SUMMARY
Lysophophatidylcholine (LPC) and lysophosphatidic acid (LPA) are potent lysolipid mediators increasingly linked with atherosclerosis and inflammation. A current model proposing that plasma LPA is produced when LPC is hydrolyzed by the enzyme autotaxin has not been rigorously investigated in human subjects. We conducted a clinical trial of eicosapentaenoic acid/docosahexaenoic acid (EPA/DHA) and aspirin ingestion in normal volunteers. Fasting blood samples were drawn at baseline and after 4-week supplementation with EPA/DHA (3.4 g/d) with and without aspirin (650 mg). Plasma LPC and LPA species and autotaxin activity were measured. EPA-LPC and DHA-LPC concentrations increased significantly with EPA/DHA supplementation whereas EPA- and DHA-LPA did not. Autotaxin activity was unaffected by any treatment, and aspirin had no effect on any endpoint. Taken together, our data demonstrate that plasma LPC, but not LPA, species can be dynamically regulated by dietary supplementation, and argue against a simple model of LPA generation via LPC hydrolysis.
INTRODUCTION
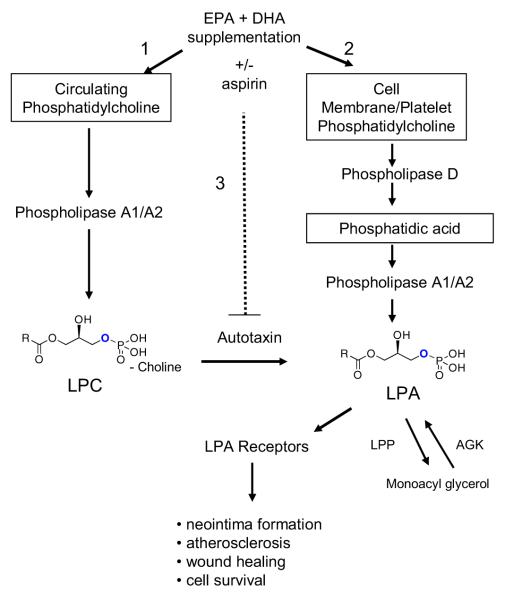
Lysophospholipids are potent lipid mediators with a diverse range of effects in a variety of tissues, and affect the growth, survival, migration and activation of many cell types.[1] Lysophosphatidic acid (LPA) and lysophosphatidylcholine (LPC) are increasingly linked with atherosclerosis by virtue of their effects on endothelial cells, monocytes and smooth muscle cells.[2-5] At the same time, LPC has demonstrated anti-inflammatory effects in animal models of sepsis.[6, 7] LPA consists of a glycerol backbone with a single acyl group at positions sn1 or sn2 and a phosphate group at position 3. It can be generated by various metabolic pathways including synthesis from LPC by hydrolysis of the choline moiety (Figure 1). Intracellular LPA is primarily generated from two sources: (i) phosphatidic acid (produced during membrane turnover or in response to extracellular signals) can be deacylated to LPA by the actions of phospholipases A1 or A2, and (ii) phosphorylation of monoacylglycerol by acyl glycerol kinase.[8, 9] Studies in vitro and in mouse models suggest that extracellular LPA is primarily generated by hydrolysis of LPC by autotaxin, an endothelial expressed enzyme, but this has not been well studied in humans.[10, 11] Plasma LPC is thought to be derived from phosphatidylcholine in lipoproteins and membrane microvesicles by acyltransferases and phospholipases [9] The discovery that autotaxin is the same enzyme as lysophospholipase D[10] was an important conceptual advance and brought together the fields of lipid biochemistry and cancer biology since autotaxin was originally identified as an autocrine factor that promoted the motility of cancer cells.[12] Antagonists of this pathway (e.g. autotaxin inhibitors or lysophospholipid receptor blockers), are under active development, and may see there way into the clinic soon.[13]
FIGURE 1. Schematic Representation of Hypothesized LPC and LPA Production Pathways.
Composite phospholipid metabolic pathways derived from in vitro and in vivo investigations. [1] EPA/DHA supplementation may affect: 1) plasma content of LPC and the pathway(s) leading to its synthesis; 2) plasma content of LPA and the pathway(s) leading to its synthesis. The dashed line to autotaxin indicates that the potential for aspirin to inhibit this pathway. LPP (lipid phosphate phosphatase) and acylglycerol kinase (AGK) regulate the balance of LPA and monoacylglycerol.
This working model of LPA and LPC metabolism, which is summarized in Figure 1, was derived almost entirely from in vitro studies and mouse models, and very little is known about how lysophospholipids are generated or catabolized in humans. Furthermore, virtually nothing is known about whether or how plasma lysophospholipid levels or composition are affected by dietary interventions or pharmacologic therapies. It has been suggested that LPC species are converted into their respective LPA species during platelet activation in rats.[14] If the distribution of circulating LPC and LPA acyl species was the same at baseline, and if both changed in the same manner after a fatty acid dietary intervention, then this would support the hypothesis that LPA was directly derived from LPC.
The acyl chains in LPC and LPA molecules vary from saturated to highly unsaturated, and from 16 to 22 carbons. Emerging evidence indicates that different LPC and LPA acyl species can have different effects on target cells, possibly reflecting subtle differences in acyl-specificity of lysophospholipid receptors.[15] For example, unsaturated LPA species induce de-differentiation and remodeling in vascular smooth muscle cells, but saturated species do not.[15, 16] If these in vitro findings apply in vivo, then determining the extent to which LPC and/or LPA species are regulated by changes in dietary fatty acid consumption may have important clinical implications.
Omega-3 fatty acids derived from fish oils (eicosapentaenoic acid {EPA} and docosahexaenoic acid {DHA}) both have anti-inflammatory and tissue protective effects that reduce risk for cardiovascular diease.[17, 18] It is currently not known whether dietary fatty acids are incorporated into plasma LPC or LPA species. In addition to EPA and DHA, aspirin has well-established cardioprotective effects due its ability to irreversibly acetylate cyclooxygenase-1 (COX-1) and inhibit platelet aggregation. A recent study found that ingestion of 100 mg/d of aspirin for 1 month reduced plasma LPA concentrations in subjects with cerebrovascular disease.[19] As aspirin’s effects on fatty acid metabolism have been ascribed to modulating cyclooxygenase (COX),[20] an effect on lysosphospholipid metabolism, which is not known to involve cyclooxygenase (COX), would be novel (Figure 1). Aspirin (through acetylation) also affects the function of other key molecules in human health including hemoglobin[21] and albumin.[22] Thus, one possibility is that aspirin may alter the activity of autotaxin directly through acetylation or indirectly: these possibilities were not examined by Li et al.[19]. Despite an extensive pre-clinical literature investigating the effects of lysophospholipids on an array of pathophysiologic processes in vitro and in vivo (which have focused primarily on cardiovascular and neoplastic diseases), few data exist regarding the metabolism of these mediators in humans. The original purpose of the study we report here was to determine the separate and combined effects of a prescription omega-3 fatty acid product and aspirin on several aspects of platelet function.[23] We have taken advantage of the availability of plasma samples from that study to examine the effects of these agents on the acyl species of both LPC and LPA in healthy adults without chronic disease. We hypothesized that the ingestion of EPA and DHA would increase concentrations of EPA and DHA species of LPC and LPA and that aspirin might reduce LPA concentrations by inhibiting autotaxin activity.
