Abstract
Objectives
We investigated the impact of polymorphisms in key renin angiotensin system genes on the association between angiotensin converting enzyme inhibitors (ACEINH) exposure and global and executive cognitive function in the Health, Aging and Body Composition study.
Design
Cohort study.
Setting
Community-based
Participants
3,075 participants: mean age: 73.6 years, 58% Caucasian, 52% women, 15% on ACEINH, 8 years of follow-up.
Measurements
The phenotypes were longitudinal change in Executive Clock Draw test-1 (CLOX1), the Digit Symbol Substitution test, and the Modified Mini Mental Status Examination. The genetic polymorphisms included the angiotensin converting enzyme insertion deletion (ACEID) in the angiotensin converting enzyme gene and the M235T and 6AG polymorphisms in the angiotensinogen gene (AGT).
Results
The 6AG and M235T polymorphisms in AGT had significant interaction with ACEINH exposure on the longitudinal change in CLOX1 scores in Caucasian participants (p=0.01 for both polymorphisms) independent of blood pressure levels. Specifically, ACEINH exposure was protective against CLOX1 score decline in carriers of the AA genotype of the 6AG and the CC genotype of the M235T (p-value for the ACEINH vs non-ACEINH groups =0.01 for 6AG and 0.005 for M235T) but not the other genotypes. These associations were not significant with other cognitive tests, with ACEID, or in African Americans.
Conclusion
ACEINH may provide a protective effect on executive function in Caucasians with AGT polymorphisms known to be associated with increased renin angiotensin system activity. If confirmed in a pharmacogenetic trial, ACEINH may have additional cognitive protection in a select group of elderly individuals.
Keywords: hypertension, cognitive function, angiotensin converting enzyme inhibitors, angiotensinogen gene
Introduction
Animal studies suggest that angiotensin converting enzyme inhibitors (ACEINH) may have a protective effect on cognition.[1, 2] In humans, this effect of ACEINH is controversial. In the Perindopril Protection Against Recurrent Stroke Study (PROGRESS), ACEINH reduced the risk of incident cognitive impairment in those with a previous history of stroke.[3] In contrast in the Hypertension in the Very Elderly Trial, treatment with an ACEINH based regimen in the very old had no effect on dementia risk or cognitive decline.[4] We have previously reported an association between ACEINH exposure and improved working memory and diminished functional decline in Alzheimer's Disease patients.[5] These inconsistent findings may be attributable to genetic variations that impact the cognitive outcomes of ACEINH.
Of the various genes in the renin angiotensin system, two key genes have been associated with renin angiotensin system activity. These include the angiotensinogen gene (AGT), which codes the angiotensinogen protein, and the angiotensin converting enzyme gene (ACE), which codes the angiotensin converting enzyme protein. Both proteins are involved in angiotensin II production; the major factor in this system with wide vascular and neurological effects in the brain.[6] In the ACE gene, the ACEID is an insertion or deletion of 287 base pairs of DNA fragments in intron 16. The DD genotype is associated with higher levels of plasma ACE.[7] The M235T polymorphism is a nucleotide change of thymine (T) to cytosine (C) in the second exon of the AGT gene. The C allele leads to an amino acid change from methionine to threonine at position 235.[8] The 6AG polymorphism is a nucleotide change of guanine (G) to adenine (A) in the promoter region of the AGT gene.[9] The A allele of the 6AG and the C allele (or the threonine amino acid) of the M235T polymorphisms are associated with higher angiotensinogen levels and possibly higher renin angiotensin system activity.[10] All three polymorphisms may be associated with cardiovascular outcomes and may modify the vascular response to ACEINH.[11] It is not known if this is also true for cognitive function outcomes of ACEINH. To our knowledge, no prior study has examined the role of these polymorphisms in modifying the cognitive outcomes of ACEINH treatment.
Seniors suffering from executive function impairments have significant difficulties in following medical advice and are more likely to develop disability.[12] Executive function exhibits close to 40% heritability according to the Aging Male Twin Study[13] and is the domain of cognitive function most vulnerable to the effects of hypertension.[14] Since renin angiotensin system is involved in cognitive function and hypertension, we hypothesized that polymorphisms in key genes that affect the renin angiotensin system activity may modify the executive function outcome related to ACEINH exposure.
Therefore, our objective was to investigate if the polymorphisms in the ACE (ACEID) and AGT (6AG and M235T) genes modify the effect of ACEINH on global and executive cognitive function decline in elderly participants in the Health, Aging and Body Composition (Health ABC) study.
Methods
Sample
Health ABC is a prospective community-based study of 3,075 well-functioning cognitively intact elderly participants (70-79 years) recruited between 1997 and 1998. We used follow-up data through year 8 (2005-2006) for this analysis. Health ABC participants were recruited from a random sample of Caucasian and African American Medicare-eligible adults living in selected ZIP code areas in Pittsburgh, PA and Memphis, TN. “Well-functioning” was defined as: self-report of no difficulty in walking a quarter of a mile, walking up 10 steps, getting in and out of bed or chairs, bathing, dressing, or eating, no need to use a cane or other assistive device, and being free of any life threatening illness. Of the sample, 42% were African American and 52% were women. For this analysis, we only included those with genotype data (141 to 144 had no usable genotype data depending on the polymorphism). By year eight, 97% of the sample was still enrolled or had known vital status.
