]
- 12.Roth S. Endogenous neuroprotection in the retina. Brain Res Bull. 2004;62(6):461–6. doi: 10.1016/j.brainresbull.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Franco PJ, Fernandez DC, Sande PH, Keller Sarmiento MI, Chianelli M, Saenz DA, Rosenstein RE. Effect of bacterial lipopolysaccharide on ischemic damage in the rat retina. Invest Ophthalmol Vis Sci. 2008;49(10):4604–12. doi: 10.1167/iovs.08-2054. [DOI] [PubMed] [Google Scholar]
- 14.Sharma RK, Netland PA, Kedrov MA, Johnson DA. Preconditioning protects the retinal pigment epithelium cells from oxidative stress-induced cell death. Acta Ophthalmol. 2009;87(1):82–8. doi: 10.1111/j.1755-3768.2008.01170.x. [DOI] [PubMed] [Google Scholar]
- 15.Thiersch M, Lange C, Joly S, Heynen S, Le YZ, Samardzija M, Grimm C. Retinal neuroprotection by hypoxic preconditioning is independent of hypoxia-inducible factor-1 alpha expression in photo receptors. Eur J Neurosci. 2009;29(12):2291–302. doi: 10.1111/j.1460-9568.2009.06781.x. [DOI] [PubMed] [Google Scholar]
- 16.Ettaiche M, Heurteaux C, Blondeau N, Borsotto M, Tinel N, Lazdunski M. ATP-sensitive potassium channels (K(ATP)) in retina: a key role for delayed ischemic tolerance. Brain Res. 2001;890(1):118–29. doi: 10.1016/s0006-8993(00)03152-8. [DOI] [PubMed] [Google Scholar]
- 17.Barnhill AE, Hecker LA, Kohutyuk O, Buss JE, Honavar VG, Greenlee HW. Characterization of the retinal proteome during rod photoreceptor genesis. BMC Res Notes. 3:25. doi: 10.1186/1756-0500-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hajkova D, Imanishi Y, Palamalai V, Rao KC, Yuan C, Sheng Q, Tang H, Zeng R, Darrow RM, Organisciak DT, Miyagi M. Proteomic changes in the photoreceptor outer segment upon intense light exposure. J Proteome Res. 9(2):1173–81. doi: 10.1021/pr900819k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Decanini A, Karunadharma PR, Nordgaard CL, Feng X, Olsen TW, Ferrington DA. Human retinal pigment epithelium proteome changes in early diabetes. Diabetologia. 2008;51(6):1051–61. doi: 10.1007/s00125-008-0991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finnegan S, Robson JL, Wylie M, Healy A, Stitt AW, Curry WJ. Protein expression profiling during chick retinal maturation: a proteomics-based approach. Proteome Sci. 2008;6:34. doi: 10.1186/1477-5956-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao BB, Phipps JA, Bursell D, Clermont AC, Feener EP. Angiotensin AT1 receptor antagonism ameliorates murine retinal proteome changes induced by diabetes. J Proteome Res. 2009;8(12):5541–9. doi: 10.1021/pr9006415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasaki T, Watanabe W, Muranishi Y, Kanamoto T, Aihara M, Miyazaki K, Tamura H, Saeki T, Oda H, Souchelnytskyi N, Souchelnytskyi S, Aoyama H, Honda Z, Furukawa T, Mishima HK, Kiuchi Y, Honda H. Elevated intraocular pressure, optic nerve atrophy, and impaired retinal development in ODAG transgenic mice. Invest Ophthalmol Vis Sci. 2009;50(1):242–8. doi: 10.1167/iovs.08-2206. [DOI] [PubMed] [Google Scholar]
- 23.Ahn J, Piri N, Caprioli J, Munemasa Y, Kim SH, Kwong JM. Expression of heat shock transcription factors and heat shock protein 72 in rat retina after intravitreal injection of low dose N-methyl-D-aspartate. Neurosci Lett. 2008;433(1):11–6. doi: 10.1016/j.neulet.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 24.Kwok MC, Holopainen JM, Molday LL, Foster U, Molday RS. Proteomics of photoreceptor outer segments identifies a subset of SNARE and Rab proteins implicated in membrane vesicle trafficking and fusion. Mol Cell Proteomics. 2008;7(6):1053–66. doi: 10.1074/mcp.M700571-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sloley S, Smith S, Algeciras M, Cavett V, Busby JA, London S, Clayton DF, Bhattacharya SK. Proteomic analyses of songbird (Zebra finch; Taeniopygia guttata) retina. J Proteome Res. 2007;6(3):1093–100. doi: 10.1021/pr060428i. [DOI] [PubMed] [Google Scholar]