PATIENTS AND METHODS
Protocol
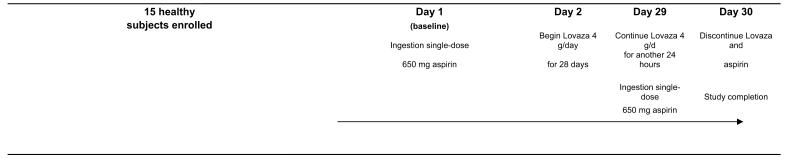
This was an open-label, four-week, sequential therapy trial in which each subject served as his/her own control. The study (see Figure 2) involved four visits: Day 1 (baseline); Day 2 (1 day after an oral dose of 650 mg of regular aspirin tablet); Day 29 (after 28 days of treatment with 4 capsules per day of prescription omega-3 fatty acids [Lovaza®, GlaxoSmithKline, Philadelphia, PA, USA]); and Day 30 (after 1 day of combined treatment with Lovaza® and 650 mg aspirin). Each 1-gram capsule of Lovaza® contains at least 900 mg of the ethyl esters of omega-3 fatty acids sourced from fish oils. These are predominantly a combination of ethyl esters of eicosapentaenoic acid (EPA - approximately 465 mg) and docosahexaenoic acid (DHA - approximately 375 mg). One day of aspirin treatment was selected because effects of aspirin on platelet aggregation are as complete 1 day after ingestion as they are 7 days after ingestion.(21) The dose of aspirin was chosen to provide robust anti-platelet effect after 24 hours. Peripheral blood was drawn into citrated tubes at each visit, and separated first into platelet rich plasma, and then in a second centrifugation, plasma was collected and frozen at −80°C.
FIGURE 2. Study Timeline.
At all study visits, subjects underwent phlebotomy for plasma LPC, LPA, autotaxin & RBC fatty acids. Phlebotomies occurred before ingestion of the study agent at each study visit
Subjects
Fifteen healthy volunteers were recruited for this study to allow for an assessment of fatty acid and aspirin effects without undue influence by factors that would alter lysophospholipid metabolism. Non-smoking male and female subjects between the ages of 21 and 60 were recruited for the study, and were taking no medications, vitamin pills, nutritional supplements or herbal preparations. Subjects could not have a history of allergic reactions to aspirin, fish or fish oils, or to non-steroidal anti-inflammatory drugs. Birth control pills were not allowed. Other exclusions included drinking more than three alcoholic beverages a day, or having any of the following conditions: an ulcer or bleeding in the stomach, liver or kidney disease, bleeding or blood clotting disorder (e.g. hemophilia), congestive heart failure, fluid retention, heart disease, high blood pressure, gout, asthma, arthritis, or nasal polyps. The study was approved by the University of South Dakota Institutional Review Board, and written informed consent was obtained from each subject. Subjects were told to notify the study coordinator by telephone if they noticed any unusual symptoms during the four-week study.
Laboratory Methods
As previously reported,[23] red blood cells (RBCs) were recovered from the RBC pellet after centrifugation at 3,000 × g for 20 min at 4°C. They were directly methylated by heating for 10 min with 14% boron trifluoride-methanol.[24] Equal portions of hexane and water were added to extract the methyl esters. The fatty acid methyl esters thus generated were analyzed by capillary gas chromatography using GC2010 (Shimadzu Corporation, Columbia, MD, USA) equipped with a SP2560, 100-m column (Supelco, Bellefonte, PA, USA). FA methyl esters were identified by comparison with a weighed standard mixture consisting of 22 fatty acids characteristic of RBCs (GLC-727, Nuchek Prep, Elysian, MN, USA). RBC EPA and DHA content are presented as a percent of total fatty acid methyl esters identified. Two RBC control pools were included with each batch to monitor analytical performance. Acceptable runs were those in which both controls fell within 2.5 standard deviations (SD). The coefficient of variation for EPA+DHA as a percent of total RBC fatty acids was 5–6%.
LPC and LPA standards were obtained from Avanti Polar Lipids Inc (Alabaster, AL). LPA and LPC were extracted using a modified Bligh and Dyer method and using direct infusion electrospray mass spectrometry methods as reported elsewhere for LPA [25] and LPC [26], with minor modifications. Briefly, LPA-17:0 and LPC-17:0 as internal standards were added (spiked) quantitatively to 400 l of plasma. Methanol, chloroform, and 0.1 N HCL 2:1:0.45 v/v/v were added to form one phase solvent system. Samples were vortexed for 30 minutes then separated into two phases by the addition of 1 ml of chloroform and 1.3 ml of 0.1 N HCl (pH ~3.0). The chloroform bottom layer was collected, dried under N2 and reconstituted with methanol / isopropanol / chloroform 4:2:1 v/v/v. Samples were analyzed on an ABI (Foster City, Ca; now Life Technologies) QTrap 2000 MS/MS using an Advion Inc. (Ithaca, NY) Nanomate nanospray (ESI) source by direct infusion using a “D” chip. Nanomate settings were constant throughout sample analyses with the gas pressure 0.3 PSI and voltage set to 1.4 kV. LPA were analyzed in negative ionization mode using MRM for the transition (M-H)− → m/z 79.0; LPC were analyzed via positive ions using (M+H)+ →m/z 184.0, where M is molecular weight of the parent species. Signals (cps) were averaged over 1 minute. Declustering potential was set to −60 for LPA and +60 for LPC. Collision energy was set at −35 V for LPA and +35 V for LPC. A two step calibration was employed. The relative signal corresponding to differences in fragmentation of the relevant MRM transitions was determined with an external standard mixture, consisting of eight equimolar components (17:0, 18:0, 18:1 20:4 as LPA and as LPC). Integrated cps average over 1 minute for each LPA and LPC analyte were calibrated and normalized to 17:0 LPA and LPC. The response factor for LPA/LPC 18:0 was used to adjust signals for saturates, 18:1 for LPA/LPC with one to three double bonds, and 20:4 for LPA/LPC with four to six double bonds. Adjusted signals were then converted to concentration using the signal for the relevant internal standards, 17:0 LPA/LPC, to adjust for ion suppression and other matrix effects affecting overall signal intensity. Pure standard LPC were analyzed to assess possible dissociation to LPA as reported recently.[27] Negligible conversion (<0.1%) of LPC to LPA were observed under our conditions, and thus neither LPA nor LPC accuracy was compromised due to aberrant reactions in the ion source.