Measures
The examiner-administered interview ascertained demographic characteristics (age, sex, education and race), health habits (smoking and alcohol use) and self-reported physical activity (weekly estimated energy expenditure from exercise and physical activity). Blood pressure recordings (sitting after 5 minute rest) were performed twice using a mercury sphygmomanometer. We used the average systolic and diastolic blood pressures measured during each examination for this analysis. Weight (balance beam scale), height (stadiometer), and radial pulse were also obtained. Information on prevalent and incident health conditions was collected by self-report. The diagnoses of heart failure, hypertension, diabetes, and coronary artery disease were based on history, exam findings and medication use. Current medication data were collected using an inventory and included all prescription and non-prescription drugs taken in the last two weeks. The data were then coded according to the Iowa Drug Information System (IDIS) (ACEINH IDIS code 24080200). Drug data were available for years 1, 2, 3, 5, 6, and 8.
For this study we used three cognitive tests administered during at least three waves of data collection. Executive function was assessed during years 3, 5 and 8 using the executive clock drawing test part 1 (CLOX1); where the subject draws a clock in response to the examiner's request. [15] The Digit Symbol Substitution test (DSST) is a sub-test from the Wechsler Adult Intelligence Scale (Wechsler, 1955) that requires timed recording of digits and symbol. It measures attention, perceptual speed, motor speed, visual scanning and memory.[16] DSST was performed in years 1, 5 and 8. The Modified Mini Mental Status Examination (3MSE) was performed in years 1, 3, 5 and 8 to assess global cognitive function. This test was developed by Teng et al. and is based on the original Mini-Mental-Status-Examination with an expanded 100-point score to provide a lower floor and a higher test ceiling. [17]
Genotyping
Blood samples for genetic analysis were collected at baseline. Genotyping was conducted using high-throughput matrix-assisted laser desorption time-of-flight mass spectrometry utilizing the MassEXTEND reaction (Sequenom). Polymerase chain reaction primers and extension primers were synthesized at BioServe.
The ACEID polymorphism was detected using polymerase chain reaction (PCR) amplification with visualization on 2% agarose gel electrophoresis. The sense-antisense primers were 5′-CTGGAGACCACTCCCATCCTTTCT-3′ AND 5′-GATGTGGCCTCA CATTCGTCAGA-3′, respectively. The PCR results were independently evaluated by two technicians. The heterozygous samples were re-amplified with a more specific primer that detects the insertion polymorphism. The AGT polymorphisms were genotyped using MassArray system (sequonom). Polymerase chain reactions were synthesized at Qiagen. Apolipoprotein (APOE) genotyping was also performed using PCR. Admixture data and European ancestry estimates for 1237 of the African American participants in Health ABC were analyzed using highly informative 1536 single nucleotide polymorphism on an Illumina platform. [18]
Statistical analysis
We conducted Hardy-Weinberg Equilibrium testing in both Caucasians and African Americans using SAS/Genetics (®Cary, NC). We identified a significant difference in allele frequencies and cognitive scores between the two racial groups and hence all analyses were conducted stratified by race. We used Mixed Models (proc mixed) for correlated repeated measures of both the outcomes and the covariates to test our hypotheses. Our phenotypes were longitudinal change in cognitive test scores (CLOX1, 3MSE, DSST) measured serially. CLOX1 and 3MSE were not normally distributed so we used a log transformation to render their distribution closer to normality. We used analysis of variance (ANOVA) to compare baseline cognitive scores between the three genotypes of the polymorphisms using data from year one for the 3MSE and DSST phenotypes and year three for the CLOX1. We used Mixed Models for repeated measures to identify the presence of significant gene by ACEINH interaction by including a gene × ACEINH term in the multivariate model. We then used the least square means obtained form these models to compare the change during the follow-up period in the cognitive scores between those on vs not on ACEINH in the three genotypes at each polymorphism. We report the % change in cognitive score over the study period as the least square mean of the cognitive score at last measurement-baseline measurement/baseline measurement. In addition, in our online supplement we report the least square mean score during the multiple measurement waves. All models were adjusted for baseline cognitive score, age, sex, educational level, systolic blood pressure (mean of two readings), BMI, diabetes mellitus, stroke (prevalent and incident), antihypertensives (other than ACEINH), and APOE haplotypes. In the analysis of the African American participants European ancestry percentage was included to minimize the effect of admixture. We modeled the covariates (age, BMI, blood pressure, diabetes, stroke and use of antihypertensives) as time-dependent measures.
Because the participant cognitive function may affect both prescribing ACEINH and the rate of cognitive decline, we performed additional analyses excluding those with low CLOX1 scores defined as less than 10 at baseline; which has been shown to be a sensitive threshold for executive dysfunction.[15]
Since participants may have started and stopped ACEINH during the study period, we included the ACEINH exposure as a time-dependent variable in the Mixed Models. In addition, we calculated the number of visits a participant was using ACEINH and compared this indicator between genotypes using ANOVA. There was no difference in this ACEINH indicator by genotypes of the three polymorphisms. We addressed multiple testing by using a Bonferroni correction for the three loci. A p-value less than 0.05/3=0.016, indicated statistical significance.
Results
Sample
Of the 3,075 participants (mean age: 73.6 ± 0.1 years) in the Health ABC study, 58% were Caucasian, 52% were women, and 15% were receiving ACEINH. Of those, 2931 to 2974 had genotyping data, depending on the polymorphism. All polymorphisms were in HWE. Online Table-A provides the allele distribution of the three polymorphisms. Allele frequencies for the AGT polymorphisms were different between the two racial groups. African Americans had greater frequency of the CC genotype of the M235T polymorphism and AA of the 6AG polymorphism. Use of ACEINH increased from 15% at baseline to 19% at year five and 27% at year eight although the absolute number of participants on ACEINH did not significantly change. In those who were using ACEINH at baseline, the median duration of use was four years and in those who started after the initial visit it was two years. There was no significant difference in ACEINH exposure by any of the polymorphisms (p=0.519 for ACEID, 0.160 for M235T and 0.119 for 6AG polymorphisms).