- 26.Dhodda VK, Sailor KA, Bowen KK, Vemuganti R. Putative endogenous mediators of precondition-ing-induced ischemic tolerance in rat brain identified by genomic and proteomic analysis. J Neu-rochem. 2004;89(1):73–89. doi: 10.1111/j.1471-4159.2004.02316.x. [DOI] [PubMed] [Google Scholar]
- 27.Focking M, Besselmann M, Trapp T. Proteomics of experimental stroke in mice. Acta Neurobiol Exp (Wars) 2006;66(4):273–8. doi: 10.55782/ane-2006-1616. [DOI] [PubMed] [Google Scholar]
- 28.Miyahara T, Kikuchi T, Akimoto M, Kurokawa T, Shibuki H, Yoshimura N. Gene microarray analysis of experimental glaucomatous retina from cynomologous monkey. Invest Ophthalmol Vis Sci. 2003;44(10):4347–56. doi: 10.1167/iovs.02-1032. [DOI] [PubMed] [Google Scholar]
- 29.Miyara N, Shinzato M, Yamashiro Y, Iwamatsu A, Kariya K, Sawaguchi S. Proteomic analysis of rat retina in a steroid-induced ocular hypertension model: potential vulnerability to oxidative stress. Jpn J Ophthalmol. 2008;52(2):84–90. doi: 10.1007/s10384-007-0507-5. [DOI] [PubMed] [Google Scholar]
- 30.Tezel G, Yang X, Cai J. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Invest Ophthalmol Vis Sci. 2005;46(9):3177–87. doi: 10.1167/iovs.05-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vazquez-Chona F, Song BK, Geisert EE., Jr Temporal changes in gene expression after injury in the rat retina. Invest Ophthalmol Vis Sci. 2004;45(8):2737–46. doi: 10.1167/iovs.03-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, Meller R, Rosenzweig HL, Tobar E, Shaw TE, Chu X, Simon RP. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotec-tive strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362(9389):1028–37. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- 33.Crosson CE, Mani SK, Husain S, Alsarraf O, Menick DR. Inhibition of Histone Deacetylase Protects the Retina from Ischemic Injury. Invest Ophthalmol Vis Sci. doi: 10.1167/iovs.09-4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng PH, Huang HS, Lee YJ, Chen YS, Ma MC. Novel role for the delta-opioid receptor in hypoxic preconditioning in rat retinas. J Neurochem. 2009;108(3):741–54. doi: 10.1111/j.1471-4159.2008.05807.x. [DOI] [PubMed] [Google Scholar]
- 35.Bantignies F, Cavalli G. Cellular memory and dynamic regulation of polycomb group proteins. Curr Opin Cell Biol. 2006;18(3):275–83. doi: 10.1016/j.ceb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 36.van der Stoop P, Boutsma EA, Hulsman D, Noback S, Heimerikx M, Kerkhoven RM, Voncken JW, Wessels LF, van Lohuizen M. Ubiquitin E3 ligase Ring1b/Rnf2 of polycomb repressive complex 1 contributes to stable maintenance of mouse embryonic stem cells. PLo S One. 2008;3(5):e2235. doi: 10.1371/journal.pone.0002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu BX, Darden AG, Laser M, Li Y, Crosson CE, Hazard ES, Ma JX., 3rd The rat Apg3p/Aut1p homolog is upregulated by ischemic preconditioning in the retina. Mol Vis. 2006;12:1292–302. [PubMed] [Google Scholar]
- 38.Fegers I, Kob R, Eckey M, Schmidt O, Goeman F, Papaioannou M, Escher N, von Eggeling F, Melle C, Baniahmad A. The tumor suppressors p33ING1 and p33ING2 interact with alien in vivo and enhance alien-mediated gene silencing. J Proteome Res. 2007;6(11):4182–8. doi: 10.1021/pr070219d. [DOI] [PubMed] [Google Scholar]
- 39.Eckey M, Hong W, Papaioannou M, Baniahmad A. The nucleosome assembly activity of NAP1 is enhanced by Alien. Mol Cell Biol. 2007;27(10):3557–68. doi: 10.1128/MCB.01106-06. [DOI] [PMC free article] [PubMed] [Google Scholar]