We used a quantitative assay for autotaxin activity using a fluorescently labeled LPC analog as substrate. Recombinant autotaxin was expressed in insect cells and used as a positive control to generate a standard curve (courtesy of Dr. Andrew Morris, Univ. of Kentucky). The LPC analog (termed FS-3, 10 uM, Echelon Biosciences)[28] uses a fluorescence “dequenching” motif, in which a fluorophore (fluorescein) that is silent because of intramolecular fluorescence resonance energy transfer to a nonfluorescing quencher (dabcyl), becomes fluorescent once enzymatic hydrolysis cleaves the substrate. The autotaxin assay was performed in triplicate in black 96-well microtiter plates using assay buffer, FS-3, and sample. The assay buffer was prepared as a 5X working solution of 140mM NaCl, 5mM KCL, 1mM CaCl2, 1mM MgCl2, 50mM TRIS with a final pH of 8. Fluorescence was analyzed after one and two hours at an incubation temperature of 37°C by a Biotex FLX-800 plate reading fluorimeter/shaker/incubator equipped with a 485/20 nm excitation filter and a 528/20nm emission filter.
Statistical Methods
The study used an open-label, four-week, sequential treatment design. Concentrations of LPC, LPA acid species, autotoxin activity were tested for normality using the Shapiro-Wilk statistic, and any that were not normally distributed based on this metric (p<.05) were log-transformed. To correlate variables within the lipid pool, red blood cell membrane fatty acids (which are typically represented as %, w/w) with LPC and LPA species (which are typically represented as a concentration), the LPC and LPA species were converted into %, w/w. In order to increase statistical power, correlations between parent fatty acids, autotaxin, and corresponding LPC and LPA species were conducted not only at each time point but also pooling all time points. In other analyses involving LPC and LPA species, these variables were represented as a concentration. Pearson correlation coefficients were used to determine the interrelationships of continuous variables, including lysophospholipid variables and autotaxin. The paired t-test or ANOVA were used to determine lysophospholipid differences between study time points. A p-value <0.05 was used to define statistical significance and was not adjusted for multiple comparisons for any analyses. This was done to prevent confusion when adjusting differently for a variety of variables, including those included in exploratory analyses. Analyses were performed using SAS software (version 9.2; SAS Institute Inc., Cary, NC, USA).
RESULTS
Fifteen volunteers were recruited and all completed the study. No subject reported any adverse clinical effects of treatment. The study participants were relatively young, most were female, had a body mass index in the overweight range, and consumed very little fish and little alcohol at the time of study enrollment (Table 1). At baseline in RBC total fatty acids, the saturated fatty acids palmitic (16:0) and stearic (18:0), and the long-chain polyunsaturated omega-6 fatty acid, arachidonic (20:4n6) acid, composed the majority of fatty acids while the fish-oil derived omega-3 fatty acids {EPA (20:5n3) and DHA (22:6n3)} comprised a very small fraction of the fatty acids, as is commonly reported in Western populations.(24) At baseline the LPC species in plasma (Table 1) found in the highest proportion (%w/w) were those containing 20:4n6, 18:1n9 (oleic acid), and 22:6n3, in decreasing order. The plasma LPA species found in the highest proportion were those containing 18:0, 16:0, and 22:6n3 in decreasing order. LPCs were found in micromolar concentrations and LPAs in nanomolar concentrations in plasma, as previously reported.[29]
TABLE 1.
Baseline Characteristics of Study Subjects
| Variable | Mean (n=15) | SD |
|---|---|---|
| Age | 33.3 | 11 |
| Male (%) | 40 | |
|
Servings of non-fried
fish per month |
1.2 | 1 |
| Alcoholic drinks/wk | 1.3 | 1.1 |
|
Body mass index
at enrollment |
27.6 | 3.4 |
|
| ||
| RBC Fatty Acids (% w/w of total) | ||
| 16:0 | 20.8 | 1.5 |
| 16:1n7 | 0.21 | 0.01 |
| 18:0 | 17.7 | 1.1 |
| 18:1n9 | 12.9 | 0.66 |
| 18:2n6 | 12.2 | 0.01 |
| 18:3n3 | 0.05 | 0.02 |
| 20:4n6 | 17.6 | 1.05 |
| 20:5n3 | 0.4 | 0.09 |
| 22:4n6 | 3.8 | 0.4 |
| 22:5n3 | 2.3 | 0.4 |
| 22:6n3 | 3.8 | 0.84 |
|
| ||
| LPC Species (% w/w of total) | ||
| 16:0 | 5.6 | 1.5 |
| 16:1n7 | 1.7 | 0.46 |
| 18:0 | 4.4 | 1.1 |
| 18:1n9 | 21.1 | 3.3 |
| 18:2n6 | 12.7 | 2.6 |
| 18:3n3 | 0.6 | 0.2 |
| 20:4n6 | 30.0 | 6.2 |
| 20:5n3 | 5.3 | 4.3 |
| 22:4n6 | 1.4 | 0.006 |
| 22:5n3 | 3.7 | 0.7 |
| 22:6n3 | 13.3 | 5.1 |
|
| ||
| LPA Species (% w/w of total) | ||
| 16:0 | 18.2 | 4.3 |
| 16:1n7 | 1.7 | 0.5 |
| 18:0 | 43.0 | 10.0 |
| 18:1n9 | 1.8 | 0.5 |
| 18:2n6 | 2.3 | .05 |