Table 1 provides the main characteristics of the overall sample, Caucasians and African Americans. Online Table B provides the main characteristics of those who were exposed and not exposed to ACEINH. Those exposed to ACEINH were more likely to be African American and have higher BMI, lower education, hypertension, diabetes and congestive heart failure.
Table 1.
Baseline characteristics of the overall sample and of the Caucasian and African American participants in the Health ABC study.
| Overall | Caucasians | African Americans | p-value | |
|---|---|---|---|---|
| N | 3075 | 1794 | 1281 | |
| Age, mean±standard error (SE), years | 73.6±0.1 | 73.7±0.1 | 73.3±0.1 | 0.0014 |
| Gender, % women | 1584 (52%) | 855 (48%) | 729 (57%) | <0.0001 |
| Body Mass Index, mean±SE, kg/m2 | 27.34±0.1 | 26.5±0.1 | 28.6±0.1 | <0.0001 |
| Education level, mean±SE, years | 13±0.1 | 14 ±0.1 | 12±0.1 | <0.0001 |
| Systolic Blood Pressure, mean±SE, mm Hg | 136±0.3 | 133±0.4 | 139±0.6 | <0.0001 |
| Diastolic Blood Pressure, mean±SE, mm Hg | 71±0.2 | 70±0.2 | 74±0.3 | <0.0001 |
| Current smoker, n (%) | 318 (10%) | 111 (6%) | 207 (16%) | <0.0001 |
| Alcohol, mean±SE drinks per day | 2.1±0.03 | 2.3±0.04 | 1.8±0.04 | <0.0001 |
| Energy expenditure, KCAL/KG/WEEK | 7.7±0.3 | 9.1±0.4 | 5.7±0.3 | <0.0001 |
| Laboratory | ||||
| LDL, mean±SE, mg/dl | 121.5±0.6 | 119.7±0.8 | 124.1±1.0 | 0.33 |
| HDL, mean±SE, mg/dl | 54.0±0.3 | 51.9±0.4 | 57.2±0.5 | 0.0006 |
| Cholesterol, mean±SE, mg/dl | 202.8±0.7 | 201.3±0.9 | 204.8±1.1 | 0.0123 |
| Morbidities, n (%): | ||||
| Hypertension | 1563 (51%) | 776 (44%) | 787 (62%) | <0.0001 |
| Diabetes Mellitus | 460 (15%) | 191 (11%) | 269 (21%) | <0.0001 |
| Coronary Artery Disease | 105 (3%) | 49 (3%) | 56 (4%) | 0.0136 |
| Congestive Heart Failure | 95 (3%) | 50 (3%) | 45 (4%) | 0.2451 |
| Stroke | 223 (7%) | 116 (7%) | 107 (8%) | 0.0419 |
| Arthritis | 1719 (57%) | 975 (55%) | 744 (59%) | 0.0299 |
| Neuropsychological test scores, mean±SE | ||||
| CLOX1 (year 3) | 10.6±0.05 | 10.9±0.06 | 10.1±0.09 | <0.0001 |
| 3MSE | 90.0±0.1 | 92.9±0.1 | 86±0.2 | <0.0001 |
| DSST | 35.2±0.3 | 40.8±0.3 | 27.2±0.4 | <0.0001 |
| CES-D | 4.7±0.1 | 4.7±0.1 | 4.8±0.2 | 0.66 |
| Medications, n (%) | ||||
| Receiving antihypertensives | 1673 (55%) | 880 (49%) | 793 (62%) | <0.0001 |
| Angiotensin converting enzyme inhibitors | 465 (15%) | 245 (14%) | 220 (17%) | 0.006 |
| Diuretics | 312 (10%) | 145 (8%) | 167 (13%) | <0.0001 |
| Beta Blockers | 410 (13%) | 272 (15%) | 138 (11%) | 0.0005 |
| Calcium channel blocker | 705 (23%) | 338 (19%) | 367 (29%) | <0.0001 |
| Angiotensin receptor blockers | 70 (2%) | 45 (3%) | 25 (2%) | 0.3176 |
| Hormonal Supplement | 349 (11%) | 264 (15%) | 85 (7%) | <0.0001 |
CLOX1: Executive clock draw test; DSST: Digit Symbol Substitution Test; 3MSE: Modified Mini Mental Status Examination; CESD: Center for Epidemiologic Studies Depression Scale
At baseline, there was no interaction between the genetic polymorphisms and ACEINH exposure in either racial group. (Table 2) However, longitudinally, both 6AG and M235T polymorphisms had significant interactions with ACEINH exposure on the change in CLOX1 scores in Caucasian participants (p=0.01 for 6AG and 0.01 for M235T after adjusting for age, sex, BMI, educational level, systolic blood pressure, stroke, APOE haplotypes, diabetes, baseline CLOX1 scores, and exposure to other high blood pressure medications). (Table 3) We describe the details of these interactions below.
Table 2.
Baseline scores on cognitive tests by genotype of the 3 polymorphisms and the interaction at baseline between the polymorphisms and ACEINH exposure.
| Caucasians | |||||
|---|---|---|---|---|---|
| ACE-ID | D/D | I/D | I/I | p-value, genotype differences | p-value, ACEINH × Gene interaction |
| CLOX1 | 11±0.1 | 10.9±0.1 | 11±0.13 | 0.78 | 0.90 |
| DSST | 41.3±0.4 | 40.7±0.4 | 40.3±0.6 | 0.16 | 0.49 |
| 3MSE | 93.0±0.24 | 93.0±0.2 | 92.7±0.3 | 0.45 | 0.37 |
| AGT6 | A/A | A/G | G/G | ||
| CLOX1 | 11.1±0.1 | 10.9±0.1 | 10.8±0.1 | 0.01 | 0.11 |
| DSST | 41.7±0.6 | 41.1±0.4 | 39.8±0.4 | 0.01 | 0.55 |
| 3MSE | 93.1±0.3 | 92.9±0.2 | 92.950.2 | 0.94 | 0.19 |
| AGTM235T | C/C | C/T | T/T | ||
| CLOX1 | 11.1±0.1 | 10.9±0.1 | 10.8±0.1 | 0.004 | 0.04 |
| DSST | 41.5±0.6 | 41.0±0.4 | 40.0±0.4 | 0.05 | 0.14 |
| 3MSE | 93.1±0.3 | 92.8±0.12 | 92.9±0.2 | 0.95 | 0.31 |
| African Americans | |||||
| ACE-ID | D/D | I/D | I/I | ||
| CLOX1 | 10.01±0.1 | 10.1±0.1 | 10.3±0.1 | 0.67 | 0.41 |
| DSST | 27.4±0.6 | 27.4±0.5 | 27.6±0.8 | 0.86 | 0.21 |
| 3MSE | 86.1±0.4 | 85.9±0.3 | 85.6±0.6 | 0.43 | 0.69 |
| AGT6 | A/A | A/G | G/G | ||
| CLOX1 | 10.0±0.1 | 10.3±0.1 | 10.4±0.4 | 0.09 | 0.76 |
| DSST | 26.9±0.4 | 28.0±0.6 | 30.0±1.9 | 0.03 | 0.69 |
| 3MSE | 85.6±0.3 | 86.6±0.4 | 87.4±1.3 | 0.005 | 0.060 |
| AGTM235T | C/C | C/T | T/T | ||
| CLOX1 | 10.1±0.1 | 10.1±0.1 | 10.6±0.3 | 0.22 | 0.97 |
| DSST | 27.0±0.4 | 27.9±0.6 | 28.3±1.7 | 0.19 | 0.45 |
| 3MSE | 85.7±0.3 | 86.2±0.4 | 87.2±1.2 | 0.04 | 0.08 |
CLOX1: Executive clock draw test; DSST: Digit Symbol Substitution Test; 3MSE: Modified Mini Mental Status Examination;; Numbers are least square means ± standard error obtained from the multivariate models after adjusting for age, gender, BMI, education, systolic blood pressure and stroke
Table 3.
Interaction between ACEINH and longitudinal cognitive function change in the Health ABC participants
| p-value for ACEINH × Gene interaction on cognitive decline | ||
|---|---|---|
| Caucasians | African Americans | |
| ACE-ID | ||
| CLOX1 | 0.39 | 0.97 |
| DSST | 0.46 | 0.14 |
| 3MSE | 0.46 | 0.14 |
| AGT6 | ||
| CLOX1 | 0.01 | 0.45 |
| DSST | 0.22 | 0.06 |
| 3MSE | 0.22 | 0.06 |
| AGTM235T | ||
| CLOX1 | 0.01 | 0.64 |
| DSST | 0.15 | 0.12 |
| 3MSE | 0.15 | 0.12 |
CLOX1: Executive clock draw test; DSST: Digit Symbol Substitution Test; 3MSE: Modified Mini Mental Status Examination; p-values are obtained from the Multivariate Mixed Models testing the hypothesis that the gene × ACEINH is significant on the longitudinal change in the cognitive test over the follow-up period. All models adjusted for age, sex, BMI, educational level, systolic blood pressure, stroke, APOE haplotypes, diabetes, baseline CLOX1 scores, and exposure to other high blood pressure medications.
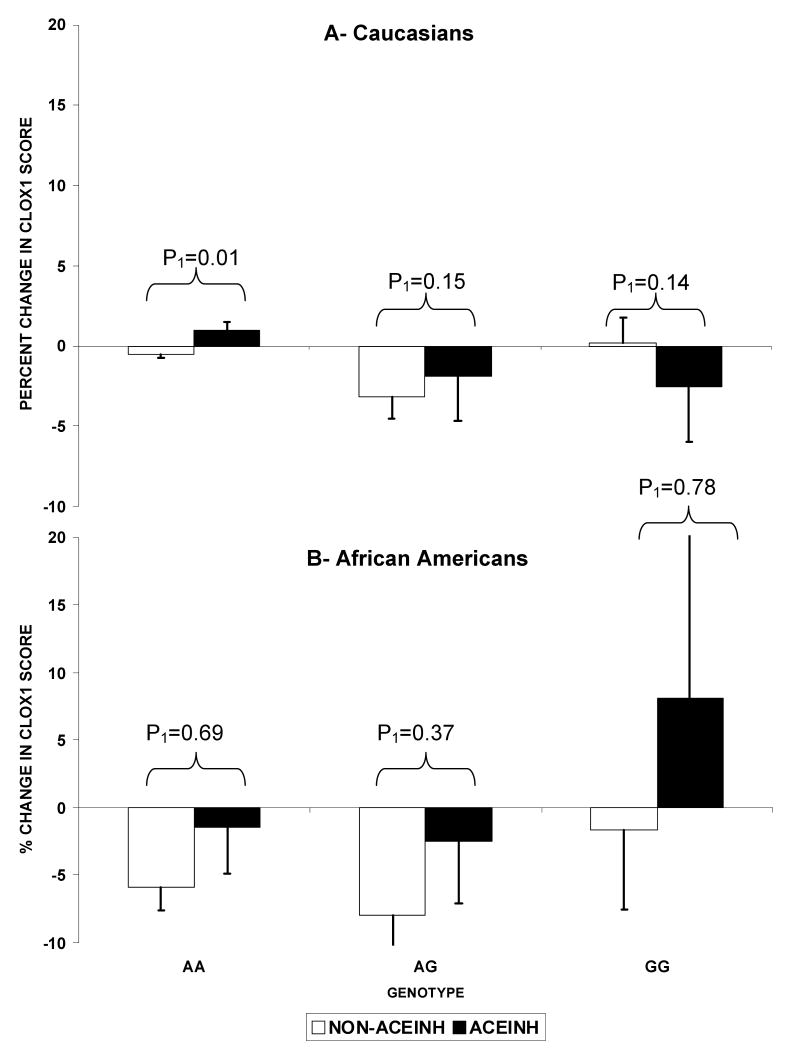