| 18:3n3 | 3.6 | 0.9 |
| 20:4n6 | 5.0 | 1.9 |
| 20:5n3 | 3.5 | 0.6 |
| 22:4n6 | 3.2 | 0.8 |
| 22:5n3 | 6.2 | 1.8 |
| 22:6n3 | 11.6 | 4.7 |
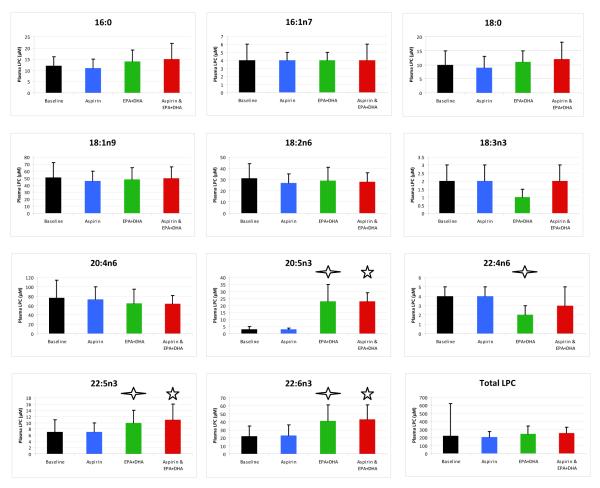
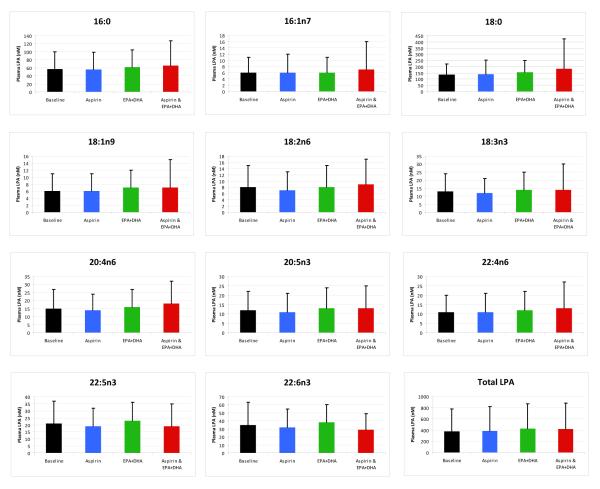
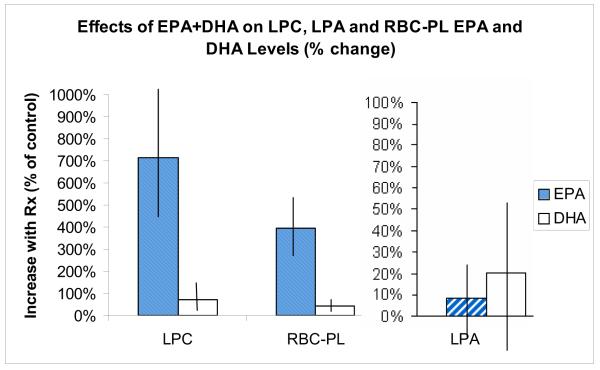
The changes in the 11 plasma LPC species over the study duration are noted in Figure 3. The ingestion of a single dose of aspirin before time points 2 and 4 had no effect on concentrations of any LPC species (p>.05). The ingestion of Lovaza 4 g/d for 28 days between days 2 and 29 significantly increased concentrations of the EPA {+20.6 μL (SD 11.7); p<.0001} and 22:6n3 {+18.5 μL (SD 12.6); p<.0001} LPC. Ingestion of the final dose of aspirin (before day 30) did not affect these LPC increases. The chronic ingestion of EPA/DHA significantly reduced the concentration of 22:4n6 LPC {−1.3 (SD 1.4); p.003}, at day 29. The changes in the 11 LPA species over the study duration are noted in Figure 4. In contrast to the effects on LPC species, no significant change in any of the LPA species, including the 20:5n3 and 22:6n3 species, was observed from baseline at any of the study time points (p>.05). The changes in ATX activity over the study time points are outlined in Table 2. No significant changes were noted after treatment with aspirin or EPA plus DHA alone or in combination. Figure 5 summarizes the effect of 28 day EPA/DHA supplementation on plasma 20:5n3 and 22:6n3 LPC and LPA species, and red blood cell total fatty acid EPA and DHA. The proportion of 20:5n3 (EPA) and 22:6n3 (DHA) in LPC significantly increased with 28-day supplementation with a concomitant increase in red blood cell membrane EPA and DHA. A similar increase in the EPA and DHA contained in LPA did not accompany these changes.
FIGURE 3. Lysophosphatidylcholine (LPC) Species Concentrations.
A diamond  indicates that a significant difference exists between LPC or LPA concentrations after 28 days of EPA+DHA (time point 3) compared to baseline. A star ☆ indicates that a significant difference (p<.05) exists between LPC or LPA concentrations at the final study visit compared to baseline. Note that the scale for each LPC and LPA varies due to differences in baseline concentrations. Error bars represent 95% confidence intervals. Differences in means were detected using the paired t-test.
indicates that a significant difference exists between LPC or LPA concentrations after 28 days of EPA+DHA (time point 3) compared to baseline. A star ☆ indicates that a significant difference (p<.05) exists between LPC or LPA concentrations at the final study visit compared to baseline. Note that the scale for each LPC and LPA varies due to differences in baseline concentrations. Error bars represent 95% confidence intervals. Differences in means were detected using the paired t-test.
FIGURE 4. Lysophosphatidic (LPA) Acid Species Concentrations.
A diamond  indicates that a significant difference exists between LPC or LPA concentrations after 28 days of EPA+DHA (time point 3) compared to baseline. A star ☆ indicates that a significant difference (p<.05) exists between LPC or LPA concentrations at the final study visit compared to baseline. Note that the scale for each LPC and LPA varies due to differences in baseline concentrations. Error bars represent 95% confidence intervals. Differences in means were detected using the paired t-test.
indicates that a significant difference exists between LPC or LPA concentrations after 28 days of EPA+DHA (time point 3) compared to baseline. A star ☆ indicates that a significant difference (p<.05) exists between LPC or LPA concentrations at the final study visit compared to baseline. Note that the scale for each LPC and LPA varies due to differences in baseline concentrations. Error bars represent 95% confidence intervals. Differences in means were detected using the paired t-test.
TABLE 2.
Autotaxin Activity
| Mean Flourescence Units/μL (SD); n=15 |
p-value Times 1-2 | p-value Times 1-3 | p-value Times 1-4 | |
|---|---|---|---|---|
| Day 1 (baseline) | 1.65 (0.18) | 0.38 | 0.11 | 0.77 |
|
| ||||
| p-value Times 2-3 | p-value Times 2-4 | |||
|
| ||||
|
Day 2 (24 hours after 650
mg aspirin) |
1.67 (0.17) | 0.08 | 0.39 | |
|
| ||||
| p-value Times 3-4 | ||||
|
| ||||
|
Day 29 (after 28 days of
Lovaza 4 g/d) |
1.59 (0.19) | 0.13 | ||
|
| ||||
|
| ||||
|
Day 30 (24 hours after
650 mg aspirin and final dose of Lovaza) |
1.64 (0.15) | |||
FIGURE 5.
The values for EPA & DHA species of LPC and LPA are represented as the % increase in the proportion of each of total LPC and LPA, respectively. The values for RBC EPA & DHA are represented as the % increase in the proportion of each of total RBC fatty acids. The vertical lines indicate 1 standard deviation.