As shown in Figure 1-A, CLOX1 score showed no decline over the follow-up period in those with the AA allele who were exposed to ACEINH compared to those not exposed (p=0.01 for the ACEINH vs non-ACEINH groups after adjusting for covariates within the AA genotype). This remained true after excluding those with low CLOX1 score at baseline (p=0.01). In the heterozygous AG carriers, this was only true after excluding those with baseline low CLOX1 scores (p=0.15 vs p=0.01 after excluding those with baseline low CLOX1 scores within the AG genotype). There was no difference in CLOX1 score change by ACEINH exposure in the GG homozygous carriers. In addition to the within genotype effect, there was an impact of ACEINH on CLOX1 change across genotypes in Caucasians. In those who were never exposed to ACEINH, homozygous and heterozygous carriers of the A allele of the 6AG polymorphisms demonstrated declines in CLOX1 score over the follow-up period whereas GG carriers showed no decline (p=0.001 for the difference in CLOX1 score change by 6AG genotypes). In contrast, those who were exposed to ACEINH demonstrated no difference between the 3 genotypes (p=0.91).
Figure 1.
Longitudinal change in CLOX1 score by AGT6 genotypes (AA, AG, GG) in the 2 ACEINH groups in Caucasians (A) and African Americans (B).
Footnote: Values are the percent change in the least square means of CLOX1 scores adjusted for baseline CLOX1, age, gender, BMI, education, systolic blood pressure, stroke, APOE, diabetes, and other antihypertensives.
*: signify that the p-value for the change within the genotype is significant (<0.01). P1 reflect the significance for the difference in change of CLOX1 scores between the ACEINH vs non-ACEINH groups (P2: excluding those with baseline low CLOX1 scores).
There was no significant interaction between ACEINH exposure and 6AG in African Americans (p=0.64). As shown in Figure1-B, although the AA and AG alleles were associated with greater declines in CLOX1 score compared to the GG allele, the difference between genotypes was not statistically significant in those exposed to ACEINH (p=0.73) or not exposed to ACEINH (p=0.93).
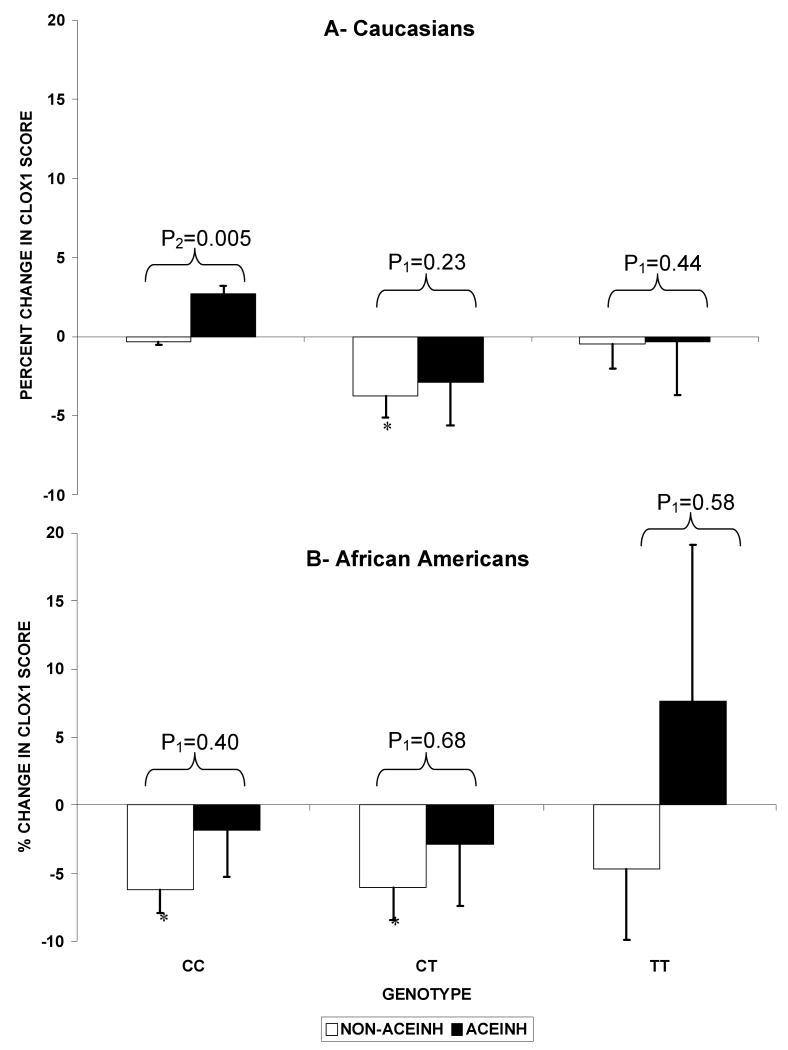
In the M235T polymorphism, shown in Figure 2-A, exposure to ACEINH was associated with a difference in the change in CLOX1 scores compared to non-exposure to ACEINH only in the CC genotype (p=0.03 for the ACEINH vs non-ACEINH groups in the CC genotype) but not the other two genotypes (TT or CT). This remained true after excluding those with low baseline CLOX1 scores (p=0.005). Across the three genotypes of the M235T polymorphism, there was a significant difference in the change in CLOX1 scores by ACEINH exposure: Caucasians who were never exposed to ACEINH with the CT genotype demonstrated greater decline in CLOX1 scores compared to those with the CC or TT genotypes. In contrast, the difference between genotypes was not present in Caucasians who were exposed to ACEINH (p=0.23). As shown in Figure2-B in African American participants, there was no significant interaction between the M235T and ACEINH exposure on CLOX1 Score change (p=0.85). There were also no differences between genotypes in CLOX1 score changes in African Americans exposed to ACEINH (p=0.44) or not exposed (p=0.99).
Figure 2.
Longitudinal change in CLOX1 score by AGTM235T genotypes (CC, CT, TT) in the 2 ACEINH groups in Caucasians (A) and African Americans (B).
Footnote: Values are the percent change in the least square means of CLOX1 scores adjusted for baseline CLOX1, age, gender, BMI, education, systolic blood pressure, stroke, APOE, diabetes, and other antihypertensives.
*: signify that the p-value for the change within the genotype is significant (<0.01). P1 reflect the significance for the difference in change of CLOX1 scores between the ACEINH vs non-ACEINH groups (P2: excluding those with baseline low CLOX1 scores).
When we investigated the association between genetic polymorphisms and cognitive function in the whole sample (i.e. those exposed and not exposed to ACEINH) stratified by race, we observed that the M235T and 6AG polymorphisms (p=0.004 for M235T and p=0.012 for 6AG) were associated with CLOX1 score in Caucasians at baseline, but not African Americans (p=0.22 for M235T and p=0.096 for 6AG). A similar association was noted longitudinally. (Table C online)
There was no interaction between ACEINH and any of the polymorphisms on DSST or 3MSE scores. The ACEID polymorphism was not associated with any cognitive function decline or interaction with ACEINH (Table 3).
Discussion
This analysis of the Health ABC data suggests that ACEINH exposure is protective against executive function decline only in individuals who have the AA genotype of the 6AG polymorphism or the CC genotype of the M235T polymorphism in the AGT gene. The AG or GG genotypes of the 6AG polymorphism and CT genotype of the M235T polymorphism in the AGT gene were associated with greater declines in CLOX1 scores only if they were not exposed to ACEINH. These associations were significant only in Caucasians.
Prior studies are not consistent regarding the effect of ACEINH on cognitive function. A recent analysis of the Woman's Health and Aging Study II suggested that treatment with ACEINH for three years was associated with a decreased risk of developing impairment in executive function.[19] Our data suggest that the observed differences in this cognitive effect may be related to an underlying genetic variability in the drug cognitive response. We have identified an AGT-by-ACEINH interaction that may provide further explanation for the discrepancy in prior studies.
As in this analysis, ACEID polymorphism did not modify the effect of ACEINH on dementia or cognitive function in the PROGRESS trial.[20] This suggests that within the renin angiotensin system, angiotensinogen may play a more important role in cognitive function than ACE.
We have observed a racial difference in the association between the polymorphisms and cognitive function as well as the interaction with ACEINH, where our results were only significant in Caucasians. Prior observations have suggested racial differences in the renin angiotensin system. For example, the association between angiotensinogen blood levels and AGT polymorphism is only present in Caucasians. [21] In a previous report, renin angiotensin system genes were associated with peripheral arterial disease only in Caucasians.[22] In the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attacks trial, ACEINH was as effective as diuretics in stroke protection in Caucasians but not in African Americans.[23] African Americans have higher angiotensinogen levels which may explain their lower sensitivity to ACEINH. [24] Race may be a proxy for factors that mediate the gene-cognitive function or the gene by ACEINH interactions observed in our study. We have found significant differences in vascular and non-vascular factors between the Caucasian and African American participants in the Health ABC study. Taken together, interpretations of the racial differences in this analysis should be considered within that context.
The mechanisms by which ACEINH may provide cognitive protection are not well established. In this analysis, ACEINH effect was independent of baseline or subsequent blood pressure and other cognitive risk factors. Therefore, ACEINH may have non-vascular effects that would lead to further brain and cognitive protection. For example, ACEINH are associated with upregulation of neprilysin, an amyloid beta degrading enzyme[25], inhibition of the Angiotensin II-related anticholinergic effect[26] and decreased inflammatory status. [27]
Our observation that ACEINH affects cognitive decline specifically in the AA genotype of 6AG or CC genotype of the M235T may be explained by the relation of these genotypes with higher angiotensinogen levels. Increased renin angiotensin system activity leads to neurovascular uncoupling which may be restored by ACEINH. Our study supports this hypothesis by showing that ACEINH exposure was associated with less executive function decline only in those with higher background renin angiotensin system activity.
Our analysis provides further evidence for an association between renin angiotensin system and cognitive function. Animal models suggest that increasing renin angiotensin system activity, by infusing angiotensin II into rats' brains, is associated with amnesia and learning disabilities.[28] In humans, renin angiotensin system gene polymorphisms linked with higher renin angiotensin system activity are associated with depression and suicide.[29] Genes in this system have also been linked with Alzheimer's disease, although not consistently.[30] A high concentration of angiotensin receptors has been noted in the frontal cortex, which is involved in executive function.[31] Our study adds evidence that the A allele of the 6AG polymorphism which is associated with higher angiotensinogen level, is also associated with greater cognitive decline. The observation in the M235T polymorphism is less consistent since only the CT genotype had greater cognitive decline compared to the TT or the CC genotypes.