An exploratory analysis of the relationship of each RBC fatty acid (e.g. 20:5n3) to its corresponding LPC and LPA species in plasma was investigated at each time point and over all time points (Table 3). In general, RBC fatty acids did not correlate significantly with corresponding LPC and LPA species at the separate time points. When the time points for each subject were pooled, however, most RBC fatty acids did correlate significantly with their respective LPC species. The RBC fatty acids did not, however, correlate with their respective LPA species. Each LPA species was positively correlated with the other LPA species (p<.05; most <.0001) and each LPC species was positively correlated with the other LPC species (p<.1; most <.001) {data not shown}.
TABLE 3.
The Association of RBC Total Fatty Acids with Corresponding LPC and LPA Species
| (Pearson correlation coefficient; p-value) | ||||
|---|---|---|---|---|
| Day 1 (Baseline) | LPC Time X n=15 |
LPC all time points n=15×4 |
LPA Time X n=15 |
LPA all time points n=15×4 |
| 16:0 | .21; 0.47 | .33; .011 | −.37; .24 | .13; .33 |
| 16:1n7 | .43; .13 | .31; .017 | .30; .30 | .06; .63 |
| 18:0 | .30: .30 | .30; .02 | −.12; .69 | −.05; .71 |
| 18:1n9 | −.04; .9 | .37; .004 | −.06; .85 | −.31; .02 |
| 18:2n6 | .10; .74 | .27; .04 | −.26; .36 | −.24; .07 |
| 18:3n3 | .24; .40 | .20; .13 | .26; .38 | .14; .31 |
| 20:4n6 | .02; .97 | .53; <.0001 | .04; .89 | .09; .52 |
| 22:4n6 | .25; .39 | .35; .007 | .15; .61 | −.07; .61 |
| 20:5n3 | .71; .005 | .95; <.0001 | .67; .009 | .19; .15 |
| 22:5n3 | .24; .40 | .37; .004 | −.04: .88 | .006; .97 |
| 22:6n3 | .27; .34 | .55; <.0001 | −.07; .81 | −.16; .22 |
|
Day 2 (24 hours after 650 mg aspirin)
| ||||
| 16:0 | .18; .52 | .33; .23 | ||
| 16:1n7 | .25; .37 | −.34; .21 | ||
| 18:0 | .49; .065 | .054; .85 | ||
| 18:1n9 | .49; .063 | −.27; .32 | ||
| 18:2n6 | .17; .54 | −.22; .42 | ||
| 18:3n3 | .26; .35 | .30; .27 | ||
| 20:4n6 | .04; .88 | .06 ;.83 | ||
| 22:4n6 | .05; .87 | .17; .56 | ||
| 20:5n3 | .70; .0036 | .14; .63 | ||
| 22:5n3 | .28; .31 | .004; .99 | ||
| 22:6n3 | .39; .15 | .06; .84 | ||
|
Day 29 (after 28 days of Lovaza 4 g/d)
| ||||
| 16:0 | .29; .29 | .20; .47 | ||
| 16:1n7 | .30; .28 | .12; .67 | ||
| 18:0 | .44; .099 | .17; .54 | ||
| 18:1n9 | .23; .41 | −.38; .22 | ||
| 18:2n6 | .08; .78 | −.38; .16 | ||
| 18:3n3 | .28; .29 | .09; .74 | ||
| 20:4n6 | .35; .21 | .20; .48 | ||
| 22:4n6 | −.25; .36 | −.16; .57 | ||
| 20:5n3 | .34; .21 | .11; .69 | ||
| 22:5n3 | −.06; .83 | .07; .81 | ||
| 22:6n3 | .23; .41 | −.28; .32 | ||
|
Day 30 (24 hours after 650 mg aspirin and final dose of Lovaza)
| ||||
| 16:0 | .55; .04 | .15; .60 | ||
| 16:1n7 | .37; .19 | .40; .16 | ||
| 18:0 | .08; .78 | −.32; .27 | ||
| 18:1n9 | .43; .12 | −.59; .026 | ||
| 18:2n6 | .40; .16 | −.003; .99 | ||
| 18:3n3 | .05; .86 | .14; .65 | ||
| 20:4n6 | .66; .01 | .04; .89 | ||
| 22:4n6 | .22; .44 | −.52; .054 | ||
| 20:5n3 | .66; .01 | .43; .12 | ||
| 22:5n3 | .14; .64 | .016; .96 | ||
| 22:6n3 | .002; 1.0 | −.48; .085 | ||
RBC total fatty acids, LPC, and LPA species were analyzed as percent (w/w) of total All variables associated with a p-value <.05 are in bold
Since LPC is thought to be the dominant precursor to LPA, the relationship of each plasma LPC species to its associated LPA species was also examined at each time point. At baseline, several LPC and LPA species were positively correlated including 16:0 (correlation coefficient 0.53; p=.04), 18:2n6 (coefficient 0.69; p=.005), 18:3n3 (coefficient 0.70; p=.004), 20:5n3 (coefficient 0.70; p=.004) species. Surprisingly, however, these positive associations were not observed at other time points including after 28 days of supplementation with EPA plus DHA (day 29). In fact, negative associations were observed between some LPC and LPA species at day 2 (e.g. 18:3n3, correlation coefficient −0.53: p=.04) and time point 4 (e.g. 22:4n6, correlation coefficient −0.54: p=.048). When LPC and LPA species concentrations were pooled across all time points, corresponding species of LPC and LPA did not significantly correlate (p>.05, data not shown). Autotaxin activity at each time point was not associated with LPC or LPA concentrations except for a few exceptions: 22:6n3-LPC was positively associated with autotaxin activity at baseline (correlation 0.55; p=.04) and 3 (correlation .65; p=.01), respectively, and 18:2n6 LPC with autotaxin activity at day 30 (correlation .54; p=.04). Autotaxin activity and LPA concentrations were not correlated (p>.05) at each time point and when data were pooled across all time points.
DISCUSSION AND CONCLUSIONS
Here we report several novel findings that will be considered in turn. First, our results demonstrate that, in association with EPA + DHA supplementation, concentrations of the EPA and DHA species of LPC increased substantively while the 22:4n-6 LPC species decreased. These data are the first demonstration, to our knowledge, that LPC molecular species are directly influenced by diet. Second, although LPC is currently thought to be the major precursor for plasma LPA, concentrations of each LPC species did not consistently correlate with their respective LPA species at all time points. Third, EPA + DHA supplementation did not alter concentrations of any LPA species, including those containing EPA and DHA. Fourth, we found no consistent relationship between plasma autotaxin activity and LPC or LPA species using a highly sensitive fluorimetric assay. Although a few LPC species positively correlated with autotaxin at different time points, these relationships are not robust due to the issue of multiple statistical testing and should be interpreted with caution. These data challenge the widely held assumption that plasma LPA species derive from LPC as a precursor, and suggest that regulation of lysophospholipid metabolism in human subjects is more complex than suggested from in vitro studies and animal models.