Our study suggests that the AGT polymorphisms are linked with executive function, but not global cognitive function decline in an elderly population. Maintaining executive function is an important aspect of successful aging. To our knowledge, this is the first report linking a genetic polymorphism in the AGT gene with executive function. These results suggest that polymorphisms in renin angiotensin system genes and executive function need to be taken into account in future cognitive and hypertension trials.
The clinical implication of our study is that executive function may be an important factor to be assessed in individuals who are candidates for or are currently receiving ACEINH treatment. Further if these findings are replicated in other populations or in a pharmacogenetic trial, then selective use of ACEINH in individuals who carry the AA genotype of the 6AG polymorphism or the CC genotype of the M235T polymorphisms may offer additional cognitive benefit beyond lowering vascular risk.
The main limitation of this analysis is confounding- by-indication, where people with higher risk for cognitive decline may not be prescribed ACEINH. Providers do not know their patients' genotypes; hence this may have minor effect on our interaction results. Nevertheless, we addressed the possibility of this confounding-by-indication by performing additional analyses where we excluded those with low cognitive scores at baseline. Our results did not change.
The genetic effect on cognitive function is relatively small. This is not uncommon for single genetic polymorphism effect. However, considering the high prevalence of hypertension and its executive function effect, the clinical and public health impact of these results are significant.
Conclusion
This analysis of the Health ABC data suggests that use of ACEINH is protective against executive function decline in individuals who have polymorphisms in the AGT gene known to be associated with greater renin angiotensin system activity. These data may explain the conflicting findings in prior studies regarding ACEINH and cognitive function. Further, if a pharmacogenetic trial confirms this genetic-drug interaction, use of ACEINH may provide additional cognitive protection in a select group of elderly individuals.
Supplementary Material
Acknowledgments
Sources of Funding: Health ABC was funded by the NIA contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106. This analysis was supported by an NIA grant (K23AG30057) to Dr. Hajjar. Dr. Yaffe is supported in part by AG031155. Dr. Lipsitz holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife. This research was also supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Author Contributions: Study concept and design IH, SS, AN, KY, ES, LL, acquisition of subjects and/or data SS, AN, KY, ES, analysis and interpretation of data IH, SS, LL, and preparation of manuscript IH, SS, AN, KY, ES, LL.
Sponsor's Role: The sponsor had no role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
Contributor Information
Ihab Hajjar, Harvard Medical School, Institute For Aging Research/Hebrew SeniorLife, Beth Israel Deaconess Medical Center, Boston, MA.
Stephen Kritchevsky, Professor of Internal Medicine, Gerontology and Geriatrics, Wake Forest University School of Medicine.
Anne B. Newman, Professor of Epidemiology and Medicine, University of Pittsburgh.
Rongling Li, Office of Population Genomics (OPG), National Human Genome Research Institute (NHGRI), National Institutes of Health (NIH).
Kristine Yaffe, Professor in Residence, Department of Psychiatry, Neurology and Epidemiology, University of California, San Francisco.
Eleanor M. Simonsick, Staff Scientist, Epidemiologist, Intramural Research Program, National Institute on Aging, National Institute of Health.
Lewis A. Lipsitz, Professor of Medicine and Chief of Gerontology, Harvard Medical School, Beth Israel Deaconess Medical Center, Institute for Aging Research/Hebrew SeniorLife.
References
- 1.Basso N, Paglia N, Cini R, et al. Effect of omapatrilat on the aging process of the normal rat. Cell Mol Biol (Noisy-le-grand) 2005;51:557–564. [PubMed] [Google Scholar]
- 2.Raghavendra V, Chopra K, Kulkarni SK. Involvement of cholinergic system in losartan-induced facilitation of spatial and short-term working memory. Neuropeptides. 1998;32:417–421. doi: 10.1016/s0143-4179(98)90065-8. [DOI] [PubMed] [Google Scholar]
- 3.Tzourio C, Anderson C, Chapman N, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069–1075. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- 4.Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): A double-blind, placebo controlled trial. Lancet Neurol. 2008;7:683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 5.Hajjar IM, Keown M, Lewis P, et al. Angiotensin converting enzyme inhibitors and cognitive and functional decline in patients with Alzheimer's disease: An observational study. Am J Alzheimers Dis Other Demen. 2008;23:77–83. doi: 10.1177/1533317507309803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazama K, Anrather J, Zhou P, et al. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res. 2004;95:1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- 7.Rigat B, Hubert C, Corvol P, et al. PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme gene (DCP1) (dipeptidyl carboxypeptidase 1) Nucleic Acids Res. 1992;20:1433. doi: 10.1093/nar/20.6.1433-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeunemaitre X, Soubrier F, Kotelevtsev YV, et al. Molecular basis of human hypertension: Role of angiotensinogen. Cell. 1992;71:169–180. doi: 10.1016/0092-8674(92)90275-h. [DOI] [PubMed] [Google Scholar]