The ingestion of the fish oil-derived long-chain polyunsaturated omega-3 fatty acids EPA and DHA is associated with substantial beneficial health effects[17, 30, 31] as are other unsaturated fats.[23] The benefits of EPA and DHA intake include the prevention of sudden cardiac death, a leading cause of death in Western populations.[32] In contrast, 3 clinical trials have generated conflicting findings regarding the effects of high-dose EPA+DHA supplementation on ICD discharges in individuals with cardiomyopathy and an implantable cardioverter defibrillator.[33-35] As no clear and convincing explanation for these divergent facts exists, a possible explanation lies in the metabolism of fatty acids. In fact, unsaturated LPA species (16:1, 18:1, and 18:2) have been implicated in the atherosclerotic process in an in vitro model by inducing vascular smooth muscle cell dedifferentiation, migration, and proliferation whereas saturated species (12:0, 14:0, 16:0, and 18:0) do not.[15] In an in vivo model, the unsaturated 18:1 LPA has been shown to induce neointimal vascular remodeling but the saturated 18:0 species has not.[16] Data from our study suggest that EPA + DHA supplementation does increase EPA- and DHA-LPC concentrations, but this does not translate directly into differences in LPA composition. The ability to increase DHA LPC with fish oil ingestion may be important given that this source of DHA appears to be its preferred carrier to the central nervous system[36] and thus an additional relevant biomarker for adequate intake of EPA and DHA.[37] Whether alterations in specific LPC species underlie any of the cardioprotective effects of omega-3 fatty acid supplementation will require further study. Our findings also suggest that the production of EPA and DHA LPA species is a highly regulated process, and that steady-state LPA levels are not influenced by the ingestion and concentration of these dietary fatty acids. Recent studies suggest that plasma LPA has a very short half-life in vivo[38]. Since we did not conduct an extensive kinetic analysis of LPC or LPA species after ingestion of the dietary supplement, we can not absolutely exclude the possibility that a very transient spike in plasma EPA or DHA LPA species occurs soon after ingestion (e.g. within minutes). However, we would question the biological relevance of such transient LPA “remodeling”, in contrast to the alterations in LPC species, which appear to be sustained. Interestingly, the proportions of 22:6n3 (DHA) LPC and LPA plasma species is higher than the proportion of DHA in red blood cell membranes (Table 1). This suggests that DHA may concentrate in lysophospholipids, providing a rich source for a variety of tissues.
In serum or plasma, LPC is formed from phospholipids via phospholipases which include circulating phospholipase A2. Oxidized LDL (which is highly atherosclerotic) is one source of the phospholipids from which LPC is derived. Current thinking is that hydrolysis of LPC by autotaxin is the major source of extracellular LPA.[1] However, we did not observe positive associations between plasma autotaxin activity and LPA concentrations at any time point. Since LPA can also be produced from phosphatidic acid via phospholipase D and other pathways, we favor the hypothesis that plasma LPA derives at least in part from an LPC- and autotaxin-independent pathway. Our results contrast with previous studies showing positive correlations between plasma LPA concentrations and serum autotaxin activity in healthy subjects.[29] and in patients with hematologic malignancies.[39] Reasons for these apparent discrepancies between studies are not immediately apparent, but could relate to differences in subject groups studied or methods of analysis. Autotaxin antagonists are under active development and entering clinical trials for patients with cancer and other diseases.[40] Future studies with these compounds should help clarify the role of autotaxin in regulation of plasma LPA levels in human subjects.
We also studied the effects of acute ingestion of a 650 mg dose of aspirin on plasma lysophospholipids and autotaxin activity. We found that aspirin did not significantly affect concentrations of LPC, LPA, or autotaxin activity, whether taken before EPA + DHA supplementation (time point 2) or after four weeks of daily EPA + DHA supplementation (time point 4). We did not analyze ATX expression (e.g. by Western blot analysis of plasma samples), thus although it remains possible that aspirin may affect ATX expression per se we conclude that this did not translate into effects on ATX activity, arguably a more relevant biological readout. In a study that used the same EPA + DHA and aspirin intervention protocol (n=10), aspirin and EPA + DHA ingestion led to reduced platelet function (in response to ADP and collagen agonists) that was more potent than for each agent alone.[23] The absence of effect of aspirin and EPA + DHA on LPA concentrations in the current study suggests that these agents do not, alone or in combination, influence LPA production. As both aspirin and LPA are known to affect platelet function,[23, 41-43] and platelet activation leads to LPA production, it is likely that the lack of effect of these agents on LPA was independent of their effects on platelet function. The ingestion of 100 mg of aspirin for 1 month has been associated with a reduction in plasma LPA in individuals with cerebrovascular disease,[19] but we did not observe acute effects of a higher dose of aspirin in our study. It is important to note that aspirin doses of 75-150 mg/d have been associated with a lower risk of vascular events than higher or lower doses.[41] In addition, a dose of 81mg/d of aspirin has been shown to increase the production of 15 epi-lipoxin A4, a metabolite of arachidonic acid with potent anti-inflammatory and tissue-protective effects, whereas doses of 325 and 650 mg do not.[44] Thus we can not exclude the possibility that lower doses of aspirin or longer durations of therapy would affect LPA metabolism in vivo. Future studies will be needed to address this possibility.
Our study has some limitations. First, the sample size was small (n=15), but the statistical power was substantially increased for sequential variable measurements by the fact that each subject acted as their own control. However, we acknowledge the possibility that reduced statistical power may have led to the lack of association between LPC, LPA species, and autotaxin activity, but note that we did not observe associations even when pooling data across all time points (n=60 data points). Second, although red blood cell content of fatty acids correlates well with dietary intake[45] and cardiac tissue content,[46] this lipid pool may not be as relevant to lysophospholipid metabolism as is the content of plasma phosphatidylcholines (PC), a known source for LPC.[8] Future studies that measure plasma PC fatty acids and lysophospholipid concentration and autotaxin activity in relevant end-organs (e.g. heart muscle or endovascular tissues) should be revealing. Since the participants were healthy adults, further research will be required to determine the effects of EPA +DHA and aspirin on lysophospholipid metabolism in diseased individuals. Current evidence supports the fact that the effects of low-dose aspirin (81-100 mg/d or less) on lipid metabolism and inflammation differ from that of higher doses.[47] In fact, we are currently analyzing changes in the LPA and LPC species described in this manuscript after a single dose of 81 mg aspirin in a separate study. We cannot completely exclude the possibility that concentrations of lysophospholipids vary significantly depending on the duration between EPA+DHA ingestion and phlebotomy. As dephosphorylation of LPA by lipid phosphate phosphohydrolase type 1 has been shown to be one potential mechanism for the rapid metabolism of LPA,[38] it is possible that a transient change in LPA due to EPA+DHA or aspirin ingestion was missed in this study. However, the main point of this research is that mean concentrations of EPA- and DHA-LPC species increase with EPA+DHA supplementation while their corresponding LPA species do not, and these conclusions are unlikely to be negated by a more consistent time-frame between dosing and phlebotomy. As the physiologic relevance of short term changes are not defined, the importance of this variation is unknown. The kinetic profile, over hours or days, of LPA concentrations in human blood after EPA+DHA ingestion would be an important focus of future research. Another limitation is the possibility that hydrolysis of the flourogenic LPC substrate used in the autotaxin activity assay in this study may be accomplished by molecules other than autotaxin and that our assay lacks specificity. However, in this study no effects on autotaxin were seen and thus this issue should not have negated the hypotheses tested.
In conclusion, our study is the first to describe the effects of EPA + DHA supplementation and acute aspirin ingestion, alone and combined, on plasma concentrations of lysophospholipid species in individuals without chronic disease. By comprehensively measuring RBC total fatty acids and plasma LPC and LPA species and autotaxin activity at each study point, we documented a complex relationship between circulating lysophospholipid species and fatty acids that would not have been readily predictable from paradigms established using in vitro or animal models. Our findings indicate that plasma LPA species in healthy subjects are not easily influenced by dietary intervention with EPA/DHA, in contrast to LPC concentrations and species. These findings may help in the interpretation of future clinical trials using autotaxin and other pathway antagonists. It should be noted that the health effects of altering blood concentrations of LPA and LPC species are currently unknown. We also conclude that plasma content of EPA and DHA LPC species may be reliable biomarkers for adequate omega-3 fatty acid intake, but LPA species may not.
ACKNOWLEDGEMENTS
None
Sources of Support This publication was made possible by Grant Number KL2 RR 024136 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. The Novel Clinical and Translational Methodologies funding mechanism within the University of Rochester’s NIH Clinical and Translational Science Institute also provided funding for this research. Additional resources were provided by R01 HL071933 (S.G.), Reliant Pharmaceuticals and by NIH Grant (P20 RR016479) from the INBRE Program of the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Aoki J. Mechanisms of lysophosphatidic acid production. Semin Cell Dev Biol. 2004;15:477–89. doi: 10.1016/j.semcdb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Wu WT, Chen CN, Lin CI, Chen JH, Lee H. Lysophospholipids enhance matrix metalloproteinase-2 expression in human endothelial cells. Endocrinology. 2005;146:3387–400. doi: 10.1210/en.2004-1654. [DOI] [PubMed] [Google Scholar]
- 3.Lucas A, Grynberg A, Lacour B, Goirand F. Dietary n-3 polyunsaturated fatty acids and endothelium dysfunction induced by lysophosphatidylcholine in Syrian hamster aorta. Metabolism. 2008;57:233–40. doi: 10.1016/j.metabol.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Panchatcharam M, Miriyala S, Yang F, Rojas M, End C, Vallant C, et al. Lysophosphatidic acid receptors 1 and 2 play roles in regulation of vascular injury responses but not blood pressure. Circ Res. 2008;103:662–70. doi: 10.1161/CIRCRESAHA.108.180778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitz G, Ruebsaamen K. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis. 2009 doi: 10.1016/j.atherosclerosis.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Chen G, Li J, Qiang X, Czura CJ, Ochani M, Ochani K, et al. Suppression of HMGB1 release by stearoyl lysophosphatidylcholine:an additional mechanism for its therapeutic effects in experimental sepsis. J Lipid Res. 2005;46:623–7. doi: 10.1194/jlr.C400018-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, et al. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med. 2004;10:161–7. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]
- 8.Aoki J, Inoue A, Okudaira S. Two pathways for lysophosphatidic acid production. Biochim Biophys Acta. 2008;1781:513–8. doi: 10.1016/j.bbalip.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Natarajan V. Lysophosphatidic acid signaling in airway epithelium: role in airway inflammation and remodeling. Cell Signal. 2009;21:367–77. doi: 10.1016/j.cellsig.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227–33. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26:5015–22. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stracke ML, Krutzsch HC, Unsworth EJ, Arestad A, Cioce V, Schiffmann E, et al. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem. 1992;267:2524–9. [PubMed] [Google Scholar]
- 13.Federico L, Pamuklar Z, Smyth SS, Morris AJ. Therapeutic potential of autotaxin/lysophospholipase inhibitors. Curr Drug Targets. 2008;9:698–708. doi: 10.2174/138945008785132439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tokumura A, Harada K, Fukuzawa K, Tsukatani H. Involvement of lysophospholipase D in the production of lysophosphatidic acid in rat plasma. Biochim Biophys Acta. 1986;875:31–8. [PubMed] [Google Scholar]