- 9.Yanai K, Saito T, Hirota K, et al. Molecular variation of the human angiotensinogen core promoter element located between the TATA box and transcription initiation site affects its transcriptional activity. J Biol Chem. 1997;272:30558–30562. doi: 10.1074/jbc.272.48.30558. [DOI] [PubMed] [Google Scholar]
- 10.Inoue I, Nakajima T, Williams CS, et al. A nucleotide substitution in the promoter of human angiotensinogen is associated with essential hypertension and affects basal transcription in vitro. J Clin Invest. 1997;99:1786–1797. doi: 10.1172/JCI119343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schelleman H, Klungel OH, Witteman JC, et al. Angiotensinogen M235T polymorphism and the risk of myocardial infarction and stroke among hypertensive patients on ACE-inhibitors or beta-blockers. Eur J Hum Genet. 2007;15:478–484. doi: 10.1038/sj.ejhg.5201789. [DOI] [PubMed] [Google Scholar]
- 12.Royall DR, Palmer R, Chiodo LK, et al. Executive control mediates memory's association with change in instrumental activities of daily living: The Freedom House Study. J Am Geriatr Soc. 2005;53:11–17. doi: 10.1111/j.1532-5415.2005.53004.x. [DOI] [PubMed] [Google Scholar]
- 13.Swan GE, Carmelli D. Evidence for genetic mediation of executive control: A study of aging male twins. J Gerontol B Psychol Sci Soc Sci. 2002;57:P133–143. doi: 10.1093/geronb/57.2.p133. [DOI] [PubMed] [Google Scholar]
- 14.Pugh KG, Kiely DK, Milberg WP, et al. Selective Impairment of Frontal-Executive Cognitive Function in African Americans with Cardiovascular Risk Factors. J Am Geriatr Soc. 2003;51:1439–1444. doi: 10.1046/j.1532-5415.2003.51463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Royall DR, Cordes JA, Polk M. CLOX: An executive clock drawing task. J Neurol Neurosurg Psychiatry. 1998;64:588–594. doi: 10.1136/jnnp.64.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wechsler D. WMS-R : Wechsler Memory Scale--Revised : manual. San Antonio: Psychological Corp. : Harcourt Brace Jovanovich; 1987. [Google Scholar]
- 17.Weissman MM, Sholomskas D, Pottenger M, et al. Assessing depressive symptoms in five psychiatric populations: A validation study. Am J Epidemiol. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 18.Reich D, Patterson N, Ramesh V, et al. Admixture mapping of an allele affecting interleukin 6 soluble receptor and interleukin 6 levels. Am J Hum Genet. 2007;80:716–726. doi: 10.1086/513206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasar S, Zhou J, Varadhan R, et al. The use of angiotensin-converting enzyme inhibitors and diuretics is associated with a reduced incidence of impairment on cognition in elderly women. Clin Pharmacol Ther. 2008;84:119–126. doi: 10.1038/sj.clpt.6100483. [DOI] [PubMed] [Google Scholar]
- 20.Harrap SB, Tzourio C, Cambien F, et al. The ACE gene I/D polymorphism is not associated with the blood pressure and cardiovascular benefits of ACE inhibition. Hypertension. 2003;42:297–303. doi: 10.1161/01.HYP.0000088322.85804.96. [DOI] [PubMed] [Google Scholar]
- 21.Winkelmann BR, Russ AP, Nauck M, et al. Angiotensinogen M235T polymorphism is associated with plasma angiotensinogen and cardiovascular disease. Am Heart J. 1999;137(4 Pt 1):698–705. doi: 10.1016/s0002-8703(99)70226-7. [DOI] [PubMed] [Google Scholar]
- 22.Li R, Nicklas B, Pahor M, et al. Polymorphisms of angiotensinogen and angiotensin-converting enzyme associated with lower extremity arterial disease in the Health, Aging and Body Composition study. J Hum Hypertens. 2007;21:673–682. doi: 10.1038/sj.jhh.1002198. [DOI] [PubMed] [Google Scholar]
- 23.Wright JT, Jr, Dunn JK, Cutler JA, et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA. 2005;293:1595–608. doi: 10.1001/jama.293.13.1595. [DOI] [PubMed] [Google Scholar]
- 24.Flack JM, Mensah GA, Ferrario CM. Using angiotensin converting enzyme inhibitors in African-American hypertensives: A new approach to treating hypertension and preventing target-organ damage. Curr Med Res Opin. 2000;16:66–79. [PubMed] [Google Scholar]
- 25.Rice GI, Jones AL, Grant PJ, et al. Circulating activities of angiotensin-converting enzyme, its homolog, angiotensin-converting enzyme 2, and neprilysin in a family study. Hypertension. 2006;48:914–920. doi: 10.1161/01.HYP.0000244543.91937.79. [DOI] [PubMed] [Google Scholar]
- 26.Barnes JM, Barnes NM, Costall B, et al. Angiotensin II inhibits the release of [3H]acetylcholine from rat entorhinal cortex in vitro. Brain Res. 1989;491:136–143. doi: 10.1016/0006-8993(89)90095-4. [DOI] [PubMed] [Google Scholar]
- 27.Marketou ME, Zacharis EA, Koukouraki S, et al. Effect of angiotensin-converting enzyme inhibitors on systemic inflammation and myocardial sympathetic innervation in normotensive patients with type 2 diabetes mellitus. J Hum Hypertens. 2008;22:191–196. doi: 10.1038/sj.jhh.1002310. [DOI] [PubMed] [Google Scholar]
- 28.Bonini JS, Bevilaqua LR, Zinn CG, et al. Angiotensin II disrupts inhibitory avoidance memory retrieval. Horm Behav. 2006;50:308–313. doi: 10.1016/j.yhbeh.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Hishimoto A, Shirakawa O, Nishiguchi N, et al. Association between a functional polymorphism in the renin-angiotensin system and completed suicide. J Neural Transm. 2006;113:1915–1920. doi: 10.1007/s00702-006-0483-9. [DOI] [PubMed] [Google Scholar]
- 30.Taylor A, Ezquerra M, Bagri G, et al. Alzheimer disease is not associated with polymorphisms in the angiotensinogen and renin genes. Am J Med Genet. 2001;105:761–764. doi: 10.1002/ajmg.10044. [DOI] [PubMed] [Google Scholar]
- 31.Lenkei Z, Palkovits M, Corvol P, et al. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: A functional neuroanatomical review. Front Neuroendocrinol. 1997;18:383–439. doi: 10.1006/frne.1997.0155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.