- 15.Hayashi K, Takahashi M, Nishida W, Yoshida K, Ohkawa Y, Kitabatake A, et al. Phenotypic modulation of vascular smooth muscle cells induced by unsaturated lysophosphatidic acids. Circ Res. 2001;89:251–8. doi: 10.1161/hh1501.094265. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida K, Nishida W, Hayashi K, Ohkawa Y, Ogawa A, Aoki J, et al. Vascular remodeling induced by naturally occurring unsaturated lysophosphatidic acid in vivo. Circulation. 2003;108:1746–52. doi: 10.1161/01.CIR.0000089374.35455.F3. [DOI] [PubMed] [Google Scholar]
- 17.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 18.Kereiakes DJ. Adjunctive pharmacotherapy before percutaneous coronary intervention in non-ST-elevation acute coronary syndromes: the role of modulating inflammation. Circulation. 2003;108:III22–7. doi: 10.1161/01.CIR.0000086951.09881.51. [DOI] [PubMed] [Google Scholar]
- 19.Li ZG, Yu ZC, Wang DZ, Ju WP, Zhan X, Wu QZ, et al. Influence of acetylsalicylate on plasma lysophosphatidic acid level in patients with ischemic cerebral vascular diseases. Neurol Res. 2008;30:366–9. doi: 10.1179/174313208X300369. [DOI] [PubMed] [Google Scholar]
- 20.Catella-Lawson F, Reilly MP, Kapoor SC, Cucchiara AJ, DeMarco S, Tournier B, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345:1809–17. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 21.Xu AS, Ohba Y, Vida L, Labotka RJ, London RE. Aspirin acetylation of betaLys-82 of human hemoglobin. NMR study of acetylated hemoglobin Tsurumai. Biochem Pharmacol. 2000;60:917–22. doi: 10.1016/s0006-2952(00)00419-6. [DOI] [PubMed] [Google Scholar]
- 22.Rendell M, Nierenberg J, Brannan C, Valentine JL, Stephen PM, Dodds S, et al. Inhibition of glycation of albumin and hemoglobin by acetylation in vitro and in vivo. J Lab Clin Med. 1986;108:286–93. [PubMed] [Google Scholar]
- 23.Larson MK, Ashmore JH, Harris KA, Vogelaar JL, Pottala JV, Sprehe M, et al. Effects of omega-3 acid ethyl esters and aspirin, alone and in combination, on platelet function in healthy subjects. Thromb Haemost. 2008;100:634–41. [PubMed] [Google Scholar]
- 24.Lopez-Lopez A, Castellote-Bargalló A, López-Sabater M. Comparison of two direct methods for the determination of fatty acids in human milk. Chromatographia. 2006;54:743–7. [Google Scholar]
- 25.Yoon HR, Kim H, Cho SH. Quantitative analysis of acyl-lysophosphatidic acid in plasma using negative ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;788:85–92. doi: 10.1016/s1570-0232(02)01031-0. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Z, Xiao Y, Elson P, Tan H, Plummer SJ, Berk M, et al. Plasma lysophosphatidylcholine levels: potential biomarkers for colorectal cancer. J Clin Oncol. 2007;25:2696–701. doi: 10.1200/JCO.2006.08.5571. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Z, Xu Y. Measurement of endogenous lysophosphatidic acid by ESI-MS/MS in plasma samples requires pre-separation of lysophosphatidylcholine. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3739–42. doi: 10.1016/j.jchromb.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferguson CG, Bigman CS, Richardson RD, van Meeteren LA, Moolenaar WH, Prestwich GD. Fluorogenic phospholipid substrate to detect lysophospholipase D/autotaxin activity. Org Lett. 2006;8:2023–6. doi: 10.1021/ol060414i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosogaya S, Yatomi Y, Nakamura K, Ohkawa R, Okubo S, Yokota H, et al. Measurement of plasma lysophosphatidic acid concentration in healthy subjects: strong correlation with lysophospholipase D activity. Ann Clin Biochem. 2008;45:364–8. doi: 10.1258/acb.2008.007242. [DOI] [PubMed] [Google Scholar]
- 30.Cohen JT, Bellinger DC, Connor WE, Kris-Etherton PM, Lawrence RS, Savitz DA, et al. A quantitative risk-benefit analysis of changes in population fish consumption. American Journal of Preventive Medicine. 2005;29:325–34. doi: 10.1016/j.amepre.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 31.American Heart Association Nutrition Committee. Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 32.Lemaitre RN, King IB, Mozaffarian D, Sotoodehnia N, Rea TD, Kuller LH, et al. Plasma phospholipid trans fatty acids, fatal ischemic heart disease, and sudden cardiac death in older adults: the cardiovascular health study. Circulation. 2006;114:209–15. doi: 10.1161/CIRCULATIONAHA.106.620336. [DOI] [PubMed] [Google Scholar]
- 33.Raitt MH, Connor WE, Morris C, Kron J, Halperin B, Chugh SS, et al. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: A randomized controlled trial. JAMA. 2005;293:2884–91. doi: 10.1001/jama.293.23.2884. [DOI] [PubMed] [Google Scholar]
- 34.Leaf A, Albert CM, Josephson M, Steinhaus D, Kluger J, Kang JX, et al. Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation. 2005;112:2762–8. doi: 10.1161/CIRCULATIONAHA.105.549527. [DOI] [PubMed] [Google Scholar]
- 35.Brouwer IA, Zock PL, Camm AJ, Bocker D, Hauer RNW, Wever EFD, et al. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: The Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) Randomized Trial. JAMA. 2006;295:2613–9. doi: 10.1001/jama.295.22.2613. [DOI] [PubMed] [Google Scholar]
- 36.Lagarde M, Bernoud N, Brossard N, Lemaitre-Delaunay D, Thies F, Croset M, et al. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J Mol Neurosci. 2001;16:201–4. doi: 10.1385/JMN:16:2-3:201. discussion 215-21. [DOI] [PubMed] [Google Scholar]
- 37.Fekete K, Marosvolgyi T, Jakobik V, Decsi T. Methods of assessment of n-3 long-chain polyunsaturated fatty acid status in humans: a systematic review. Am J Clin Nutr. 2009 doi: 10.3945/ajcn.2009.27230I. [DOI] [PubMed] [Google Scholar]
- 38.Tomsig JL, Snyder AH, Berdyshev EV, Skobeleva A, Mataya C, Natarajan V, et al. Lipid phosphate phosphohydrolase type 1 (LPP1) degrades extracellular lysophosphatidic acid in vivo. Biochem J. 2009;419:611–8. doi: 10.1042/BJ20081888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masuda A, Nakamura K, Izutsu K, Igarashi K, Ohkawa R, Jona M, et al. Serum autotaxin measurement in haematological malignancies: a promising marker for follicular lymphoma. Br J Haematol. 2008;143:60–70. doi: 10.1111/j.1365-2141.2008.07325.x. [DOI] [PubMed] [Google Scholar]
- 40.Prestwich GD, Gajewiak J, Zhang H, Xu X, Yang G, Serban M. Phosphatase-resistant analogues of lysophosphatidic acid: agonists promote healing, antagonists and autotaxin inhibitors treat cancer. Biochim Biophys Acta. 2008;1781:588–94. doi: 10.1016/j.bbalip.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antithrombotic Trialists’ Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, et al. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–55. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris WS. Expert opinion: omega-3 fatty acids and bleeding-cause for concern? Am J Cardiol. 2007;99:44C–6C. doi: 10.1016/j.amjcard.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 44.Chiang N, Hurwitz S, Ridker PM, Serhan CN. Aspirin has a gender-dependent impact on antiinflammatory 15-epi-lipoxin A4 formation: a randomized human trial. Arterioscler Thromb Vasc Biol. 2006;26:e14–7. doi: 10.1161/01.ATV.0000196729.98651.bf. [DOI] [PubMed] [Google Scholar]
- 45.Harris WS, von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Preventive Medicine. 2004;39:212–20. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 46.Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, et al. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–9. doi: 10.1161/01.CIR.0000142292.10048.B2. [DOI] [PubMed] [Google Scholar]
- 47.Patrono C, Rodriguez L.A. Garcia, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353:2373–83. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]